Abstract
Background/Aims:
American Indian adults have some of the highest alcohol abstinence rates compared to the overall U.S. population. Despite this, many American Indian people are more likely to concurrently use alcohol and illicit drugs and are less likely to participate and remain in outpatient treatment for alcohol and other drug use compared to the general U.S. population. There is limited knowledge about effective interventions targeting alcohol and drug co-addiction among American Indian adults. Contingency management (CM) is a behavioral intervention designed to increase drug abstinence by offering monetary incentives in exchange for drug and alcohol negative urine samples. We aim to evaluate and describe a culturally-tailored CM intervention to increase alcohol and other drug abstinence among American Indian adults residing in a Northern Plains reservation.
Methods:
This 2×2 factorial, randomized controlled trial currently includes 114 American Indian adults with alcohol and/or drug-dependence who are seeking treatment. Participants were randomized into 1 of 4 groups that received: 1) CM for alcohol, 2) CM for other drug, 3) CM for both substances, or 4) no CM for either substance. We present descriptive, baseline data to characterize the sample and describe the modified CM approach that is specific to the community wherein this trial was being conducted.
Results:
The sample is 49.1% male, with an average age of 35.8 years (SD= 10.4 years). At baseline, 43.0% of the sample tested positive for ethyl glucuronide, 50.9% of participants self-reported methamphetamine as their most used drug, 36.8% self-reported cannabis and 12.3% self-reported prescription opiates as their most used drug. Among randomized participants, CM targeting co-addiction in American Indians 3 47.4% tested positive for cannabis, 28.1% tested positive for methamphetamine, 16.7% tested positive for amphetamines, and 2.1% tested positive for opiates.
Conclusions:
This is the first study to examine a culturally tailored CM intervention targeting co-addiction of two substances among American Indian adults. By establishing a tribal-university partnership to adapt, implement, and evaluate CM, we will increase the literature on evidence-based addiction treatments and research, while improving trust for addiction interventions among American Indian communities through ongoing collaboration. Moreover, results have implications for the use of CM as an intervention for co-addiction in any population.
Keywords: Contingency management, American Indians, substance use disorder treatment, co-addiction, ethyl glucuronide, randomized clinical trial
Background
American Indian communities are diverse and distinct. For instance, tribal communities in the Southwest have lower lifetime prevalence of alcohol use disorders compared to tribal communities in the Northern Plains region (9.8% vs. 16.6%).1 In addition, American Indian adults have some of the highest alcohol abstinence prevalence compared to the overall U.S. population.2–5 Despite this variation between tribal communities in prevalence of alcohol use disorders, overall American Indians have a higher prevalence of alcohol use disorders than other races.1, 6 These disparities can be traced back to decades of historical trauma and a variety of historical and sociocultural factors stemming from colonization.7, 8 Even with the variation in alcohol use among tribal communities, alcohol is the most frequently misused substance, with a use rate of 10.7% for American Indian adults compared with 7.6% for the general population.9 As a likely consequence, 11.7% of all American Indian adult deaths are alcohol related, and the age-adjusted death rate for American Indian adults is approximately twice that of the U.S. population.10 Additionally, an estimated 5% of American Indian adults, compared to 2.9% of the general U.S. population, had a substance use disorder in the past year.9, 11–13
Co-addiction of two or more substances is critically understudied, yet, it is common in virtually all substance use disorder treatment settings.14 In 2015, 2.7 million people over the age of 12 met the criteria for both an alcohol use disorder and illicit drug disorder in the past year.15 American Indian adults are more likely to concurrently use alcohol and an illicit drug compared to other ethnic groups in the U.S., 9, 16 but are less likely than the general U.S. population to participate in and remain in outpatient or residential treatment for alcohol and other drug use.17,18 Nevertheless, there is limited knowledge about effective interventions targeting co-occurring alcohol and other drug use among all adults, and particularity among American Indian adults, despite the association between lack of accessible treatment and related health inequities.1
To our knowledge, there are only two published randomized controlled trials of alcohol and drug use among American Indian and Alaska Native adults.19–21 One study was a pharmacological trial of naltrexone for alcohol misuse20 while the other study integrated cultural activities into a driving while intoxicated program among first time offenders.21 Both randomized controlled trials found promising results within the treatment groups. The dearth of randomized controlled trials among American Indian/Alaska Native communities is likely due to many factors. Often cited is the cultural incongruence within tribal communities of providing services to some individuals, while withholding services to other individuals in need. Another factor is that randomized controlled trials do not always capture the potential mediating and moderating effects of local context and culture.22, 23
However, it may still be feasible to implement randomized controlled trials with and among tribal communities if successful tribal-university partnerships are developed through a community engaged approach. Our group has partnered with several tribal communities to conduct two randomized controlled trials of contingency management (CM) in American Indian/Alaska Native communities. One such partnership resulted in the HONOR study, which targets alcohol use only and includes both rural and urban American Indian/Alaska Native communities in multiple sites across the western U.S. and Alaska. The methods describing the HONOR study have been discussed in our previous work.24
CM is a behavioral intervention designed to increase alcohol and drug abstinence by offering escalating monetary incentives in exchange for objective evidence of alcohol or drug abstinence (i.e., alcohol or drug negative urine samples).25 CM is rooted in basic behavioral age science, specifically in operant conditioning. Operant conditioning is a type of learning process where behavior is modified via consequences. Similarly, CM conceptualizes substance use as an operant behavior that is sustained at least partially by environmental factors, thus making it receptive to behavioral interventions.26 Therefore, CM shapes behavior either by providing tangible reinforcements (e.g., monetary incentives, vouchers) in exchange for evidence of desired behavior (e.g., abstinence) or by withholding those reinforcements in cases of undesired behavior (e.g., drinking).27 Presently in substance use disorder trials, reinforcements are generally dependent on objective evidence of the desired behavior (e.g., biochemically-verified alcohol or drug abstinence, treatment attendance, medication adherence). In addition, CM is also a verifiably cost-effective treatment option for many substance use disorders, including for marijuana, cocaine, stimulants, opioids, and alcohol-dependence.28–32 CM treatment costs are further decreasing as novel technology-based CM interventions (e.g. computer or mobile-based CM interventions) are increasingly being delivered, reducing costs by allowing remote intervention delivery.33, 34
In Non-American Indian/Alaska Native populations CM has been effective in promoting abstinence from benzodiazepines,35 cocaine,36 nicotine,37 opiates,38, 39 marijuana,40, 41 alcohol,42, 43 and methamphetamine,44 with multiple meta-analyses supporting the efficacy of CM.45–47 One primary meta-analysis of controlled studies of psychosocial treatments for addiction observed that CM interventions demonstrate larger reductions in drug use compared to other modalities.46 CM interventions have been used in several large randomized controlled trials, as well as in clinical practices throughout the U.S.48–50 and the United Kingdom.51 In both our randomized controlled trials of CM in American Indian/Alaska Native communities, strong community and university partnerships have developed allowing us to implement these studies in partnership. We believe that the characteristics of the intervention, such as its confidential, non-judgmental, and positive strength-based focus, its feasibility and potential for cultural tailoring were other reasons that communities were interested in seeing if CM might be an effective treatment for their communities.
The trial we describe here is a randomized controlled trial that will compare three different CM interventions to a control group that receives rewards not contingent on alcohol or drug abstinence. This study focuses on treating alcohol and drug co-addiction in one rural reservation community in a Northern Plains reservation community.
This clinical trial design also addresses a common methodological barrier by using a CM paradigm based on a superior alcohol measure, ethyl glucuronide urine tests.52–54 Breath tests cannot detect alcohol use between clinical assessments, hence their use may lead to inconsistent delivery of interventions.27, 55 However, ethyl glucuronide is a well-researched metabolite of alcohol52,56–62 that can be detected in urine for up to three days,52–54 and it is available as a relatively low-cost immunoassay. This method is increasingly being used to test for alcohol use in forensic, employment screening, and treatment settings.61 The ethyl glucuronide urine test can be conducted by using an on-site analyzer, allowing immediate test results and delivery of CM reinforcers. Many participants rely on the CM incentives to afford basic necessities and therefore allowing for immediate, same-day prize draws is a significant benefit for participants.
Study aims
The primary objective of this clinical trial is to determine if CM is an effective intervention for treating alcohol and drug co-addiction in 114 American Indian adults diagnosed with alcohol dependence who also use other drugs. Our specific aims are to: 1) determine if participants randomized to CM conditions use less alcohol and drugs than those in the non- contingent control group; 2) determine if the intervention is disproportionately effective for participants receiving CM for both alcohol and other drug abstinence, compared to participants in the single-substance CM groups or the non-contingent control group; 3) determine group differences in secondary addiction-related outcomes (e.g., cravings, illicit drug use) and alcohol- and drug-associated health-impairing behaviors (e.g., HIV-risk behavior, nicotine use). At the time of submission, we have completed recruitment and treatment and follow-up assessments are ongoing. Therefore, in this article we describe the design and methodology of this study and characterize the study sample at baseline.
Methods
Design
Data collection for this study includes two phases: 1) the study period (weeks 1–12, including a baseline assessment), and 2) the 3-month follow-up period (weeks 16–36). Participants are asked to abstain only from their most used drug if they are in the CM for Drug only or CM for Alcohol and Drug group. Participants’ most used drug was identified at screening based on self-report. At baseline, we collected urine tests and self-reported current alcohol and drug use. We also assessed participant mental and general health status, success of the randomization, and other relevant factors associated with patient health. In addition to age and race, a Follow-Up Locator Form is completed at baseline and then at regular intervals thereafter to assist in follow-up efforts. This form was developed in our previous research to track participants with substance use disorders. Participants also complete brief self-report questionnaires on alcohol and drug use at each visit. Participants visit the treatment facility and provide urine samples every Monday, Wednesday, and Friday during the 12-week study period after they provide consent and complete the baseline interview. We include additional 30-minute data collection visits to assess longitudinal measures at weeks 4, 8, and 12. During the follow-up period, participants are scheduled for 3 visits at 4-week, 8-week, and 12-week intervals (weeks 16, 24, and 36). Each follow-up visit takes approximately 30 minutes during which we collect urine samples, self-reported drug and alcohol data, and information on health and lifestyle behaviors. This study was approved by the Washington State University Institutional Review Board and Tribal Institutional Review Board with jurisdiction.
Setting
This study is being conducted on one American Indian reservation located in the Northern Plains region. This tribal reservation experiences patterns of alcohol consumption and drug misuse, not too unlike several other neighboring regions and cities. Treatment as usual includes an outpatient addiction treatment program, as well as services offered through probation. Community members also have access to other socials services and cultural services and supports. We are not naming the community to protect community confidentiality.
The Northern Plains site has over 10,000 enrolled tribal members, with approximately half of the members residing on or near the reservation. The tribal health department has two clinics offering medical, dental, mental health, substance misuse, laboratory, pharmacy services, as well as a center offering treatment for alcohol and drug use disorders.
Participants
We recruited n=114 individuals with co-occurring alcohol dependence and drug-misuse. Addiction treatment clinicians and staff gave potential participants a study brochure and a form asking if they would like to be contacted with further information. Research coordinators then contacted interested participants to explain the study in greater detail and screen for alcohol and drug use in the last 30 days. Patients deemed eligible were scheduled for an initial in-person study interview. At the interview, participants provided informed consent before baseline data collection and randomization.
Eligibility
Our eligibility criteria were as follows: 1) self-reported American Indian race; 2) seeking alcohol misuse or dependence and drug misuse or dependence treatment on a participating reservation; 3) age 18–65 years; 4) a Diagnostic and Statistical Manual, fourth edition diagnosis of current alcohol dependence; 5) current drug misuse, defined as using drugs without a prescription at least once in the past 30 days; 6) ability to read and speak English; and 7) ability to provide written informed consent. The variables of race, age, alcohol dependence, and drug misuse were determined based on patient self-report at the screening interview. Diagnoses of alcohol dependence and drug misuse were made using the MINI Neuropsychiatric Interview63 coupled with self-report. Our exclusion criteria included: 1) significant risk of dangerous alcohol withdrawal or expression of concern by the participant, research project leader, and/or healthcare provider about dangerous withdrawal; 2) a Diagnostic and Statistical Manual, fourth edition diagnosis of drug dependence; 3) significant risk of dangerous drug withdrawal and/or self-reported or medically documented severe withdrawal from drugs in the 6 months before study entry; 3) any medical or psychiatric condition, such as organic brain disorder, dementia, or psychotic disorder, that the research project leader determines would compromise safe study participation; and 4) receiving drugs under the direction of a physician for pain management or another medical condition for which drug abstinence is contraindicated. Final determination of eligibility was made by the research project leader based on medical chart review, patient interviews, and clinician input.
Intervention adaptation
We conducted five provider interviews and one focus group from the tribal community to review the CM protocol. The focus group included a health researcher with experience working on alcohol and/or substance misuse in American Indian populations; an expert in CM interventions; adult patients receiving treatment for addiction; and other tribal community members. The group reviewed the overall CM approach, focusing especially on the reinforcement strategy. We devoted much of the meeting to discussing selection of reinforcement items, as well as the manner in which reinforcement is delivered to participants, to ensure that the process is compatible with cultural values related to gift-giving in each community. Additionally, the group reviewed study procedures and data collection questionnaires, and recommended changes to increase cultural relevance and acceptability. We modified strategies and materials based on group recommendations. After modification, we sent updated descriptions and protocols to group members for final review and approval. Each focus group participant received $20 for his or her time and effort.
Randomization
Based on well-established protocols for randomized trials of CM,64, 65 participants were assigned to treatment groups following a 2 × 2 factorial design, using a stratified block randomization procedure, balanced on confounding factors that might affect outcomes. Characteristics at the baseline interview that were balanced include 1) sex, 2) site, 3) positive urine test for alcohol, 4) positive urine test for drugs, 5) positive urine test for both alcohol and drugs, and 6) negative urine test for both alcohol and drugs. After a participant provided informed consent and completed baseline data collection, the research coordinator notified a designated study investigator after the information necessary for randomization was entered in the online database, REDCap (Research Electronic Data Capture),66 hosted by the university. This investigator then used the randomization table to randomly assign the participant to a treatment condition and notified the research coordinator.
Study intervention
Contingency management.
Participants randomized into the 3 CM groups receive escalating reinforcement for alcohol and/or drug abstinence. Testing occurs 3 times per week (Monday, Wednesday, and Friday) during in-person visits. Consistent with previous CM interventions,48, 49 participants who do not provide a urine sample on the required days are considered as missing, and therefore are treated as a positive sample, unless they previously established an agreement with research staff. Urine tests for cannabis, methamphetamine, amphetamines, and cocaine are conducted via QuickScreen urine cup (Confirm Biosciences, San Diego, CA) and results are available almost immediately. Urine tests for alcohol and opioids are conducted via a benchtop ThermoFisher Indiko analyzer (Fremont, CA). The magnitude of reinforcement increases across consecutive weeks for which all three of the participant’s urine tests are negative for relevant compounds. Alcohol-only and drug-only CM groups receive reinforcement contingent on negative tests for alcohol or drugs, respectively. The alcohol-and-drug CM group receive reinforcement contingent on negative urine tests for both substances.
At every CM study visit, each participant with a negative result is invited to draw chips out of a bag containing 500 chips. Fifty percent of the chips say “Good job!” or a similar encouraging phrase (no prize), 41.8% of the chips result in a small prize ($1 value), 8% result in a large prize ($20 value), and 0.2% result in a jumbo prize ($80 value). All chips are replaced after all draws, so that odds of drawing any given chip are the same each time. The algorithm for number of draws is as follows: three draws for the first week of negative urine tests, with one additional draw added for each consecutive week of negative testing. Positive or missing urine tests result in no prize draws for that study visit, with the number reset to three at the first subsequent study visit with a negative test. After three consecutive negative tests (approximately 1 week) following a reset, the participant is returned to the highest number of draws earned before the reset. The maximum possible number of draws at any given week is 14, for participants who test negative for all 12 weeks of the study period. This escalating schedule with a reset contingency has been found to decrease the probability of relapse once abstinence has been initiated.44, 67, 68 Because up to four days may be needed for alcohol and drug metabolites to completely clear the system, participants are informed that up to two study appointments after substance use may be needed to assess regained abstinence by urine testing.
Non-contingent control.
Compensation for participants in the non-contingent control group follows a well-established protocol used by other studies to isolate the effect of a CM intervention in large randomized trials.64, 65 The algorithm for this process is continually updated to accommodate the ongoing inflow of additional information from subsequent urine tests and newly enrolled CM group participants over time. This results in a compensation scheme for non-contingent control group participants that is approximately equal to the average total number of prize draws across all three CM groups over the duration of the study period. This is a commonly employed method in CM research in order to isolate the true effect of CM and hold reinforcement density across the arms equivalent.69–73 While it is true that this procedure does tend to increase abstinence from baseline among most participants, this provides a robust control condition to understand the true effects of CM. Compensation for non-contingent control group participants is dependent only on providing all three urine samples for a given week, regardless of whether the urine tests are negative for alcohol and/or drugs.
Types of reinforcers and maximum treatment earnings.
Prizes are displayed in a locked storage cabinet in the on-site study office. Types of prizes were selected based on our recent studies, as well as feedback from our key informant group of American Indian leaders, clinicians, and adults receiving addiction treatment services. Typical prizes include ($1 value) toiletries and art supplies; ($20 value) gift cards, mp3 players, and clothing; and ($80 value) DVD players and tablets. The maximum value of reinforcers available to participants who remained continuously abstinent was approximately $688.
Primary outcomes
Urine samples are collected and analyzed for ethyl glucuronide and opioids by using non-quantitative ThermoFisher Indiko analyzer (Fremont, CA) at each study visit. Previous CM studies targeting alcohol dependence have used alcohol breath tests that are limited to detecting use for only 12 hours.55, 74, 75
We determine recent alcohol use by an ethyl glucuronide threshold of 150 ng/mL. This threshold reflects alcohol consumption for up to three days, depending on the amount of alcohol consumed.76 Participants are reminded verbally, by signs, and reminder cards that they should abstain from using alcohol-containing products. We determine recent drug use by detection of metabolites in urine using the QuickScreen urine cup (Confirm Biosciences, San Diego, CA) with the following criteria: opioids (morphine > 2,000ng/mL), amphetamine (d-amphetamine > 1,000ng/mL), methamphetamine (d-methamphetamine > 1,000ng/mL), cocaine (benzoylecgonine > 300 ng/mL), and cannabis (tetrahydrocannabinol > 50ng/mL).
Secondary outcomes
Secondary outcomes comprise self-reported data on alcohol and drug use. The Alcohol Timeline FollowBack is used to measure the frequency and amount of daily drinking and drug use. At each study visit, participants report the number of standard drinks consumed since their last study visit (interval varying from 2 to 3 days during treatment periods to 1 month during baseline and follow-up). To improve the accuracy of self-report, this instrument is administered after urine samples are collected and prizes are administered. The Addiction Severity Index, Native American Version,77 is used monthly to measure severity of self-reported addiction-related problems and quality of life. This measure assesses demographics and alcohol and drug use severity, as well as the impact of alcohol and drug use on psychiatric, legal, medical, and family domains. We assess cravings with 10 cm visual analog scales anchored at 0 (no craving) and 100 (most intense craving possible) for alcohol and drugs. We also collect secondary outcome data on other health behaviors that frequently correlate with alcohol and drug use. The Cigarette Timeline FollowBack is used to assess daily number of cigarettes smoked.78 HIV risk behavior is assessed using the brief HIV Risk Behavior Scale79, 80 to characterize changes in HIV risk behavior secondary to CM-associated reductions in substance misuse. We measure health-related quality of life with the Short-Form Health Survey, a well-established measure81 that has been used to asses health-related quality of life in American Indian populations,82 as well as with the American Indian Enculturation Scale.
Analytic plan
Descriptive statistics.
We calculated percentages for categorical variables and means with standard deviations for continuous variables separately by study group. We assessed success of the randomization process by comparing the baseline distribution of variables across the 4 study groups, using one-way Analysis of Variance (ANOVA) for continuous factors, and chi square tests for categorical factors. For each primary and secondary biochemical outcome, we created binary indicators of abstinence at each urine test. We also calculated the number of days for each participant from baseline to their first negative test, and the duration in days of the longest period of abstinence. We followed similar procedures to create summary descriptive variables for self-reported secondary outcomes.
Primary analyses.
We will perform an intention-to-treat analysis for outcomes comparing treatment groups. Primary outcomes will be biochemically verified alcohol and/or drug abstinence measured longitudinally for a total of 24 weeks. Our analytic approach is based on previous randomized trials of CM as a treatment for illicit drug dependence.44, 48, 49 Using a 2×2 factorial design, we will evaluate the independent main effects of CM for alcohol and CM for drug abstinence, and their interaction, to support our a priori hypothesis that CM for both conditions will be more effective than CM for just one or the other. We will also perform survival analysis of time-to-event data to examine group differences in duration of abstinence and time to relapse. We will use longitudinal modeling techniques that allow for missing data, as long as values are assumed to be “missing at random,” and that allow for estimation of time-varying and time-invariant covariates. If any outcome measures fail to satisfy the assumptions of parametric tests, data may be transformed (e.g., square root or arcsine transformations for percent days abstinent) to adjust for differences in variability or for skewness, or nonparametric tests, such as the Kruskal-Wallis test, may be used. All inferential results will be presented as point estimates with 95% confidence intervals, and we will use an alpha error rate of 0.05 as the threshold for statistical significance.
Missing data.
To minimize missing data, we thoroughly collect data when participants are available. Missing data that occur despite our efforts are handled consistent with previous large-scale investigations of CM for illicit drug use.44, 48, 49 In addition, we note that use of random effects modeling techniques within the general latent variable modeling framework allows for missing data and estimates parameters from existing data in a manner that adjusts the estimation process to account for bias resulting from missing data.
Power.
The choice of sample size is based on statistical power calculations for the primary aim of reducing alcohol and drug use. Previous studies have demonstrated medium to large effect sizes (0.40 standard deviation units or higher) for CM procedures in reducing substance use compared to standard therapy.36, 83 Based on results of our recently completed CM trial for drug dependence, we anticipate at least a medium effect size of 0.30 for the secondary analyses in this study. Assuming a within-participant correlation of r = 0.4 and a 2-tailed test with alpha set at 0.05, we expect at least 85% power to detect an effect size of 0.25 with n=120, our planned sample size. This is a conservative estimate, because we will be using longitudinal analysis techniques that will make use of as many as 40 urine tests (1 at baseline, 3 urine test per week for 12 weeks, and 3 follow-up tests) on each individual, significantly increasing our ability to detect smaller effects.
Results
Baseline characteristics
One hundred and forty-two potential participants were screened. Of these individuals, 114 met study criteria and were randomized (see Figure 1). The sample was 49.1% male, with an average age of 35.8 years (SD = 10.4 years), and 53.5% of the sample had a high school degree or higher. At baseline, 50.9% of all participants self-reported methamphetamine as their most used drug, 36.8% self-reported cannabis as their most used drug, and 12.3% self-reported prescription opiates as their most used drug. Among randomized participants, 47.4% tested positive for cannabis, 43.0% tested positive for alcohol, 28.1% tested positive for methamphetamines, 16.7% tested positive for amphetamines, and lastly, 2.1% tested positive for opiates. Baseline, alcohol and drug, and clinical characteristics by group are presented in Tables 1 and 2.
Figure 1.
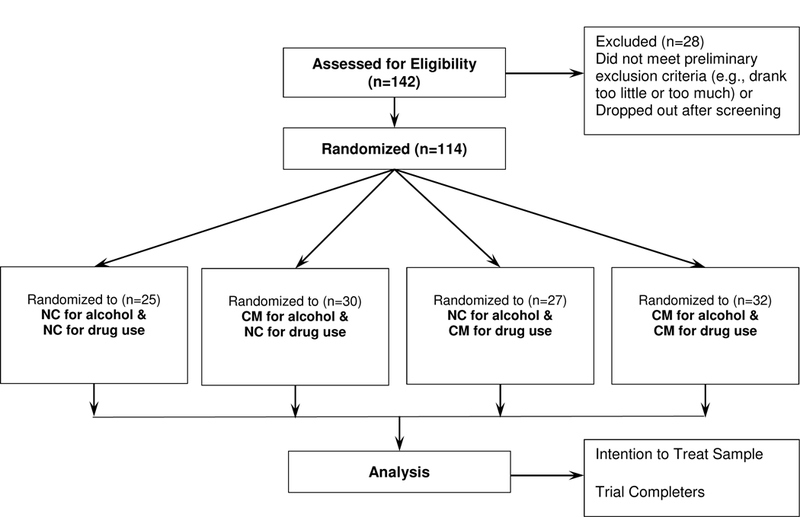
Overview of study procedures and CONSORT flow diagram. CM = contingency management, NC = non-contingent
Table 1.
Baseline Characteristics of the Sample
| All Participants | CM for Alcohol | CM for Drug | CM for Alcohol and Drugs |
Non-Contingen | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=114 | n=30 | n=27 | n=32 | n=25 | ||||||||||||||||
| n | % | M | SD | n | % | M | SD | n | % | M | SD | n | % | M | SD | n | % | M | SD | |
|
Demographic Characteristics |
||||||||||||||||||||
| Age | 114 | 35.8 | 10.4 | 30 | 36.8 | 10.7 | 27 | 37.6 | 10.7 | 32 | 34.9 | 11.4 | 25 | 33.9 | 8.2 | |||||
| Male | 56 | 49.1% | 14 | 46.7% | 14 | 51.9% | 16 | 50.0% | 12 | 48.00% | ||||||||||
| Housing (< one year) | 51 | 44.7% | 11 | 36.7% | 7 | 59.3% | 10 | 31.3% | 14 | 56.00% | ||||||||||
| Medical services* | 29 | 25.4% | 6 | 20.0% | 25.9% | 10 | 31.3% | 6 | 24.00% | |||||||||||
| Education | ||||||||||||||||||||
| < high school | 53 | 46.5% | 19 | 63.3% | 11 | 40.7% | 11 | 34.4% | 12 | 48.00% | ||||||||||
| High school | 25 | 21.9% | 5 | 16.7% | 3 | 11.1% | 9 | 28.1% | 8 | 32.00% | ||||||||||
| > high school | 36 | 31.6% | 6 | 20.0% | 13 | 48.1% | 12 | 37.5% | 5 | 20.00% | ||||||||||
| Employment | ||||||||||||||||||||
| Unemployed | 95 | 83.3% | 24 | 80.0% | 24 | 88.9% | 23 | 71.9% | 24 | 96.00% | ||||||||||
|
Alcohol and drug use at baseline |
||||||||||||||||||||
|
Self-report most used drug |
||||||||||||||||||||
| Cannabis | 42 | 36.8% | 11 | 36.7% | 8 | 29.6% | 11 | 34.4% | 12 | 48.00% | ||||||||||
| Methamphetamines | 58 | 50.9% | 16 | 53.3% | 15 | 55.6% | 15 | 46.9% | 12 | 48.00% | ||||||||||
| Prescription opiates | 14 | 12.3% | 3 | 10.0% | 4 | 14.8% | 6 | 18.8% | 1 | 4.00% | ||||||||||
| Positive at baseline | ||||||||||||||||||||
| Alcohol (EtG+) | 49 | 43.0% | 13 | 43.3% | 11 | 40.7% | 15 | 46.9% | 10 | 40.00% | ||||||||||
| Amphetamines | 19 | 16.7% | 4 | 13.3% | 4 | 14.8% | 7 | 21.9% | 4 | 16.00% | ||||||||||
| Cannabis | 54 | 47.4% | 14 | 46.7% | 9 | 33.3% | 17 | 53.1% | 14 | 56.00% | ||||||||||
| Cocaine | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | ||||||||||
| Methamphetamines | 32 | 28.1% | 6 | 20.0% | 10 | 37.0% | 9 | 28.1% | 7 | 28.00% | ||||||||||
| Opiates | 2 | 2.1% | 1 | 3.1% | 0 | 0.0% | 1 | 3.1% | 0 | 0.0% | ||||||||||
| Days alcohol use (last 30 days) |
114 | 13.5 | 9.6 | 30 | 12.9 | 9.5 | 27 | 11.5 | 8.8 | 32 | 13.6 | 9.3 | 25 | 16.3 | 10.6 | |||||
| Days heavy alcohol use (last 30 days)* |
113 | 12.1 | 9.1 | 30 | 9.3 | 6.4 | 27 | 10 | 8.4 | 32 | 13.3 | 9.4 | 24 | 16.2 | 10.7 | |||||
Medical service use in the last 30 days.
ethyl glucuronide.
Table 2.
Clinical Characteristics of the Sample
| All Participants | CM for Alcohol | CM for Drug | CM for Alcohol and Drugs |
Non-Contingen | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=114 | n=30 | n=27 | n=32 | n=25 | ||||||||||||||||
| n | % | M | SD | n | % | M | SD | n | % | M | SD | n | % | M | SD | n | % | M | SD | |
| Clinical Characteristics | ||||||||||||||||||||
| AIE Scale* | ||||||||||||||||||||
| Total | 114 | 50.1 | 17.9 | 30 | 49.3 | 16.6 | 27 | 53.2 | 21.4 | 32 | 51.3 | 19 | 25 | 46.3 | 13.4 | |||||
| Fagerstrom | ||||||||||||||||||||
| Total* | 88 | 2.8 | 2.7 | 22 | 2.0 | 2.6 | 21 | 2.3 | 2.7 | 27 | 3.3 | 2.6 | 18 | 3.7 | 3.0 | |||||
| BSI | ||||||||||||||||||||
| GSI Total | 114 | 1.4 | .69 | 30 | 1.5 | .68 | 27 | 1.3 | .73 | 32 | 1.4 | .56 | 25 | 1.5 | .82 | |||||
| ASI | ||||||||||||||||||||
| Medical | 114 | .32 | .88 | 30 | .23 | .32 | 27 | .42 | 1.3 | 32 | .22 | .32 | 25 | .43 | 1.3 | |||||
| Employment | 114 | .84 | .69 | 30 | .82 | .24 | 27 | .76 | .19 | 32 | .93 | 1.3 | 25 | .84 | .21 | |||||
| Alcohol | 114 | .44 | .23 | 30 | .41 | .21 | 27 | .37 | .24 | 32 | .48 | .21 | 25 | .48 | .26 | |||||
| Drugs | 114 | .18 | .12 | 30 | .21 | .11 | 27 | .14 | .09 | 32 | .19 | .13 | 25 | .18 | .11 | |||||
| Legal | 114 | .15 | .41 | 30 | .11 | .19 | 27 | .26 | .77 | 32 | .13 | .18 | 25 | .11 | .21 | |||||
| Family | 114 | .27 | .18 | 30 | .26 | .16 | 27 | .26 | .18 | 32 | .27 | .18 | 25 | .27 | .19 | |||||
| Psychiatric | 114 | .35 | .26 | 30 | .38 | .20 | 27 | .32 | .19 | 32 | .36 | .36 | 25 | .35 | .25 | |||||
| HRBS | ||||||||||||||||||||
| Risky sexual behavior | 113 | 5.2 | 4.9 | 29 | 4.7 | 4.9 | 27 | 4.7 | 4.9 | 32 | 5.9 | 5.3 | 25 | 5.2 | 4.3 | |||||
| Socrates | ||||||||||||||||||||
| Alcohol Recognition | 114 | 27.8 | 5.6 | 30 | 28.5 | 5.0 | 23 | 27.3 | 5.0 | 32 | 27.6 | 6.7 | 25 | 27.9 | 5.7 | |||||
| Alcohol Ambivalence | 114 | 15.8 | 3.1 | 30 | 15.6 | 3.0 | 23 | 15.8 | 2.9 | 32 | 16.0 | 3.4 | 25 | 15.6 | 3.2 | |||||
| Alcohol Taking Steps | 114 | 32.4 | 4.9 | 30 | 32.6 | 4.0 | 23 | 33.0 | 4.5 | 32 | 31.3 | 5.6 | 25 | 32.8 | 5.4 | |||||
| Drug Recognition | 114 | 28.2 | 5.2 | 30 | 28.8 | 5.7 | 17 | 26.6 | 4.6 | 32 | 28.8 | 5.1 | 25 | 28.3 | 5.2 | |||||
| Drug Ambivalence | 114 | 16.0 | 2.6 | 30 | 16.3 | 2.6 | 17 | 15.8 | 2.2 | 32 | 16.3 | 2.9 | 25 | 15.6 | 2.6 | |||||
| Drug Taking Steps | 114 | 32.7 | 5.0 | 30 | 32.9 | 4.3 | 17 | 32.6 | 5.1 | 32 | 32.8 | 5.8 | 25 | 32.5 | 5.0 | |||||
| Treatment phase visits | 93 | 10.9 | 11.2 | 23 | 11.7 | 10.8 | 23 | 9.2 | 12.2 | 26 | 9.5 | 9.4 | 21 | 14.3 | 12.5 | |||||
AIE Scale = American Indian Enculturation Scale
Fagerstrom total is for participants that indicated non-ceremonial smoking of tobacco.
Participants currently in the treatment phase at the time of analyses were not included.
Discussion
The proposed research is innovative and has the potential to improve public health. To our knowledge, this is the first CM randomized controlled trial intervention targeting alcohol and drug co-addiction in American Indian adults. CM has proven efficacious for all populations in which it has been used; yet no previous study of CM has focused specifically on treating alcohol and drug use among American Indian adults. Should this trial prove successful, and given the great cost inflicted by alcohol addiction and drug misuse among American Indian communities, a simple culturally-adapted program of CM focused on substance misuse may be an effective strategy for tribal communities. A CM program can easily be added to most treatment systems for substance misuse disorders with moderate effort and expense relative to other treatment strategies, many of which require extensive training and financial resources. By establishing a tribal-university partnership to adapt, implement, and evaluate this trial, we can increase our knowledge of evidence-based addiction treatments and research, while improving trust for addiction interventions among American Indian communities through ongoing collaboration.
Lessons learned
We experienced substantial challenges with recruitment and implementation of this trial which required us to significantly modify recruitment and research design (See Table 3 for summary). For example, this trial was originally designed to focus on co-use of alcohol and prescription opioids in two American Indian communities, due to challenges both these communities were facing with prescription drug abuse nearly five years ago. After grant funding was obtained, one of the participating communities decided not to participate and a subsequent site was unable to recruit sufficient numbers of participants (<10 participants in one year), in part because of leadership changes and other site issues out of our control that negatively impacted the community and made recruitment challenging. Moreover, there was a significant amount of lag time (approximately 2.25 years) between when the grant for this research was submitted and when it was officially awarded so that we could begin re-contacting sites in order to assess their level of interest. Therefore, the trial was carried out in only one community. Despite this change we successfully recruited from this one community, meeting our initial recruitment goals for the study. Importantly, the primary drugs of abuse in this community are methamphetamine and cannabis. Therefore, we modified the study procedures to allow for the inclusion of alcohol and any other drug (amphetamines, cannabis, cocaine, methamphetamines, opiates), and we lowered the required number of drinking days from at least five times within the past month to at least one occasion of four or more drinks to capture heavy drinking and again, emphasizing the primary drugs of abuse, methamphetamine and cannabis.
Table 3.
Summary of Study Barriers and Lessons Learned
| Barriers | Changes to the trial | Lessons Learned |
|---|---|---|
|
Substances of focus: |
Trial was originally designed to focus on co-use of alcohol and prescription opioids but the primary drugs of abuse in the participating community were methamphetamine and cannabis. We modified the study procedures to allow for the inclusion of alcohol and any other drug and we lowered the required number of drinking days to capture heavy drinking. |
Important to be flexible to the current needs of participating communities. |
| Recruitment: | Initial efforts relying primarily on clinician referrals were ineffective. We worked with study interventionists to develop new methods for recruitment, including: radio, newspaper, and Facebook advertisements, as well as regular attendance at community events, and word of mouth recruitment. We also modified the protocol to address attrition, including using multiple offices located across the community to conduct study interviews as well as obtaining IRB approval for study staff to provide transportation. |
Word of mouth recruitment, (i.e., referrals from current participants) was the most effective strategy in this trial. In addition, knowledgeable and trusted community interventionists are crucial to successfully recruit participants. Study recruitment significantly improved when the university-based study team agreed to significant modifications to recruitment population based on feedback received from the community- based researchers. |
| Site drop-out | Commited additional resources as soon as possible to site where recruitment is more successful, and continued to develop novel recruitment methods based on community feedback, trial and error, etc. |
Develop several alternative sites for data collection, especially when the collaboration with a site is new for a given project, and with a difficultto- recruit population, such as those suffering from co-addiction in rural locations. |
|
Obtaining approval from multiple entities |
Submitted study protocol to review several times to multiple university and tribal entities. |
Plan and allow ample time for study review by multiple entities. |
|
Lack of transportation |
Obtained IRB approval for study staff to provide transportation. | Providing transportation to remote participants improved recruitment and attrition. |
Contingency management intervention targeting co-addiction of alcohol and drugs among American Indian adults: Design, methodology, and baseline data
Another challenge we faced was recruitment. Initial efforts relying primarily on clinician referrals were ineffective. We worked with study interventionists to develop new methods for recruitment. These included, radio, newspaper, and Facebook advertisements, as well as regular attendance at community events, and word of mouth recruitment. It is our experience that word of mouth recruitment, (i.e., referrals from current participants) was the most effective strategy for recruitment. Without our knowledgeable and trusted community interventionists we would not have been able to successfully recruit participants. We also made modifications to the protocol to address attrition, including using multiple offices located across the community to conduct study interviews as well as obtaining institutional review board approval for study staff to provide transportation.
In conclusion, this randomized controlled trial of CM for alcohol and other drugs, demonstrates that randomized controlled trials of adapted behavioral interventions can be conducted in partnership with American Indian communities. However, this work presents unique challenges, such as needing to obtain approval from multiple entities, challenges finding qualified study staff who are also trusted community members, distrust of research, and lack of transportation. The success of this study in terms of recruitment, is a direct result of the strong reciprocal partnership that developed between our university and community-based university research teams. Study recruitment significantly improved when the university-based study team agreed to significant modifications to recruitment population based on feedback received from the community-based researchers.
Acknowledgments
We thank our community partners for their continuing support and partnership throughout all stages of this project.
Funding
This work was supported by the National Institute on Minority Health and Health Disparities, research grant P20MD006871, Principal Investigators McPherson and Buchwald.
Footnotes
Declaration of conflicting interests
Drs. McPherson and Roll have received research funding from the Bristol-Myers Squibb Foundation. Dr. McPherson has received research funding from Ringful Health, LLC., the Orthopedic Specialty Institute, and consulted for Consistent Care company. This funding is in no way related to the investigation reported here.
References
- 1.Gone J and Trimble J. American Indian and Alaska Native mental health: diverse perspectives on enduring disparities. Annu Rev Clin Psychol 2012; 8: 131–160. [DOI] [PubMed] [Google Scholar]
- 2.Spicer P, Beals J, Croy C, et al. The prevalence of DSM-III-R alcohol dependence in two American Indian populations. Alcohol Clin Exp Res 2003; 27: 1785–1797. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Results from the 2013 national survey on drug use and health: Summary of national findings Rockville, MD: Substance Abuse and Mental Health Services Administration, 2014. [Google Scholar]
- 4.Cunningham JK, Solomon TA and Muramoto ML. Alcohol use among Native Americans compared to whites: examining the veracity of the ‘Native American elevated alcohol consumption’ belief. Drug Alcohol Depend 2016; 160: 65–75. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health. Alcohol use and alcohol use disorders in the United States: main findings from the 2001–2002 national epidemiologic survey on alcohol and related conditions Bethesda, MD: National Institute of Alcohol Abuse and Alcoholism, 2006. [Google Scholar]
- 6.US Department of Health and Human Services. Trends in Indian Health 2014 Edition U.S. Department of Health and Human Services Indian Health Service, Office of Public Health Support, Division of Program Statistics, 2014. [Google Scholar]
- 7.Duran E Healing the soul wound: counseling with American Indian and other native peoples New York, NY: Teachers College Press, 2006. [Google Scholar]
- 8.Duran E and Duran B. Native American postcolonial psychology Albany, New York: State Univeristy of New York Press, 1995. [Google Scholar]
- 9.National Survey on Drug Use and Health. The NSDUH Report: Substance use among American Indian or Alaska Native Adolescents, 2011. [Google Scholar]
- 10.Naimi TS, Cobb N, Boyd D, et al. Alcohol-attributable deaths and years of potential life lost among American Indians and Alaska Natives—United States, 2001–2005. MMWR Morb Mortal Wkly Rep 2008; 57: 938–941. [PubMed] [Google Scholar]
- 11.Mitchell CM, Beals J, Novins DK, et al. Drug use among two American Indian populations: prevalence of lifetime use and DSM-IV substance use disorders. Drug Alcohol Depend 2003; 69: 29–41. [DOI] [PubMed] [Google Scholar]
- 12.Beals J, Novins DK, Spicer P, et al. Help seeking for substance use problems in two American Indian reservation populations. Psychiatr Serv 2006; 57: 512–520. [DOI] [PubMed] [Google Scholar]
- 13.Beals J, Spicer P, Mitchell CM, et al. Racial disparities in alcohol use: Comparison of 2 American Indian reservation populations with national data. Am J Public Health 2003; 93: 1683–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Midanik LT, Tam TW and Weisner C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend 2007; 90: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2015 national survey on drug use and health. (HHS Publication No. SMA 16–4984, NSDUH Series H-51) Rockville, MD: Substance Abuse and Mental Health Services Administration, 2016. [Google Scholar]
- 16.Substance Abuse and Mental Health Services Administration. The NSDUH Report: Substance use among American Indian or Alaska Native adults Rockville, MD: Office of Applied Studies, 2010. [Google Scholar]
- 17.Feldstein SW, Venner KL and May PA. American Indian/Alaska Native alcohol-related incarceration and treatment. Am Indian Alsk Native Ment Health Res 2006; 13: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaghan RC. Risk factors associated with dropout and readmission among First Nations individuals admitted to an inpatient alcohol and drug detoxification program. CMAJ 2003; 169: 23–27. [PMC free article] [PubMed] [Google Scholar]
- 19.Greenfield BL and Venner KL. Review of substance use disorder treatment research in Indian country: Future directions to strive toward health equity. Am J Drug Alcohol Abuse 2012; 38: 483–492. [DOI] [PubMed] [Google Scholar]
- 20.O’Malley SS, Robin RW, Levenson AL, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res 2008; 32: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodall WG, Delaney HD, Kunitz SJ, et al. A randomized trial of a DWI intervention program for first offenders: intervention outcomes and interactions with antisocial personality disorder among a primarily American-Indian sample. Alcohol Clin Exp Res 2007; 31: 974–987. [DOI] [PubMed] [Google Scholar]
- 22.Gone J and Alcantara C. Identifying effective mental health interventions for American Indians and Alaska Natives: a review of the literature. Cultur Divers Ethnic Minor Psychol 2007; 13: 356–363. [DOI] [PubMed] [Google Scholar]
- 23.Nebelkopf E, King J, Wright S, et al. Growing roots: Native American evidence-based practices. J Psychoactive Drugs 2011; 43: 263–268. [DOI] [PubMed] [Google Scholar]
- 24.McDonell MG, Nepom JR, Leickly E, et al. A culturally-tailored behavioral intervention trial for alcohol use disorders in three American Indian communities: rationale, design, and methods. Contemp Clinical Trials 2016; 47: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigelow GE and Silverman KE. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST and Silverman KE (eds) Motivating behavior change among illicit-drug abusers: Research on contingency management interventions Washington DC: American Psychological Association, 1999, pp.15–31. [Google Scholar]
- 26.Stanger C and Budney AJ. Contingency management approaches for adolescent substance use disorders. Child Adolesc Psychiatr Clin N Am 2010; 19: 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins ST and Petry NM. Contingency management. Incentives for sobriety. Alcohol Res Health 1999; 23: 122–127. [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy SM, McDonell MG, McPherson S, et al. An economic evaluation of a contingency-management intervention for stimulant use among community mental health patients with serious mental illness. Drug Alcohol Depend 2015; 153: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olmstead TA and Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine-or opioid-dependent outpatients. Drug Alcohol Depend 2009; 102: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olmstead TA, Sindelar JL, Easton CJ, et al. The cost-effectiveness of four treatments for marijuana dependence. Addiction 2007; 102: 1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olmstead TA, Sindelar JL and Petry NM. Clinic variation in the cost-effectiveness of contingency management. Am J Addict 2007; 16: 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olmstead TA, Sindelar JL and Petry NM. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug Alcohol Depend 2007; 87: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budney AJ, Stanger C, Tilford JM, et al. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol Addict Behav 2015; 29: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurti AN, Davis D, Redner R, et al. A review of the literature on remote monitoring technology in incentive-based interventions for health-related behavior change. Transl Issues Psychol Sci 2016; 2: 128–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stitzer ML, Bigelow GE and Liebson I. Reinforcement of drug abstinence: A behavioral approach to drug abuse treatment. NIDA Res Monogr 1979; (25): 68–90. [DOI] [PubMed] [Google Scholar]
- 36.Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994; 51: 568–576. [DOI] [PubMed] [Google Scholar]
- 37.Stitzer ML, Rand CS, Bigelow GE, et al. Contingent payment procedures for smoking reduction and cessation. J Appl Behav Anal 1986; 19: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins ST, Stitzer ML, Bigelow GE, et al. Contingent methadone delivery: effects on illicit-opiate use. Drug Alcohol Depend 1986; 17: 311–322. [DOI] [PubMed] [Google Scholar]
- 39.Robles E, Stitzer ML, Strain EC, et al. Voucher-based reinforcement of opiate abstinence during methadone detoxification. Drug Alcohol Depend 2002; 65: 179–189. [DOI] [PubMed] [Google Scholar]
- 40.Budney AJ, Higgins ST, Radonovich KJ, et al. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000; 68: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 41.Kadden RM, Litt MD, Kabela-Cormier E, et al. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav 2007; 32: 1220–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller PM. A behavioral intervention program for chronic public drunkenness offenders. Arch Gen Psychiatry 1975; 32: 915–918. [DOI] [PubMed] [Google Scholar]
- 43.Higgins ST and Silverman KE. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions Washington DC: American Psychological Association, 1999. [Google Scholar]
- 44.Roll JM. Contingency management: an evidence-based component of methamphetamine use disorder treatments. Addiction 2007; 102: 114–120. [DOI] [PubMed] [Google Scholar]
- 45.Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008; 165: 179–187. [DOI] [PubMed] [Google Scholar]
- 46.Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 2006; 101: 1546–1560. [DOI] [PubMed] [Google Scholar]
- 47.Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006; 101: 192–203. [DOI] [PubMed] [Google Scholar]
- 48.Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry 2005; 62: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 49.Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006; 63: 201–208. [DOI] [PubMed] [Google Scholar]
- 50.Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. J Consult Clin Psychol 2008; 76: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pilling S, Strang J and Gerada C. Psychosocial interventions and opioid detoxification for drug misuse: summary of NICE guidance. BMJ 2007; 335: 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol 2009; 44: 55–61. [DOI] [PubMed] [Google Scholar]
- 53.Wurst FM and Metzger J. The ethanol conjugate ethyl glucuronide is a useful marker of recent alcohol consumption. Alcoholism Clin Exp Res 2002; 26: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 54.Litten RZ, Bradley AM and Moss HB. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcohol Clin Exp Res 2010; 34: 955–967. [DOI] [PubMed] [Google Scholar]
- 55.Helmus TC, Saules KK, Schoener EP, et al. Reinforcement of counseling attendance and alcohol abstinence in a community-based dual-diagnosis treatment program: a feasibility study. Psychol Addict Behav 2003; 17: 249–251. [DOI] [PubMed] [Google Scholar]
- 56.Wurst FM, Kempter C, Metzger J, et al. Ethyl glucuronide: a marker of recent alcohol consumption with clinical and forensic implications. Alcohol 2000; 20: 111–116. [DOI] [PubMed] [Google Scholar]
- 57.Wurst FM, Seidl S, Ladewig D, et al. Ethyl glucuronide: on the time course of excretion in urine during detoxification. Addict Biol 2002; 7: 427–434. [DOI] [PubMed] [Google Scholar]
- 58.Wurst FM, Skipper GE and Weinmann W. Ethyl glucuronide—the direct ethanol metabolite on the threshold from science to routine use. Addiction 2003; 98: Suppl 2: 51–61. [DOI] [PubMed] [Google Scholar]
- 59.Wurst FM, Tabakoff B, Alling C, et al. World Health Organization/International Society for Biomedical Research on Alcoholism study on state and trait markers of alcohol use and dependence: back to the future. Alcohol Clin Exp Res 2005; 29: 1268–1275. [DOI] [PubMed] [Google Scholar]
- 60.Wurst FM, Vogel R, Jachau K, et al. Ethyl glucuronide discloses recent covert alcohol use not detected by standard testing in forensic psychiatric inpatients. Alcohol Clin Exp Res 2003; 27: 471–476. [DOI] [PubMed] [Google Scholar]
- 61.Skipper GE, Weinmann W, Thierauf A, et al. Ethyl glucuronide: a biomarker to identify alcohol use by health professionals recovering from substance use disorders. Alcohol Alcohol 2004; 39: 445–449. [DOI] [PubMed] [Google Scholar]
- 62.Kip MJ, Spies CD, Neumann T, et al. The usefulness of direct ethanol metabolites in assessing alcohol intake in nonintoxicated male patients in an emergency room setting. Alcohol Clin Exp Res 2008; 32: 1284–1291. [DOI] [PubMed] [Google Scholar]
- 63.Sheehan D, Lecrubier Y, Sheehan H, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59: Suppl 20: 22–33; quiz 34–57. [PubMed] [Google Scholar]
- 64.Kosten T, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend 2003; 70: 315–325. [DOI] [PubMed] [Google Scholar]
- 65.Oliveto A, Poling J, Sevarino KA, et al. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depend 2005; 79: 157–165. [DOI] [PubMed] [Google Scholar]
- 66.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roll JM and Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend 2000; 58: 103–109. [DOI] [PubMed] [Google Scholar]
- 68.Roll JM, Higgins ST and Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. J Appl Behav Anal 1996; 29: 495–504; quiz 504–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonell MG, Leickly E, McPherson S, et al. A randomized controlled trial of ethyl glucuronide-based contingency management for outpatients with co-occurring alcohol use disorders and serious mental illness. Am J Psychiatry 2017; 174: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roll JM, Chudzynski J, Cameron JM, et al. Duration effects in contingency management treatment of methamphetamine disorders. Addict Behav 2013; 38: 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonell MG, Srebnik D, Angelo F, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am J Psychiatry 2013; 170: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chudzynski J, Roll JM, McPherson S, et al. Reinforcement schedule effects on long-term behavior change. Psychol Rec 2015; 65: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orr MF, Lederhos Smith C, Finlay M, et al. Pilot investigation: randomized-controlled analog trial for alcohol and tobacco smoking co-addiction using contingency management. Behav Pharmacol 2018; 29: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ries RK, Dyck DG, Short R, et al. Outcomes of managing disability benefits among patients with substance dependence and severe mental illness. Psychiatr Serv 2004; 55: 445–447. [DOI] [PubMed] [Google Scholar]
- 75.Petry NM, Martin B, Cooney JL, et al. Give them prizes and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000; 68: 250–257. [DOI] [PubMed] [Google Scholar]
- 76.McDonell MG, Skalisky J, Leickly E, et al. Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients. Drug Alcohol Depend 2015; 157: 184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carise D, Wicks K, McLellan A, et al. Addiction severity index 5th edition–North Dakota State adaptation for use with Native Americans. Treatment Research Institute at University of Pennsylvania, 1998. [Google Scholar]
- 78.Sobell LC and Sobell MB. Alcohol timeline followback. In: Rush JA, Pincus HA and Measures TFftHoP (eds) Handbook of psychiatric measures Washington DC: American Psychiatric Association, 2000, pp.477–479. [Google Scholar]
- 79.Barry D, Weinstock J and Petry NM. Ethnic differences in HIV risk behaviors among methadone-maintained women receiving contingency management for cocaine use disorders. Drug Alcohol Depend 2008; 98: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darke S, Hall W, Heather N, et al. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS 1991; 5: 181–185. [DOI] [PubMed] [Google Scholar]
- 81.Ware J Jr, Kosinski M and Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 82.Quandt SA, Graham CN, Bell RA, et al. Ethnic disparities in health-related quality of life among older rural adults with diabetes. Ethn Dis 2007; 17: 471–476. [PMC free article] [PubMed] [Google Scholar]
- 83.Higgins ST, Delaney DD, Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry 1991; 148: 1218–1224. [DOI] [PubMed] [Google Scholar]


