Abstract
Radiotherapy plays an important role in curative and palliative cancer treatment. As a novel radiation delivery technique, stereotactic radiotherapy (SRT) utilizes 3D-conformal treatment planning, high-precision beam delivery technology and patient specific position verification to target tumors, often in one to five high-dose fractions. Currently, there is no consensus about best SRT practices in veterinary radiotherapy. The objective of this study was to document the breadth of perspectives, techniques and applications of SRT in veterinary medicine. We conducted an online survey of ACVR members specializing in radiation oncology to assess how, when and why SRT is being used. Both SRT users and non-users completed the survey. The overall response and survey completion rates were 54% (67/123) and 87% (58/67), respectively. Fifty-five percent of respondents reported providing SRT at their facility, with a median of 4.5 canine cases and 1 feline case per month. Delivery methods included C-arm linear accelerator with multi-leaf collimator, helical tomotherapy and CyberKnife. Non-pituitary intracranial tumors, pituitary tumors and sinonasal tumors were the most common cancers treated using SRT in both species. The most common fractionation scheme was 3 fractions of 10 Gy/fraction. The results of this survey suggest common availability of SRT in veterinary radiation facilities. These results provide valuable information regarding current SRT practices in veterinary medicine, and may provide an initial step towards standardizing methods and establishing consensus guidelines.
Keywords: radiotherapy, radiosurgery, hypofractionated, intensity-modulated, oncology
Introduction
Radiation therapy has played a significant role in the treatment of cancer in veterinary patients. The various technologies utilized to deliver radiation therapy have evolved over time, beginning with non-computerized manual radiation therapy to newer methods such as intensity-modulated radiation therapy, image-guided radiotherapy, and the focus of this study, stereotactic radiation therapy (SRT). SRT is a novel method of radiation delivery that integrates 3D-imaging, advanced computerized treatment planning and specialized beam delivery technologies so that conformal hypofractionated radiation protocols can be delivered in a relatively short period of time, whilst sparing normal tissue. The goal of delivering large radiation doses to the tumor is to achieve a greater antitumor biological effect than can be achieved through conventional fractionation.1–3 Another advantage of SRT is the convenience of completing radiation therapy in a limited number of fractions usually within one week, compared to conventional approaches that often involve daily treatments over multiple weeks.
In 2001, a study reported that 80% of veterinary radiation plans were manually calculated whilst 20% were computer planned.4 Since that time, there has been rapid advancement in the technology used to plan and deliver radiation treatments. A more recent survey of veterinary radiation practices performed in 2010 indicated that 92% of facilities used 3D computerized planning with a median of 50% of plans being computer generated (range: 20–100%).5 In the 2010 survey, 28% of facilities indicated the capacity to deliver SRT but details of the planning, quality assurance and treatment techniques utilized were not reported.5 Over the past few years, a number of manuscripts have been published suggesting a more widespread adoption of SRT.6–21
There is limited knowledge regarding current SRT practices in veterinary medicine. To continue advancing SRT in veterinary oncology, a better understanding of how it is being used is needed. Differences in practices among clinicians and institutions represent potential hurdles to the widespread implementation of SRT in clinical practice, particularly with respect to interpretation and repeatability of reported outcomes. To address this need, an online survey of American College of Veterinary Radiology (ACVR) members specializing in radiation oncology was conducted to assess how, when and why SRT is being used. We hypothesized that there is wide variation in SRT usage among veterinary radiation oncology clinicians. We aimed to characterize the variation in terms of equipment types, treatment planning techniques, treatment indications, fractionation schemes and quality assurance methods.
Materials and Methods
Survey questions were developed to assess SRT usage in veterinary medicine. Questions were distributed to participants via online survey using Qualtrics software (Qualtrics®, Provo, Utah). The survey was available from August 16th 2016 to October 22nd 2016. Prior to distribution, six clinicians involved in SRT planning and delivery at our facility completed the survey with the aim of providing feedback. The survey design consisted of initial questions regarding demographics and general radiation therapy equipment questions that all participants were asked. All participants were asked to provide their definition of the term ‘stereotactic radiotherapy’. Skip logic was then used to direct respondents to specific questions based on whether or not they use SRT techniques in their practice. This allowed participants to be designated as SRT users and non-SRT users. Non-SRT users were asked a total of 12 questions, while SRT users were asked a total of 36 questions. Question format included both closed and open-ended questions. Many questions allowed more than one response. Open text space was provided for participants who selected a response of ‘other’, for non-multiple choice questions that required free text, or for participants who required free text to justify their selection.
The survey questions specific to SRT users were divided into sections on personnel, radiation system/technology, delivery techniques, treatment indications, treatment planning techniques including target delineation, fractionation schemes, patient position verification, machine quality assurance, treatment plan quality assurance, medical physics support, and overall satisfaction with SRT and other utilized radiation therapy technologies. Non-SRT users also answered questions regarding satisfaction with radiation technologies they employed. Additionally, non-SRT participants were asked why SRT had not been adopted, and if applicable, what may motivate them to adopt SRT in the future. Both SRT and non-SRT participants had an open text comment section at the end of the survey to provide feedback, clarify answers or ask questions.
Selection and Description of Subjects
Participants for the online survey were identified using the ACVR member directory, which provided 123 members listed under the specialty of Radiation Oncology in August 2016. An email was sent to each member requesting survey participation and providing an anonymous, individualized link to access the survey. Individuals listed in the member directory included board certified veterinarians (single, dual and triple board-certified Diplomates), veterinarians trained in radiation oncology without board certification and radiation oncology residents. The survey was distributed online to ACVR members in the United States, Canada, Europe and Australia/Asia Pacific. Both private practices and facilities associated with a university were represented.
Data Recording and Analysis
Data was collected for each question from completely and partially completed surveys. Analysis was performed based on number of survey respondents for each individual question. Many questions allowed multiple selections when answering. These data are presented by providing the number of respondents selecting a specific option as the numerator and the denominator indicating the total number of respondents answering the question. For questions with multiple components requiring numerical data input, it was assumed that if at least one answer was given, all non-answered components were zero. When a respondent reported a range of numeric values, the mean of the range was used for analysis. Data were maintained and analyzed using Qualtrics® survey software and exported to spreadsheet (Microsoft Excel 2016 (Seattle, WA)) and statistical (R version 3.4.2, R Foundation for Statistical Computing (http://www.R-project.org)) software for further analysis. Descriptive statistics were calculated, including counts, percentages, medians, and ranges and/or interquartile ranges (IQRs). Fisher exact tests were used to assess SRT use in academia versus private practice, to compare personnel involved in delivering radiation therapy between practices using and not using SRT, and to compare use of PTV contouring in practices using planar-only versus volumetric imaging. A linear mixed effects model was used to assess satisfaction with SRT versus non-SRT delivery techniques among SRT users, with a random effect to account for intra-subject dependence between responses. The definitions of SRT provided in free text format were analyzed based on frequency of equivalent or near equivalent key terms, and then grouped according to the defining terms used in the American Association of Physicists in Medicine (AAPM) Task Group 101 (TG 101) report22 The key terms used from TG 101 included: high target dose, accurate radiation delivery, conformity and dose heterogeneity.
Results
The overall response rate for the survey was 54% (67/123) and the completion rate was 87% (58/67).
Characteristics of Survey Respondents
The majority of survey respondents were located in the United States (58/66, 88%), while responses also came from Canada (2/66, 3%), Europe (5/66, 8%), and Australia/Asia Pacific (1/66, 2%). Respondents classified their veterinary facilities as private practices (34/67, 51%), academic (university-based) (31/67, 46%) or both (2/67, 3%). Eighty-five percent (56/66, 85%) of respondents held board certification in Radiation Oncology. Remaining respondents included nine DVMs without board certification (9/66, 14%) and one respondent with board certification in Diagnostic Imaging (1/66, 2%). The median number of years that participants have worked in radiation therapy was 10 (IQR: 5–14.5). The most common types of radiotherapy (RT) offered at the practices of the respondents are shown in Table 1. Fifty nine percent (39/66) of respondents reported having intensity-modulated RT capabilities (IMRT). Of those with IMRT, 92% (36/39) performed SRT at their facility.
Table 1.
The types of radiation therapy provided by Stereotactic Radiation Therapy and non-Stereotactic Radiation Therapy users.a
| Radiation Therapy Type | Number of Responses (% of 66 Respondents) |
|---|---|
| 3D-conformal | 58 (88%) |
| Non-computerized (manual) | 47 (71%) |
| Intensity-modulated | 39 (59%) |
| Stereotactic | 36 (55%) |
| Image-guided with on-board planar imaging (KV or MV) | 32 (48%) |
| Image-guided with on-board CT or camera guidance | 24 (36%) |
| Cobalt-60 | 0 |
| Proton beam | 0 |
| Radiation therapy not provided at facility | 1 (2%) |
| Other | 14 (21%) |
“Other” types of radiation therapy provided include electron beam therapy, high-dose rate brachytherapy, Strontium-90, and image-guided with port films.
One respondent reported a future upgrade to image-guided radiation therapy. KV=kilovoltage and MV=megavoltage.
Stereotactic Radiation Therapy Use
Of 66 respondents providing information on SRT usage, 36 (55%) reported performing SRT at their facility (“SRT users”), 22 (33%) did not provide SRT but referred cases to other facilities for SRT, and 8 (12%) neither provided SRT nor referred for it. Seventeen respondents reported that they performed remote treatment planning and, among these individuals, 41% (7/17) have created SRT plans for delivery at a distant practice. Table 2 shows the study population characteristics for SRT and non-SRT users. SRT use was more prevalent in academia (including combined academic/private facilities) than in private practice-only facilities (22/32, 69% vs 14/34, 41%, p=0.03). The median number of years of SRT experience reported by 31 respondents was 4 (IQR: 2–5.5). The median number of canine patients treated using SRT was 4.5 canine patients per month (Range: 1–20 cases/month) and 1 feline patient per month (Range: 0–8 cases/month). Other species treated by SRT included rabbits, ferrets, reptiles and goats.
Table 2.
Demographics for Stereotactic Radiation Therapy Users and Non-Stereotactic Radiation Therapy Users are shown in regard to geographic location, practice type and board certification(s) of respondents.b
| Number of SRT Users (% of Respondents) | Number of Non-SRT Users (% of Respondents) | |
|---|---|---|
| Geographic Location | ||
| United States | 31 (54%) | 26 (46%) |
| Canada | 2 (100%) | 0 (0%) |
| Europe | 2 (40%) | 3 (60%) |
| Australia/Asia Pacific | 1 (100%) | 0 (0%) |
| Practice Type | ||
| Academic | 20 (67%) | 10 (33%) |
| Private | 14 (41%) | 20 (59%) |
| Academic and Private | 2 (100%) | 0 (0%) |
| Board Certification | ||
| Radiation Oncology (only) | 18 (62%) | 11 (38%) |
| Radiation Oncology + Other | 14 (54%) | 12 (46%) |
| Non-Radiation Oncology | 0 (0%) | 1 (100%) |
| DVM Without Board Certification | 4 (44%) | 5 (56%) |
SRT, stereotactic radiation therapy.
The percentages of SRT users given in parenthesis is based on 36 respondents while the percentages of non-SRT users given in parenthesis is based on 29 respondents for geographic location and board certification and 30 respondents for practice type.
Personnel Involved in Radiotherapy Delivery
For SRT and non-SRT users, participants were asked which of the following personnel categories are actively involved RT delivery: radiation oncologist, radiologist, DVM (non-boarded and non-resident), medical physicist, anesthesiologist, resident (radiation oncology, radiology or anesthesia), human trained radiation therapist, veterinary technician, anesthesia certified technician, or other. Being actively involved in RT delivery was defined as being involved in radiation treatment planning, quality assurance and/or radiation delivery.
All SRT users (36/36) and 90% (26/29) of non-SRT users reported that a board-certified radiation oncologist was involved in RT delivery. All SRT users (34/34) had a medical physicist in some capacity compared to sixty-two percent of non-SRT users (18/29). SRT users were more likely than non-SRT users to have a boarded anesthesiologist involved in delivering radiation (12/36=33% versus 0/29=0%, p<0.001).
Defining Stereotactic Radiation Therapy
To determine how clinicians characterize SRT in veterinary medicine, all participants (SRT and non-SRT users) were asked to define SRT in an open-ended, free text survey question. Sixty-four respondents (96%) provided SRT definitions. Table 3 shows the terms or concepts used by the respondents, and Table 4 shows how these terms were grouped for analysis. Twenty-two respondents (34%) defined SRT in accordance with three of four major terms used by the AAPM,22 including high tumor dose, accuracy of radiation delivery, and conformity. The number of respondents dropped to only two (3%) when dose heterogeneity was added as a defining term. Five respondents who included treatment intent as part of their definition of SRT used the term ‘ablative’, and one also referred to palliation as the goal of SRT. Thirty-five respondents defined SRT by the number of fractions in a protocol, with most listing between 1 and 3 fractions. Daily treatments were reported most frequently followed by every other day treatment. Twelve respondents said that SRT includes protocols with greater than 3 fractions, and 3 respondents said that protocols may include greater than 5 fractions. Two respondents suggested that the term SRT can be used to describe conventionally fractionated schedules for highly accurate and conformal plans. Six respondents commented that the current use of SRT in veterinary medicine is different than in human medicine with respect to treatment intent, indications, and radiation prescriptions.
Table 3.
Respondent SRT Definitions Analyzed for Most Frequent Terms and Concepts
| SRT Definition Term or Concept | Number of Respondents (% of 64 Total Respondents) |
|---|---|
| Hypofractionation | 41 (64%) |
| Conformal | 36 (56%) |
| Number of fractions | 35 (55%) |
| Accuracy | 25 (39%) |
| Image-guidance | 24 (37%) |
| High target dose | 24 (37%) |
| Target localization | 16 (25%) |
| Steep dose gradient | 15 (23%) |
| Margin definition | 11 (17%) |
| Body location | 9 (14%) |
| Precision delivery | 6 (9%) |
| Tumor size/shape limitations | 5 (8%) |
| Goal/treatment intent | 5 (8%) |
| Specialized treatment planning | 4 (6%) |
| Delivery system | 4 (6%) |
| Dose heterogeneity | 3 (5%) |
| High dose rate | 2 (3%) |
Table 4.
Respondent Stereotactic Radiation Therapy Terms and Concepts Grouped According to American Association of Physicists in Medicine Definition.c
| AAPM Defining Terms of SRT22 | Corresponding Terms or Concepts from Table 3 used by Respondents | Number of Respondents (% of 64 Total Respondents) | |
|---|---|---|---|
| A | High target dose | High target dose and/or hypofractionation | 49 (77%) |
| B | Accurate radiation delivery | Image-guidance and/or accuracy and/or target localization and/or precision delivery | 45 (70%) |
| C | Conformity | Conformal and/or steep dose gradient | 38 (59%) |
| D | Dose heterogeneity | Dose heterogeneity | 3 (3%) |
| A + B + C | 22 (34%) | ||
| A + B + C +D | 2 (2%) | ||
SRT, stereotactic radiation therapy; AAPM, American Association of Physicists in Medicine.
The number of respondents using each of the AAMP’s four criteria for defining SRT was analyzed.
Stereotactic Radiation Therapy Delivery Systems, Treatment Techniques
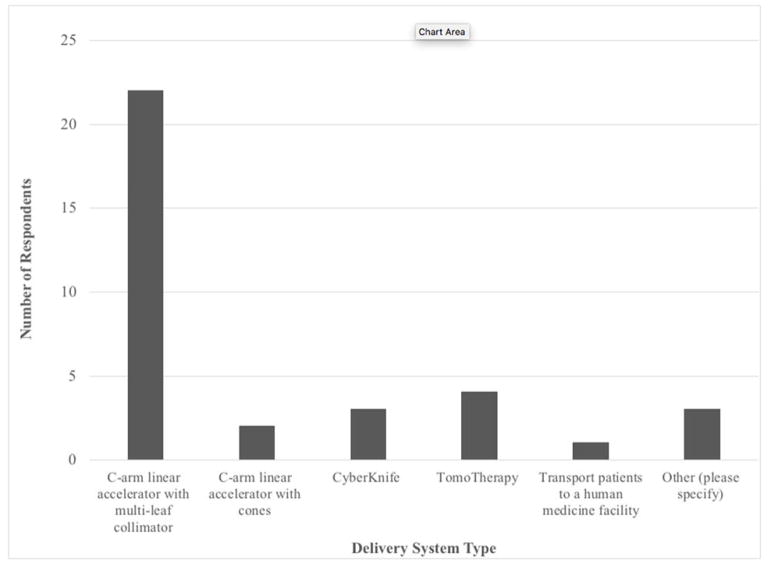
The most common system used to deliver SRT was a C-arm linear accelerator equipped with a multileaf collimator (21/32, 66%). Less frequently utilized systems included helical tomotherapy (4/32, 13%) (Accuray, Sunnyvale, CA), CyberKnife (3/32, 9%) (Accuray, Sunnyvale, CA), and C-arm linear accelerator with cones (2/32, 6%) (Figure 1). A majority of SRT users cited dynamic delivery as the technique used for SRT (17/32, 53%). Segmental delivery (6/32, 19%), helical tomotherapy (4/32, 13%), CyberKnife (4/32, 13%), volumetric modulated arc therapy (3/32, 9%), and non-modulated arc therapy (3/32, 9%) were less common techniques. Seventy-five percent (24/32) reported using only coplanar beam geometries, while 25% (8/32) reported having used non-coplanar beams.
Figure 1. Radiation Delivery Systems Used for SRT.
Radiation delivery systems used for SRT are reported. Of the three respondents reporting “other,” two did not specify and one stated linear accelerator with no other specifications.
Stereotactic Radiation Therapy-treated Tumor Types
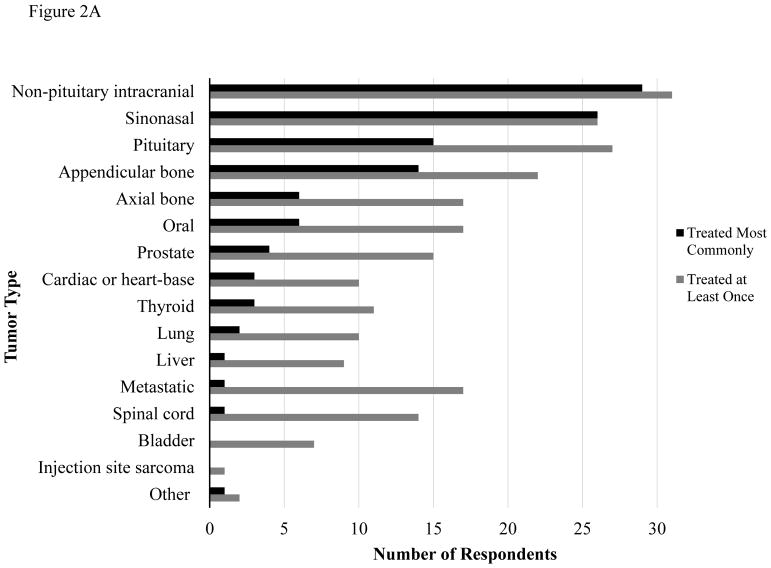
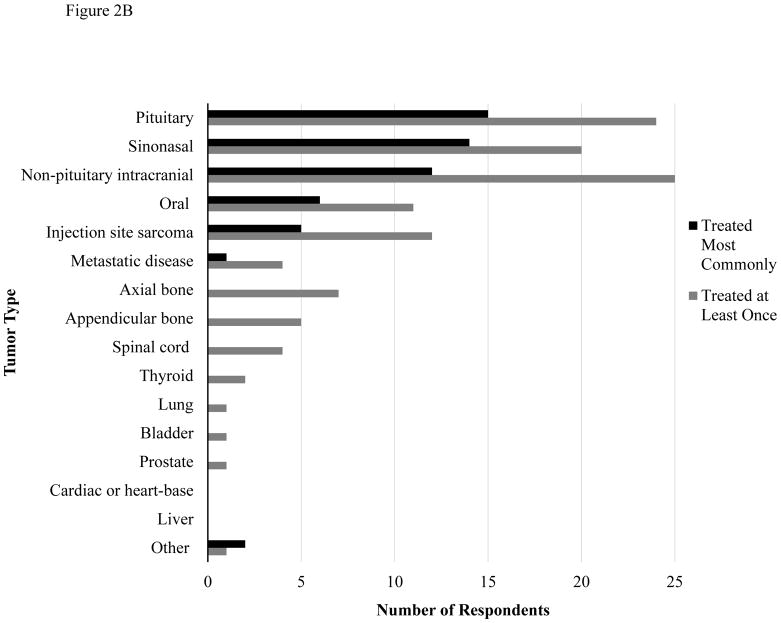
Respondents were asked to list the most common tumor types treated with SRT in their practice, as well as the tumor types that they have treated at least once (Figure 2A for dogs and Figure 2B for cats). The most frequently treated tumor types in both canine and feline patients included non-pituitary intracranial tumors, sinonasal tumors and pituitary tumors. Respondents also indicated the settings in which a tumor type would be treated with SRT, including treatment of gross disease, incomplete resection with residual microscopic disease, and re-treatment of previously irradiated tumors. SRT was most frequently employed for treatment of gross disease or re-treatment of previously irradiated tumors. There were six respondents who reported treating residual microscopic disease following incomplete resection for at least one tumor type. Treatment of microscopic disease was reported most commonly for non-pituitary intracranial tumors (4/6), metastatic disease (3/6) and sinonasal tumors (3/6). One respondent treated microscopic disease for every tumor type provided except injection site sarcoma. This individual did not define SRT in terms of hypofractionation or high target dose and was using 18–19 fractions of 2.5–3 Gy for most tumor types. Conversely, the five other respondents who treat microscopic disease considered SRT to be high dose and hypofractionated.
Figure 2.
Figure 2A. Tumor Types Treated with SRT in Dogs
Figure 2B. Tumor Types Treated with SRT in Cats
Tumor types treated with SRT in dogs (Fig 2A) and cats (Fig 2B). “Other” tumor types treated included soft tissue sarcomas (dogs), periorbital tumors (cats), anal sac adenocarcinoma, renal tumors and retroperitoneal tumors (unspecified if dog or cat).
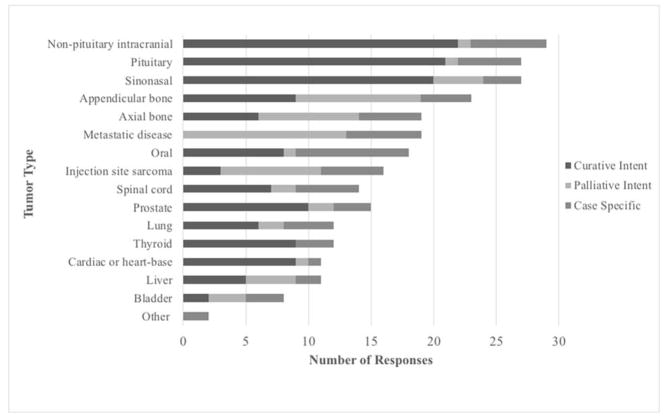
Respondents classified treatment intent for a tumor type as curative, palliative, or case specific (Figure 3). Curative intent was most commonly cited as the treatment goal for the majority of tumor types, particularly for non-pituitary intracranial tumors, pituitary tumors and sinonasal tumors. Tumor types with nearly equal numbers of respondents considering SRT treatment curative or palliative included axial and appendicular bone tumors, liver tumors and bladder tumors. Bladder tumors were the only tumor type where the majority of respondents cited they would not treat with SRT (23/30, 77%). Ten or greater respondents indicated they would not treat oral tumors, spinal cord tumors, injection site sarcomas, thyroid tumors, cardiac or heart-based tumors, bladder tumors, and prostate tumors.
Figure 3. Treatment Goal by Tumor Type.
For a given tumor type, respondents reported the typical treatment goal. For “other” tumor types, one respondent reported case-specific treatment goals for periorbital tumors and one respondent reported case-specific for soft tissue sarcomas.
Stereotactic Radiation Therapy Treatment Planning
When performing SRT treatment planning, respondents determined normal tissue dose constraints based on available human literature and guidelines (31/32, 97%), available veterinary literature (23/32, 72%), and clinical experience (24/32, 75%). Respondents also provided typical target volume contouring methods utilized for SRT cases, in terms of gross tumor volume (GTV - extent of tumor visible on imaging), clinical target volume (CTV – possible microscopic disease) and planning target volume (PTV – margin to account for random and systemic delivery error). Twenty-eight percent (9/32) contoured GTV, CTV and PTV, and 28% (9/32) only contoured GTV. In terms of CTV contouring, 9% (3/32) always contoured CTV, 50% (16/32) sometimes contoured CTV, and 19% (3/32) never contoured CTV. For a given tumor type, 50% (16/32) stated that the CTV may differ in cases treated with SRT compared to a standardly fractionated protocol. As well, 62% (20/32) always contoured a PTV (20/32) and 10% (3/32) never contoured a PTV. Interestingly, SRT users with only planar imaging capabilities were less likely than those with volumetric or both planar and volumetric imaging to contour a PTV at least sometimes (8/13=62% versus 18/19=95%, respectively; p=0.03).
Stereotactic Radiation Therapy Treatment Protocols
Treatment protocols, including fraction number, dose in Gray (Gy) per fraction, and schedule provided by respondents for each tumor type are listed in Supplement 1. The treatment protocols for each tumor type were analyzed in terms of the mode for fraction number and dose per fraction (Table 5). The range for fraction number was 1–5, with the exception of two respondents (2/30) who utilized up to 20 fractions for multiple tumor types. The maximum Gy per fraction reported was 25 Gy, which was used by one respondent for appendicular bone tumors, and by another respondent for metastatic disease.
Table 5.
Stereotactic Radiation Therapy Protocols, by Tumor Typed
| Tumor Type | Number of Fractions (Mode) | Gy Per Fraction (Mode) | Number of Responses |
|---|---|---|---|
| Liver | 3 | 8 | 10 |
| Lung | 3 | 8 | 12 |
| Non-pituitary Intracranial | 3 | 8 | 29 |
| Pituitary | 3 | 8 | 27 |
| Spinal Cord | 3 | 8 | 14 |
| Bladder | 3 | 9 | 6 |
| Appendicular Bone | 3 | 10 | 21 |
| Axial Bone | 3 | 10 | 18 |
| Cardiac or Heart-based | 3 | 10 | 10 |
| Metastatic Disease | 3 | 10 | 16 |
| Oral | 3 | 10 | 15 |
| Prostate | 3 | 10 | 15 |
| Sinonasal | 3 | 10 | 27 |
| Thyroid | 3 | 10 | 11 |
| Injection Site Sarcoma | 3 | 12 | 12 |
SRT, stereotactic radiation therapy; Gy, gray.
SRT users provided treatment protocols for select tumor types. For each tumor type, the modes for number of fractions and dose per fraction in Gray are provided along with the number of responses for that tumor type.
Patient Immobilization and Respiratory Motion Management
Seventy-six percent (22/29) reported utilizing various approaches at least once in their practice to control for respiratory motion. The most commonly used approach was contouring an Internal Target Volume (ITV – a margin applied to the CTV to account for uncertainty in the size, shape or position of the CTV)23 or a larger PTV (14/22, 64%). Other techniques including abdominal compression, advanced anesthetic ventilation (e.g. jet ventilation), real-time tumor tracking, respiratory gating, respiratory paralysis or 4D CT were used at least once by 86% (19/22) of respondents reporting control of respiratory motion. No modification for respiratory motion was reported by 24% (7/29).
Patient Position Verification for Stereotactic Radiation Therapy
The three most common patient imaging tools available on delivery systems used for SRT were megavoltage portal imaging (20/32, 63%), kilovoltage portal imaging (15/32, 47%) and kilovoltage cone beam CT (14/32, 44%). Respondents described when volumetric (CT) or planar (KV or MV) imaging would be used for image-guided localization in SRT patients, and if it was dependent on the tumor location. Fifty percent (13/26) reported the use of volumetric imaging exclusively or almost exclusively regardless of tumor type when SRT is used. Thirty-one percent (8/26) utilized only planar imaging, with all specifying only having planar capabilities. Additionally, two of these respondents (2/8) specified that only certain tumor types (head and neck) were considered for SRT due to lack of volumetric imaging. Three (3/26) respondents routinely used different imaging protocols based on the tumor site. The following distinctions were made by these respondents: planar imaging for bony targets with PTV and volumetric for soft tissue or bony targets without PTV; planar imaging for bony tumors and volumetric for soft tissue or some bony tumors; planar imaging for intracranial tumors and concurrent planar and volumetric for extra-cranial tumors. Two respondents utilized both planar and volumetric imaging together for all tumor types. Respondents also reported which personnel perform position verification prior to SRT delivery. Personnel included veterinary radiation oncologist (30/30, 100%), veterinary radiation oncology resident (10/13, 77%), human medicine radiation therapist (11/16, 67%), veterinary technician (4/26, 15%) and medical physicist (2/24, 8%). None of the 30 respondents reported that patient position verification was not always performed.
Stereotactic Radiation Therapy Quality Assurance
All respondents (29/29) reported that their treatment unit undergoes quality assurance in accordance with Task Group 142 or Task Group 148 (helical tomotherapy). Furthermore, 92% (22/24) reported their on-board imaging system undergoes quality assurance in accordance with Task Group 142. Quality assurance on SRT treatment plans was done utilizing diode array (16/30, 53%), point dosimetry (phantom and ionization chamber) (10/30, 33%), on board imaging (portal) dosimetry based (6/30, 20%), radiographic film (4/30, 13%), and gafchromic film (3/30, 10%). Seventeen percent (5/30) reported that quality assurance was not always performed on SRT plans. The majority of respondents reported use of a single modality with 30% (9/30) stating use of two or more modalities. Furthermore, respondents reported criteria deemed acceptable for an SRT plan to “pass” quality assurance testing: a minimum of 95% gamma for a 3-mm distance to agreement and a 3% absolute dose difference (7/29, 24%), a minimum of 95% gamma for a 2-mm distance to agreement and a 2% absolute dose difference (9/29, 31%), a minimum of 95% gamma for a 1-mm distance to agreement and a 1% absolute dose difference (2/29, 7%). Thirty-one percent (9/29) did not know which benchmark was utilized but all of these respondents cited a medical physicist involved in plan quality assurance amongst other roles. Some respondents also indicated that their quality assurance acceptance criteria varied by case and selected more than one category.
Stereotactic Radiation Therapy User Satisfaction Assessment
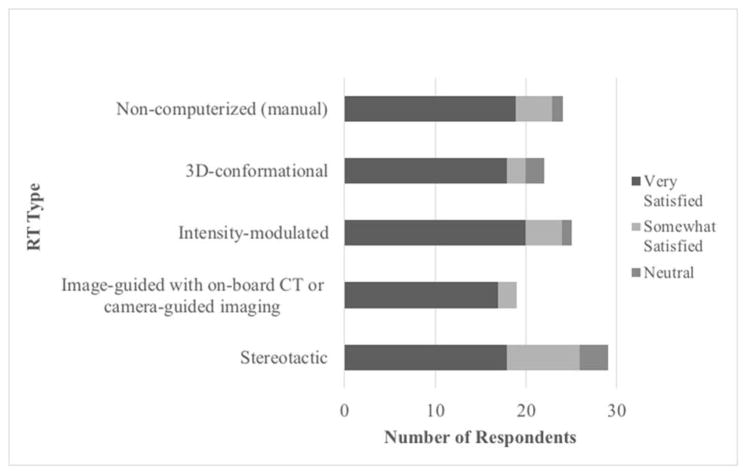
SRT users were asked to report overall satisfaction with five different radiation therapy techniques (Figure 4). Sixty-two percent (18/29) were “very satisfied” with SRT, 28% (8/29) were “somewhat satisfied”, and 10% (3/29) were “neutral.” Seventy-nine percent of respondents (19/24) were “very satisfied” with non-computerized (manual) RT, 82% (18/22) with 3D-conformational RT, 80% (20/25) with IMRT and 89% (17/19) with image-guided radiation (IGRT) with on-board CT or camera-guided imaging. Interestingly, amongst SRT users, there was less overall satisfaction with SRT than with other non-SRT forms of radiation therapy (p=0.01).
Figure 4. Overall Satisfaction Assessment by SRT Users of Various Radiation Therapy Types.
SRT users provided overall satisfaction assessment with different radiation therapy types. No respondent reported being “somewhat dissatisfied” or “very dissatisfied” with any radiation therapy type.
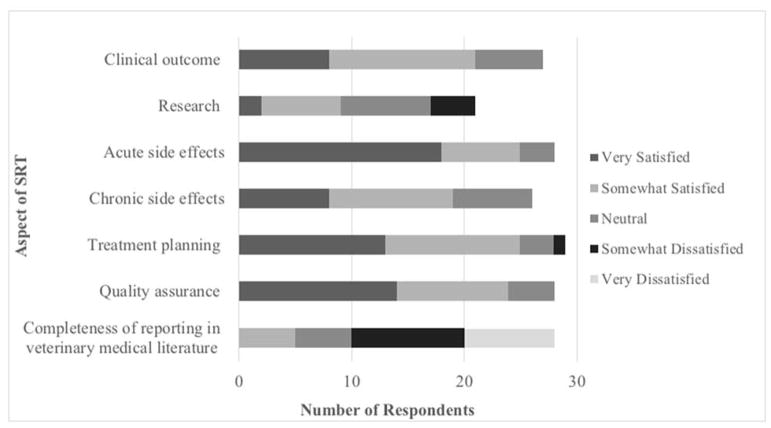
SRT users were asked to indicate their level of satisfaction with specific aspects of SRT, (Figure 5). A majority of SRT users were satisfied in regard to acute side effects, quality assurance, and treatment planning. Conversely, dissatisfaction was reported with completeness of reporting of SRT in veterinary medicine literature and research.
Figure 5. SRT User Satisfaction with SRT.
SRT users reported their level of satisfaction with various aspects of SRT.
Non- Stereotactic Radiation Therapy User Satisfaction with Non- Stereotactic Radiation Therapy Techniques and Plans to Adopt Stereotactic Radiation Therapy
Among respondents not currently performing SRT at their facility, satisfaction of alternative radiation therapy techniques was assessed. The radiation techniques with the highest number of “very satisfied” users were IGRT with on-board CT or camera-guided imaging (2/3, 67%), 3D- conformational RT (18/29, 62%) followed by non-computerized (manual) RT (15/28, 54%), and IMRT (4/9, 44%). The main reasons SRT was not adopted was a lack of necessary equipment (27/30, 90%), followed by a lack of data suggesting clinical benefit (7/30, 23%) and satisfaction with non-SRT techniques (6/30, 20%). Eighty-three percent (25/30) of respondents reported considering adopting SRT in the future. The most commonly reported motivations to adopt SRT were convenience for the client (22/25, 88%), competitive advantage in the veterinary oncology marketplace (18/25, 72%), potential improved clinical outcome for patients (14/25, 56%), opportunity for re-treatment of previous irradiated tumors (11/25, 44%), and clinic financial profit (10/25, 40%).
Comments from Non- Stereotactic Radiation Therapy Users
Some non-SRT users (6 out of 8 non-SRT users providing comments) expressed concern about the current use of SRT in veterinary medicine. Concerns included inappropriate selection of cases (based on tumor size, shape and location), minimal published information on dose, fractionation, planning, radiobiological research into normal tissue tolerance or response, and high costs of utilizing the technology. Concerns regarding how SRT was presented to clients was also reported. In particular, it was stated that the term “curative intent” can be misleading to clients, that prognosis may be overstated and that client education regarding the potential for late side effects may be insufficient. Respondents also requested clearer definitions of SRT and improved transparency amongst the radiation oncology community in order to improve SRT research and the use of scientific data to guide clinical decisions.
Comments from Stereotactic Radiation Therapy -Users
Similar to non-SRT users, SRT participants expressed a desire to further share data regarding SRT side effects, treatment outcomes, client satisfaction and to carefully evaluate the advantages and disadvantages of providing this approach in order to provide better veterinary care. Concern was also raised regarding misuse of SRT in less appropriate tumors/sites or for financial motivation rather than patient benefit. The need for SRT treatments to be supported by scientific data was also emphasized.
Discussion
This is the first published survey of SRT use in veterinary medicine. It provides important information about current standards of practice and the perspectives of respondents who offer SRT. The overall survey response rate was 54%, similar to that of a 2010 survey of veterinary radiation facilities.5 In our survey, 59% of respondents reported having IMRT capabilities and the majority (92%) of IMRT users perform SRT. Overall, 55% (n=36) of respondents identified as SRT users, suggesting an increase in SRT use compared to 2010 when 28% of facilities indicated the capacity to deliver SRT. Our survey was distributed to individuals, not facilities, so some facilities may be represented more than once. SRT users had a median of 4 years of experience with SRT. They were more likely to work in academia than in private practice. The most common SRT delivery system was a C-arm linear accelerator equipped with a multileaf collimator (69%) using dynamic IMRT delivery. Helical tomotherapy and Cyberknife were also represented. Dogs were more commonly treated with SRT than cats, which likely reflects a higher canine caseload at most facilities.
The survey showed that within the veterinary community there is a lack of consensus regarding how SRT should be defined. SRT was most commonly characterized in terms of hypofractionation, conformity and number of fractions with most respondents listing between 1-3 fractions. In 2010, a task group established by the American Association of Physicists in Medicine (AAPM)22 stated that in human medicine SRT can be distinguished from conventional radiation therapy by the use of hypofractionated/high dose, highly conformal and accurately delivered beams. It stated that dose distribution to the tumor is considered to be inhomogeneous in order to obtain rapid fall-off from the target and to minimize normal tissue toxicity.22 Only two survey respondents included all of these concepts cited by the AAPM when asked to provide their definition of SRT. About 1/3 of respondents defined SRT using three of the four AAPM terms, including high target dose, conformity and accurate radiation delivery. Interestingly, two respondents said that fractionation is not relevant to the definition of SRT, and that conventionally fractionated protocols apply. Some respondents commented that SRT in veterinary medicine differs from that in human radiotherapy. These results show disparity in the definition of SRT in veterinary medicine, and speaks to the importance of standardized reporting of SRT treatments in the literature to ensure effective communication among clinicians.24,25
Despite the lack of SRT definition consensus, it is notable that the majority of respondents utilized similar SRT treatment protocols in terms of number of fractions, dose per fraction and treatment schedule. The most common dose prescription for all but one tumor type was three fractions of 8–10 Gy/fractions given daily or every other day. The survey demonstrated key differences in terms of other aspects of SRT, including tumor types treated, perspectives about treatment intent (curative vs. palliative), quality assurance standards, acceptance criteria and contouring techniques. With respect to disparity in tumor delineation, survey findings showed that fifty percent of SRT users modify the CTV according to the treatment protocol being used (SRT vs. conventional fractionation), which underlines the subjectivity of CTV contouring and its potential impact on clinical outcomes. Despite the variable use of a PTV, survey findings showed that SRT users appreciated the importance of patient position verification to optimize precision in treatment delivery. All respondents reported that patient position verification was always performed at their facility. Although volumetric imaging was generally preferred by SRT users when available, only 59% of SRT users had on-board CT capabilities. It was surprising therefore that SRT users with only planar imaging were less likely than those with volumetric or both planar and volumetric imaging to contour a PTV. When less precise position verification imaging tools are used, it is customary to include a PTV to prevent a geographic miss of the target, especially when highly conformal SRT treatments are used. Also potentially compromising of target coverage, 24% of respondents reported that respiratory motion is not accounted for in their practice.
Due to the complexity of SRT, collaborative studies as they were performed in the past, in which the description of treatment details are limited, will not be possible in the SRT era. Given the disparity in how SRT is applied in veterinary medicine, standardization and description of every step from patient simulation to planning to machine quality assurance will be required for future collaborative SRT efforts. Pooling of data over prolonged time periods for retrospective studies or between institutions will also be problematic unless consensus guidelines are developed and followed.
Use of high dose, hypofractionated radiation in SRT makes it critical that case selection is appropriate. Non-pituitary intracranial tumors, pituitary tumors and sinonasal tumors were the most common cancers treated using SRT in both dogs and cats. Given the low metastatic rate and well-defined nature of these tumors, they are seemingly reasonably suited to aggressive, highly conformal local therapy using SRT. While SRT was most frequently employed to treat gross disease, some respondents reported SRT treatment of microscopic disease after incomplete surgical resections or in the treatment of highly locally invasive tumor types such as injection site sarcoma and bladder tumors. Selecting SRT for treatment of microscopic disease is an emerging application in the human radiation oncology in the setting of CNS metastasis26–30 but its utility in other microscopic settings is unclear.
Thorough and routine quality assurance (QA) of linear accelerators, treatment plans and imaging systems is a necessary measure to minimize error and avoid accidental delivery of high dose radiation to critical organs.31 All respondents (100%) delivering SRT in this study reported routine machine QA and most (92%) reported routine on-board imaging QA. However, 17% of SRT respondents indicated that QA is not always performed on SRT plans. Future collaborative studies of SRT in veterinary medicine will require standardization of case selection, contouring, machine, plan and imaging QA. Establishment or utilization of a body similar to the Imaging and Radiation Oncology Core, whose mission is to provide integrated radiation oncology and diagnostic imaging quality control programs and provide site credentialing support for National Cancer Institute trials, would be a positive step towards multi-institutional collaboration in veterinary radiation oncology.
Amongst SRT users, overall satisfaction with SRT was lower when compared to satisfaction with other RT types. It is important to differentiate if dissatisfaction stems from innate flaws with SRT technology that have led to poor clinical outcome vs. insufficient data to guide when and how to apply the technology. Along these lines, the majority of respondents reported dissatisfaction in terms of completeness of reporting SRT planning, dosing, and delivery in veterinary medical literature. This was in line with SRT user comments that expressed a desire to share data regarding SRT side effects and treatment outcomes to determine advantages and disadvantages of SRT and to identify indications for use. This highlights the importance of developing standardized reporting guidelines and nomenclature to optimize the evaluation and use of SRT in veterinary medicine.
While our survey provided a broad overview of current SRT practices, certain limitations do exist. Only 54% of the 123 people who were contacted responded to the survey. Some respondents started the survey but did not complete it. Completion rate was 87%. Response and completion rates may have been reduced due to the length of the survey. Questions often had multiple components, including open-ended responses or questions possibly requiring some investigation, such as number of canine and feline SRT cases per month. Furthermore, distribution of the survey was by individual rather than facility; most questions were designed to be answered by individuals and may not have been applicable to the practice of the entire facility. This approach could have lead to over-representation of groups who practice together. With these limitations, the data may not fully reflect the status of SRT in veterinary medicine.
The survey relied on respondents’ own definition of SRT to decide whether or not they provide the technology, which then dictated which survey questions were asked. Hence, survey results including prevalence of SRT may be influenced by the varying interpretations of what constitutes SRT.
The aim of this survey was to gain an understanding of current veterinary SRT practices. It revealed consistencies and important differences in our community’s perspectives and approaches to SRT. The study findings emphasize the importance of working collectively to establish best practice guidelines and to continue research efforts to better understand how SRT can be optimized.
Supplementary Material
Supplement 1. Stereotactic Radiation Therapy Treatment protocols for different tumor types. Treatment protocols include dose (Gray (Gy)/fraction), number of fractions and treatment schedule (Note: EOD=every other day). Each respondent is assigned a number that is displayed in the first column of the table on each page.
Acknowledgments
Funding Support: Merial Veterinary Summer Scholars Program 2016, University of Wisconsin-Madison School of Veterinary Medicine; Clinical and Translational Science Award (CTSA) program through NIH National Center for Advancing Translational Services (NCATS), grant UL1TR000427.
Footnotes
List of Author Contributions
- Conception and Design: Elizabeth M. Dunfield, Neil I. Christensen, Michelle M. Turek
- Acquisition of Data: Elizabeth M. Dunfield, Neil I. Christensen, Michelle M. Turek
- Analysis and Interpretation of Data: Elizabeth M. Dunfield, Neil I. Christensen, Kevin A. Buhr, Michelle M. Turek
- Drafting the Article: Elizabeth M. Dunfield, Neil I. Christensen, Michelle M. Turek
- Revising Article for Intellectual Content: Elizabeth M. Dunfield, Neil I. Christensen, Kevin A. Buhr, Michelle M. Turek
- Final Approval of the Completed Article: Elizabeth M. Dunfield, Neil I. Christensen, Kevin A. Buhr, Michelle M. Turek
References
- 1.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6. Philadelphia (PA): Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 3.Prasanna A, Ahmed MM, Mohiuddin M, Coleman CN. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. J Thorac Dis. 2014;6:287–302. doi: 10.3978/j.issn.2072-1439.2014.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEntee MC. A survey of veterinary radiation facilities in the United States during 2001. Vet Radiol Ultrasound. 2004;45:476–479. doi: 10.1111/j.1740-8261.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 5.Farrelly J, McEntee MC. A survey of veterinary radiation facilities in 2010. Vet Radiol Ultrasound. 2014;55:638–643. doi: 10.1111/vru.12161. [DOI] [PubMed] [Google Scholar]
- 6.Dolera M, Malfassi L, Mazza G, et al. Feasibility for using hypofractionated stereotactic volumetric modulated arc radiotherapy (vmat) with adaptive planning for treatment of thymoma in rabbits: 15 cases. Vet Radiol Ultrasound. 2016;57:313–2. doi: 10.1111/vru.12321. [DOI] [PubMed] [Google Scholar]
- 7.Dolera M, Malfassi L, Pavesi S, et al. Volumetric-modulated arc stereotactic radiotherapy for canine adrenocortical tumours with vascular invasion. J Small Anim Pract. 2016;57:710–717. doi: 10.1111/jsap.12592. [DOI] [PubMed] [Google Scholar]
- 8.Dolera M, Malfassi L, Bianchi C, et al. Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet Comp Oncol. 2017;1:236–12. doi: 10.1111/vco.12316. [DOI] [PubMed] [Google Scholar]
- 9.Dolera M, Malfassi L, Bianchi C, et al. Frameless stereotactic volumetric modulated arc radiotherapy of brachial plexus tumours in dogs: 10 cases. Br J Radiol. 2017;90:20160617–10. doi: 10.1259/bjr.20160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolera M, Malfassi L, Pavesi S, et al. Stereotactic Volume Modulated Arc Radiotherapy in Canine Meningiomas: Imaging-Based and Clinical Neurological Posttreatment Evaluation. J Amer Anim Hosp Assoc. 2018;54:77–84. doi: 10.5326/JAAHA-MS-6488. [DOI] [PubMed] [Google Scholar]
- 11.Hansen KS, Zwingenberger AL, Théon AP, Pfeiffer I, Kent MS. Treatment of MRI-Diagnosed Trigeminal Peripheral Nerve Sheath Tumors by Stereotactic Radiotherapy in Dogs. J Vet Intern Med. 2016;8:1–9. doi: 10.1111/jvim.13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gieger TL, Nolan MW. Linac-based stereotactic radiation therapy for canine non-lymphomatous nasal tumours: 29 cases (2013–2016) Vet Compar Oncol. 2017;25:475–478. doi: 10.1111/vco.12334. [DOI] [PubMed] [Google Scholar]
- 13.Glasser SA, Charney S, Dervisis NG, et al. Use of an image-guided robotic radiosurgery system for the treatment of canine nonlymphomatous nasal tumors. J Amer Anim Hosp Assoc. 2014;50:96–104. doi: 10.5326/JAAHA-MS-6024. [DOI] [PubMed] [Google Scholar]
- 14.Griffin LR, Nolan MW, Selmic LE, Randall E, Custis J, LaRue S. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Vet Compar Oncol. 14:e158–e170. doi: 10.1111/vco.12129. [DOI] [PubMed] [Google Scholar]
- 15.Nolan MW, Griffin LR, Custis JT, LaRue SM. Stereotactic body radiation therapy for treatment of injection-site sarcomas in cats: 11 cases (2008–2012) J Amer Vet Med Assoc. 2013;15:526–531. doi: 10.2460/javma.243.4.526. [DOI] [PubMed] [Google Scholar]
- 16.Kubicek L, Vanderhart D, Wirth K, et al. Association between computed tomographic characteristics and fractures following stereotactic radiosurgery in dogs with appendicular osteosarcoma. Vet Radiol Ultrasound. 2016;57:321–330. doi: 10.1111/vru.12351. [DOI] [PubMed] [Google Scholar]
- 17.Kubicek L, Milner R, An Q, et al. Outcomes and prognostic factors associated with canine sinonasal tumors treated with curative intent cone-based stereotactic radiosurgery (1999–2013) Veterinary Radiol Ultrasound. 2016;57:331–340. doi: 10.1111/vru.12349. [DOI] [PubMed] [Google Scholar]
- 18.Mariani CL, Schubert TA, House RA, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol. 2015;13:409–423. doi: 10.1111/vco.12056. [DOI] [PubMed] [Google Scholar]
- 19.Rancilio NJ, Bentley RT, Plantenga JP, Parys MM, Crespo BG, Moore GE. Safety and feasibility of stereotactic radiotherapy using computed portal radiography for canine intracranial tumors. Vet Radiol Ultrasound. 2018;59:212–220. doi: 10.1111/vru.12579. [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa H, Ehrhart EJ, Charles JB, Custis JT, LaRue SM. Assessment of predictive molecular variables in feline oral squamous cell carcinoma treated with stereotactic radiation therapy. Vet Comp Oncol. 2016;14:39–57. doi: 10.1111/vco.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwingenberger AL, Pollard RE, Taylor SL, Chen RX, Nunley J, Kent MS. Perfusion and volume response of canine brain tumors to stereotactic radiosurgery and radiotherapy. J Vet Intern Med. 2016;30:827–835. doi: 10.1111/jvim.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 23.International Commission of Radiation Units and Measurements. J ICRU. 1. Vol. 10. Oxford: Oxford University Press; 2010. Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT), ICRU Report 83. [Google Scholar]
- 24.Keyerleber MA, McEntee MC, Farrelly J, Podgorsak M. Completeness of reporting of radiation therapy planning, dose, and delivery in veterinary radiation oncology manuscripts from 2005 to 2010. Vet Radiol Ultrasound. 2012;53:221–230. doi: 10.1111/j.1740-8261.2011.01882.x. [DOI] [PubMed] [Google Scholar]
- 25.Christensen NI, Forrest LJ, White PJ, Henzler M, Turek MM. Single institution variability in intensity modulated radiation target delineation for canine nasal neoplasia. Vet Radiol Ultrasound. 2016;57:639–645. doi: 10.1111/vru.12398. [DOI] [PubMed] [Google Scholar]
- 26.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foreman PM, Jackson BE, Singh KP, et al. Postoperative radiosurgery for the treatment of metastatic brain tumor: Evaluation of local failure and leptomeningeal disease. J Clin Neurosci. 2018;49:48–55. doi: 10.1016/j.jocn.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kepka L, Tyc-Szczepaniak D, Bujko K, et al. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: Results from a randomized trial. Radiother Oncol. 2016;121:217–224. doi: 10.1016/j.radonc.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan A, Salmaan A, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soliman H, Ruschin M, Angelov L, et al. Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2018;100:436–432. doi: 10.1016/j.ijrobp.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 31.Arkans MM, Gieger TL, Nolan MW. Misadministration of radiation therapy in veterinary medicine: a case report and literature review. Vet Comp Oncol. 2017;15:237–246. doi: 10.1111/vco.12161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Stereotactic Radiation Therapy Treatment protocols for different tumor types. Treatment protocols include dose (Gray (Gy)/fraction), number of fractions and treatment schedule (Note: EOD=every other day). Each respondent is assigned a number that is displayed in the first column of the table on each page.