1. Introduction
Social stress exposure can lead to a wide range of neurologic behavioral disturbances relevant to psychiatric disorders [1]. Social defeat of an intruder confronted by a resident is a potent social stressor in rodents. This model promotes several behavioral, endocrine and physiological changes in the intruder animal, which may result in anxiety- or depressive-like behaviors [2,3]. Social stress produces neuroendocrine responses characterized by increased activity of the hypothalamus-pituitary-adrenal axis which is mediated by a cascade of hormones, such as CRF, adrenocorticotropic hormone and glucocorticoids [4]. Although these mechanisms are protective, prolonged exposure to stress may engender pathophysiological consequences [5].
The effects of CRF and the structurally related Urocortins are mediated by two CRF receptor subtypes: CRF type 1 (CRFR1) and type 2 (CRFR2). Additionally, CRF and Urocortins interact with a CRF binding protein (CRFBP), which binds CRF with high affinity (Ki values for CRF and Urocortin 1 are 217 and 77.2 pM, respectively) compared to CRF receptors [6,7]. CRF is bound with a 40-fold higher affinity for CRFR1 than CRFR2, while CRFR2 and the CRFBP are bound with high affinity to Urocortin in rats [8,9]. Although the CRFBP function in the brain is largely unknown, distinct mechanisms have been proposed. One possibility is that CRFBP is responsible for capturing the CRF available extracellularly, preventing CRF receptor activation [10]. Alternatively, there is evidence that the function of the CRFBP may be more complex and may extend beyond mere sequestration of CRF or CRF-like ligands [11]. Based on ex vivo experiments in mice, Ungless and colleagues proposed that CRF enhances N-methyl-D-aspartate (NMDA) receptor activity through activation of CRFR2 in neurons of the ventral tegmental area (VTA), an effect that requires the action of the CRFBP [11]. Similarly, our group recently demonstrated that the co-infusion of astressin-2B, a CRFR2 antagonist, with a sub-effective dose of CRF6–33 in the VTA attenuates alcohol binge drinking in mice [12]. These studies not only indicate that the CRFBP may actively participate in CRF signaling, but also suggest that the CRFBP may act in combination with CRFR2. More evidence, however, is needed to clarify the role of this binding protein in the brain, specifically in discrete regions involved in the stress regulation.
The BNST connects the amygdaloid complex with the hypothalamus and has been implicated in the control of neuroendocrine functions and behavioral responses to stress [13,14]. Both, inhibitory and facilitatory roles of the BNST in these responses have been reported, depending on the type of the aversive stimulus and subregion of the BNST [15]. For example, the anterior portion has been described as an important site of action for extrahypothalamic CRF in the modulation of anxiety-like behaviors [16]. In vivo experiments showed that intracerebroventricular (i.c.v.) infusion of CRF increased startle responses and this effect can be blocked by CRF antagonism or lesions of the anterior BNST in rodents [17,18]. Moreover, intra-BNST CRF increased anxiety-like behaviors in the elevated plus-maze [19], produced place conditioning aversion to a CRF-paired environment [19], and reinstated cocaine seeking in rats during drug abstinence [20]. The BNST contains CRF-producing neurons which seem to be sensitive to stress [21,22]. In fact, exposures to corticosterone and the pharmacological stressor yohimbine upregulate CRF mRNA expression in the BNST [23,24].
Exposure to intermittent episodes of social defeat seems to reduce responses to hedonic stimuli; defeated animals show a lack of sexual interest [25], decreased preference for sweet solutions [26,27], reduction of locomotor and exploratory behaviors in novel environments [27], and reduced preference for social interaction [28]. The current experiments were designed to test the hypothesis that four brief encounters with an aggressor rat can elicit dysregulated behaviors in adult Wistar rats, including anxiety- or depressive-like symptoms. Further, we investigated the role of BNST CRFBP in the modulation of stress-induced anxiety responses.
2. Methods
2.1. Animals
Adult male Wistar rats were obtained at approximately postnatal day (PND) 50 and housed at the Animal Experimental Unit of Hospital de Clínicas de Porto Alegre (Porto Alegre, RS, Brazil) for 2 weeks before starting the experiments. At PND 70, rats were singly housed in custom-built acrylic cages (15 × 25 × 20 cm). Separate male Wistar rats, weighing 465 ± 10 g, with a reliable history of aggressive behavior in confrontations with intruders, termed stimulus ‘resident’ rats, were housed in pairs with sterile female Wistar rats in large custom-built acrylic cages (46 × 71 × 46 cm). Resident rats and intruder rats were kept in separate rooms with controlled environmental conditions: 21 ± 1° C temperature, 40–60 % humidity and 12 h/12 h light-dark cycle (lights on at 7:00 AM). The cages were lined with sawdust bedding and rats had free access to food and water. A total of N = 50 rats was used in this study (n = 7 residents; n = 7 females paired with residents; n = 3 ovariectomized females for social interaction test; n = 33 intruders). This study was carried out in accordance with the Brazilian Federal Law No11.794/2008 for the scientific use of animals. The protocol was approved by the Ethics Committee on Animal Use of Animal Experimentation Unit from Hospital de Clínicas de Porto Alegre.
2.2. Drug
The selective CRFBP antagonist CRF6–33 (Tocris Bioscience; Ellisville, MD, USA) was dissolved in sterile saline solution (NaCl 0.9%) at concentrations of 0.25 and 0.5 µg in 0.2 µL.
2.3. Procedures
The stress protocol was conducted following procedures established by Miczek and colleagues [26,29]. Rats were subjected to four brief intermittent episodes of social defeat stress over the course of 10 days, or served as contemporary home cage controls. After concluding the stress protocol, rats were tested for sweet solution preference, had bilateral cannulae implanted into the BNST, received local injections of CRF6–33 and were examined for social approach to an unfamiliar ovariectomized female (Figure 1.a). All procedures occurred during the first four hours of the dark phase. Rats were monitored daily for health conditions and were weighed before the first and the last social defeat session.
Figure 1 –
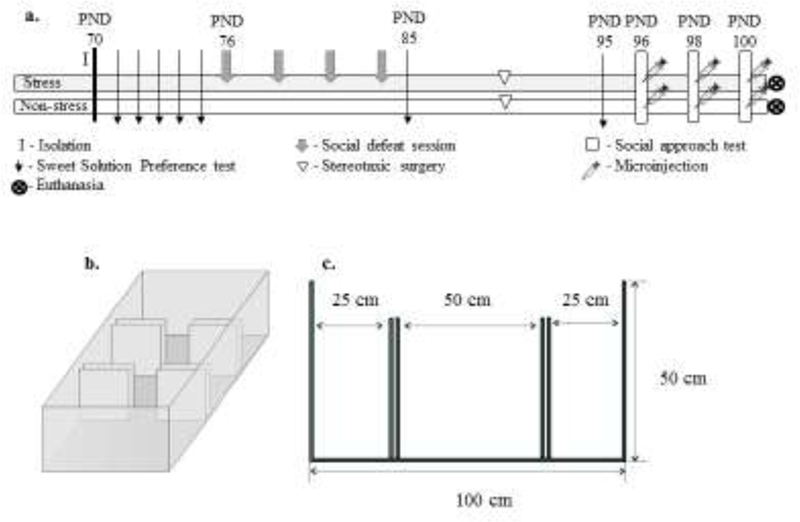
a. Experimental procedure. Stress: rats exposed to intermittent episodes of social defeat; Non-stress: control animals; n = 13–14 rats per group; b. Schematic representation of the social approach apparatus in a three-dimensional view; c. Length and height of the chambers and walls. The central chamber has openings that allow the rat to move freely throughout the whole apparatus. After the habituation period (5 min), an unfamiliar ovariectomized female was placed inside a small acrylic cage in one of the side chambers and an identical empty cage was placed in the opposite side chamber. The walls were removed and the rat could move freely throughout the three chambers. The positions of the female and the empty cage were alternated between side chambers and upper and lower corners between sessions.
2.4. Intermittent episodes of social defeat stress
Intruders were exposed to a different resident for four sessions with 72 h interval between them [30]. The protocol consists of three phases: in the first phase, ‘pre-defeat’, the female was removed and a protective cage containing the intruder was placed into the large resident home cage for 10 min. The intruder was threatened and investigated by the resident through the perforated acrylic walls of the protective cage. In the second phase, ‘defeat phase’, the intruder was removed from the protective cage and directly confronted the aggressor resident. The fight was terminated after the intruder displayed supine posture for 5 s, or 5 min after the resident’s first biting attack, or after 5 bites, whichever occurred first. In the third phase, ‘subordination’, the intruder was placed back into the protective cage within the resident’s home cage for 10 min [30,31]. Control rats were handled and weighed, without being submitted to the stress protocol.
2.5. Preference for sweet solution
At PND 85 and 95, rats were removed from their home cages and assessed for sweet solution preference (0.8% saccharin and sodium cyclamate; Zero·Cal, Hypermarcas; São Paulo, SP, Brazil) during one hour in a test cage (24 × 38 × 15 cm) in an appropriated room for behavioral testing. First, at PND 71, rats were exposed overnight to one bottle containing the sweet solution and another bottle containing water. During the following four consecutive days, PND 72–75, rats were given a daily 2-bottle choice (sweet solution and water) for one hour, one week before the stress protocol (baseline condition). To counteract side preference, the position of the bottles was switched between trials. Animals were not food or water deprived, before or after the procedure. Fluid consumption was measured by weighing the bottles, and preference was expressed as percentage of total intake, calculated by dividing the total amount of sweet solution consumed by the total fluid intake (water plus sweet solution) [32]. An empty “drip” cage served as control for evaporation and spillage due to handling of bottles.
2.6. Stereotaxic Surgery
Rats were anesthetized with isoflurane prior to and during the surgery. Pre-surgical analgesia was induced with tramadol (20 mg/kg, i.p.) and local anesthesia with bupivacaine (8 mg/kg, intradermal). Rats were placed on a stereotaxic frame (Kopf Instruments; Tujunga, CA, USA) and guide cannulae were bilaterally implanted into the BNST (from bregma, AP: 1.0; ML: +/− 2.5; DV: −8.0 with 10° angle) [33,34]. Guide cannulae were fixed with stainless steel screws and dental cement, and were sealed with custom made dummy cannulae. Immediately after surgery, rats received dipyrone (500 mg/kg, i.p.), and subsequent pain control was provided with tramadol (20 mg/kg, i.p.), every 12 h for 2 days. Surgeries were conducted at least 5 days before the first infusion.
2.7. Intra-BNST CRF6–33 infusions
Starting ten days after the last social defeat encounter (PND 96), rats received intra-BNST CRF6–33 three times, according to a design that counterbalanced saline and drug doses (once a day, with 48 h intervals between the infusions). Microinjections were performed with an infusion pump (Insight EFF 311; Ribeirão Preto, SP, Brazil) and were delivered bilaterally and simultaneously at a constant volume of 0.2 μL/side over a period of 2 min. The injector needles extended 1 mm beyond the tip of the guide cannulae and were left in place for 1 additional minute after the end of the infusion to avoid reflux and allow for diffusion. During the microinjection procedure, rats were allowed to move freely in a small cage (30 × 20 × 13 cm). Infusions started five minutes before the social approach test. Doses were scheduled as follows: saline solution, a lower dose of CRF6–33 (0.25 μg/0.2 μL), or a higher dose of CRF6–33 (0.5 μg/0.2 μL). These doses have been previously used [12].
2.8. Social Approach Test
The social approach apparatus consists of an acrylic box (40 × 50 × 120 cm) with removable walls separating the box into three chambers (Figure 1.b-c). The walls have openings that allow the animal to move freely throughout the whole apparatus. Rats were initially confined to the middle chamber for 5 min. After this habituation period, an unfamiliar ovariectomized female was placed inside a small acrylic cage (15 × 25 × 20 cm) in one of the side chambers. An identical empty cage was placed in the opposite side chamber. Then, the walls were removed, allowing the rat to explore all three chambers over a period of 10 min. The small cages did not allow physical contact between the rats, preventing the female from any aggressive interaction. The location of the female and the empty cage were alternated between the upper and lower corners of the side chamber in consecutive trials. All sessions were video recorded and total distance travelled was measured using a behavioral tracking system (ANY-Maze; Wood Dale, IL, USA); additional behaviors were coded using event-logging software [35]. During the habituation period distance traveled was measured for 5 min, and frequency and duration of entries into the side chambers containing the unfamiliar female (interaction zone) or the empty cage (object zone) were measured for 10 min [36]. The apparatus was cleaned with 70% alcohol between trials. Animals were tested three times, with a 48 h-interval between sessions.
2.9. Histology
One day after the last social approach test (PND 101), rats were deeply anesthetized with an overdose of isoflurane 5% for more than 3 min and were perfused with 0.9% saline followed by 4% buffered paraformaldehyde solution prior to brain removal. The fixed brains were sliced in 80 µm coronal sections using a cryostat (Microm HM 505 E; Waltham, MA, USA). Slices were mounted on glass slides and stained with hematoxylin-eosin, and injector placements were verified using light microscopy, according to the rat brain atlas [34]. Diagrammatic representations of bilateral BNST injection sites are shown in Figure 2. Rats with injector tracks that did not reach the BNST were excluded from the analysis (~18%).
Figure 2 –
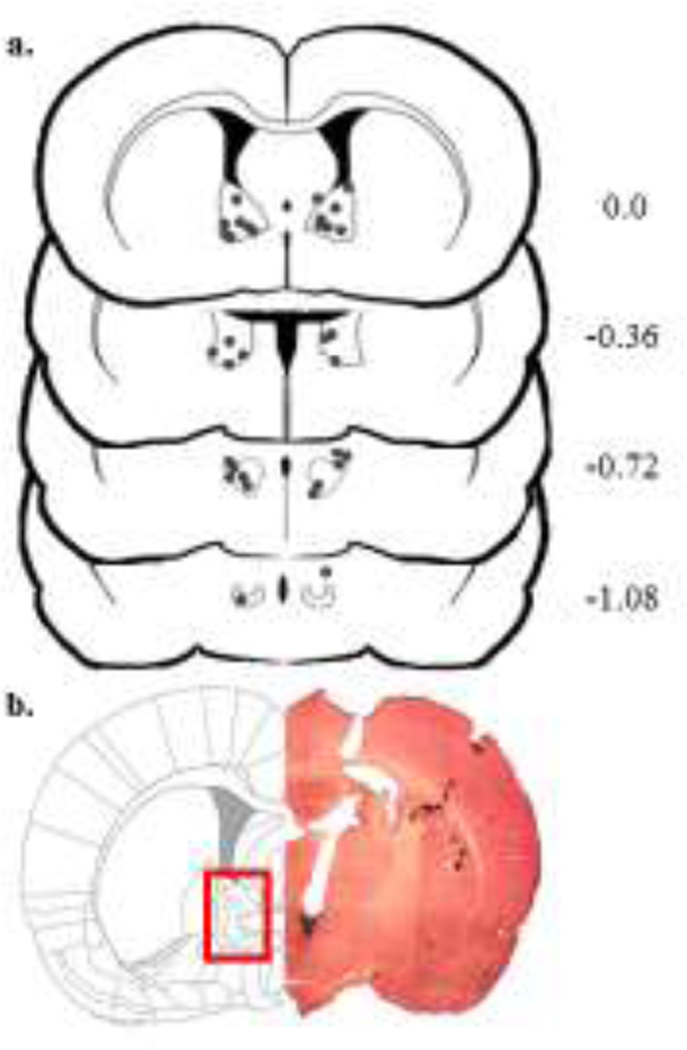
a. Correct cannulae placements into the bed nucleus of the stria terminalis; b. Diagram and representative photomicrograph after hematoxylin-eosin staining. The number of points in the figures is less than the total number of animals because of overlapping injection sites.
2.10. Statistical analysis
To assess the effects of social stress on preference for sweet solution, a two-way repeated measures analysis of variance (ANOVA) was conducted (Statistica 6.0, Dell Software; Round Rock, TX, USA) using stress vs. non-stress as the condition factor, and baseline and two additional observation times as the sessions factor. To assess social approach, frequency of entries and time spent on either interaction zone (IZE and IZT) or object zone (OZE and OZT) were analyzed. A two-way repeated measures ANOVA was performed, followed by a priori hypothesis driven one-way ANOVA to evaluate the effects of CRF6–33 treatments (0.25 μg/0.2 μL, and 0.5 μg/0.2 μL) using each condition (stress or non-stress) as a single factor. When indicated by a significant main effect, post hoc comparisons were performed using Bonferroni correction for multiple comparisons. T-tests were conducted to compare stressed vs. non-stressed groups treated with saline. The statistical significance was set at p < 0.05.
3. Results
3.1. Effects of intermittent social defeat stress on sweet solution preference and total distance travelled
Intermittent exposure to social defeat stress did not produce significant effects on preference for sweet solution as revealed by two-way repeated measures ANOVA (F (1, 20) = 0.33, p > 0.05). Moreover, rats did not show significant differences in preference across sessions (F (1, 20) = 0.16, p > 0.05) (Table 1). Intermittent exposure to social stress did not affect total distance travelled (F (1, 23) = 0.97, p > 0.05), as well as the treatments (F (1, 23) = 0.09, p > 0.05) (Figure 3e).
Table 1 –
Data and summary of repeated measures ANOVA for preference (mean ± SEM) for sweet solution during baseline and two additional measurements. The significance threshold was set at p < 0.05 for all comparisons.
| Sweet Solution Preference (%) | |||
|---|---|---|---|
| PND 72–75 (Baseline) | PND 85 | PND 95 | |
| Non-stressed | 93.0 ± 3.0 | 96.0 ± 3.0 | 96.0 ± 7.0 |
| Stressed | 93.0 ± 2.0 | 94.0 ± 3.0 | 90.0 ± 6.0 |
Figure 3 -.

Effects of corticotropin releasing factor (CRF) fragment 6–33, a CRF binding protein antagonist, administered into the bed nucleus of the stria terminalis of rats exposed to intermittent social defeat stress or non-stressed controls. a. Number of entries into the interaction zone; b. Time spent in the interaction zone; c. Number of entries into the object zone; d. Time spent in object zone. The interaction zone contained an unfamiliar female placed inside a small acrylic cage and the object zone contained an empty small acrylic cage. Data are mean ± SEM; * stressed vs. non-stressed rats treated with saline; # saline infusion vs. CRF6–33 0.25 µg/0.2 µl in stressed rats; p < 0.05, n = 13–14 rats per group. e. Total distance travelled measured in meters. The animals were tested during the habituation period (5 min) in the central chamber of the three-chamber apparatus. The central chamber measures 0.5 × 0.5 m. Data are mean ± SEM; n = 12 rats per group
3.2. Effects of intra-BNST CRF6–33 on social approach
The two-way repeated measures ANOVA did not reveal significant differences between conditions (IZE: F (1, 75) = 1.28; IZT: F (1, 75) = 0.98; OZE: F (1, 75) = 0.30; OZT: F (1, 75) = 2.12; p > 0.05 in all cases), treatments (IZE: F (2, 75) = 1.52; IZT: F (2, 75) = 0.64; OZE: F (2, 75) = 0.13; OZT: F (2, 75) = 0.78; p > 0.05 in all cases), and interactions between conditions and treatments (IZE: F (2, 75) = 2.10; IZT: F (2, 75) = 1.82; OZE: F (2, 75) = 0.09; OZT: F (2, 75) = 0.14; p > 0.05 in all cases).
Pairwise comparisons between conditions (stress vs. non-stress) after saline infusions showed that socially stressed animals presented lower levels of social interaction compared to non-stressed rats (IZE: t = 2.39 and IZT: t = 2.46; p < 0.05 in both cases) (Figure 3a-b). As the two-way repeated measures ANOVA failed to demonstrate significant differences between conditions we performed a one-way ANOVA based on a priori hypotheses. Stressed rats treated with intra-BNST CRF6–33 entered the interaction zone significantly more than saline-treated animals (IZE: F (2, 39) = 4.14, p < 0.05). Post hoc analysis indicated that this difference is related to an increase in social approach promoted by the low dose of CRF6–33 (Figure 3b). Drug treatments did not alter the time spent in the interaction zone (IZT: F (2, 39) = 1.91, p > 0.05) (Figure 3b) or the exploratory behaviors of non-stressed animals (IZE: F (2, 36) = 0.07; IZT: F (2, 36) = 0.20; OZE: F (2, 36) = 0.20; OZT: F (2, 36) = 0.17; p > 0.05 in all cases) (Figure 3).
General exploratory behaviors were not altered by exposure to intermittent social stress in saline-treated animals (OZE: t = 0.48; OZT: t = 0.99; p > 0.05 in both cases) (Figure 3c-d). Moreover, intra-BNST CRF6–33 did not affect the exploration of a new object (OZE: F (2, 39) = 0.01; OZT: F (2, 39) = 0.72; p > 0.05 in all cases) (Figure 3c-d), as well as activity in the central chamber (data not shown), indicating that the drug has a selectively action on social approach.
4. Discussion
The current experiments provide the first evidence for selective improvements on social behaviors after exposure to brief intermittent episodes of social defeat stress following microinjection of the CRFBP antagonist CRF6–33 into the BNST. The drug did not affect general activity or exploration. The lack of changes on sweet solution preference across sessions and between non-stressed controls and stressed rats suggests that the deficits in social approach exhibited by stressed animals are not related to the development of depressive-like symptoms.
To investigate the stress effect on behaviors with hedonic motivation, we tested the preference of the animals for a palatable sweet solution prepared with 0.8% saccharin and sodium cyclamate. This non-stressful, non-invasive procedure, allows multiple tests without compromising the animal’s behavior [32]. Classically, the reduction in preference for sweet solutions and palatable food has been interpreted as an index of anhedonia, the lack or disruption of the ability to experience pleasure [37]. Anhedonia is considered one of the core symptoms of major affective disorders according to the DSM-5 [38]. In this study, we did not find differences in the preference for sweet solution after acute experience of social defeat or 10 days after the last session of social stress. It seems that chronic exposure to social stress is required in order to engender hedonic and motivational deficits in rats [37,39], although under specific conditions acute social defeat stress may produce suppression of preference for sweet solutions [40]. Thus, the lack of changes in preference for sweet solution found in the present study is in accordance with evidence contrasting the effects of chronic and intermittent exposures to stress [26].
Stressed animals were less likely to approach a conspecific female in the three-chamber social approach and interaction test 10–13 days after the last confrontation. Deficits in social interaction have been reported after intermittent exposure to social defeat, with impaired interactions persisting up to seven weeks after the last social defeat experience [41]. Reduced social interaction is associated with several psychiatric conditions, such as depression, social anxiety and autism spectrum disorders, which are characterized by dysfunctional reciprocal social interactions and altered coping with social environments [42,43]. It is tempting to suggest that the neuroadaptations promoted by social defeat stress may lead to difficulties in coping with social challenges in defeated animals. In a non-threatening environment, impaired social interaction may be interpreted as a maladaptive effect as well as a sign of anxiety [43,44].
In our study, the antagonism of CRFBP located in the BNST selectively re-established social approach in stressed rats, without modifying general exploratory behaviors. We used the same doses of CRF6–33 reported as effective in a previous study (0.25 and 0.5 µg) [12]. Our data showed that CRF6–33 promoted a significant increase in the frequency of entries in the interaction zone in socially stressed animals to levels similar to non-stressed controls.
It is worth mentioning that CRF6–33 has already been tested in behavioral cognitive and pre-clinical studies and has also shown performance-enhancing effects. Animals tested in the Morris water maze exhibited significant improvements on performance after i.c.v. infusion of CRF6–33 [45,46]. Additionally, these animals presented an increase in duration of searching the target quadrant compared to vehicle-treated animals, indicating an enhancement of spatial learning [46]. However, in the same set of experiments, a similar effect was seen after infusion of CRF1–41, a CRFR agonist, indicating that the improvements reported may be due an increase in the availability of CRF due to CRFBP antagonism.
There is evidence, however, supporting the idea that CRFBP is not only a sequestering protein, but may also possess additional functions [11,12]. Increasing data indicate that CRFR2 and CRFBP seem to act together, at least in the VTA, to promote the CRF effects [11]. More precisely, Slater and colleagues [47] showed that CRFBP forms a protein complex with the CRFR2α isoform. These authors suggest that the CRFBP could act as an escort protein regulating the access of CRFR2α located intracellularly to the cell surface. These findings strengthen the idea of a synergic mechanism of action between the CRFBP and CRFR2.
Interestingly enough, the CRFBP has been demonstrated to present spontaneous cleavage, originating two distinct fragments: a 27 kD and a 10 kD [50]. Haas-Koffler and colleagues [51] verified recently that the CFRBP (full length) can stably be expressed on the plasma membrane and only the 10 kD fragment is able to potentiate CRF signaling properties. Thus, taking into account that the 27kD fragment retains the active picomolar affinity binding site for CRF [52], it is possible that the distinct role of the CRF6–33 may rely on the fact that blocking the binding site of the 27 kD fragment would suppress the CRFBP sequestrating action and induce an increase in free CRF levels. On the other hand, the 10 kD fragment could act potentiating the CRFR2α. Further studies, however, will help to clarify the significance of the CRFBP fragments in in vivo experiments.
The BNST is known as one of the most complex structures in the central nervous system [15], whose heterogeneous nature is due to its division into different subregions and cell types, known to modulate anxiety in opposing ways [15]. The BNST is densely connected with the hypothalamus, amygdala, midbrain, and lower brainstem regions, with different afferents [53,54] and distinct projections [55,56]. Moreover, BNST nuclei connect each other intensely, and the γ-aminobutyric acid (GABA) seems to be the primary neurotransmitter, although a small number of glutamatergic neurons are also located in its ventral portion. BNST neurons also express a variety of neuropeptides, including CRF, enkephalin, neuropeptide Y, neurotensin and somatostatin [57]. In fact, CRF is produced by anterolateral BNST neurons and seems to act in this same region, potentiating anxiety-like behavior and stress response [18,21,22]. In our study, CRF6–33 infusions occurred mostly in the anterior portion of the BNST. Interestingly, the inhibition of CRF neurons in the anterior BNST promoted anxiolytic-like effects in the elevated plus-maze and open field tests [58]. Contrarily to our results, however, Klampfl and colleagues [59] reported no effect of CRF6–33 into the anterior BNST, and anxiogenic-like effects accompanied by increased locomotion when CRF6–33 was injected into the posterior BNST. Nonetheless, important differences between these studies are that Klampfl and colleagues [59] used female rats from a different strain and during their lactating period.
A limitation of the present study is that we did not measure the content of CRF in the BNST. However, results from our group indicate that repeated social defeat stress promotes increase in CRF levels in the BNST of mice [60]. Direct injection of CRF in BNST subregions will also help to identify specificities of the CRF mechanisms, and future studies using CRFR1 and CRFR2 antagonists will bring light to the role of the CRFBP in the central nervous system.
5. Conclusions
The present study brings new findings about the involvement of the CRF system in anxiety-related states, implicating the BNST CRFBP in the modulation of social behaviors in male rats previously exposed to a social stressor. Further studies will help to elucidate how the CRFBP promotes its action in discrete brain regions, as well as the role of stress in this circuitry, and whether the CRFBP could be considered a target candidate for the development of novel therapeutic treatments of stress-related disorders.
Highlights.
Intermittent social defeat did not promote anhedonic-like symptoms
Intra-BNST CRF6–33 infusions restored social approach impaired by social defeat
CRF mechanisms in the BNST modulate anxiety responses in stressed rats
6. Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) and Fundo de Incentivo à Pesquisa e Eventos (FIPE-HCPA/UFRGS). L. Albrechet-Souza was supported by CAPES–Brazil (Program CSF-PAJT 88887.096822/2015–00). R. M. M. de Almeida was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 475176/2012–0). The authors would like to thank Andre Gehling, Andreo Rysdyk, Eneida Rocha, Gabriela Jung, Giovana Brum, Luane Landau and Matheus Gallas for assistance in data collection and Fernanda Valiati, Tuane Garcez, Daniela Campagnol e Marta Cioato for providing helpful technical assistance during the development of this study
Funding: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes-Brazil); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundo de Incentivo à Pesquisa e Eventos (FIPE-HCPA/UFRGS).
Abbreviations
- BNST
bed nucleus of the stria terminalis
- CRF
corticotropin releasing factor
- CRFBP
CRF Binding Protein
- CRFR
CRF receptor
- EPSC
Excitatory postsynaptic currents
- i.c.v.
intracerebroventricular
- IZE
Interaction Zone - Entries
- IZT
Interaction Zone – Time
- NMDA
N-methyl-D-aspartate
- OZE
Object Zone - Entries
- OZT
Object Zone - Time
- PND
postnatal days
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].de Kloet ER, Joëls M, Holsboer F, Stress and the brain: from adaptation to disease, Nat. Rev. Neurosci 6 (2005) 463–475. doi:10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- [2].Blanchard RJ, Caroline Blanchard D, Aggressive behavior in the rat, Behav. Biol 21 (1977) 197–224. doi:10.1016/S0091-6773(77)90308-X. [DOI] [PubMed] [Google Scholar]
- [3].Meerlo, Overkamp, Daan, Van Den Hoofdakker RH, Koolhaas, Changes in behaviour and body weight following a single or double social defeat in rats, Stress Amst. Neth 1 (1996) 21–32. [DOI] [PubMed] [Google Scholar]
- [4].de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M, Brain corticosteroid receptor balance in health and disease, Endocr. Rev 19 (1998) 269–301. doi:10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- [5].McEwen BS, Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators, Eur. J. Pharmacol 583 (2008) 174–185. doi:10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eckart K, Jahn O, Radulovic J, Tezval H, van Werven L, Spiess J, A single amino acid serves as an affinity switch between the receptor and the binding protein of corticotropin-releasing factor: Implications for the design of agonists and antagonists, Proc. Natl. Acad. Sci 98 (2001) 11142–11147. doi:10.1073/pnas.211424998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huising MO, Vaughan JM, Shah SH, Grillot KL, Donaldson CJ, Rivier J, Flik G, Vale WW, Residues of corticotropin releasing factor-binding protein (CRF-BP) that selectively abrogate binding to CRF but not to urocortin 1, J. Biol. Chem 283 (2008) 8902–8912. doi:10.1074/jbc.M709904200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE, Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors, Proc. Natl. Acad. Sci 98 (2001) 2843–2848. doi:10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bale TL, Vale WW, CRF and CRF Receptors: Role in Stress Responsivity and Other Behaviors, Annu.Rev.Pharmacol.Toxicol 44 (2004) 525–557. doi:10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- [10].Karolyi IJ, Burrows HL, Ramesh TM, Nakajima M, Lesh JS, Seong E, Camper SA, Seasholtz AF, Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice, Proc. Natl. Acad. Sci 96 (1999) 11595–11600. doi:10.1073/pnas.96.20.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A, Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons, Neuron 39 (2003) 401–407. doi:10.1016/S0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- [12].Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA, Corticotropin releasing factor binding protein and CRF2 receptors in the ventral tegmental area: modulation of ethanol binge drinking in C57BL/6J mice, Alcohol. Clin. Exp. Res 39 (2015) 1609–1618. doi:10.1111/acer.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crestani CC, Alves FHF, Gomes FV, Resstel LBM, Correa FMA, Herman JP, Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review, Curr. Neuropharmacol 11 (2013) 141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Davis M, Walker DL, Miles L, Grillon C, Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety, Neuropsychopharmacology 35 (2009) 105–135. doi:10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Daniel SE, Rainnie DG, Stress modulation of opposing circuits in the bed nucleus of the stria terminalis, Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41 (2016) 103–125.doi:10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Henckens M.J. a. G., Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, Kuehne C, Kolarz A, Eder M, Deussing JM, Justice NJ, Yizhar O, Chen A, CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery, Mol. Psychiatry (2016). doi:10.1038/mp.2016.133. [DOI] [PubMed] [Google Scholar]
- [17].Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V, Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress., J. Mol. Neurosci 42 (2010) 327–340. doi:10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee Y, Davis M, Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex., J. Neurosci 17 (1997) 6434–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF, Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes, Psychopharmacology (Berl.) 186 (2006) 122–132. doi:10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Erb S, Stewart J, A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking., J. Neurosci. Off. J. Soc. Neurosci 19 (1999) RC35–RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cummings S, Elde R, lls J, Lindall A, Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study, J. Neurosci. Off. J. Soc. Neurosci 3 (1983) 1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dabrowska J, Hazra R, Guo J-D, DeWitt S, Rainnie DG, Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis, Front. Neurosci 7 (2013). doi:10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Makino S, Gold PW, Schulkin J, Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus, Brain Res 657 (1994) 141–149. doi:10.1016/0006-8993(94)90961-X. [DOI] [PubMed] [Google Scholar]
- [24] .Funk D, Li Z, Lê AD, Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking, Neuroscience 138 (2006) 235–243. doi:10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- [25].Nocjar C, Zhang J, Feng P, Panksepp J, The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus, Neuroscience 218 (2012) 138–153. doi:10.1016/j.neuroscience.2012.05.033 [DOI] [PubMed] [Google Scholar]
- [26].Miczek KA, Nikulina EM, Shimamoto A, Covington HE, Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats, J. Neurosci. Off. J. Soc. Neurosci 31 (2011) 9848–9857. doi:10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Olivares EL, Silva-Almeida C, Pestana FM, Sonoda-Côrtes R, Araujo IG, Rodrigues NC, Mecawi AS, Côrtes WS, Marassi MP, Reis LC, Rocha FF, Social stressinduced hypothyroidism is attenuated by antidepressant treatment in rats, Neuropharmacology 62 (2012) 446–456. doi:10.1016/j.neuropharm.2011.08.035. [DOI] [PubMed] [Google Scholar]
- [28].Fanous S, Hammer RP Jr, Nikulina EM, Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions, Neuroscience 167 (2010) 598–607. doi:10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miczek KA, A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine, Psychopharmacology (Berl.) 60 (1979) 253–259. doi:10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- [30].Tornatzky W, Miczek KA, Long-term impairment of autonomic circadian rhythms after brief intermittent social stress, Physiol. Behav 53 (1993) 983–993. doi:10.1016/00319384(93)90278-N. [DOI] [PubMed] [Google Scholar]
- [31].Vasconcelos M, Stein DJ, de Almeida RMM, Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade, Trends Psychiatry Psychother 37 (2015) 51–66. doi:10.1590/2237-6089-2014-0034. [DOI] [PubMed] [Google Scholar]
- [32].Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U, Anhedonia and motivational deficits in rats: Impact of chronic social stress, Behav. Brain Res 162 (2005) 127–134. doi:10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [33].Lee Y, Fitz S, Johnson PL, Shekhar A, Repeated Stimulation of CRF Receptors in the BNST of Rats Selectively Induces Social but not Panic-Like Anxiety, Neuropsychopharmacology 33 (2008) 2586–2594. doi:10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Paxinos G, Watson C, The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition, Academic Press, 2006. [Google Scholar]
- [35].Friard O, Gamba M, BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations, Methods Ecol. Evol 7 (2016) 1325–1330. doi:10.1111/2041-210X.12584. [Google Scholar]
- [36].Nadler JJ, Moy SS, Dold G, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN, Automated apparatus for quantitation of social approach behaviors in mice, Genes Brain Behav 3 (2004) 303–314. doi:10.1111/j.1601183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- [37].Willner P, Muscat R, Papp M, Chronic mild stress-induced anhedonia: A realistic animal model of depression, Neurosci. Biobehav. Rev 16 (1992) 525–534. doi:10.1016/S01497634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- [38].American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, American Psychiatric Association, 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 (accessed May 17, 2016). [Google Scholar]
- [39].Rygula R, Papciak J, Popik P, Trait pessimism predicts vulnerability to stress-induced anhedonia in rats, Neuropsychopharmacology 38 (2013) 2188–2196. doi:10.1038/npp.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Furay AR, McDevitt RA, Miczek KA, Neumaier JF, 5-HT1B mRNA expression after chronic social stress, Behav. Brain Res 224 (2011) 350–357. doi:10.1016/j.bbr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fanous S, Terwilliger EF, Hammer RP Jr., Nikulina EM, Viral depletion of VTA BDNF in rats modulates social behavior, consequences of intermittent social defeat stress, and long-term weight regulation, Neurosci. Lett 502 (2011) 192–196. doi:10.1016/j.neulet.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Heimberg RG, Hofmann SG, Liebowitz MR, Schneier FR, Smits JAJ, Stein MB, Hinton DE, Craske MG, Social anxiety disorder in DSM-5: Review, Depress. Anxiety 31 (2014) 472–479. doi:10.1002/da.22231. [DOI] [PubMed] [Google Scholar]
- [43].Mehling MH, Tassé MJ, Severity of autism spectrum disorders: current conceptualization, and transition to DSM-5, J. Autism Dev. Disord 46 (2016) 2000–2016. doi:10.1007/s10803-016-2731-7. [DOI] [PubMed] [Google Scholar]
- [44].File SE, Seth P, A review of 25 years of the social interaction test, Eur. J. Pharmacol 463 (2003) 35–53. doi:10.1016/S0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- [45].Stewart CA, Morris RGM, The watermaze, Behav. Neurosci 1 (1993) 107–122. [Google Scholar]
- [46].Behan DP, Heinrichs SC, Troncoso JC, Liu X-J, Kawas CH, Ling N, De Souza EB, Displacement of corticotropin releasing factor from its binding protein as possible treatment for Alzheimer’s disease, Nature 378 (1995) 284–287. doi:10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- [47].Slater PG, Cerda CA, Pereira LA, Andrés ME, Gysling K, CRF binding protein facilitates the presence of CRF type 2α receptor on the cell surface, Proc. Natl. Acad. Sci 113 (2016) 4075–4080. doi:10.1073/pnas.1523745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF, Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress, Nat. Genet 24 (2000) 410– 414. doi:10.1038/74263. [DOI] [PubMed] [Google Scholar]
- [49].Coste SC, Heard AD, Phillips TJ, Stenzel-Poore MP, Corticotropin-releasing factor receptor type 2-deficient mice display impaired coping behaviors during stress, Genes Brain Behav 5 (2006) 131–138. doi:10.1111/j.1601-183X.2005.00142.x. [DOI] [PubMed] [Google Scholar]
- [50].Woods RJ, Kemp CF, David J, Sumner IG, Lowry PJ, Cleavage of Recombinant Human Corticotropin-Releasing Factor (CRF)-Binding Protein Produces a 27-Kilodalton Fragment Capable of Binding CRF, J. Clin. Endocrinol. Metab 84 (1999) 2788–2794. doi:10.1210/jcem.84.8.5898. [DOI] [PubMed] [Google Scholar]
- [51].Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, Lasek AW, Magill M, Schwandt ML, Momenan R, Hodgkinson CA, Bartlett SE, Swift RM, Bonci A, Leggio L, Defining the role of corticotropin releasing factor binding protein in alcohol consumption, Transl. Psychiatry 6 (2016) e953. doi:10.1038/tp.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW, Corticotropin Releasing Factor (CRF) Binding Protein: A Novel Regulator of CRF and Related Peptides, Front. Neuroendocrinol 16 (1995) 362–382. doi:10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- [53].McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M, Cortical afferents to the extended amygdala, Ann. N. Y. Acad. Sci 877 (1999) 309–338. doi:10.1111/j.17496632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- [54].Dong H-W, Petrovich GD, Swanson LW, Topography of projections from amygdala to bed nuclei of the stria terminalis, Brain Res. Rev 38 (2001) 192–246. doi:10.1016/S0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- [55].Dong H-W, Petrovich GD, Swanson LW, Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat, Brain Res 859 (2000) 1–14. doi:10.1016/S0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- [56].Dong H-W, Petrovich GD, Watts AG, Swanson LW, Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain, J. Comp. Neurol 436 (2001) 430–455. doi:10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- [57].Walter A, Mai JK, Lanta L, Görcs T, Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain, J. Chem. Neuroanat 4 (1991) 281–298. doi:10.1016/0891-0618(91)90019-9. [DOI] [PubMed] [Google Scholar]
- [58].Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K, Diverging neural pathways assemble a behavioural state from separable features in anxiety, Nature 496 (2013) 219–223. doi:10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Klampfl SM, Schramm MM, Stinnett GS, Bayerl DS, Seasholtz AF, Bosch OJ, Brain CRF-binding protein modulates aspects of maternal behavior under stressful conditions and supports a hypo-anxious state in lactating rats, Horm. Behav 84 (2016) 136–144. doi:10.1016/j.yhbeh.2016.06.009. [DOI] [PubMed] [Google Scholar]
- [60].Albrechet-Souza L, Viola TW, Grassi-Oliveira R, Miczek KA, Almeida D, M RM, Corticotropin Releasing Factor in the Bed Nucleus of the Stria Terminalis in Socially Defeated and Non-stressed Mice with a History of Chronic Alcohol Intake, Front. Pharmacol 8 (2017). doi:10.3389/fphar.2017.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]


