Abstract
Background.
We used ultrasound molecular imaging to evaluate the therapeutic effects of anti-oxidant therapy with EUK-207, which has superoxide dismutase and catalase activities, on suppressing high risk atherosclerotic features.
Methods.
Mice with age-dependent atherosclerosis produced by deletion of the LDL-receptor and Apobec-1 were studied at 20 and 40 weeks of age. EUK-207 or vehicle was administered for the preceding 8 weeks. Therapy for 28 weeks was also studied for 40 week-old mice. Ultrasound molecular imaging of the thoracic aorta was performed with contrast agents targeted to endothelial P-selectin, von Willebrand factor (VWF) A1-domain, and platelet GPIbα; or control agent. Aortic plaque area and macrophage content were assessed by histology.
Results.
In 20 week-old DKO mice, EUK-207 compared to sham therapy produced only non-significant trends for reduction in molecular imaging signal for endothelial P-selectin, VWF A1-domain and platelet adhesion. At 40 weeks, EUK-207 given for 8 or 28 weeks significantly (p<0.05) reduced signal for all three endothelial-associated events essentially to background levels, with the exception of GPIbα signal after 8 weeks (p=0.06). On aortic histology, EUK-207 therapy for 8 weeks did not affect plaque area or macrophage content at either age. However, EUK-207 for 28 weeks almost completely suppressed plaque development (350±258 vs 4±6 ×103 μm2, p=0.014) and macrophage content (136±103 vs 3±2 × 103 μm2, p=0.002) compared to control mice at 40 weeks.
Conclusions.
Molecular imaging can be used to assess vascular responses to anti-oxidants and has demonstrated that certain anti-oxidants reduce vascular endothelial activation and platelet adhesion, but reductions in plaque size and macrophage content occurs only with long-duration therapy that is started early.
Keywords: Anti-oxidants, Atherosclerosis, Contrast ultrasound, Platelets, Reactive oxygen species
Non-invasive imaging is increasingly used in the process of drug development to assess efficacy and safety of drugs in animal models and clinical trials. Molecular imaging in particular has been valuable in pre-clinical research for confirming on-target efficacy of candidate drugs and for dose optimization, and in clinical trials for selecting patients who are most likely to benefit from therapy.1 In atherosclerotic disease, ultrasound molecular imaging has been used for the assessment of endothelial inflammatory activation, including expression of adhesion molecules which happens early in atherogenesis.2,3 One of the major contributors towards endothelial activation in atherosclerosis is oxidative stress which occurs directly through reactive oxygen species (ROS),4–8 and through oxidatively-modified LDL.8,9 ROS also have been shown to suppress cleavage of Von Willebrand factor (VWF) multimers on the endothelial surface,10 which can lead to arterial platelet adhesion and promotion of plaque progression.11
Plaque formation in atherosclerotic mouse models is inhibited by gene alterations that reduce ROS generation, and is increased by deletion of antioxidant enzymes including certain superoxide dismutase (SOD) isoforms6,7,12 In humans, carotid intimal thickening is lower in humans with genetic alteration that reduce ROS production.13 These observations have established vascular ROS as a therapeutic target in atherosclerosis, although anti-oxidant drugs that are safe and effective in humans have not been identified. When evaluating new anti-oxidants, molecular imaging of the endothelial responses are likely to provide important data on in vivo efficacy. In this study we used contrast-enhanced ultrasound (CEU) molecular imaging to test whether potent multi-functional antioxidant therapy could suppress high-risk plaque features, particularly when started early in atherosclerosis. The salen-Mn compound EUK-207 was studied in a murine model based on its multifunctional antioxidant actions as a combined SOD and catalase mimetic.14 In vivo molecular imaging of endothelial phenotype was used to Atkinson T, et al. 6assess whether EUK-207 reduces endothelial adhesion molecule expression, endothelial-associated VWF in its active form (exposure of its A1 binding domain for platelet GPIbα), and platelet-endothelial adhesion.
METHODS
Animals and Study Design
The study was approved by Animal Care and Use Committee at Oregon Health and Science University. Mice with susceptibility to age-related atherosclerosis through gene-targeted deletion or “double knockout” (DKO) of the LDL-receptor and apolipoprotein-B mRNA editing enzyme catalytic polypeptide 1 (Apobec-1) on a C57Bl/6 background were studied.15 Mice were fed a chow diet ad libitum. Evaluation of plaque by molecular imaging and histology was performed at either 20 weeks of age (equivalent to end of first quarter of average lifespan or 20 human years) when DKO mice have early intramural atherosclerosis (fatty streaks) of the proximal aorta; or at 40 weeks of age (just over half of average lifespan or close to 45 human years) at which time there are moderate to severe macrophage-rich intraluminal plaques.2 To evaluate the effects of short-term therapy at early or late-stages of disease, mice were treated with EUK-207 or vehicle for 8 weeks starting at either 12 weeks (EUK-207 n=12, control n=11) or 32 weeks (EUK-207 n=9, control n=10) of age for later study at 20 and 40 wks. To evaluate long-term therapy, EUK-207 was given for 28 weeks starting at 12 weeks (n=8) and studied at 40 weeks. Non-invasive imaging studies were performed pre-therapy (baseline) and post-therapy. On each study day, mice were anesthetized with inhaled isoflurane (1.0 to 1.5 %) and a jugular vein was cannulated for intravenous administration of contrast material.
EUK-207 Treatment
EUK-207 (9 mg/mL in 5% mannitol) (provided by Bernard Malfroy-Camine, MindSet Rx, Inc., Arlington, MA) was loaded into an osmotic minipump (Model 1004, ALZET Osmotic Pumps, Cupertino, CA) programmed for an infusion rate of 0.11 μl/hr over 28 days for a calculated dose rate of approximately 1 mg/kg/day. Sham therapy was performed with 5% mannitol alone. Mice were anesthetized with inhaled isoflurane and pumps were placed in the ventral hindlimb subcutaneous tissue via a small incision which was subsequently closed. Pumps were exchanged every 4 weeks using the same site unless significant scar tissue was encountered. There were no pump failures.
Targeted microbubble preparation
Full methods for preparation of microbubbles targeted to platelet GPIbα (MBPlt), “activated” VWF (MBVWF), or P-selectin (MBP) are provided in the on-line supplement. Briefly, biotinylated lipid-shelled decafluorobutane microbubbles were prepared and surface conjugation of targeting ligands was performed using: recombinant VWF A1 domain for MBPlt,16 a GPIbα peptide for MBVWF,3 a monoclonal antibody (RB40.34, BD Biosciences, San Jose, CA) for MBP. Control non-targeted microbubbles (MB) were prepared using a non-specific non-binding control monoclonal antibody (mAb) (R3–34, BD Biosciences).
In Vivo Molecular Imaging.
Contrast-enhanced ultrasound (CEU) molecular imaging of the ascending aorta and proximal aortic arch in long-axis was performed with a linear-array probe (15L8 probe; Sequoia, Siemens Medical Systems, Mountain View, CA) using multi-pulse phase-inversion and amplitude-modulation imaging at 7 MHz, a dynamic range of 55 dB, and a mechanical index of 0.97. Images were acquired 8 min after intravenous injection of targeted or control microbubbles (1×10 6) to allow decay of almost all of the signal from freely circulating microbubbles in the blood pool.17 Signal from retained microbubbles alone was determined as previously described by acquiring the first ultrasound frame and then digitally subtracting several averaged frames obtained after complete destruction of microbubbles in the imaging field to eliminate signal from the low concentration of freely-circulating microbubbles in the blood pool.18 Intensity was measured from a region-of-interest that was standardized by encompassing the entire ascending aorta from just beyond the sinuses to just beyond the origin of the brachiocephalic artery. Region selection was guided by high frequency fundamental 2-D imaging at 14 MHz performed by switching from 7 MHz contrast-specific imaging at the end of each video capture. Since low-grade arterial attachment of non-targeted MBs occurs from vascular inflammation in an age-dependent fashion in DKO mice,3 all data were expressed as a difference in signal from control agent (MB).
Assessment of LV Function and Aortic Compliance
Echocardiography (Vevo 2100, Visualsonics Inc., Toronto Canada) was performed using a high-frequency (30 MHz) phased-array probe. Short-axis images at the LV mid-papillary muscle level were acquired and LV systolic function was quantified by: (i) fractional shortening of the LV; and (ii) global circumferential strain values calculated by averaging strain speckle-tracking strain values for all visualized regions on a 6-segment model. M-mode imaging of the mid ascending aorta was used to assess aortic diameter change (D) over the cardiac cycle. Pulse wave transit time (PTT) was performed to measure aortic stiffness.19 This method relies on the increase in flow velocity (reduction in transit time) in rigid non-compliant arteries due to lack of distension and less conversion from kinetic to potential energy. PTT was measured by the time delay of the onset of systolic pressure rise on pulse-wave Doppler in the proximal ascending aorta and the femoral artery approximately 0.5 mm from the pudendoepigastric trunk. Measurements were averaged for 3 cardiac cycles and were not normalized to BP.
Histology
Full methods are provided in the on-line supplement. Briefly, perfusion-fixed short axes from the proximal ascending aorta to the distal aortic arch were obtained from EUK-207 or sham-treated mice at 20 or 40 weeks of age. Plaque area was determined by Masson’s trichrome. Fluorescent immunohistochemistry for Mac-2 was used for macrophage staining.
Statistical Analysis
Data were analyzed using Prism (version 5.0, GraphPad Software). Comparisons between pre- and post-therapy values were made by paired Student’s t-test. Group-wise differences were assessed by Mann-Whitney U test for data that were determined to be non-normally distributed by D’Agostino and Pearson omnibus test. For data with normal distribution, group-wise differences were assessed by one-way ANOVA with post-hoc Student’s t-test and Bonferroni’s correction when appropriate for multiple comparisons. Differences were considered significant at p<0.05.
RESULTS
Cardiovascular and Vascular Functional Imaging
Because oxidative stress associated with hyperlipidemia and inflammation can contribute to LV remodeling and changes in myocardial contractile function,20 echocardiographic evaluation of LV function was performed in control and EUK-207 mice at 20 weeks or 40 weeks of age. There were no major treatment-related differences in either mid-ventricular fractional shortening on 2-dimensional imaging or in global circumferential strain on speckle-tracking echocardiography (Figure 1). Treatment with EUK-207 produced a modest improvement of pulse-transit time in the DKO mice at 40 weeks of age that were treated with long-duration therapy of 28 weeks, but not with short duration therapy of 8 weeks (Figure 2).
Figure 1.

Echocardiographic data (mean±SEM) for peak circumferential left ventricular strain for DKO mice at (A) 20 weeks, and (B) 40 weeks of age; and fractional shortening at (C) 20 weeks, and (D) 40 weeks of age. Data at 40 weeks includes mice receiving EUK-207 for 8 or 28 weeks.
Figure 2.

Images illustrate (A) aortic diameter measurements by M-mode echocardiography of the ascending aorta, and (B) pulsed-wave Doppler from the ascending aorta (Ao) and femoral artery (FA) illustrating the temporal delay (gated to the ECG) for measuring pulse transit time. Pulse transit time (PTT) data is shown for DKO mice at (C) 20 weeks, and (D) 40 weeks of age where PTT is lower (velocity faster) in stiffer vessels.
Molecular Imaging of Vascular Phenotype
On molecular imaging of the proximal ascending aorta in DKO mice at 20 weeks of age, short-term (8-week) therapy with EUK-207 compared to sham-treated controls produced only non-significant trends for lower signal enhancement for microbubbles targeted against endothelial P-selectin, VWF A1 binding domain reflecting endothelial-associated activated VWF, and GPIbα reflecting platelet adhesion to the vascular wall (Figure 3). At 40 weeks of age, treatment with EUK-207 resulted in a significant reduction in signal for all three vascular markers of disease, irrespective of whether the duration of therapy was short (8 weeks) or long (28 weeks). Only signal for platelet GPIbα was of borderline significance.
Figure 3.

Thoracic aorta CEU Molecular imaging data (mean±SEM) for DKO mice at (A) 20 weeks of age, (B) 40 weeks of age after 8 weeks of therapy; and (C) 40 weeks of age after 28 weeks of therapy. Data are normalized by subtracting background signal intensity from control MBs which, for some conditions, stochastically resulted in “negative” values when there was no selective enhan cement. *p<0.01 for the targeted agent versus control MB. (D) Images illustrate 2-D ultrasound of the thoracic aorta (left panels) and background-subtracted color-coded (color scale at bottom) CEU images of platelet adhesion (MBP) (right panels) for 40 week-old DKO control-treated mice, or mice treated with EUK-207 for 8 or 28 weeks.
Plaque Histology
Consistent with previous studies with DKO mice, sham-treated mice there was an age-dependent increase in aortic plaque area between 20 and 40 weeks of age (10,386±4,614 vs 133,063±28,522 μm2, p<0.0001). When given for short duration (8 weeks), EUK-207 had minimal effect on plaque size for 20 and 40 week-old DKO mice (Figure 4 and 5). However, therapy for 28 weeks resulted in the near complete absence of plaque development in both the aortic root and distal ascending aorta. Interobserver variability in measurement of plaque area was excellent (ICC=0.98 [95% Confidence Interval: 0.94 to 0.99], r2=0.97). There was a significant age-dependent increase in plaque macrophage content (Mac-2) in sham-treated control mice, and nearly complete absence of intramural macrophages in 40 week mice treated with EUK-207 for 28 weeks.
Figure 4.

Histology data (mean±SEM) of plaque area from DKO mice at (A) 20 weeks of age and (B) 40 weeks of age; and of plaque macrophage area by Mac-2 staining at (C) 20 weeks of age and (D) 40 weeks of age.
Figure 5.
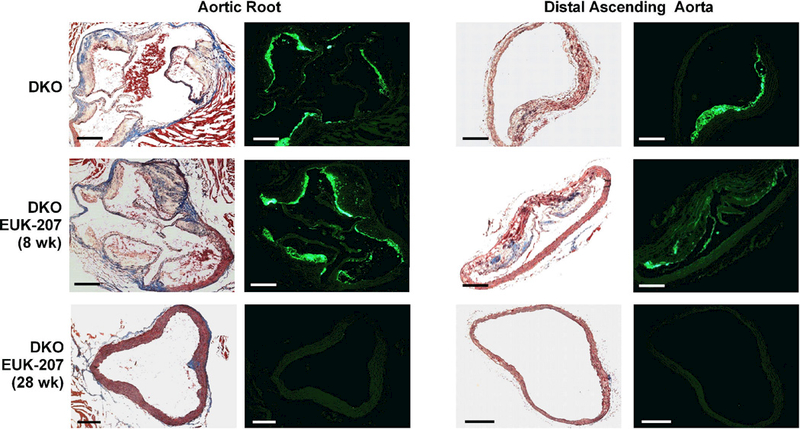
Examples of histology from the aortic root at the level of the aortic sinuses, or from the distal ascending aorta from 40 week-old DKO vehicle-treated mice, or mice treated with EUK-207 for 8 or 28 weeks. For each location in the aorta, images show plaque area by Masson’s staining (left panels) and Mac-2 macrophage immunofluorescent (green) staining (right panels). Scale bar = 200 μm.
DISCUSSION
Atherosclerosis is recognized to be an indolent disease process that, in humans, develops over decades in response to chronic vascular inflammatory, mitogenic and pro-thrombotic processes. Short-term therapy with anti-inflammatory or antithrombotic therapies can prevent recurrent acute atherothrombotic events in late-stage disease. However, it is widely believed that chronic long-term suppression of disease-related pathways is needed to permanently arrest or even prevent the development of atherosclerotic plaques. However, convincing data of the short-and long-term effects of antioxidant therapy have been limited by the paucity of in vivo methods for directly examining biologic changes that occur in the vessel wall in response to novel anti-oxidant therapies that can then be correlated with histology. In this study, CEU molecular imaging was applied to evaluate the beneficial effects of a multifunctional anti-oxidant, EUK-207 on the vascular endothelium.
Our results indicate that the short-term beneficial effects of EUK-207 to reduce endothelial activation and platelet-endothelial interactions leads to a profound reduction in plaque size and inflammation when therapy is continued for a long duration. Initiation of therapy late in the disease process had a profound effect to reduce endothelial activation and platelet adhesion; however there were no effects on plaque size or macrophage content either because 10 weeks was not enough time to show morphologic change, or because a reduction of endothelial activation is not sufficient by itself to promote advanced plaque regression.
The selection of EUK-207 as a therapeutic compound in this study was based on the notion that potent anti-oxidant therapy could reduce not only plaque inflammation, but also platelet-endothelial interactions that have been implicated in promoting plaque progression and monocyte recruitment even in early disease.11,21 Oxidative stress in atherosclerosis is generated from a variety of sources. Resident vascular cells produce free radicals through the enzymatic activity of Nox complexes, endothelial xanthine-oxidase, uncoupled endothelial nitric oxide synthase, and mitochondrial respiration.6,8,22 Inflammatory cells and platelets also contribute to vascular oxidative stress.23 The suppression or quenching of anti-oxidant enzyme families such as SOD and catalase can also contribute to plaque oxidative burden.6
There are many pathways by which oxidative stress leads to plaque progression and unstable plaque phenotype. In this study we used molecular imaging to specifically evaluate vascular endothelial events triggered by oxidative stress. Vascular inflammation occurs through signaling of specific receptors such (Lox-1, toll-like receptors, and scavenger receptors)6,9 or inhibition of anti-inflammatory processes.22 As a result, there is upregulation of adhesion molecules including P-selectin, ICAM-1, and VCAM-1 that are involved in leukocyte recruitment; and the endothelial production of pro-inflammatory chemokines.4,5
Platelet recruitment is yet another pro-atherosclerotic consequence of oxidative stress which occurs, in part, from endothelial events. Increased ROS can result in loss of the normal anti-platelet activities of both NO and prostacyclin.22 Reviewed elsewhere, adverse oxidative events in atherosclerosis can lead to platelet recruitment through interaction of endothelial VWF with the GPIbα component of the platelet GPIb/GPV/GPIX complex.11 This may occur, in part from oxidative inhibition of proteases that cleave endothelial-associated VWF.10,24 The platelet-endothelial interactions that ensue can amplify inflammation through a variety of signaling pathways or through direct platelet-mediated recruitment of monocytes.11
Based on the extensive pro-atherogenic endothelial effects of ROS discussed above, we used CEU molecular imaging to assess the beneficial effects of EUK-207 on selectin expression, endothelial-associated VWF, and endothelial recruitment of platelets. CEU is uniquely suited for this application for several reasons. First, it uses targeted MBs that are confined to the intravascular compartment, thereby giving us confidence that signal was from events occurring at the interface of the endothelium and blood pool. For MBs targeted to platelets and VWF, we used recombinant biologic versions of the endogenous binding receptors that are able to ligate particles at physiologic shear. Finally, MBs that do not attach are rapidly cleared from the blood pool, thereby providing the opportunity to image multiple endothelial targets in a relatively rapid fashion. The targeted MB agents used in this study have been validated previously.2,3,16 We were also leveraging information from prior validation studies that demonstrated that the markers of endothelial activation in this study are expressed at a high level at all stages of atherosclerosis, even early, consistent with the notion that macrophage accumulation occurs very gradually in response to a consistent high level of endothelial activation.2,3,16 In this study, imaging of these same molecular processes was used in DKO mice as a readout for the endothelial effects of EUK-207 which has been shown previously to mitigate other oxidatively-mediated diseases such as injury radiation injury and ischemia-reperfusion injury.25–27 Our data indicates the presence of beneficial short- and long-term effect of EUK-207 on endothelial phenotype, particularly in late-stage disease when endothelial abnormalities tend to be more severe. It should be noted that “negative” intensity values for normalized signal intensity (control - targeted signal) are likely because in the absence of any targeted attachment, the presence of targeting ligands can stochiometrically prevent non-specific retention of microbubbles.28
Plaque histology yielded important information on the effect of EUK-207 on plaque development and content. When paired with molecular imaging data, histology also provided insight into the temporal relationship between the modification of endothelial phenotype and plaque progression. Our findings indicate that potent anti-oxidant therapy can dramatically reduce endothelial activation, yet the effects on plaque size and content with short-term therapy are modest. On the other hand, long-term therapy when started early can have profound effects on preventing plaque development.
With regards to clinical implications, our findings suggest that: (i) potent anti-inflammatory therapies will be most effective when given at early stages of disease to very high-risk individuals; and (ii) trials evaluating the effect of new anti-oxidant therapies in patients are likely to require extended periods of follow-up. This latter issue provides a strong basis for molecular imaging of endothelial cell phenotype or other forms of vascular inflammation imaging. These techniques may be helpful in clinical trials by demonstrating beneficial early vascular responses to anti-oxidant therapies with compounds such as EUK-207 to ensure “on-target” cellular and molecular effects before any expected effects on plaque size or composition. There is precedence in this strategy, such as the recent demonstration of lack of effect of dalcetrapib on positron emission tomography imaging of inflammation in patients with atherosclerotic disease, which has been useful for understanding lack of therapeutic benefit in clinical trials.29
There are limitations of the study that must be acknowledged. Experience with vitamin-based anti-oxidants have taught us that the effects of oxidative stress in atherosclerosis and the benefits of anti-oxidants found in murine models do not guarantee replication in humans.30 However, the mechanism of action of EUK-207 is profoundly different from that of vitamin-based anti-oxidants as EUK-207 targets both ROS and reactive nitrogen species, and acts catalytically, which conveys distinct advantages over compounds which are more specific and act in a stochiometric way. We also cannot state with certainty how much of the beneficial effects of EUK-207 on plaque size and content were attributable to its effects on the endothelium. We did not directly compare molecular imaging signal to immunohistochemistry for P-selectin and VWF since these molecules are normally stored in endothelial Weibel-Palade bodies which are present on histology. Platelet signal has already been validated against histology.3 It is important to realize that a murine models do not necessarily fully capitulate human disease based on location of disease, shear forces, the temporal course of disease development, and the contrived genetic nature of disease susceptibility which starts from birth. Finally, we have not necessarily proven that any of the processes that were imaged on molecular imaging are responsible for EUK207’s beneficial effects on plaque growth. This will be proven only by showing lack of additional effect in animals with genetic deletion of P-selectin, or animals treated with GPIbα antagonists.
In summary, the results from this study indicate that the SOD and catalase mimetic EUK-207 acts to reduce endothelial P-selectin expression, and to suppress platelet-endothelial interactions by inhibiting endothelial-associated VWF. The beneficial anti-inflammatory and anti-platelet endothelial effects of EUK-207 occurred with even short duration of therapy, yet meaningful effects on reducing plaque size and content were found only with long-term therapy, indicating a slow cumulative effect of anti-oxidant therapy. These findings are also important for understanding how the beneficial effects of potent anti-oxidant on plaque morphometry may only be manifest with long-term therapy, whereas effects discoverable on molecular imaging of endothelial phenotype may be seen much earlier.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Lindner is supported by grants R01-HL078610 and R01-HL130046 from the NIH, and a grant (14-14NSBRI1-0025) from the NASA National Space Biomedical Research Institute. Dr. Atkinson was supported by grant T32-HL-94294-5 from the NIH. EUK-207 was provided for this study by Bernard Malfroy-Camine, Ph.D., MindSet Rx, Inc.
ABBREVIATIONS
- CEU
Contrast-enhanced ultrasound
- DKO
Double knockout
- GPIb
Glycoprotein-Ib
- ICAM-1
Intercellular adhesion molecule-1
- LOX-1
Lectin-like oxidized LDL receptor
- MB
Microbubble(s)
- Nox
NADPH oxidase
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- VCAM-1
Vascular cell adhesion molecule-1
- VWF
Von Willebrand factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no other pertinent disclosures for other authors.
REFERENCES
- 1.Lindner JR and Link J. Molecular Imaging in Drug Discovery and Development. Circ Cardiovasc Imaging 2018;11:e005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol 2010;30:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim CY, Liu YN, Atkinson T, Xie A, Foster T, Davidson BP, et al. Molecular Imaging of Platelet-Endothelial Interactions and Endothelial von Willebrand Factor in Early and Mid-Stage Atherosclerosis. Circ Cardiovasc Imaging 2015;8:e002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunsch C and Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 1999;85:753–66. [DOI] [PubMed] [Google Scholar]
- 5.Tummala PE, Chen XL and Medford RM. NF- kappa B independent suppression of endothelial vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 gene expression by inhibition of flavin binding proteins and superoxide production. J Mol Cell Cardiol 2000;32:1499–508. [DOI] [PubMed] [Google Scholar]
- 6.Forstermann U, Xia N and Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ Res 2017;120:713–735. [DOI] [PubMed] [Google Scholar]
- 7.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, et al. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 2001;108:1513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madamanchi NR, Vendrov A and Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 2005;25:29–38. [DOI] [PubMed] [Google Scholar]
- 9.Xu S, Ogura S, Chen J, Little PJ, Moss J and Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cellular and molecular life sciences : CMLS 2013;70:2859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Fu X, Wang Y, Ling M, McMullen B, Kulman J, et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood 2010;115:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu MD, Atkinson TM and Lindner JR. Platelets and von Willebrand factor in atherogenesis. Blood 2017;129:1415–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B and Miller FJ, Jr. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 2011;216:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, et al. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol 2013;33:406–12. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A 2003;100:8526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell-Braxton L, Veniant M, Latvala RD, Hirano KI, Won WB, Ross J, et al. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat Med 1998;4:934–8. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging 2013;6:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr CL, Qi Y, Davidson B, Chadderdon S, Jayaweera AR, Belcik JT, et al. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol 2011;31:2526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007;116:276–84. [DOI] [PubMed] [Google Scholar]
- 19.Oliver JJ and Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 2003;23:554–66. [DOI] [PubMed] [Google Scholar]
- 20.Csonka C, Sarkozy M, Pipicz M, Dux L and Csont T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxid Med Cell Longev 2016;2016:3863726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 2002;196:887–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubos E, Handy DE and Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci 2008;13:5323–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak WN, Deng J, Ruan XZ and Xu Q. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler Thromb Vasc Biol 2017;37:e41–e52. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Chen J, Ling M, Lopez JA, Chung DW and Fu X. Hypochlorous acid generated by neutrophils inactivates ADAMTS13: an oxidative mechanism for regulating ADAMTS13 proteolytic activity during inflammation. J Biol Chem 2015;290:1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem 2011;11:359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doctrow SR, Lopez A, Schock AM, Duncan NE, Jourdan MM, Olasz EB, et al. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J Invest Dermatol 2013;133:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao F, Fish BL, Szabo A, Doctrow SR, Kma L, Molthen RC, et al. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res 2012;178:468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher NG, Christiansen JP, Klibanov A, Taylor RP, Kaul S and Lindner JR. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J Am Coll Cardiol 2002;40:811–9. [DOI] [PubMed] [Google Scholar]
- 29.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378:1547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Violi F, Micheletta F and Iuliano L. Antioxidants and atherosclerosis. Eur Heart J Suppl 2002;4:B17–B21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


