Abstract
Introduction:
Renal disease is common amongst people living with HIV. However, there is limited information on the incidence and risk factors associated with renal dysfunction among this population in Asia.
Methods:
We used data from the TREAT Asia HIV Observational Database. Patients were included if they started antiretroviral therapy (ART) during or after 2003, had a serum creatinine measurement at ART initiation (baseline) and had at least two follow-up creatinine measurements taken ≥3 months apart. Patients with a baseline estimated glomerular filtration rate (eGFR) ≤60ml/min/1.73m2 were excluded. Chronic kidney disease was defined as two consecutive eGFR values ≤60ml/min/1.73m2 taken ≥3 months apart. Generalized estimating equations were used to identify factors associated with eGFR change. Competing risk regression adjusted for study site, age and sex, and cumulative_incidence plots were used to evaluate factors associated with CKD.
Results:
Of 2,547 patients eligible for this analysis, tenofovir was being used by 703 (27.6%) at baseline. Tenofovir use, high baseline eGFR, advanced HIV disease stage and low nadir CD4 were associated with a decrease in eGFR during follow up. CKD occurred at a rate of 3.4 per 1000 patient/years. Factors associated with CKD were tenofovir use, old age, low baseline eGFR, low nadir CD4, and protease inhibitor use.
Conclusions:
There is an urgent need to enhance renal monitoring and management capacity among at-risk groups in Asia and improve access to less nephrotoxic antiretrovirals.
Keywords: Renal function, Antiretroviral therapy, eGFR, HIV AIDS, Asia, Tenofovir (TDF)
Introduction
Antiretroviral therapy (ART) has been responsible for achieving near normal life expectancy amongst people living with HIV (PLHIV) [1]. However, as PLHIV age, the morbidity contributed by non-communicable diseases has increased. Renal disease is common amongst PLHIV with etiologies varying from HIV infection, immune complex disease, chronic kidney disease (CKD) associated with comorbidities like diabetes and hypertension, and exposure to nephrotoxic medications, including antiretroviral drugs like tenofovir (TDF) and atazanavir (ATV)[2].
Tenofovir is widely used as one component of a nucleoside backbone in ART and has been recommended by the World Health Organization (WHO). TDF has been associated with renal toxicity ranging from Fanconi’s syndrome, acute kidney injury (AKI), and reduction in estimated glomerular filtration rate (eGFR), sometimes progressing to CKD[3–6]. There is limited information on the incidence and risk factors associated with renal dysfunction amongst PLHIV in Asia. We previously reported an incidence of TDF-associated renal dysfunction of 1.75 per 100 years in the TREAT Asia HIV Observational Database (TAHOD) cohort[7]. However, we did not compare renal dysfunction amongst PLHIV using TDF and other non-TDF based regimens. In this analysis, we describe the long-term changes in eGFR amongst patients using TDF and non-TDF based ART, and report the incidence and risk factors associated with CKD amongst patients on ART in our regional cohort.
Methods
Patient selection and baseline data
The study population consisted of HIV-infected patients enrolled in TAHOD before September 30, 2016. This cohort contributes to the International Epidemiology Databases to Evaluate AIDS (IeDEA) global consortium and has been previously described.[8] Recruitment started in 2003. In September 2016, TAHOD included data from 8,984 adults (≥18 years of age) that had ever received care from one of 20 clinics in Cambodia (n=1), China (n=1), Hong Kong (n=1), India (n=2), Indonesia (n=2), Japan (n=1), Malaysia (n=2), Philippines (n=1), Singapore (n=1), South Korea (n=1), Taiwan (n=1), Thailand (n=4) or Vietnam (n=2). These sites are predominantly public or university-based HIV referral clinics. Ethics approval was obtained at the sites, TREAT Asia/amfAR (coordinating centre), and the Kirby Institute (data management and statistical analysis centre). Patient consent is deferred to the individual participating sites and their institutional review boards.
Individuals were included in this analysis if they started ART during or after 2003, had a serum creatinine measurement at ART initiation (baseline) and had at least two follow-up creatinine measurements that were taken ≥3 months apart. The window period for baseline creatinine was between three months before ART initiation to 1.5 months after ART initiation. Values observed closest to ART initiation were used. ART was defined as a regimen containing ≥3 antiretroviral drugs. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.[9] Patients with a baseline eGFR ≤60 ml/min/1.73m2 were excluded.
CKD was defined as two consecutive eGFR measurements ≤ 60 ml/min/1.73m2 taken ≥3 months apart.[10] Hypertension was defined as two consecutive systolic blood pressure measurements >140 mmHg or two consecutive diastolic blood pressure measurements >90 mmHg. Diabetes was defined as documentation of two consecutive fasting blood glucose measurements ≥7 mmol/L. Patients were considered hepatitis B co-infected if they had any record of a positive hepatitis B surface antigen test, and hepatitis C co-infected if they had any record of a positive hepatitis C antibody test. Nephrotoxic opportunistic infection prophylaxis included amphotericin, cotrimoxazole, acyclovir, foscarnet and pentamidine.
Statistical analysis
Follow up was censored at: 1) the time of TDF cessation for individuals using TDF at ART initiation; 2) the time of TDF initiation for those not using TDF at ART initiation; or 3) the last recorded clinic visit whilst still eligible for inclusion “(including the date 12 months after the final clinic visit for those lost to follow up, the date of death, or the date of CKD diagnosis [for the change in eGFR analysis only]).
The Kruskal-Wallis test was used to compare time on ART and rates of creatinine monitoring among TDF and non-TDF users.
eGFR analysis
Generalized estimating equations adjusted for study site were used to identify factors associated with change in eGFR. Follow up in this analysis was additionally censored at the time of CKD onset. Creatinine measurements taken after baseline were evaluated at three (±1.5) month intervals up to five years of follow-up. If, for any given time interval, multiple eGFR values were available for a patient, the value taken closest to the 3-monthly time point was used. The adjusted effect of TDF on changes in eGFR was calculated by modelling TDF exposure and time on ART as an interaction term.
CKD analysis
Competing risk regression adjusted for study site, age and sex, and cumulative incidence plots were used to evaluate factors associated with CKD. Age and sex were included in our final model of CKD regardless of statistical significance, as they have previously been shown to be important factors in the development of chronic renal disease[11] [12]. Loss to follow-up and death were considered competing risks. Loss to follow-up was defined as not having been seen in clinic for >12 months without documentation of transfer. Rates of loss to follow-up and death were compared among TDF and non-TDF users using univariate competing risk regression whereby CKD and loss to follow-up or death (whichever was not the outcome of interest) were considered competing risks.
In all analyses, age, sex, HIV exposure category (mode of acquisition of HIV: heterosexual, homeosexual, IDU), baseline hypertension, hepatitis B surface antigen/hepatitis C antibody positivity (at any point during TAHOD follow-up), baseline eGFR, nadir CD4 count and study site were considered as fixed covariates. Protease inhibitor use, nephrotoxic opportunistic infection prophylaxis, CD4 cell count, HIV viral load and Centers for Disease Control (CDC) status were evaluated as time-updated covariates.
Covariates were considered for inclusion in our final models if one or more categories exhibited a univariate p-value <0.2 and retained if one or more categories exhibited an adjusted p-value <0.05. Patients with missing data were included in all analyses, but coefficients and hazard ratios for missing categories are not reported. Stata (StataCorp, College Station, TX) version 14.1 was used for all statistical analysis.
Results
Patient characteristics
Of 7,103 adults who initiated ART after 2002, 3,873 (54.5%) had a baseline creatinine measurement available and 3,757 (52.9%) had a baseline eGFR >60 ml/min/1.73m2. Of those with adequate baseline renal function, 2,547 (66.0%) had at least two follow-up creatinine measurements taken ≥3 months apart and were therefore eligible for this analysis. TDF was being used by 703 (27.6%) eligible patients. The median (interquartile range [IQR]) duration of follow-up was 3.9 (2.0-6.1) years (TDF 3.2 [IQR 1.6-4.8] years vs non-TDF 4.3 [IQR 2.2-6.6] years, p<0.01). Creatinine monitoring occurred at a median rate of 2.6 (IQR 1.7-4.3) measurements/patient/year and did not differ significantly between TDF and non-TDF users (TDF 2.5 [IQR 1.8-5.4] vs non-TDF 2.6 [IQR 1.7-4.1], p=0.15). Baseline characteristics of the study population are shown in Table 1.
Table 1 –
Baseline Characteristics
| Baseline Characteristics | All (n=2,547) | Non-tenofovir users (n=1,844) | Tenofovir users (n=703) | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | Median (IQR) | 35.6 | (30.5, 42.3) | 35.7 | (30.6, 42.3) | 35.4 | (30.4, 42.0) |
| Sex | Male | 1,796 | 70.5% | 1,302 | 70.6% | 494 | 70.3% |
| Height (cm) | Median (IQR) | 165 | (158, 171) | 165 | (158, 170) | 166 | (159, 171) |
| n | 2,348 | 91.2% | 1,696 | 92.0% | 652 | 92.7% | |
| Weight (kg) | Median (IQR) | 56.0 | (49.0, 64.7) | 56.0 | (48.5, 63.7) | 57.5 | (50.2, 66.5) |
| n | 2,224 | 86.4% | 1,603 | 86.9% | 621 | 88.3% | |
| eGFR (ml/min/1.73m2) | Median (IQR) | 104.1 | (89.0, 115.4) | 103.3 | (88.2, 115.2) | 106.6 | (91.9, 116.0) |
| HIV exposure | Heterosexual | 1,533 | 60.2% | 1,128 | 61.2% | 405 | 57.6% |
| Homosexual | 635 | 24.9% | 419 | 22.7% | 216 | 30.7% | |
| IDU | 148 | 5.8% | 109 | 5.9% | 39 | 5.6% | |
| Other | 231 | 9.1% | 188 | 10.2% | 43 | 6.1% | |
| Hypertension | Yes | 113 | 4.4% | 76 | 4.1% | 37 | 5.3% |
| Diabetes | Yes | 51 | 2.0% | 43 | 2.3% | 8 | 1.1% |
| Hepatitis B surface antigen status | Negative, % tested | 2,098 | 90.3% | 1,571 | 92.0% | 527 | 85.6% |
| Positive, % tested | 225 | 9.7% | 136 | 8.0% | 89 | 14.4% | |
| Unknown | 224 | 8.8% | 137 | 7.4% | 87 | 12.4% | |
| Hepatitis C antibody status | Negative, % tested | 1,865 | 86.8% | 1,404 | 86.1% | 461 | 89.0% |
| Positive, % tested | 284 | 13.2% | 227 | 13.9% | 57 | 11.0% | |
| Unknown | 398 | 15.6% | 213 | 11.6% | 185 | 26.3% | |
| CDC category | 1 | 1,283 | 50.4% | 860 | 46.6% | 423 | 60.2% |
| 2 | 415 | 16.3% | 310 | 16.8% | 105 | 14.9% | |
| 3 | 849 | 33.3% | 674 | 36.6% | 175 | 24.9% | |
| Nadir CD4 cell count (cells/mm3) | Median (IQR) | 139 | (46, 228) | 122 | (38, 210) | 183 | (79, 276) |
| n | 2,515 | 97.7% | 1,818 | 98.6% | 697 | 99.1% | |
| HIV viral load (log10 copies/mL) | Median (IQR) | 4.9 | (4.3, 5.4) | 4.9 | (4.3, 5.4) | 4.9 | (4.3, 5.3) |
| n | 1,609 | 62.5% | 1,043 | 56.6% | 566 | 80.5% | |
| Protease inhibitor use | Yes | 475 | 18.7% | 269 | 14.6% | 206 | 29.3% |
Values are n (%total) unless otherwise indicated. eGFR, estimated glomerular filtration rate; CDC, Centers for Disease Control and Prevention; IQR, interquartile range; IDU, intravenous drug use
Changes in eGFR over time
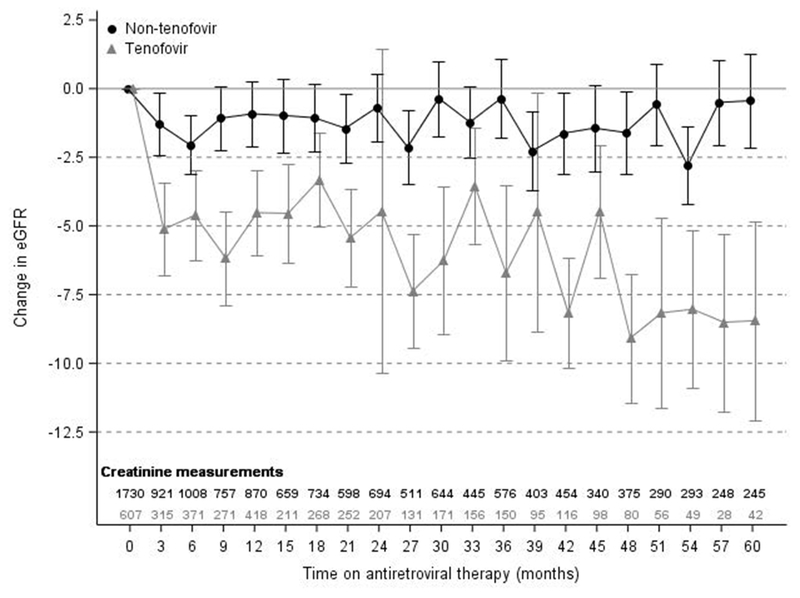
Supplementary Table 1 shows factors associated with eGFR change over time. In our multivariate model, lower baseline eGFR (61-89 ml/min/1.73m2) was associated with increased eGFR of 16.6 ml/min/1.73m2 (95% CI 15.3-17.9 ml/min/1.73m2, p<0.01) as compared to baseline eGFR of >89 ml/min/1.73m2. More advanced CDC stage (stage 3 vs Stage 1) was associated with decline in eGFR of 1.6 ml/min/1.73m2 (95% CI −3.0 to −0.2 ml/min/1.73m2, p=0.02) and lower nadir CD4 cell count (<100 cells/mm3 versus >200 cells/mm3) was associated with a decline of 3.6 ml/min/1.73m2 (95%CI −5.1 to −2.2 ml/min/1.73m2, p<0.01) in eGFR during follow up. The adjusted coefficients for the interaction between TDF and time on ART are shown in graphical form in Figure 1.
Figure 1 – Adjusted mean change in eGFR by tenofovir use.
Estimates are adjusted for baseline eGFR, current CDC stage, nadir CD4 cell count and study site. Error bars represent 95% confidence interval around the mean. eGFR, estimated glomerular filtration rate (ml/min/1.73m2)
Chronic kidney disease
Overall, 37 cases of CKD occurred over 10,900.5 years of follow up at a rate of 3.4 [95%CI 2.5 – 4.7) events per 1000 patient/years (see Figure 2). Factors associated with CKD are shown in Table 2. Loss to follow up occurred in 128 patients at a rate of 11.7 (95%CI 9.9-14.0) events per 1000 patient/years (15.4 [95%CI 11.1-21.2] among tenofovir users vs. 10.7 [95%CI 8.7-13.2] among non-tenofovir users, p<0.01). Death occurred in 17 patients at a rate of 1.6 (95%CI 1.0-2.5) events per 1000 patient/years (0.4 [95%CI 0.1-3.0] among tenofovir users vs. 1.9 [95%CI 1.2-3.1] among non-tenofovir users, p=0.12).
Figure 2 – Cumulative incidence curve showing the unadjusted risk of chronic kidney disease during antiretroviral therapy by tenofovir use.
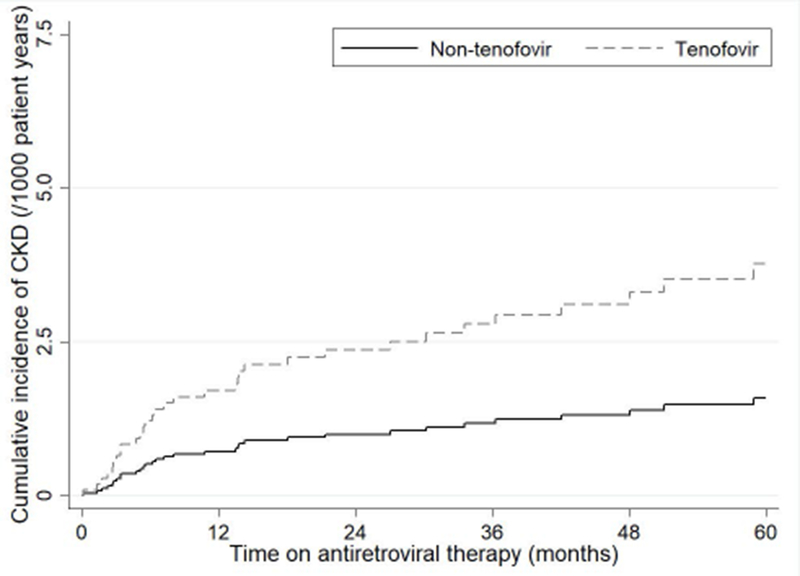
CKD, chronic kidney disease
Table 2 –
Factors associated with chronic kidney disease on antiretroviral therapy
| Covariate | CKD | Patient years follow up | Rate per 1000pt/yrs (95%CI) | Univariate HR (95%CI) | p | Multivariate HR (95%CI) | p |
|---|---|---|---|---|---|---|---|
| Overall | 37 | 10900.5 | 3.39 (2.46 - 4.68) | ||||
| Current tenofovir use^ | |||||||
| No | 24 | 8494.6 | 2.83 (1.89 - 4.22) | 1.00 | 1.00 | ||
| Yes | 13 | 2405.9 | 5.40 (3.14 - 9.31) | 2.38 (1.10 - 5.12) | 0.03 | 2.59 (1.06 - 6.33) | 0.04 |
| Baseline age^ | |||||||
| Per 5 years older | 37 | 10900.5 | 3.39 (2.46 - 4.68) | 1.40 (1.19 - 1.64) | <0.01 | 1.34 (1.12 - 1.61) | <0.01 |
| Sex^ | |||||||
| Male | 24 | 7604.9 | 3.16 (2.12 - 4.71) | 1.00 | 1.00 | ||
| Female | 13 | 3295.5 | 3.94 (2.29 - 6.79) | 1.46 (0.69 - 3.09) | 0.33 | 1.68 (0.80 - 3.55) | 0.17 |
| Baseline weight (kg) | |||||||
| <55 | 13 | 4152.2 | 3.13 (1.82 - 5.39) | 1.00 | |||
| 55 - 65 | 12 | 3323.7 | 3.61 (2.05 - 6.36) | 1.17 (0.53 - 2.61) | 0.69 | ||
| >65 | 4 | 2146.2 | 1.86 (0.70 - 4.97) | 0.53 (0.17 - 1.68) | 0.28 | ||
| Unknown | 8 | 1278.4 | 6.26 (3.13 - 12.51) | - | |||
| Baseline eGFR^ | |||||||
| 61-89 ml/min/1.73m2 | 22 | 2986.2 | 7.37 (4.85 - 11.19) | 4.25 (2.09 - 8.63) | <0.01 | 2.69 (1.29 - 5.62) | 0.01 |
| >89 ml/min/1.73m2 | 15 | 7914.3 | 1.90 (1.14 - 3.14) | 1.00 | 1.00 | ||
| HIV exposure | |||||||
| Heterosexual | 26 | 6623.5 | 3.93 (2.67 - 5.77) | 1.00 | |||
| Homosexual | 5 | 2634.6 | 1.90 (0.79 - 4.56) | 0.22 (0.07 - 0.67) | 0.01 | ||
| IDU/Other | 6 | 1642.4 | 3.65 (1.64 - 8.13) | 0.72 (0.27 - 1.92) | 0.52 | ||
| Hypertension | |||||||
| No | 34 | 10451.8 | 3.25 (2.32 - 4.55) | 1.00 | |||
| Yes | 3 | 448.7 | 6.69 (2.16 - 20.73) | 2.82 (0.69 - 11.56) | 0.15 | ||
| Hepatitis B surface antigen status | |||||||
| Negative | 27 | 8812.2 | 3.06 (2.10 - 4.47) | 1.00 | |||
| Positive | 1 | 950.1 | 1.05 (0.15 - 7.47) | 0.35 (0.05 - 2.57) | 0.30 | ||
| Unknown | 9 | 1138.2 | 7.91 (4.11 - 15.20) | - | |||
| Hepatitis C antibody status | |||||||
| Negative | 25 | 7771.5 | 3.22 (2.17 - 4.76) | 1.00 | |||
| Positive | 3 | 1155.4 | 2.60 (0.84 - 8.05) | 1.03 (0.24 - 4.44) | 0.97 | ||
| Unknown | 9 | 1973.6 | 4.56 (2.37 - 8.76) | - | |||
| Current CDC category | |||||||
| Stage 1 | 13 | 4908.2 | 2.65 (1.54 - 4.56) | 1.00 | |||
| Stage 2 | 7 | 2090.0 | 3.35 (1.60 - 7.03) | 1.07 (0.30 - 3.75) | 0.92 | ||
| Stage 3 | 17 | 3902.3 | 4.36 (2.71 - 7.01) | 1.68 (0.83 - 3.41) | 0.15 | ||
| Nadir CD4 cell count (cells/mm3)^ | |||||||
| >200 | 8 | 3270.4 | 2.45 (1.22 - 4.89) | 1.00 | 1.00 | ||
| 100 - 200 | 8 | 3284.7 | 2.44 (1.22 - 4.87) | 1.21 (0.43 - 3.42) | 0.72 | 1.35 (0.46 - 3.94) | 0.58 |
| <100 | 21 | 4179.2 | 5.02 (3.28 - 7.71) | 2.53 (1.08 - 5.93) | 0.03 | 3.07 (1.33 - 7.06) | 0.01 |
| Unknown | 0 | 166.2 | 0.00 (0.00 - 0.00) | - | - | ||
| Current CD4 cell count (cells/mm3) | |||||||
| >500 | 7 | 3211.3 | 2.18 (1.04 - 4.57) | 1.00 | |||
| 350 - 500 | 5 | 2776.8 | 1.80 (0.75 - 4.33) | 0.75 (0.25 - 2.23) | 0.60 | ||
| <350 | 24 | 4809.5 | 4.99 (3.34 - 7.44) | 1.63 (0.68 - 3.91) | 0.27 | ||
| Unknown | 1 | 102.9 | 9.72 (1.37 - 69.00) | - | |||
| Current HIV viral load^ | |||||||
| Undetectable | 20 | 7749.8 | 2.58 (1.66 - 4.00) | 1.00 | |||
| Detectable | 11 | 1244.4 | 8.84 (4.90 - 15.96) | 2.24 (0.89 - 5.60) | 0.09 | ||
| Unknown | 6 | 1906.3 | 3.15 (1.41 - 7.01) | - | |||
| Current protease inhibitor use^ | |||||||
| No | 20 | 8818.8 | 2.27 (1.46 - 3.52) | 1.00 | 1.00 | ||
| Yes | 17 | 2081.7 | 8.17 (5.08 - 13.14) | 5.59 (2.53 - 12.34) | <0.01 | 5.22 (2.36 - 11.56) | <0.01 |
| Current nephrotoxic opportunistic infection prophylaxis use | |||||||
| No | 20 | 8282.9 | 2.41 (1.56 - 3.74) | 1.00 | |||
| Yes | 17 | 2617.5 | 6.49 (4.04 - 10.45) | 2.41 (1.08 - 5.38) | 0.03 |
All models were adjusted for study site but HRs for sites are not shown.
Included in final model.
IDU and other categories combined as no outcomes in IDU group. eGFR, estimated glomerular filtration rate; IDU, intravenous drug use; CDC, Centres for Disease Control and Prevention; CKD, chronic kidney disease; CI, confidence interval; HR, hazard ratio
Discussion
Our study documents a significant risk of decline in eGFR and development of CKD on a TDF-based regimen across PLHIV in Asia. Several cohort studies have shown the association between exposure to some ARVs and development of CKD. For instance, the D:A:D cohort has documented a higher incidence rate of CKD with each year of exposure to TDF, Atazanavir/ritonavir (ATV/r) and Lopinavir/ritonavir (LPV/r) but not with other boosted Protease Inhibitors (PIs). Amongst PLHIV with a baseline eGFR >90 ml/min/1.73m2, rates of progression to a eGFR <70 ml/min/1.73m2 were high with TDF, ATV/r and LPV/r, and this risk increased with each year of exposure to these ARVs[13]. The overall incidence of CKD in the above cohort was 1.76 per 1000 person-years of follow-up. The incidence of CKD in our cohort (3.4 per 1000 person years) is substantially higher than that reported in the DAD cohort although we also included patients with baseline eGFR of 60-90/ml/min/1.73m2 in our analysis. However, another DAD study with comparable inclusion criteria demonstrated a higher rate of CKD viz 10.5 per 1000 person-years[14] . Few other studies have also documented higher incidence of CKD amongst PLHIV[15, 16] .
Numerous reports have documented higher incidence of CKD in Asian PLHIV compared to European and US cohorts. In a study from India, PLHIV on a TDF-based regimen had higher and faster decline in the eGFR compared to PLHIV in the United Kingdom[17]. In a 12-year observational cohort in Tokyo, incidence of CKD was 20.6 per 1000 years of follow up and was strongly associated with use of TDF[4]. CKD has been reported amongst 7.3% of Vietnamese PLHIV on ART[18]. Apart from TDF use, other risk factors including low body weight, age, male sex, duration of ART and concomitant comorbidities such as hypertension, were strongly associated with CKD in these studies. Interestingly in our study we found lower baseline eGFR, nadir CD4 counts, uncontrolled viremia and current PI/r use associated with incident CKD. Although we did not have adequate power to determine which PI/r was associated with CKD, previous analyses have indicated that atazanavir causes nephrolithiasis and is associated with development of interstitial nephritis [19, 20], switching from ATV/r or LPV/r to DRV/r is associated with improvement in kidney function[21], and use of a concomitant PI/r with TDF amplifies the renal toxicity of the later due to inhibition of Multi-resistant Protein 4 (MRP-4) efflux channels in the proximal tubular cells by ritonavir leading to TDF accumulation in the cells[22].
Nadir CD4 counts were significantly associated with CKD, suggesting that initiating ART early may help in reducing this risk. For example, early initiation of ART in the Strategic Timing for Antiretroviral Therapy (START) study was associated with a modestly higher eGFR and lower risk of proteinuria [23]. In addition, TDF use may be best avoided amongst patients with lower baseline eGFR as is recommended by some guidelines[24].
Estimated GFR can be affected by ethnicity and body composition. We used the CKD-EPI equation to estimate eGFR in our cohort. In a Thai cohort, re-expressed MDRD formula with Thai racial correction factor was most accurate in estimating GFR[25]. However, in the absence of any gold standard, CKD-EPI has been used to estimate GFR reasonably precisely in most studies across diverse ethnicities [26, 27].
Currently TDF is a recommended component of the nucleoside backbone in the 2016 WHO Consolidated ARV guidelines. As TDF use is scaled up in Asia, significant numbers of PLHIV may develop renal toxicity, decline in eGFR or progress to CKD. Recovery from the decline in eGFR after TDF discontinuation is slow and irreversible amongst a third of PLHIV[28]. As such, it is especially important that monitoring for renal toxicity is urgently strengthened. Monitoring with dipstick for proteinuria underestimates urinary protein excretion on TDF since TDF causes tubular proteinuria and the dipsticks measure albuminuria[29]. In addition, capacities to manage renal toxicity, including access to dialysis, needs to be further strengthened. Risk scores have been developed for predicting PLHIV at risk for renal disease using the D:A:D data[30]. Simpler scoring systems/calculators need to be developed for Asian populations that can be used at the primary health care level. Finally access to antiretrovirals like abacavir (ABC), often used as a substitute for TDF in cases of renal toxicity, and Tenofovir Alafenamide (TAF), are needed. Compared to TDF, TAF is associated with lower risk of renal and bone toxicity[31, 32].
Our study has several limitations. We did not use other markers for defining CKD, including proteinuria and radiologic evidence, as this data was not uniformly collected across sites. Including these would however, have been likely to increase the documented incidence of CKD in the study population. We also do not have access to determine true GFR in routine clinic settings across Asia as there may be substantial difference between measured calculated eGFR. Due to the small number of patients with documented diabetes, we did not include diabetes as a covariate. Key strengths of this analysis include a large heterogeneous PLHIV study population (largest study of CKD amongst PLHIV from Asia to date), and robust statistical methods.
Conclusion
Compared with other studies we observed a low prevalence of CKD in this cohort of Asian individuals, however progression to CKD was substantially associated with exposure to TDF. There is an urgent need to build up renal monitoring and management capacities across Asia and improve access to less nephrotoxic ARVs like TAF.
Supplementary Material
Acknowledgements
The TREAT Asia HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
The TREAT Asia HIV Observational Database
PS Ly* and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang* ‡, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China;
MP Lee* , PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy* , S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
S Pujari* , K Joshi , S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India;
TP Merati* , DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
E Yunihastuti* , D Imran and A Widhani, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
J Tanuma* , S Oka and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan;
JY Choi*, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
BLH Sim* , YM Gani, and R David, Hospital Sungai Buloh, Sungai Buloh, Malaysia;
A Kamarulzaman* , SF Syed Omar, S Ponnampalavanar and I Azwa, University Malaya Medical Centre, Kuala Lumpur, Malaysia;
R Ditangco* , E Uy and R Bantique, Research Institute for Tropical Medicine, Muntinlupa City, Philippines;
WW Wong* †, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan;
OT Ng*, PL Lim, LS Lee and PS Ohnmar, Tan Tock Seng Hospital, Singapore;
A Avihingsanon* , S Gatechompol, P Phanuphak and C Phadungphon, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Kiertiburanakul* , A Phuphuakrat, L Chumla and N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
R Chaiwarith* , T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai, Thailand;
P Kantipong* and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
KV Nguyen* , HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
DD Cuong* , NV An and NT Luan, Bach Mai Hospital, Hanoi, Vietnam;
AH Sohn* , JL Ross* and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law* , A Jiamsakul* and DC Boettiger, The Kirby Institute, UNSW Sydney, Sydney, Australia.
* TAHOD Steering Committee member; † Steering Committee Chair; ‡ co-Chair
References
- 1.Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoldi A, De Crignis E, Miserocchi A, Bon I, Musumeci G, Longo S, et al. HIV and kidney: a dangerous liaison. New Microbiol 2017; 40(1):1–10. [PubMed] [Google Scholar]
- 3.Zuniga M, Galindo A, Galaz MI, Vivanco M, Romero P, Balboa P, et al. [Tenofovir-associated Fanconi`s syndrome and rickets in a HIV infected girl]. Rev Chil Pediatr 2017; 88(1):148–152. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S, Nishijima T, Kawasaki Y, Kurosawa T, Mutoh Y, Kikuchi Y, et al. Effect of Tenofovir Disoproxil Fumarate on Incidence of Chronic Kidney Disease and Rate of Estimated Glomerular Filtration Rate Decrement in HIV-1-Infected Treatment-Naive Asian Patients: Results from 12-Year Observational Cohort. AIDS Patient Care STDS 2017; 31(3):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamzah L, Jose S, Booth JW, Hegazi A, Rayment M, Bailey A, et al. Treatment-limiting renal tubulopathy in patients treated with tenofovir disoproxil fumarate. J Infect 2017; 74(5):492–500. [DOI] [PubMed] [Google Scholar]
- 6.Tanuma J, Jiamsakul A, Makane A, Avihingsanon A, Ng OT, Kiertiburanakul S, et al. Renal Dysfunction during Tenofovir Use in a Regional Cohort of HIV-Infected Individuals in the Asia-Pacific. PLoS One 2016; 11(8):e0161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanuma J, Jiamsakul A, Makane A, Avihingsanon A, Ng OT, Kiertiburanakul S, et al. Renal Dysfunction during Tenofovir Use in a Regional Cohort of HIV-Infected Individuals in the Asia-Pacific. PLoS One 2016; 11(8):e0161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr 2005; 38(2):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. In. [DOI] [PubMed]
- 11.Prakash S, O’Hare AM. Interaction of aging and chronic kidney disease. Semin Nephrol 2009; 29(5):497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iseki K Gender differences in chronic kidney disease. Kidney Int 2008; 74(4):415–417. [DOI] [PubMed] [Google Scholar]
- 13.Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV 2016; 3(1):e23–32. [DOI] [PubMed] [Google Scholar]
- 14.Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010; 24(11):1667–1678. [DOI] [PubMed] [Google Scholar]
- 15.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis 2008; 197(11):1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasch MG, Engsig FN, Feldt-Rasmussen B, Kirk O, Kronborg G, Pedersen C, et al. Renal function and incidence of chronic kidney disease in HIV patients: a Danish cohort study. Scand J Infect Dis 2012; 44(9):689–696. [DOI] [PubMed] [Google Scholar]
- 17.Pujari SN, Smith C, Makane A, Youle M, Johnson M, Bele V, et al. Higher risk of renal impairment associated with tenofovir use amongst people living with HIV in India: a comparative cohort analysis between Western India and United Kingdom. BMC Infect Dis 2014; 14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizushima D, Tanuma J, Kanaya F, Nishijima T, Gatanaga H, Lam NT, et al. WHO antiretroviral therapy guidelines 2010 and impact of tenofovir on chronic kidney disease in Vietnamese HIV-infected patients. PLoS One 2013; 8(11):e79885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoriello D, Al-Nabulsi M, Reddy A, Salamera J, D’Agati VD, Markowitz GS. Atazanavir-Associated Crystalline Nephropathy. Am J Kidney Dis 2017. [DOI] [PubMed] [Google Scholar]
- 20.Milburn J, Jones R, Levy JB. Renal effects of novel antiretroviral drugs. Nephrol Dial Transplant 2017; 32(3):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jose S, Nelson M, Phillips A, Chadwick D, Trevelion R, Jones R, et al. Improved kidney function in patients who switch their protease inhibitor from atazanavir or lopinavir to darunavir. AIDS 2017; 31(4):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol 2007; 71(2):619–627. [DOI] [PubMed] [Google Scholar]
- 23.Achhra AC, Mocroft A, Ross M, Ryom-Nielson L, Avihingsanon A, Bakowska E, et al. Impact of early versus deferred antiretroviral therapy on estimated glomerular filtration rate in HIV-positive individuals in the START trial. Int J Antimicrob Agents 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59(9):e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Praditpornsilpa K, Avihingsanon A, Chaiwatanarat T, Chaiyahong P, Wongsabut J, Ubolyam S, et al. Comparisons between validated estimated glomerular filtration rate equations and isotopic glomerular filtration rate in HIV patients. AIDS 2012; 26(14):1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocroft A, Ryom L, Reiss P, Furrer H, D’Arminio Monforte A, Gatell J, et al. A comparison of estimated glomerular filtration rates using Cockcroft-Gault and the Chronic Kidney Disease Epidemiology Collaboration estimating equations in HIV infection. HIV Med 2014; 15(3):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristelli MP, Cofan F, Rico N, Trullas JC, Manzardo C, Aguero F, et al. Estimation of renal function by CKD-EPI versus MDRD in a cohort of HIV-infected patients: a cross-sectional analysis. BMC Nephrol 2017; 18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose S, Hamzah L, Campbell LJ, Hill T, Fisher M, Leen C, et al. Incomplete reversibility of estimated glomerular filtration rate decline following tenofovir disoproxil fumarate exposure. J Infect Dis 2014; 210(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sise ME, Hirsch JS, Canetta PA, Herlitz L, Mohan S. Nonalbumin proteinuria predominates in biopsy-proven tenofovir nephrotoxicity. AIDS 2015; 29(8):941–946. [DOI] [PubMed] [Google Scholar]
- 30.Mocroft A, Lundgren JD, Ross M, Law M, Reiss P, Kirk O, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med 2015; 12(3):e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raffi F, Orkin C, Clarke A, Slama L, Gallant J, Daar E, et al. Brief Report: Long-Term (96-Week) Efficacy and Safety After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in HIV-Infected, Virologically Suppressed Adults. J Acquir Immune Defic Syndr 2017; 75(2):226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Post FA, Yazdanpanah Y, Schembri G, Lazzarin A, Reynes J, Maggiolo F, et al. Efficacy and safety of emtricitabine/tenofovir alafenamide (FTC/TAF) vs. emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) as a backbone for treatment of HIV-1 infection in virologically suppressed adults: subgroup analysis by third agent of a randomized, double-blind, active-controlled phase 3 trial. HIV Clin Trials 2017; 18(3):135–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.