Abstract
Objectives
Contextual factors can transform how we experience pain, particularly if pain is associated with other positive outcomes. Here we test a novel meaning-based intervention: Participants were given the opportunity to choose to receive pain on behalf of their romantic partners, situating pain experience in a positive, prosocial meaning context. We predicted that the ventromedial prefrontal cortex (vmPFC), a key structure for pain regulation and generation of affective meaning, would mediate the transformation of pain experience by this prosocial interpersonal context.
Methods
We studied fMRI activity and behavioral responses in 29 heterosexual female participants during (1) a baseline pain challenge and (2) a task in which participants decided to accept a self-selected number of additional pain trials in order to reduce pain in their male romantic partners (“Accept Partner-Pain” condition).
Results
Enduring extra pain for the benefit of the romantic partner reduced pain-related unpleasantness (t=−2.54, p=.016) but not intensity, and increased positive thoughts (t=3.60, p=.001) and pleasant feelings (t=5.39, p<.0005). Greater willingness to accept one’s partner pain predicted greater unpleasantness reductions (t=3.94,p=.001) and increases in positive thoughts (r=.457, p=.013). The vmPFC showed significant increases (q<.05 FDR-corrected) in activation during Accept-Partner-Pain, especially for women with greater willingness to relieve their partner’s pain (t=2.63, p=.014). Reductions in brain regions processing pain and aversive emotion significantly mediated reductions in pain unpleasantness (q<.05 FDR-corrected).
Conclusions
The vmPFC has a key role in transforming the meaning of pain, which is associated with a cascade of positive psychological and brain effects, including changes in affective meaning, value, and pain-specific neural circuits.
Keywords: Meaning of pain, Prosocial, Empathy for pain, Romantic couples, fMRI, brain
Introduction
Pain is a primary motivator and driver of learning. Pain is driven in part by nociceptive input from the periphery to the brain, but it is also deeply imbued with and influenced by meaning—the value and implications afforded by the situational context in which it occurs(1). Historically, pain has been linked with both positive and negative social meanings. On the one hand, pain can signal punishment for betrayal, transgression and wrongdoing. On the other hand, it has been linked to purification, sacrifice, and affirmations of faith(2,3). The meaning we attribute to pain may, in some cases, flip the emotional quality of the experience from unpleasant to pleasant(4,5). A paradigmatic first-person example of this “pleasant pain” experience was eloquently documented in Viktor Frankl’s book “Man’s Search for Meaning” (6) when he describes the joy and deep relief of being so sick and weak that he was allowed to refrain from strenuous physical work in the freezing Auschwitz winter.
Pain is associated with strong negative meaning in multiple clinical conditions, including disease progression and failures of medical interventions(7,8). Such negative meaning enhances patients’ threat responses(9,10), increasing pain-related distress and potentially pain itself, both in adults and children(3,11). Importantly, anxiety and chronic psychosocial stress early in life and during adulthood have powerful pain amplifying effects(12–14). On the contrary, positively re-interpreting clinical pain can reduce pain(11,15,16). A range of successful educational interventions aim at changing patients’ interpretation of pain(17) by emphasizing the notion that it is a protective mechanism and, in many patients with chronic pain, no longer an indicator of tissue damage(18–23). The same negative and positive effects of interpretation and meaning of pain have been observed in healthy subjects during experimental manipulations(24–28). The analgesic effects of associating pain with positive consequences are also commonly observed during competitive sports and mountaineering challenges, in which pain endurance is associated with reward and achievement, and with the joy and relief that comes with overcoming obstacles(4,27,28). Finally, among other types of meaning, the desire to protect loved ones and relieve their suffering is a primary goal that leads many individuals to willingly endure pain and other hardships(29). Therefore, the way we interpret physical pain and primarily aversive experiences can transform the way we experience them and the way we fear their consequences, which could have relevant implications for the management of chronic stress and adversity.
Importantly, the brain mechanisms underlying such changes in pain experience due to attribution of positive meaning remain largely unknown. In addition, most investigations of pain meaning and context involve manipulating implications for the self(5,30–32), but we don’t know whether associating pain with prosocial meaning (positive consequences for others) may also transform the experience and brain processing of pain. We reasoned that the positive meaning associated with voluntarily taking on pain in place of a close other—here, participants’ romantic partners—might be a powerful way to attenuate pain and pain-related brain processes. Alternatively, however, the “Accept-Partner-Pain” might instead increase pain and brain-evoked pain responses, since participants may expect increased pain during this condition, which predicts increased pain in previous studies (33–35).
Among brain areas, the ventromedial prefrontal cortex (vmPFC) may be particularly important for meaning-based modulation of pain(36). It is strategically positioned to integrate information from affective, sensory, self-related and social-cognitive brain networks(37–42). This multi-system integration hub has been postulated to serve a fundamental role in conceptual processing(43) and in generating affective meaning and flexible responses when there is critical need for engaging conceptual representations about the situation and the self (reviewed in(36), see also((44,45)). Also, the vmPFC is frequently engaged in assigning value to actions and events when value is based on conceptual thought(46–49). VmPFC activity has been associated with finding positive meaning in otherwise negative experiences(44). In the context of pain processing, the vmPFC has been hypothesized to translate social information about pain into self-relevant expectations and affective meaning(31), and activation in healthy individuals is associated with reduced pain across multiple studies(50). For example, Leknes and colleagues(4,28) showed that the vmPFC was activated during a “relative pain relief” condition in which a medium-intensity painful stimulus was the best of two possible outcomes, compared with a condition in which it was the worst of the two outcomes(4). This “relative pain relief” condition was associated with strong reductions in pain. Overlapping activation in vmPFC was also observed during appetitive reward and pain relief(28).
Here we studied brain and behavioral responses in 29 heterosexual female participants during an fMRI task in which they voluntarily decided to endure more or less additional pain to reduce pain in their romantic partners (“Accept-Partner-Pain” condition). Unbeknownst to the participants, they all received the same intensity and number of noxious stimuli in both the “Baseline” control condition and the “Accept Partner-Pain” condition—the only difference was in the social meaning associated with the painful stimuli. We hypothesized that (i) greater voluntary willingness to endure extra pain to reduce one’s romantic partner’s pain would be associated with increases in positive thoughts and feelings and reductions in pain intensity and/or unpleasantness; (ii) vmPFC activation would increase during the Accept-Partner-Pain condition, in line with its role in representing affective meaning; and (iii) finally, in line with expected pain unpleasantness reductions, we expected activation reductions in pain-processing regions and in a multi-region neural signature that was developed to specifically track physical pain(52). We expected these effects to be modulated by one’s willingness to endure extra pain for the sake of their loved other. Confirmation of these hypotheses would indicate that pro-socially transforming the meaning of pain would be not only beneficial for others but also for those deciding to endure the extra suffering, both at the psychological and brain levels. This pro-social transformation of pain could have a profound impact on promoting and educating in a culture of altruism and compassion, which would contribute to overall well-being in stressful or aversive environments.
Materials and Methods
Participants
The study included 29 healthy women (mean age of 24.65 ± 6.72 years) with no history of psychiatric, neurological, or pain disorders and no current pain symptoms, who were in a committed and monogamous romantic relationship for at least 3 months, as described previously(53). All participants and their male partners gave written informed consent that was approved by the institutional review board of the University of Colorado Boulder and were paid for their participation. One additional participant was not able to complete the fMRI session due to excessive delays in the procedure. The timeframe of data collection for this study was October 2012 to June 2013.
Procedures
All participants and their partners first underwent a short pain calibration session to assure normal pain sensitivity and familiarize them with the heat pain stimulation. This procedure ensured that the stimulus we used (47°C, 11-second stimuli, 7.5-second plateau temperature) was within the tolerable, yet painful, range for all subjects. During the main fMRI session, we assessed brain and behavioral responses during two experimental conditions of interest (“Baseline” condition (a) and “Accept-Partner-Pain” condition (b)), following an a-b-b-a run experimental design (Figure 1). During runs 1 and 4 (Baseline condition) participants were told they were to experience the first half of the total amount of painful stimulations that the computer had assigned to them (no explicit manipulation of affective meaning involved). Then, right before the onset of run 2, participants were asked to decide what amount (25% - 75%) of painful stimulations they were willing to accept to reduce pain in their romantic partner, using a visual analogue scale. Experimenters and MRI technicians were blind to their decision. Right after making this decision, the female participant was told that she was going to experience the first half of the total number of painful thermal stimulations that she had decided to endure instead of her partner and that those trials were directly removed from her partner’s subsequent sequence of painful stimulations. Right before run 3 the participant received the instruction that she was going to receive the second half of those painful stimulations she had decided to endure instead of her romantic partner (prosocial meaning manipulation for both runs 2 and 3). Then, right before run 4 (again Baseline condition), we reminded the female participants that they were about to receive the second half of the painful stimulations that the computer had assigned to them. Figure 1 provides a complete representation of this task structure and the trial timing. Unbeknownst to the participants, the study was designed such that both conditions (Accept-Partner-Pain and Baseline) consisted of 8 heat pain trials each (47°C, 11-second stimuli, 7.5-second plateau temperature); therefore, the number of painful stimulations and the temperature was identical for both conditions and the only difference relied on the different affective meaning of pain for each condition (neutral or negative for the Baseline condition, whereas prosocially helping/positive for the Accept-Partner-Pain condition). Heat painful stimulations were administered to the volar surface of the participants’ left forearm using an MRI-compatible PATHWAY ATS (Advance Thermal Stimulation) thermode with 16-mm diameter (Medoc Ltd., Ramat Yishai, Israel). Importantly, the thermode was moved in a random manner to a different (pre-marked) location in the volar forearm after each run. After each pain stimulus (trial), participants rated, using a computerized visual analogue scale (VAS), pain intensity (“how intense was the painful stimulus?”, ranging from 0, “not intense at all” to 100, “the most intense imaginable”) and pain unpleasantness (“how unpleasant was the painful stimulus?”, i.e., how much did the stimulus “bother” you?, from 0, “not at all unpleasant” to 100, “the most unpleasant imaginable”). We conducted two multi-level general linear models (GLMs) in order to test for (i) condition effects (Accept-Partner-Pain vs. Baseline), (ii) habituation/sensitization effects of run order and (iii) habituation/sensitization effects of trial order, on ratings of pain intensity (GLM model 1) and ratings of pain unpleasantness across trials (GLM model 2) (see results section and Supplementary Figure 1, Supplemental Digital Content).
Figure 1. Experimental design: graphic representation of run and trial structure.
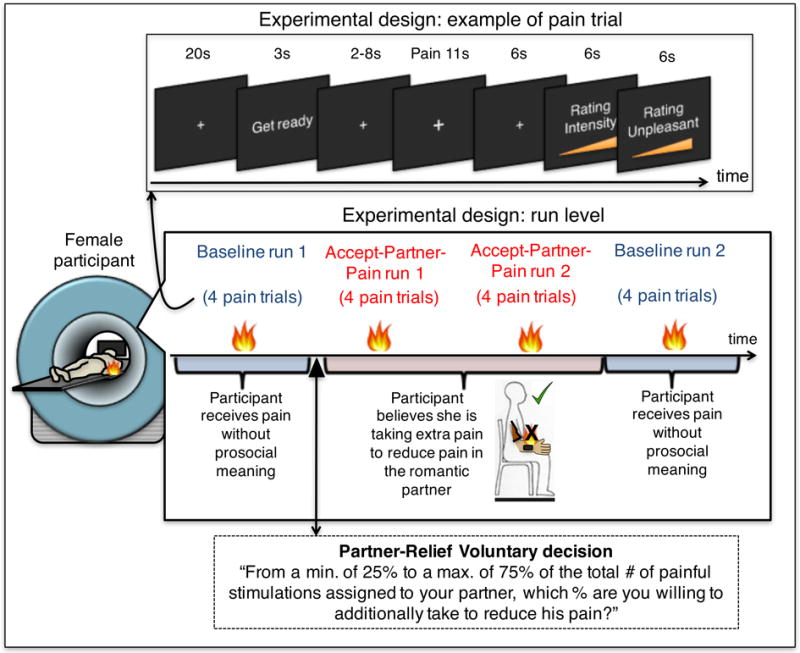
The top panel represents the trial structure. The medium panel represents the structure of the task at the run level. The fMRI task included four runs following an a-b-b-a experimental design, in which condition “a” corresponds to the Baseline control condition (the participant believes she is being exposed to the pain that the computer assigned to her), and condition “b” corresponds to the “Accept-Partner-Pain” condition, in which the participant believes she is voluntarily enduring additional pain to reduce pain in her romantic partner (prosocial meaning condition). As the black arrow indicates, right before the first “Accept-Partner-Pain” run the participant decides the percentage of the total number of painful stimulations she is willing to voluntarily endure to alleviate her romantic partner.
All of the 29 women believed that we were going to administer pain subsequently to their romantic partners because they had already seen their romantic partners in pain right before the start of this fMRI task during a pain empathy paradigm(53).
At the end of each run we collected run-level measures of positive thoughts (“How positive were your thoughts?” from 0, “not positive at all” to 100, “the most positive imaginable”?), pleasant feelings and unpleasant feelings (“how pleasant/unpleasant were your feelings?” from 0, “no pleasant/unpleasant at all” to 100, “the most pleasant/unpleasant imaginable”), using a computerized VAS.
Further, although we do not use these measures in the current study, we collected measures of perceived closeness with the romantic partner, quality of the romantic relationship and emotional empathic tendency as reported in our previous paper on a separate experimental task of this study (c.f.,(53)).
Study design
We used a within-subjects a-b-b-a design. Each condition consisted of 8 trials divided into two runs per condition (4 trials each) (Figure 1). During condition “a” the participant received pain without prosocial meaning; during condition “b” the participant believed she was voluntarily taking extra pain to reduce pain in her romantic partner.
We performed trial-by-trial multi-level general linear model analyses to assess the effects of condition (Baseline vs. Accept-Partner Pain) on pain ratings accounting for potential habituation/sensitization across trials within run (tested on the same skin site) and across runs (‘Run number, tested on different skin sites). Separate models were run for trial-by-trial pain intensity and pain unpleasantness ratings, as these were the two outcomes we tested. Supplementary Figure 1 illustrates the results (Supplemental Digital Content).
Analyses of fMRI data
MRI acquisition and preprocessing
Functional brain activity was measured using a Siemens TrioTim 3T scanner, covering the brain in 26 interleaved transversal slices (3.4mm isomorphic voxels), with a T2* weighted EPI GRAPPA sequence (TR = 1.3s, TE = 25ms, flip angle = 50°, FOV = 220mm). SPM8 was used for preprocessing for functional images, using a standard pipeline for motion correction, slice-time correction, spatial normalization to MNI space, and spatial smoothing of images using an 8mm FWHM Gaussian kernel. For spatial normalization, T1 structural MPRAGE images (1mm isomorphic voxels) were first coregistered to the mean functional image and then normalized to the SPM template using unified segmentation. Preprocessed functional images were resampled at a voxel size of 2×2×2mm. Regarding motion correction, translation and rotation estimates (x, y, z) were less than 2 mm or 2°, respectively, for all participants.
First level single-subject fMRI analyses
We used a GLM analysis approach as implemented in SPM8 software to estimate, for each subject, brain responses to pain during (a) single trials for the Baseline and Accept-Partner Pain conditions to be used in the whole-brain multilevel mediation model and (b) an average brain response to pain and pain anticipation during the Baseline and Accept-Partner-Pain conditions to be used to compute Neurologic Pain Signature (NPS(52)) pattern expression for each subject and condition.
For both Baseline and Accept-Partner-Pain conditions, either single-trial pain regressors or a regressor modeling all pain trials for each condition were created, by convolving each painful stimulation period with a canonical hemodynamic response function. The model also included regressors modeling the anticipatory periods and the rating periods. The remaining “rest” period served as an implicit baseline. Lastly, the model included 24 motion regressors (3 translation and 3 rotation regressors, plus their first and second derivatives). Parameter estimates were calculated at each voxel using the general linear model. A high-pass filter was used to remove low-frequency signal fluctuations (1/180 Hz). We calculated single-trial pain contrast images for each participant, for the 8 Baseline and 8 Accept-Partner-Pain (vs. implicit baseline) trials. The individual contrast images were carried forward to a whole-brain multilevel mediation model computed using publicly available software (https://github.com/canlab/MediationToolbox/blob/master/mediation_toolbox/mediation_brain.m).
Signature responses
For each female participant we computed a single scalar value representing their expression of the NPS pattern for the Baseline and Accept-Partner-Pain contrast images (as explained in detailed in previous articles, e.g., (Lopez-Sola M, Koban L, Krishnan A, Wa…; López-Solà et al. 2017).
Multilevel Whole-Brain Mediation analyses
First level contrast images for the single-trial pain period regressors for each subject were carried forward to a multilevel mediation analysis model. To avoid that single-trial estimates could be driven by movement artifacts or other sources of noise, trial estimates with variance inflation factor of 5 or more were excluded from further analysis(53,54). We then tested relationships between condition (Accept-Partner-Pain vs. Baseline), single-trial pain-evoked brain activation, and pain unpleasantness ratings across individual trials using multilevel mediation analysis(35) (Figures 4 and 5 for illustration). Multilevel mediation analysis identifies three steps in a potential mechanistic pathway underlying Accept-Partner-Pain effects: (1) brain regions that show activity increases or decreases during Accept-Partner-Pain (Path a), (2) brain regions that predict changes in pain unpleasantness (Path b), when controlling for Path a, and (3) brain regions that formally mediate the relationship between condition (Accept-Partner-Pain vs. Baseline) and reductions in pain unpleasantness (Path a*b), thus significantly reducing the strength of the direct Path c. Resulting activation maps were thresholded at q<0.05 FDR-corrected within an extensive whole-brain gray-matter mask including 352,328 voxels (corresponding to a voxel threshold of p=.001) and across mediation paths (53,69). To facilitate interpretation of the functional maps, adjacent voxels were displayed at thresholds of p=.005 and p=.01 uncorrected.
Results
Female participants experienced four runs of 47 degree-Celsius noxious heat trials: A Baseline pain run, two Accept-Partner-Pain condition runs, and a final Baseline run (Figure 1). Each run consisted of 4 trials. Right before the Accept-Partner-Pain runs, we told them that the more painful stimulations they decided to accept on behalf of their romantic partner—from a minimum of 25% of the total number of painful trials that had been assigned to their partners to a maximum value of 75%–the more we would reduce the number of painful stimulations that the partner would subsequently receive. The experimenters were blind to the decision, which did not influence the actual amount of pain the participants received (to avoid confounds with stimulus history). We compared pain-related brain responses and self-reported experience during “Accept-Partner-Pain” vs. “Baseline” runs (Figure 1). Each female participant had previously been exposed to the same pain herself and had also observed her romantic partner in pain through a mirror system installed in the scanner during separate fMRI tasks reported elsewhere (see (53)). All participants reported to have believed the veracity of these instructions (i.e., that their partners were to be subsequently exposed to the remaining painful trials).
On average, women decided to take 61.75% of the painful stimulations that had been assigned to their partner (range: 25%-75%), with thirteen out of the 29 women choosing to take the maximum 75% of the partner’s pain trials. Importantly, women’s sensitivity to pain during baseline did not explain the degree of partner’s pain acceptance, r=−.014, p=.943 for baseline pain intensity and r=−.133, p=.493 for baseline pain unpleasantness.
We performed trial-by-trial multi-level general linear model analyses to assess the effects of condition (Baseline vs. Accept-Partner-Pain) on pain ratings (intensity and unpleasantness) accounting for potential habituation/sensitization across trials within run (tested on the same skin site) and across runs (‘Run number, tested on different skin sites). Separate models were run for trial-by-trial pain intensity and pain unpleasantness ratings, as these were the two outcomes we tested. We found a significant effect of condition on pain unpleasantness controlling for run position (in the overall sequence) and within-run trial position (order): Accept-Partner-Pain (vs. Baseline) significantly reduced pain-evoked unpleasantness (t = 2.57; p= .015; Supplementary Figure 1). Pain intensity did not show a significant effect (t=1.16, p=.25) of Accept-Partner-Pain vs. Baseline after controlling for run position and trial position. As expected from previous work (55), we found a significant within-run pain habituation effect, t = 7.12 p <.00005 for the intensity model, and t = 6.20, p<.00005 for the unpleasantness model. Women who decided to take more additional pain to relieve their partners’ pain (by median split, as nearly half the sample opted for the maximum amount) showed greater unpleasantness reductions during the Accept-Partner-Pain condition (t=3.94, p=.001) (Figure 2) than women who took less pain from their partners. The correlation between the continuous variable representing the percentage of pain that the female participant decided to take on and unpleasantness reductions was also statistically significant (r=.390, p=.036, Figure 2 legend).
Figure 2. Behavioral pain-related effects of ”Accept-Partner-Pain” vs. “Baseline” conditions.
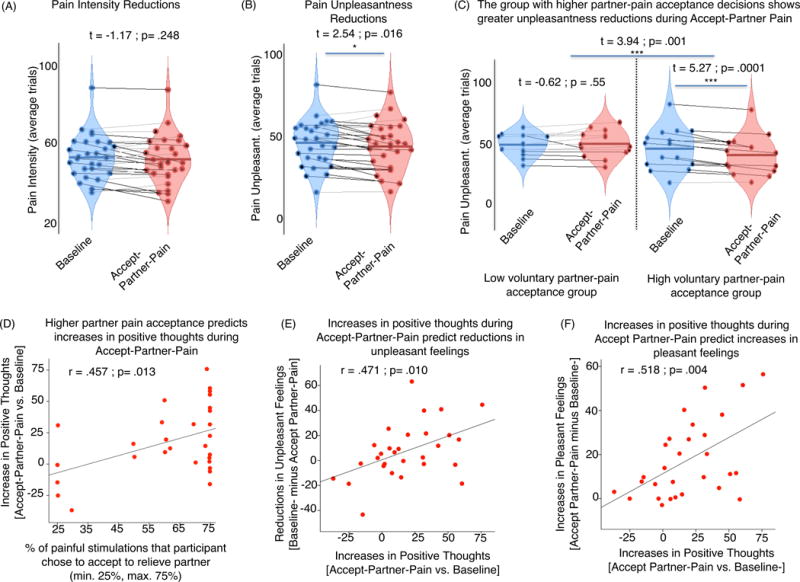
Red violin plots in panels (A), (B), and (C) indicate responses during ”Accept-Partner-Pain”, whereas blue violin plots correspond to “Baseline” control condition measures. Each dot in the violin plot figures represents one subject. Lines connect responses given by the same subjects. Black lines indicate subjects for whom responses go in the hypothesized direction. Gray lines indicate responses going in the opposite direction. For the analysis shown in panel (C), we also computed and report the correlation effect between partner-pain acceptance decisions and pain unpleasantness reductions during the Accept-Partner-Pain condition (r=.390, p=.036). Panels (D), (E), and (F) correspond to significant correlations.
Compared to Baseline, the Accept-Partner-Pain condition also evoked significant increases in positive thoughts (t=3.60, p=.001) and pleasant feelings (t=5.39, p<.0005), indicating a shift in meaning-related thoughts and feelings. Those who accepted greater amounts of partner pain showed greater increases in positive thoughts during Accept-Partner-Pain vs. Baseline (r=.457, p=.013). Further, greater increases in positive thoughts predicted greater increases in pleasant feelings (r=.518, p=.004) and greater reductions in unpleasant feelings (r=.471, p=.010).
In sum, the behavioral findings suggest that prosocially transforming the meaning of pain evokes significant reductions in pain unpleasantness, and that more prosocial women experienced larger reductions in pain unpleasantness and increased positive cognitive and emotional responses during pain.
Neurologic Pain Signature (NPS) during Baseline and Accept-Partner-Pain
We next investigated how the experimental conditions affected the response of the NPS, a brain measure validated to sensitively and specifically respond to pain(52). As shown in Figure 3A, NPS responses were selective for evoked pain periods (not pain anticipation), but they were similar across both conditions, Baseline and Accept-Partner-Pain (Figure 3A). Though there was no significant main effect of Accept-Partner-Pain vs. Baseline, participants reporting above-median increases in positive thoughts showed significant NPS reductions during Accept-Partner-Pain vs. Baseline (t=2.48, p= .01) and such reductions were also significantly greater for those with above-median than for those with below-median increases in positive thoughts (t=4.90, p=.00003). Indeed, participants with below-median increases in positive thoughts showed significant NPS increases during Accept-Partner-Pain vs. Baseline (t=−2.11, p=.04) (Figure 3B). This effect was also statistically significant when assessed using the continuous variable correlation (see Figure 3B legend, r=.580, p=.001). Therefore, greater reductions in pain-specific processing were predicted by greater engagement in positive thoughts, which were in turn predicted by more prosocial pain-acceptance decisions. Conversely, engaging in less positive thoughts and more unpleasant feelings may increase pain-specific processing when taking extra pain to reduce it in another.
Figure 3. Neurologic pain signature (NPS) responses during Baseline (blue) and Accept-Partner-Pain (red) conditions.
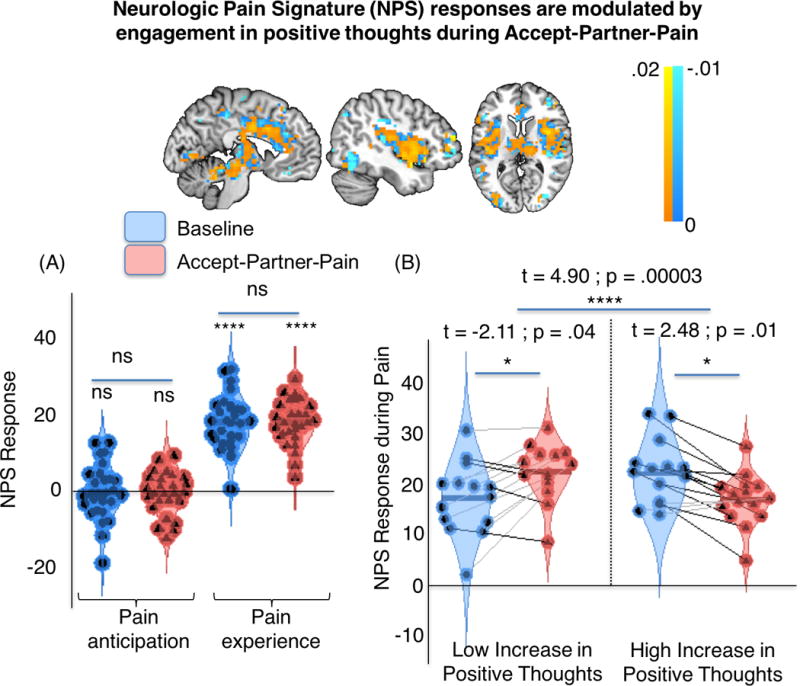
The top panel represents the NPS positive (warm colors) and negative (cold colors) voxel weights for reference. Red violin plots indicate NPS responses during ”Accept-Partner-Pain”, whereas blue violin plots correspond to “Baseline” NPS measures. Top: The NPS pattern, displayed in neurological orientation (right side of the axial image corresponds to right brain hemisphere). (A) NPS responses for Anticipation and Pain periods. Blue: baseline; red: Accept-Partner-Pain. (B) Pain-evoked NPS responses for separate participant subgroups defined based on differences in positive thoughts for [Accept-Partner-Pain – Baseline]. Blue: baseline; red: Accept-Partner-Pain. Acceptance-related NPS reductions were found for individuals with high increases in positive thoughts. Increases in positive thoughts (tested as a continuous variable) were also correlated with reductions in NPS responses during pain (r = .580, p =.001). ****: p<.0001; *: p < .05, two-tailed; ns: non-significant.
Brain mediators of pain unpleasantness reductions during Accept-Partner-Pain
To understand the brain pathways underlying the psychosocial modulation of pain, we conducted a whole-brain multilevel mediation analysis in which we assessed the potential brain regions mediating the effects of condition on pain unpleasantness. In this analysis, condition (Accept-Partner-Pain vs. Baseline) was the independent variable (X), trial-by-trial pain-period activity served as a set of mediating variables (M), and pain unpleasantness was the outcome (Y).
In agreement with our hypothesis, path a—testing activity changes during Accept-Partner-Pain vs. Baseline—showed a q < 0.05 FDR-corrected significant pain-evoked activation increase in the vmPFC during Accept-Partner-Pain vs. Baseline (peak voxel: (x, y, z = 10, 58, 2), z = 7.58). Furthermore, greater prosocial pain acceptance predicted greater increases in vmPFC activation (computed using the mask of significant voxels around the peak voxel reported above) during Accept-Partner-Pain (t=2.63, p=.014; Figure 4B). Other regions also showed significant FDR-corrected activation increases during the Accept-Partner-Pain condition, including the right thalamus (peak voxel: (8, −26, 8), z = 9.04) and the cerebellum (peak voxel: (4, −42, −44), z = 8.86).
Figure 4. Whole-brain multilevel mediation: path (a) effects.
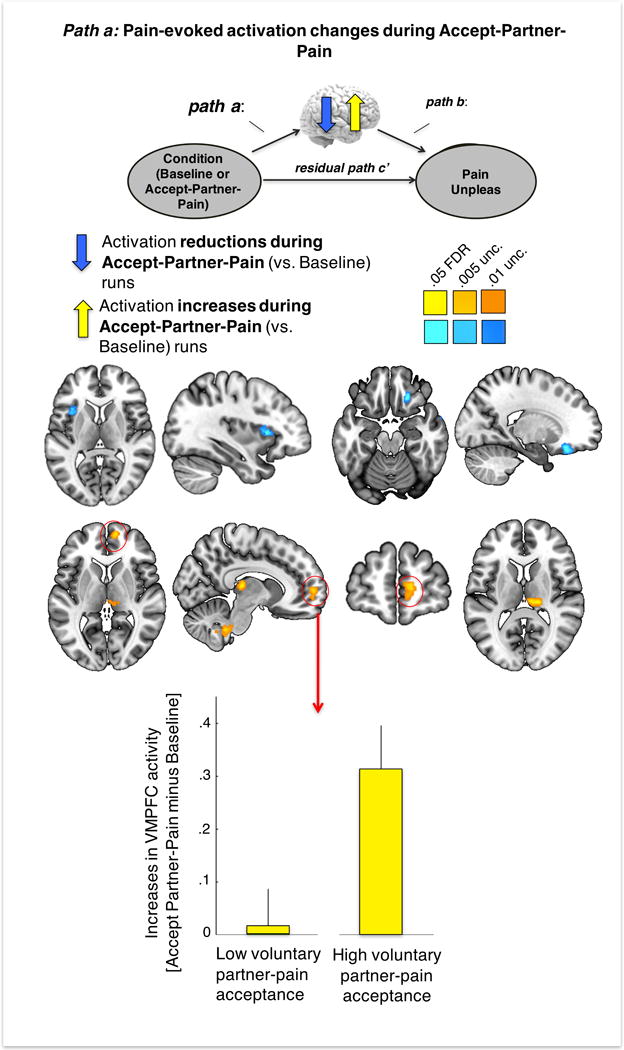
Path (a): brain effects of condition (Accept-Partner-Pain vs. Baseline) on brain activation. Brain image maps display significant (q<.05 FDR corrected) brain activation reductions in the left anterior insula and activation increases in the VMPFC, right thalamus and cerebellum during Accept-Partner-Pain (vs. Baseline). The yellow bars represent the effect of high vs. low voluntary partner-pain acceptance decisions (median split) on VMPFC pain-evoked activation increases during Accept-Partner-Pain (vs. Baseline). Brain images are displayed in neurological orientation (right side of the axial image corresponds to right brain hemisphere).
The Accept-Partner-Pain condition also produced FDR-corrected reductions in pain-evoked activation (negative Path a) in the left anterior insula (aINS, peak voxel: (−36, 16, 6), z = −6.91) and the right orbitofrontal cortex (rOFC, peak voxel: (20, 30, −18), z = −9.54) (see Figure 4A, blue).
Turning to the overall mediation analyses, which jointly test effects of Accept-Partner-Pain on brain responses and trial-by-trial brain response correlations with pain unpleasantness, we observed a significant path (a*b) effect consistent with activation reductions in pain-processing regions including aINS/mid-insula (midINS) (peak voxels: left insula, (−34, 10, 8), z = 11.62 and right insula, (38, 24, 4), z = 13.42), basal ganglia (peak voxel: (−28, −4, 2), z = 12.70), primary somatosensory/motor contralateral to the site of stimulation (peak voxel: (−48, −18, 48), z= 10.50), the anterior mid cingulate cortex (MCC, peak voxel: (−2, 28, 36), z = 15.67) extending to the pre-supplementary motor area (pre-SMA), and the right lateral prefrontal cortex (peak voxel: (44, 34, 34), z = 16.93). These are shown in Figure 5. Therefore, brain activation reductions in these regions (on a trial-by-trial level) during Accept-Partner-Pain significantly predicted greater reductions in pain unpleasantness.
Figure 5. Whole-brain multilevel mediation: mediating path (a*b) effects.
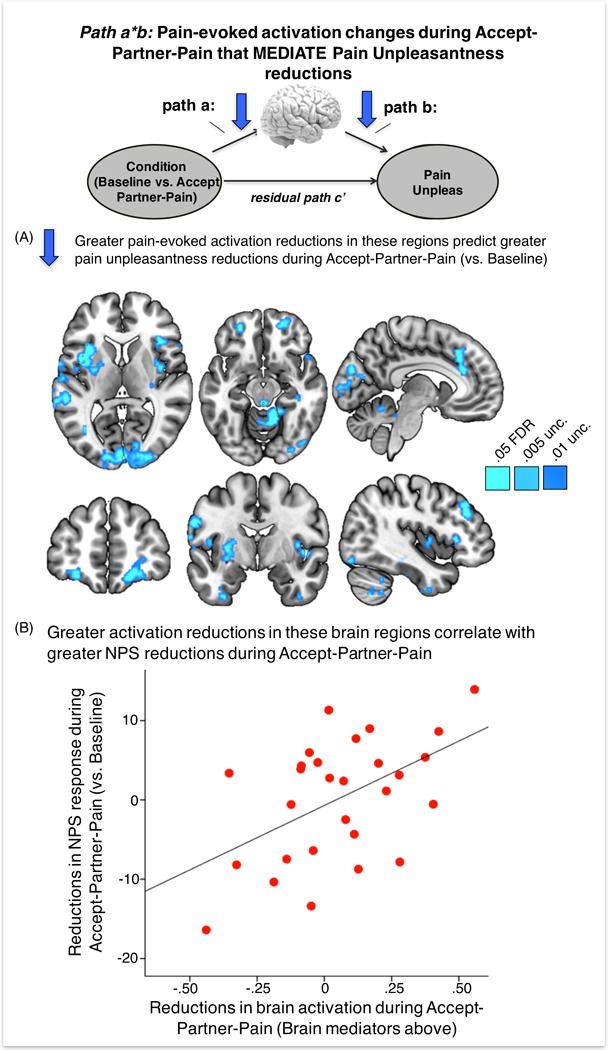
Path (a*b): significant (q<.05 FDR corrected) brain mediators of condition on pain unpleasantness, i.e., greater brain activation reductions during Accept-Partner-Pain (vs. Baseline) in these regions mediated greater pain unpleasantness reductions in the bilateral anterior and mid insula, anterior mid cingulate cortex (aMCC), bilateral orbitofrontal cortex, periaqueductal gray, right lateral prefrontal cortex, visual and cerebellar regions. Panel (B) shows a significant correlation between greater average brain activation reductions in these regions and reductions in pain-evoked NPS response during Accept-Partner-Pain (vs. Baseline). Brain images are displayed in neurological orientation (right side of the axial image corresponds to right brain hemisphere).
The map of FDR-corrected brain mediators also included other regions that are not typically activated during painful stimulation per se, such as the bilateral orbitofrontal cortex (OFC) (peak voxels: (24, 56, −10), z = 10.41 and (−28, 50, −12), z = 8.59), the primary visual cortex (peak voxel: (4, −92, 2), z = 14.43), the left hippocampus (peak voxel: (−36, −36, −4), z = 11.34) and inferior temporal cortices (peak voxel left: (−34, −4, −40), z =9.62; peak voxel right: (32, 20, −34), z = 7.14). Separate individual differences analyses showed that greater activation reductions in the entire mask of significant brain mediators during Accept-Partner-Pain vs. Baseline correlated with greater NPS reductions. The two regions showing significant activation reductions during Accept-Partner-Pain, i.e., the right OFC and left aINS are part of the significant mediating regions in path a*b. However, the vmPFC did not show a significant mediation effect in this model, indicating that there was no direct effect from the vmPFC on the reduction of pain unpleasantness during Accept-Partner-Pain.
Figure 6 shows a summary of brain and behavioral effects associated with voluntarily taking additional pain to relieve the romantic partner’s pain. First, greater willingness to voluntarily accept extra pain for the benefit of the romantic partner (a decision that takes place before any other measures are collected) predicts greater increases in positive thoughts and greater reductions in unpleasant feelings as well as greater activation increases in the vmPFC. Activity changes in vmPFC and lateral OFC—associated with affective meaning, expectation, and punishment value, respectively, among other psychological processes—correlate with pain-evoked activation reductions in core pain-processing regions. These activation reductions correlated with greater reductions in NPS expression, a brain marker sensitive and specific for pain(52), and with greater reductions in pain unpleasantness. NPS reductions also correlated with increases in pleasant feelings, which in turn correlated with increases in positive thoughts.
Figure 6. Summary illustration of the relationships between pain-evoked brain responses and subjective experience during Accept-Partner-Pain (vs. Baseline) conditions.
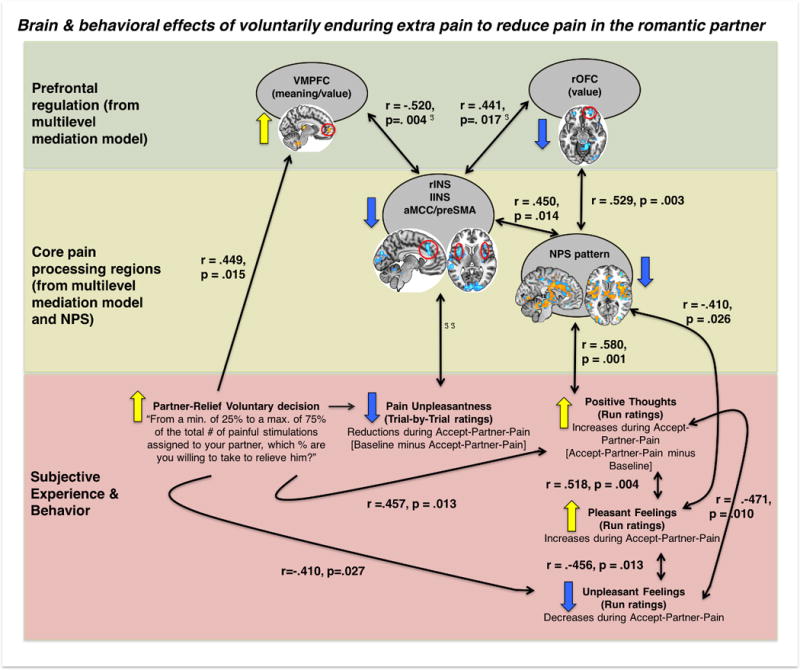
Black double-headed arrows indicate significant correlations. Single-headed arrows are used when there is one variable preceding another variable in time, e.g., “partner-relief voluntary decision” precedes positive thoughts and VMPFC activation. ℑ vmPFC (ventromedial prefrontal cortex, greater stimulus-evoked activation during Accept-Partner-Pain) and rOFC (right orbitofrontal cortex, reduced pain-evoked activation) changes were associated with reduced noxious stimulus-evoked activation reductions in core pain-processing regions, such as aMCC (anterior mid cingulate cortex)/preSMA (pre-supplementary motor area) (VMPFC: r=−.715 p<.0005; rOFC = .364, p=.052) and insulae (left insula and VMPFC: r=−.520, p=.004; right insula and VMPFC: r=−.494, p=.006; right insula and rOFC r=.654, p<.0005; left insula and rOFC =.441, p=.017). ℑ ℑ indicate significant (q<.05 FDR corrected) mediation (path a*b) results, summarized in figure 5.
In sum, the greater the willingness to take a loved one’s pain, the greater the involvement of vmPFC conceptual meaning-related systems during the Accept-Partner-Pain condition and the greater the engagement of positive thoughts and feelings. The vmPFC may contribute to reducing pain-evoked activation in core pain-processing regions and NPS responses during the Accept-Partner-Pain condition, which are also associated with reductions in pain unpleasantness and increases in positive thoughts and pleasant feelings.
Discussion
Prosocial decision-making has been associated with increases in happiness and reductions in impact of stress from young children to adults (56–61). Here, we observed that voluntarily deciding to accept painful stimulation to prevent a close other from experiencing pain is associated with increases in positive thoughts and pleasant feelings, reductions in unpleasant feelings, reductions in the unpleasantness of evoked pain, and reductions in pain-related brain responses in those who made the most prosocial choices. Interpreting our pain as having positive consequences for our loved ones can significantly reduce its aversive characteristics. These results expand on previous findings, which have shown that associating pain with positive direct consequences to oneself reduces pain reports and increases pain tolerance (11,26,27).
The vmPFC is thought to be central for generating affective meaning(36) and maintaining representations of imagined or desired rewards for loved or similar others (62–65). In line with these prior results, we observed increases in vmPFC activation during voluntary pain-acceptance. Greater activation of the vmPFC was predicted by greater willingness to take pain and was associated with greater increases in positive thoughts during the partner relief condition. Moreover, acting as a benefactor evoked brain activation reductions in regions traditionally associated with processing of ‘pain affect’ and pain unpleasantness in previous studies(66,67). Specifically, reductions in the aMCC, aINS, and the lateral OFC (LOFC), were significant mediators of reductions in pain unpleasantness. Activation reductions in these regions on average correlated with reduced NPS responses (across subjects), a brain measure that specifically tracks pain in multiple studies(52,53,68,69). Pain-evoked NPS responses were strongly reduced specifically in women who reported more positive thoughts during the partner relief condition. Since positive thoughts were predicted by generous choices, which in turn correlated with greater benefactor well-being, it is likely that generosity (i.e., greater prosocial decisions in this case) plays an important initial role in promoting the effects of prosocial positive meaning of pain, possibly by inducing a “warm glow” experience(70).
The vmPFC findings in our study complement prior observations indicating an important role for this region in (i) instantiating positive meaning and representation of positive bias(44,71,72), (ii) representing vicarious rewards in similar others(62–65) contributing to “extraordinary empathy”, altruistic motivation, generous choices and empathic care(62,73–78), and, more broadly, (iii) promoting social emotions(79–83) and cognitive-affective representations of the state of the self and others (84–86)(87–90). In this line, patients with lesions affecting the vmPFC are abnormally insensitive to guilt and display significant empathy and theory of mind deficit(89,91,92) and less generous behaviors(82,92–95). Importantly, transcranial direct current stimulation (tDCS) of the vmPFC increases trustworthiness and altruism, experimentally supporting a core role for this region in promoting cooperative behavior(75). Our findings further resonate with a larger body of work that suggests an important role of the vmPFC in the regulation of pain and negative emotions(50,96–100).
In our study, the LOFC shows the opposite context-induced behavior as the vmPFC. Specifically, the left LOFC shows significant reductions in pain-evoked activation during the partner relief condition. Interestingly, lower activation of the LOFC at anatomical locations similar to ours has been correlated with lower levels of punishment attributed to a stimulus(101), though lateral OFC is associated with many other value-related processes as well. Our observations may be interpreted in line with a potentially less punishing value associated with painful stimulation when experiencing it for the benefit of a loved one.
Besides the engagement of medial prefrontal and orbitofrontal regions, it is worth focusing on the effects of the prosocial meaning of pain manipulation in core pain processing brain regions. We found activation reductions in affective/evaluative components of the brain response to pain (aMCC, aINS), but not in regions more directly involved in the processing of the sensory input, such as the parietal operculum (second somatosensory cortex, SII) or posterior insula. These brain findings are in agreement with the observed reductions in pain-evoked unpleasantness without significant modifications in perceived intensity(105), and with the overall null effect on NPS pain-specific responses. Yet, a very robust interaction effect exists between NPS response and change in positive thoughts during the partner relief condition. This suggests that only those with a strong increase in positive thoughts show reduction in NPS pain-specific responses.
Our study may support a role for the vmPFC in activating and maintaining neural representations of the imagined positive consequences of our pains in loved others, and integrating those with representations of self-related internal states, thereby altering the narrative and meaning of such states (e.g. pain) and their subjective experience. Future studies may further characterize the specific conceptual representation that may be engaged and maintained by the vmPFC, and inform about the specific psychosocial computations distinctly taking place in vmPFC vs. OFC regions when flexibly changing the meaning of pain in complex social situations. It would be important to establish the relative contribution of personality, upbringing and social-cultural and stress-related factors in predicting vmPFC engagement and its influence on prosocial behavior. Future research may disentangle the effects of compassion meditation training, particularly at a younger age, in increasing prosocial behavior and its overarching beneficial effects in pain-related contexts. Training to increase prosocial behaviors in a pain-related context may not only favor positive interaction dynamics in romantic couples, but may provide a complementary therapeutic tool for chronic pain patients by engaging meaning, relative value and pain-specific processing circuits.
Supplementary Material
Acknowledgments
Source of Funding
This work was supported by grant R01DA035484 (T.D.W.). Dr. López-Solà’s work was in part supported by a Beatriu de Pinos-A Postdoctoral Fellowship (2010_BP_A_00136) from the Government of Catalunya, Spain.
List of abbreviations
- aINS
Anterior insula
- FDR
false discovery rate
- fMRI
functional magnetic resonance imaging
- MCC
midcingulate cortex
- midINS
mid-insula
- NPS
neurologic pain signature
- OFC
orbitofrontal cortex
- SMA
supplementary motor area
- vmPFC
ventro-medial prefrontal cortex
Footnotes
Conflicts of Interest
All authors declare that they have no competing interests.
Matlab code implementing the analyses presented here is available at wagerlab.colorado.edu.
References
- 1.Henderson SW. The unnatural nature of pain. JAMA: the journal of the American Medical Association. 2000;283:117. [PubMed] [Google Scholar]
- 2.Merskey H. Some features of the history of the idea of pain. Pain. 1980;9:3–8. doi: 10.1016/0304-3959(80)90024-X. [DOI] [PubMed] [Google Scholar]
- 3.Koffman J, Morgan M, Edmonds P, Speck P, Higginson IJ. Cultural meanings of pain: a qualitative study of Black Caribbean and White British patients with advanced cancer. Palliative medicine. 2008;22:350–59. doi: 10.1177/0269216308090168. [DOI] [PubMed] [Google Scholar]
- 4.Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: when relative relief renders pain pleasant. Pain. 2013;154:402–10. doi: 10.1016/j.pain.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jepma M, Wager TD. Multiple potential mechanisms for context effects on pain. Pain. 2013;154:629–31. doi: 10.1016/j.pain.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frankl VE. Man’s Search For Meaning. Simon and Schuster; 1985. p. 221. [Google Scholar]
- 7.Ferrell BR, Dean G. The meaning of cancer pain. Seminars in oncology nursing. 1995;11:17–22. doi: 10.1016/s0749-2081(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 8.Cormie PJ, Nairn M, Welsh J. Guidelines: Control of Pain in Adults with Cancer: Summary of SIGN Guidelines. BMJ: British Medical Journal. 2008;337:1106–9. doi: 10.1136/bmj.a2154. [DOI] [PubMed] [Google Scholar]
- 9.Strang P. Existential consequences of unrelieved cancer pain. Palliative medicine. 1997;11:299–305. doi: 10.1177/026921639701100406. [DOI] [PubMed] [Google Scholar]
- 10.Strang P. Cancer pain–a provoker of emotional, social and existential distress. Acta oncologica. 1998;37:641–44. doi: 10.1080/028418698429973. [DOI] [PubMed] [Google Scholar]
- 11.Smith WB, Gracely RH, Safer MA. The meaning of pain: cancer patients’ rating and recall of pain intensity and affect. Pain. 1998;78:123–29. doi: 10.1016/S0304-3959(98)00122-5. [DOI] [PubMed] [Google Scholar]
- 12.You DS, Meagher MW. Childhood Adversity and Pain Sensitization. Psychosomatic medicine. 2016;78:1084–93. doi: 10.1097/PSY.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 13.Gordon JL, Johnson J, Nau S, Mechlin B, Girdler SS. The Role of Chronic Psychosocial Stress in Explaining Racial Differences in Stress Reactivity and Pain Sensitivity. Psychosomatic medicine. 2017;79:201–12. doi: 10.1097/PSY.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsur N, Defrin R, Ginzburg K. Posttraumatic Stress Disorder, Orientation to Pain, and Pain Perception in Ex-Prisoners of War Who Underwent Torture. Psychosomatic medicine. 2017;79:655–63. doi: 10.1097/PSY.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 15.Lewandowski W, Good M, Draucker CB. Changes in the meaning of pain with the use of guided imagery. Pain management nursing: official journal of the American Society of Pain Management Nurses. 2005;6:58–67. doi: 10.1016/j.pmn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Forsberg JT, Martinussen M, Flaten MA. The Placebo Analgesic Effect in Healthy Individuals and Patients: A Meta-Analysis. Psychosomatic medicine. 2017;79:388–94. doi: 10.1097/PSY.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 17.Moseley GL, Butler DS. Fifteen Years of Explaining Pain: The Past, Present, and Future. The journal of pain: official journal of the American Pain Society. 2015;16:807–13. doi: 10.1016/j.jpain.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Moseley GL. Joining forces–combining cognition-targeted motor control training with group or individual pain physiology education: a successful treatment for chronic low back pain. The Journal of manual & manipulative therapy. 2003;11:88–94. [Google Scholar]
- 19.Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. European journal of pain. 2004;8:39–45. doi: 10.1016/S1090-3801(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 20.Moseley GL, Nicholas MK, Hodges PW. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. The Clinical journal of pain. 2004;20:324–30. doi: 10.1097/00002508-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Clarke CL, Ryan CG, Martin DJ. Pain neurophysiology education for the management of individuals with chronic low back pain: systematic review and meta-analysis. Manual therapy. 2011;16:544–49. doi: 10.1016/j.math.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Van Oosterwijck J, Nijs J, Meeus M, Truijen S, Craps J, Van den Keybus N, Paul L. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. Journal of rehabilitation research and development. 2011;48:43–58. doi: 10.1682/jrrd.2009.12.0206. [DOI] [PubMed] [Google Scholar]
- 23.Meeus M, Nijs J, Van Oosterwijck J, Van Alsenoy V, Truijen S. Pain Physiology Education Improves Pain Beliefs in Patients With Chronic Fatigue Syndrome Compared With Pacing and Self-Management Education: A Double-Blind Randomized Controlled Trial. Archives of physical medicine and rehabilitation. 2010;91:1153–59. doi: 10.1016/j.apmr.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Arntz A, Claassens L. The meaning of pain influences its experienced intensity. Pain. 2004;109:20–25. doi: 10.1016/j.pain.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Moseley GL, Arntz A. The context of a noxious stimulus affects the pain it evokes. Pain. 2007;133:64–71. doi: 10.1016/j.pain.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain. 2013;154:361–67. doi: 10.1016/j.pain.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Leknes S, Bastian B. The Benefits of Pain. Review of Philosophy and Psychology. 2014;5:57–70. [Google Scholar]
- 28.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PloS one. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruddick S. Maternal Thinking: Toward a Politics of Peace. Beacon Press; 1995. p. 291. [Google Scholar]
- 30.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Koban L, Jepma M, Geuter S, Wager TD. What’s in a word? How instructions, suggestions, and social information change pain and emotion. Neuroscience and biobehavioral reviews. 2017 doi: 10.1016/j.neubiorev.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koban L, Wager TD. Beyond conformity: Social influences on pain reports and physiology. Emotion. 2016;16:24–32. doi: 10.1037/emo0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Current opinion in anaesthesiology. 2007;20:435–39. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- 34.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nature medicine. 2010;16:1277–83. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 35.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:12964–77. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in cognitive sciences. 2012;16:147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckner RL, Carroll DC. Self-projection and the brain. Trends in cognitive sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 39.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenbaum G, Takahashi Y, Liu T-L, McDannald MA. Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 42.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain research bulletin. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 43.Constantinescu AO, O’Reilly JX, Behrens TEJ. Organizing conceptual knowledge in humans with a gridlike code. Science. 2016;352:1464–68. doi: 10.1126/science.aaf0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doré BP, Boccagno C, Burr D, Hubbard A, Long K, Weber J, Stern Y, Ochsner KN. Finding Positive Meaning in Negative Experiences Engages Ventral Striatal and Ventromedial Prefrontal Regions Associated with Reward Valuation. Journal of cognitive neuroscience. 2017;29:235–44. doi: 10.1162/jocn_a_01041. [DOI] [PubMed] [Google Scholar]
- 45.Rangel A, Hare T. Neural computations associated with goal-directed choice. Current opinion in neurobiology. 2010;20:262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:6225–31. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learning & memory. 2007;14:520–24. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo C-W, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, Atlas LY, Wager TD. Quantifying cerebral contributions to pain beyond nociception. Nature communications. 2017;8:14211. doi: 10.1038/ncomms14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashar YK, Chang LJ, Wager TD. Brain Mechanisms of the Placebo Effect: An Affective Appraisal Account. Annual review of clinical psychology. 2017;13:73–98. doi: 10.1146/annurev-clinpsy-021815-093015. [DOI] [PubMed] [Google Scholar]
- 52.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. The New England journal of medicine. 2013;368:1388–97. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Sola M, Koban L, Krishnan A, Wager TD. When pain really matters: A vicarious-pain brain marker tracks empathy for pain in the romantic partner. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Koban L, Kross E, Woo C-W, Ruzic L, Wager TD. Frontal-Brainstem Pathways Mediating Placebo Effects on Social Rejection. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:3621–31. doi: 10.1523/JNEUROSCI.2658-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jepma M, Jones M, Wager TD. The dynamics of pain: evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain. The journal of pain: official journal of the American Pain Society. 2014;15:734–46. doi: 10.1016/j.jpain.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raposa EB, Laws HB, Ansell EB. Prosocial Behavior Mitigates the Negative Effects of Stress in Everyday Life. Clinical psychological science. 2016;4:691–98. doi: 10.1177/2167702615611073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson SK, Layous K, Cole SW, Lyubomirsky S. Do unto others or treat yourself? The effects of prosocial and self-focused behavior on psychological flourishing. Emotion. 2016;16:850–61. doi: 10.1037/emo0000178. [DOI] [PubMed] [Google Scholar]
- 58.Park SQ, Kahnt T, Dogan A, Strang S, Fehr E, Tobler PN. A neural link between generosity and happiness. 2017;8:15964. doi: 10.1038/ncomms15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunn EW, Aknin LB, Norton MI. Spending money on others promotes happiness. Science. 2008;319:1687–88. doi: 10.1126/science.1150952. [DOI] [PubMed] [Google Scholar]
- 60.Aknin LB, Hamlin JK, Dunn EW. Giving leads to happiness in young children. PloS one. 2012;7:e39211. doi: 10.1371/journal.pone.0039211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aknin LB, Barrington-Leigh CP, Dunn EW, Helliwell JF, Burns J, Biswas-Diener R, Kemeza I, Nyende P, Ashton-James CE, Norton MI. Prosocial spending and well-being: cross-cultural evidence for a psychological universal. Journal of personality and social psychology. 2013;104:635–52. doi: 10.1037/a0031578. [DOI] [PubMed] [Google Scholar]
- 62.Shimada S, Matsumoto M, Takahashi H, Yomogida Y, Matsumoto K. Coordinated activation of premotor and ventromedial prefrontal cortices during vicarious reward. Social cognitive and affective neuroscience. 2016;11:508–15. doi: 10.1093/scan/nsv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varnum MEW, Shi Z, Chen A, Qiu J, Han S. When “Your” reward is the same as “My” reward: self-construal priming shifts neural responses to own vs. friends’ rewards. NeuroImage. 2014;87:164–69. doi: 10.1016/j.neuroimage.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 65.Apps MAJ, Rushworth MFS, Chang SWC. The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron. 2016;90:692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:705–15. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villemure C, Schweinhardt P. Supraspinal pain processing: distinct roles of emotion and attention. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2010;16:276–84. doi: 10.1177/1073858409359200. [DOI] [PubMed] [Google Scholar]
- 68.Krishnan A, Woo C-W, Chang LJ, Ruzic L, Gu X, López-Solà M, Jackson PL, Pujol J, Fan J, Wager TD. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife [Internet] 2016:5. doi: 10.7554/eLife.15166. Available from: [DOI] [PMC free article] [PubMed]
- 69.López-Solà M, Woo C-W, Pujol J, Deus J, Harrison BJ, Monfort J, Wager TD. Towards a neurophysiological signature for fibromyalgia. Pain. 2017;158:34–47. doi: 10.1097/j.pain.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andreoni J. Impure Altruism and Donations to Public Goods: A Theory of Warm-Glow Giving? Economic journal. 1990;100:464–77. [Google Scholar]
- 71.D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in human neuroscience. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blair KS, Otero M, Teng C, Jacobs M, Odenheimer S, Pine DS, Blair RJR. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. NeuroImage. 2013;78:103–10. doi: 10.1016/j.neuroimage.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waytz A, Zaki J, Mitchell JP. Response of dorsomedial prefrontal cortex predicts altruistic behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:7646–50. doi: 10.1523/JNEUROSCI.6193-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage. 2010;51:1468–75. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 75.Zheng H, Huang D, Chen S, Wang S, Guo W, Luo J, Ye H, Chen Y. Modulating the Activity of Ventromedial Prefrontal Cortex by Anodal tDCS Enhances the Trustee’s Repayment through Altruism. Frontiers in psychology. 2016;7:1437. doi: 10.3389/fpsyg.2016.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hutcherson CA, Bushong B, Rangel A. A Neurocomputational Model of Altruistic Choice and Its Implications. Neuron. 2015;87:451–62. doi: 10.1016/j.neuron.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janowski V, Camerer C, Rangel A. Empathic choice involves vmPFC value signals that are modulated by social processing implemented in IPL. Social cognitive and affective neuroscience. 2013;8:201–8. doi: 10.1093/scan/nsr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashar YK, Andrews-Hanna JR, Dimidjian S, Wager TD. Empathic Care and Distress: Predictive Brain Markers and Dissociable Brain Systems. Neuron. 2017;94:1263–73.e4. doi: 10.1016/j.neuron.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basile B, Mancini F, Macaluso E, Caltagirone C, Frackowiak RSJ, Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Human brain mapping. 2011;32:229–39. doi: 10.1002/hbm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 81.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:951–56. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:2188–92. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu H, Cai Q, Shen B, Gao X, Zhou X. Neural substrates and social consequences of interpersonal gratitude: Intention matters. Emotion. 2017;17:589–601. doi: 10.1037/emo0000258. [DOI] [PubMed] [Google Scholar]
- 84.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in cognitive sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 85.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of cognitive neuroscience. 2012;24:1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooper JC, Kreps TA, Wiebe T, Pirkl T, Knutson B. When giving is good: ventromedial prefrontal cortex activation for others’ intentions. Neuron. 2010;67:511–21. doi: 10.1016/j.neuron.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adolphs R. The social brain: neural basis of social knowledge. Annual review of psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain: a journal of neurology. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 90.Decety J, Cowell JM. The complex relation between morality and empathy. Trends in cognitive sciences. 2014;18:337–39. doi: 10.1016/j.tics.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “Affective Theory of Mind” Is Associated with Right Ventromedial Prefrontal Damage. Cognitive and behavioral neurology: official journal of the Society for Behavioral and Cognitive Neurology. 2005;18:55. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- 92.Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. Journal of cognitive neuroscience. 2003;15:324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- 93.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature neuroscience. 1999;2:1032–37. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 94.Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–11. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moretto G, Sellitto M, di Pellegrino G. Investment and repayment in a trust game after ventromedial prefrontal damage. Frontiers in human neuroscience. 2013;7:593. doi: 10.3389/fnhum.2013.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woo C-W, Roy M, Buhle JT, Wager TD. Distinct Brain Systems Mediate the Effects of Nociceptive Input and Self-Regulation on Pain. In: Posner M, editor. PLoS biology. Vol. 13. 2015. p. e1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ochsner KN, Gross JJ. Cognitive Emotion Regulation: Insights from Social Cognitive and Affective Neuroscience. Current directions in psychological science. 2008;17:153–58. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nature reviews Neuroscience. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- 100.Rainville P. Brain mechanisms of pain affect and pain modulation. Current opinion in neurobiology. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 101.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 102.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional …. Journal of Neuroscience [Internet] 2003 doi: 10.1523/JNEUROSCI.23-25-08771.2003. Available from: http://www.jneurosci.org/content/23/25/8771.short. [DOI] [PMC free article] [PubMed]
- 103.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural brain research. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 104.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:6610–14. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159–71. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


