Abstract
Aims:
Patients with Crohn′s disease (CD) are shown to have abnormal changes in brain structures. This study aimed to further investigate whether these patients have abnormal brain activities and network connectivity.
Methods:
Sixty patients with CD and 40 healthy controls (HCs) underwent resting-state functional magnetic resonance imaging (fMRI) scans. Amplitude of low-frequency fluctuation (ALFF) and seed-based functional connectivity (FC) were used to assess differences in spontaneous regional brain activity and functional connectivity.
Results:
Compared to the HCs, patients with CD showed significantly higher ALFF values in hippocampus and parahippocampus (HIPP/paraHIPP), anterior cingulate cortex, insula, superior frontal cortex and precuneus. The ALFF values were significantly lower in secondary somatosensory cortex (S2), precentral gyrus, and medial prefrontal cortex. Functional connectivities between left HIPP and left inferior temporal cortex, and right middle cingulate cortex, HIPP, and fusiform area were significantly lower. The functional connectivities between right HIPP and right inferior orbitofrontal cortex and left HIPP were also significantly lower.
Conclusion:
Patients with CD showed higher or lower spontaneous activity in multiple brain regions. Altered activities in these brain regions may collectively reflect abnormal function and regulation of visceral pain and sensation, external environmental monitoring, and cognitive processing in these patients. Lower functional connectivity of the hippocampus-limbic system was observed in these patients. These findings may provide more information to elucidate the neurobiological mechanisms of the disease.
Keywords: Crohn’s disease, Resting-state functional MRI, Amplitude of low-frequency fluctuation, Functional connectivity
Introduction
Crohn′s disease (CD), a type of inflammatory bowel disease (IBD), is a chronic gastrointestinal inflammatory disease of unknown etiology. The major signs and symptoms include abdominal pain, chronic diarrhea, weight loss and fatigue. The disease typically has progressive course and causes intestinal damage or even disability in severe cases (Torres et al. 2016). Currently, there is no definitive treatment for CD. The condition typically requires lifelong medication and supportive care. Recent years have witnessed a steady increase in the incidence of IBD in Asia; China has the highest incidence of IBD in Asia, which places a heavy burden on health care resources and affects social productivity (Ng et al., 2013). Because chronic pain, inflammation, and brain-gut interaction are known to involve various brain networks (Vermeulen et al. 2014), characterizing specific changes in brain network and activity in CD patients is important for understanding the mechanism of the disease and controlling the symptoms.
Functional abnormality in the central nervous system and the brain-gut axis was recently shown to play a key role in the pathogenesis and development of CD (Al & Aziz, 2014; Bonaz & Bernstein, 2013). Studies have shown abnormal brain activity in CD patients in remission. Specifically, altered resting-state brain activity was observed in brain regions involved in visceral sensation, motility, emotional awareness, attention and cognition (Bao et al. 2016). However, different brain activities may be related to different symptoms and pathogenesis of CD. A previous study showed that abnormal brain activity may be related to abdominal pain sensation in CD patients. Among patients with CD in remission, those with and without abdominal pain showed differences in resting-state brain activity; further, activity profile in specific brain areas were related to the severity of the disease (Bao et al., 2016). Researchers have also investigated functional brain activity evoked by psychological stress in patients with CD in remission. They found altered neural activity in hippocampus, amygdala, and insula in CD patients (Agostini et al. 2013). However, functional brain activity in patients with CD is not well characterized. The key regions with abnormal brain activity in these patients have not been fully elucidated and findings from previous studies need to be further validated.
Resting-state functional magnetic resonance imaging (rs-fMRI) has emerged as a useful technique to study the changes in functional activities in the resting state of brain. Amplitude of low frequency fluctuations (ALFF), which detect the amplitude of blood oxygen level dependent (BOLD) signal relative to the baseline (Zang et al., 2007), can be used to reflects the level of spontaneous activity at each voxel. Functional connectivity (FC), which reflects synchronous spontaneous fluctuations between brain regions, can provide information about functional integrity of brain networks (Fox & Raichle, 2007). The combination of these two methods allows assessment of the intrinsic activity of a specific brain region and its network associated with a disease. Having been widely used in the study of functional gastrointestinal disorders (Ma et al., 2015; Zhou et al., 2013), neurological disorders (Li et al., 2016; Van Hees et al., 2014), psychosis (Li et al., 2014; Zhang et al., 2014), and other medical conditions, this method shows promise in investigating functional activity of the resting brain. To date, no study has been conducted on resting-state brain activity indexed by ALFF and FC in patients with CD. Therefore, these approaches may help elucidate the characteristics of resting-state brain activity in CD patients, and provide useful information on the pathophysiological mechanisms of CD.
In this study, we used ALFF and FC to explore resting-state brain activity in patients with CD in remission. We hypothesized that there would be differences in ALFF and FC in certain brain regions between patients with CD in remission and healthy controls, and that neuroimaging results would be associated with the duration of disease.
Methods
Subjects
This study was approved by the Ethics Committee of the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated with Shanghai University of Traditional Chinese Medicine. All subjects signed informed consent forms.
Sixty patients with CD were recruited from the outpatient clinics for inflammatory bowel diseases at the Shanghai Research Institute of Acupuncture and Meridian, and the Endoscopy Center of Zhongshan Hospital affiliated with Fudan University. All subjects underwent systematic and gastrointestinal screening, including colonoscopy and intestinal mucosal biopsy. CD Endoscopic Index of Severity (CDEIS) of each subject was scored by an expert gastroenterologist. To assess the level of inflammation, C-reactive protein, erythrocyte sedimentation rate and platelet counts for each subject were measured and recorded two weeks prior to fMRI scans.
Inclusion criteria: right-handed (as determined by Edinburgh handedness inventory) (Oldfield, 1971); age: 18–50 years; minimum education level: 6 years of education; remission lasting more than 6 months; CDAI score ≤ 150; CDEIS score < 3.
Exclusion criteria: abnormal levels of inflammation (C reactive protein > 10 mg/L; erythrocyte sedimentation rate > 20 mm/h; platelets > 300×109/L); history of CD-related abdominal surgery; history of treatment with glucocorticoids, anti TNF-α drugs, psychotropic or opioid drugs in the past 3 months; pregnant or breastfeeding women; patients with current or previous history of neurological or psychiatric conditions, head trauma or loss of consciousness; claustrophobia; and patients with metallic implants in the body.
Forty gender, age, and education matched healthy subjects (HCs) were recruited from the Shanghai University of Traditional Chinese Medicine via a newspaper advertisement. They did not suffer from any gastrointestinal disorders or pain and did not receive any medications. In addition, they had negative results on colonoscopy performed within the preceding year as part of routine physical examination.
All subjects were evaluated by an experienced gastroenterologist. To rule out psychiatric or neurological disorders, psychiatric examinations were performed by an experienced psychiatrist, based on a structured psychiatric interview tool from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV).
Symptom assessment
The condition of CD patients was assessed by the Crohn′s disease activity index (CDAI) (Best, Becktel, & Singleton, 1979); the quality of life was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ) (Irvine et al., 1994); emotional distress was assessed using the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983).
MRI data acquisition
All MRI data were obtained from a 3T magnetic resonance scanner (Siemens, TRIO, Erlangen, Germany) in the Department of Radiology at the Shanghai Mental Health Center. All subjects were supine on the scanner and were told to relax, keep their eyes closed and not fall asleep or think. A standard sponge pad was used to fill the gap between the head and the coil to prevent head movement and to protect the ears from the noise of scanner.
A set of high-resolution 3D T1-weighted structural images was obtained prior to functional san (TR/TE: 2300 ms/2.98 ms; field of view (FOV): 256 × 256 mm2; matrix size: 256 × 256; flip angle: 9°; in-plane resolution: 1 × 1 mm2; slice thickness: 1.0 mm with no gaps and 176 slices). Functional images were acquired with a single-shot gradient–recalled echo planar imaging (EPI) sequence (TR/TE: 2000 ms/30 ms; 180 time points; FOV: 240 × 240 mm2; matrix size: 64 × 64; flip angle: 90°; in-plane resolution: 3.75 × 3.75 mm2, 32 sagittal slices and slice thickness: 5mm with no gaps.
Image data preprocessing
The imaging data processing assistant for resting-state fMRI (DPARSF, V3.1, http://www.restfmri.net) was used to analyze imaging data (Chao-Gan & Yu-Feng, 2010), which is based on SPM 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12), and the resting state fMRI data analysis toolkit (REST, V1.8, http://restfmri.net/forum/ REST_V1.8) (Song et al., 2011). The first ten volumes of each functional time series were discarded to allow for signals equilibrium and for participants’ adaptation to the scanning noise. The remaining images were corrected for temporal difference in the acquisition of the different slices and then realigned to the first volume for head-motion correction. Subjects with head motion exceeded 2 mm translation or 2 degrees rotation in any direction were excluded. The corrected images were further spatially normalized to the Montreal Neurological Institute (MNI) 152 template and resliced with isotropic 3 mm × 3 mm × 3 mm voxel size and spatially smoothed with a 6-mm full-width at half maximum (FWHM) Gaussian kernel. After the linear trend of time courses removal, the imaging data were then temporally band pass filtered (0.01–0.1Hz) to remove the effects of low-frequency drift and high-frequency noise. The Friston 24 head-motion parameters, white matter signal, and cerebral spinal fluid signal were regressed out as nuisance covariates (Fair et al., 2008).
Amplitude of low-frequency fluctuation (ALFF)
ALFF maps were calculated using REST v1.8 software (http://restfmri.net/forum/REST_V1.8) (Song et al., 2011). First, the preprocessed time series was transformed to frequency domain using fast Fourier transform (FFT) to obtain power spectrum. Then, the square root was calculated at each frequency of the power spectrum and averaged across 0.01–0.1 Hz at each voxel. This averaged square root was taken as the ALFF. To reduce the global effects of variability across the subjects, the ALFF of each voxel was divided by the global mean ALFF value to standardize data across subjects (Zang et al., 2007).
Functional Connectivity (FC)
CD is an autoimmune disease and hippocampus is thought to play an important role in neuroimmune regulation (Lathe, 2001). Previous studies (Agostini et al. 2013; Bao et al. 2016a; Bao et al. 2016b; Bao et al. 2015) have shown differences in gray matter structures and functional activity in the hippocampal cortex of patients with CD. This study also found abnormal ALFF values in bilateral hippocampal cortex. Thus, the detected bilateral hippocampus clusters from the ALFF analysis were used as the regions of interest (ROIs). The MNI coordinates of the center of the 6-mm spherical ROI were determined by the peak t-score detected at the hippocampus. For functional connectivity analysis, the mean time series was extracted from the seed region and correlated with the time series of each voxel of the whole brain for each subject. The correlation coefficients were transformed into z values using Fisher’s r-to-z transformation to improve normality. An entire brain z-score map was created for each subject (Wei et al., 2016). In this study, the global mean timecourse was not regressed out in the model.
Statistical analyses
Clinical and demographic characteristic measures were analyzed with Statistical Product and Service Solutions (SPSS) 16.0 software [SPSS Inc. Chicago, IL]. The data are presented as mean ± standard deviation.
In this study, we only focused on the group-difference-related positive intrinsic connectivity, which was used to identify abnormal FC connectivity within this specific brain network. A two sample t-test was performed to assess the differences of ALFF and FC between the two groups within the brain network, with gender, age, anxiety score of HADS (HADS-A) and the depression score of HADS (HADS-D) as covariates. The inclusion of anxiety and depression as covariates was to exclude the effects of negative emotions on the results and to focus on the impact of the disease itself on brain activity. The statistical significance was set at voxel-wise P < 0.001 uncorrected with an extent threshold of cluster-wise false discovery rate (FDR) (P < 0.05 at cluster lever, cluster size >45). ROI-wise analysis was used to investigate the relationship between ALFF alteration and disease duration. Pearson’s correlation was calculated in each group at a threshold of P < 0.05 with the Bonferroni correction. The ROIs included all nine brain regions showing different ALFF values in CD patients. The MNI coordinates of the center of the 6-mm spherical ROI were determined by the peak t-score of all detected ROIs.
Results
Clinical and demographic characteristics
Clinical and demographic characteristics of study subjects are summarized in Table 1. CD patients and HCs did not differ significantly with respect to demographic characteristics including gender, age, height, and weight (P > 0.05). The CD patients had significantly higher HADS-A and HADS-D scores than the HCs (both P <0.01). None of the subjects were smokers. 51.7% of the patients presented with abdominal pain. Of these, 28 were treated with mesalazine, 11 were treated with azathioprine, four were treated with mesalazine plus azathioprine, and 17 did not receive any medication.
Table 1.
Demographic and clinical characteristics of the study population.
| CD Patients (n = 60) |
Healthy controls (n = 40) |
P value | ||
|---|---|---|---|---|
| Sex (male/female) | 43/17 | 27/13 | 0.656 | |
| Age (years) | 30.80 ± 7.62 | 30.68 ± 5.78 | 0.930 | |
| Height (cm) | 169.80 ± 6.94 | 169.68 ± 7.03 | 0.930 | |
| Weight (kg) | 56.48 ± 9.04 | 59.33 ± 6.07 | 0.85 | |
| HADS-A | 5.85 ± 3.46 | 3.25 ± 1.98 | 0.000 | |
| HADS-D | 4.57 ± 3.68 | 2.95 ± 1.83 | 0.012 | |
| Disease duration (months) | 77.70 ± 50.40 | — | — | |
| CDAI | 76.61 ± 43.93 | — | — | |
| IBDQ | 173.53 ± 26.29 | — | — | |
| PLT | 220.93 ± 43.21 | — | — | |
| ESR | 10.93 ± 5.58 | — | — | |
| CRP | 4.48 ±2.84 | — | — | |
| CDEIS | 1.14 ± 0.73 | — | — | |
| Montreal classification | ||||
| Age at | — | — | ||
| A1 | 6 | |||
| diagnosis | ||||
| A2 | 53 | — | — | |
| A3 | 1 | — | — | |
| Location | L1 | 14 | ||
| L2 | 12 | — | — | |
| L3 | 34 | — | — | |
| L4 | 0 | — | — | |
| Behavior | B1 | 20 | — | — |
| B2 | 4 | — | — | |
| B3 | 17 | — | — | |
| B1P | 5 | — | — | |
| B2P | 1 | — | — | |
| B3P | 13 | — | — | |
Data presented as mean (± standard deviation).
CD, Crohn’s disease; CDAI, CD Activity Index; CDEIS, CD Endoscopic Index of Severity; CRP, C-reactive protein; ESR, eosinophil sedimentation rate; HADS-A or -D, Hospital Anxiety and Depression Scores; IBDQ, In-flammatory Bowel Disease Questionnaire; PLT, platelet levels.
Difference in ALFF values between the CD patients and HCs
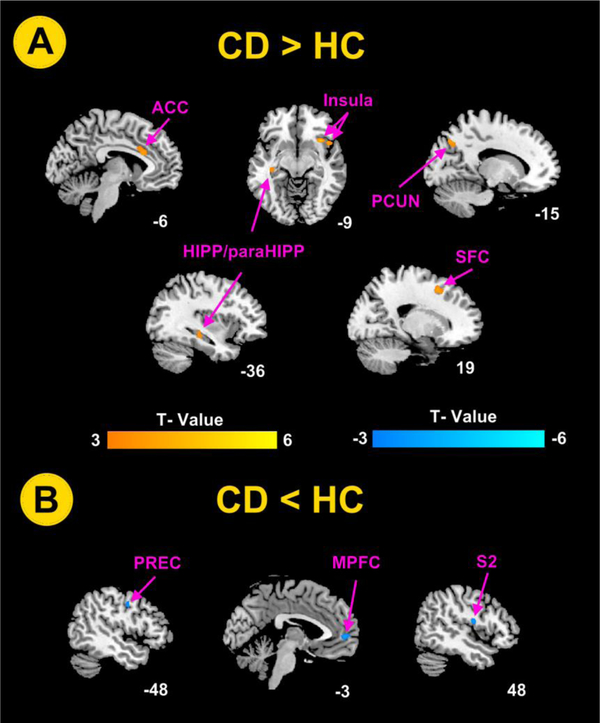
As showed in Table 2, compared to the HCs, the CD patients showed higher ALFF values in bilateral hippocampus and parahippocampus, left anterior cingulate cortex (ACC) and precuneus, and right insula and superior frontal cortex (Figure 1A). The CD patients also showed lower ALFF values in left precentral gyrus, left medial prefrontal cortex (MPFC), and right secondary somatosensory cortex (S2) (Figure 1B).
Table 2.
Brain regions with significant differences in ALFF values between CD patients and HCs.
| MNI | |||||||
|---|---|---|---|---|---|---|---|
| Regions | Hem | BA | t value | Voxels | |||
| X | Y | Z | |||||
| CD > HC | |||||||
| Insula | R | 47 | 36 | 18 | −9 | 4.76 | 60 |
| ACC | L | 24 | −6 | 21 | 24 | 3.73 | 111 |
| HIPP/paraHIPP | L | 20 | −36 | −24 | −6 | 3.92 | 50 |
| HIPP/paraHIPP | R | 37 | 24 | −30 | −6 | 3.87 | 53 |
| Precuneus | L | 19 | −15 | −69 | 39 | 3.96 | 87 |
| Superior frontal cortex | R | 8 | 18 | 18 | 54 | 4.63 | 91 |
| CD < HC | |||||||
| Precentral gyrus | L | 6 | −48 | −3 | 39 | −3.83 | 52 |
| MPFC | L | 10 | −3 | 54 | 6 | −3.89 | 132 |
| S2 (Rolandic-Oper) | R | 48 | 48 | −9 | 18 | −3.99 | 63 |
The results employed age, gender, anxiety and depression as covariates. The statistical threshold was set at P<0.05 (false discovery rate corrected) at cluster level and the cluster size > 45. ACC, anterior cingulate cortex; BA, Brodmann area; CD, Crohn’s disease; Hem, hemisphere; MPFC, medial prefrontal cortex; HCs, healthy controls; HIPP / paraHIPP, hippocampal / parahippocampal cortex.
Figure 1.
Brain regions exhibited significant differences in amplitude of low frequency fluctuations (ALFF) values between CD patients and healthy control subjects using age, gender, anxiety and depression as covariates. The statistical significance was set at voxel-wise P < 0.001 uncorrected with an extent threshold of cluster-wise false discovery rate (FDR) (P < 0.05 at cluster lever, cluster size >45). (A) Brain regions exhibit significantly higher ALFF values in CD patients; (B) Brain regions exhibit significantly lower ALFF values in CD patients. ACC, anterior cingulate cortex; CD, Crohn’s disease; MPFC, medial prefrontal cortex; HC, healthy control; HIPP / paraHIPP, hippocampal / parahippocampal cortex; PCUN, precuneus; PREC, precentral gyrus; S2, secondary somatosensory cortex; SFC, superior frontal cortex.
There was no significant correlation between the abnormal ALFF values and disease duration in the patients with CD (P>0.05 for all).
Difference in functional connectivity of hippocampus between the CD patients and HCs.
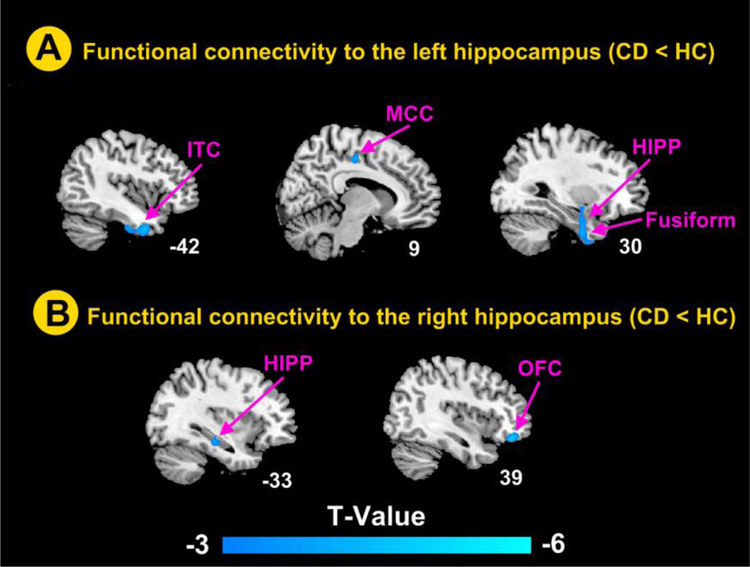
As showed in Table 3, compared to HCs, the left hippocampus of the CD patients had lower functional connectivity with the left inferior temporal cortex, and right MCC, hippocampus and fusiform (Figure 2A). The right hippocampus of the CD patients had lower functional connectivity with the right inferior orbitofrontal cortex and left hippocampus (Figure 2B).
Table 3.
Brain regions showing significantly lower functional connectivity to bilateral hippocampus between CD patients and HCs.
| Regions | Hem | BA | MNI | T | Voxels | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Value | ||||
| Left hippocampus | |||||||
| MCC | R | 23 | 9 | −21 | 36 | −3.83 | 67 |
| Hippocampus | R | 36 | 30 | −3 | −30 | −3.75 | 49 |
| Fusiform | R | 20 | 30 | −3 | −39 | −3.87 | 147 |
| Inferior temporal cortex | L | 20 | −42 | 3 | −36 | −4.29 | 104 |
| Right hippocampus | |||||||
| OFC | R | 47 | 39 | 42 | −18 | −4.53 | 87 |
| Hippocampus | L | 20 | −33 | −24 | −15 | −3.67 | 52 |
The results employed age, gender, anxiety and depression as covariates. The statistical threshold was set at P<0.05 (false discovery rate corrected) at cluster level and cluster size > 45. BA, Brodmann area; CD, Crohn’s disease; Hem, hemisphere; HCs, healthy controls; MCC, middle cingulate cortex; OFC, orbitofrontal cortex.
Figure 2.
Brain regions showed significant differences in the hippocampus-related connectivity between CD patients and healthy control subjects using age, gender, anxiety and depression as covariates. The statistical significance was set at voxel-wise P < 0.001 uncorrected with an extent threshold of cluster-wise false discovery rate (FDR) (P < 0.05 at cluster lever, cluster size >45). (A) Brain regions showing significantly lower connectivity to the left hippocampus in CD patients; (B) Brain regions showing significantly lower connectivity to the right hippocampus in CD patients. CD, Crohn’s disease; HC, healthy control; HIPP, hippocampus; ITC, inferior temporal cortex, MCC, middle cingulate cortex; OFC, orbitofrontal cortex.
Discussion
The results showed significant differences in ALFF values in multiple brain regions in CD patients, including higher ALFF values in hippocampus and parahippocampus, ACC, precuneus, insula, and superior frontal cortex, and lower ALFF values in precentral gyrus, MPFC, and S2. Functional connectivity in hippocampus-limbic system in CD patients was also significantly lower than that in HCs. Together, abnormal activities and connectivity in these brain regions may suggest abnormal brain functions related to the regulation of visceral sensation, pain processing, default mode network (DMN), and neuro-immunity in these patients.
ALFF differences between patients with CD and HCs
In this study, CD patients showed different spontaneous fluctuations in brain regions including insula, ACC, MPFC, precentral gyrus, S2 and hippocampal cortex, all of which are important components of visceral sensory and pain networks (Mayer, Naliboff, & Craig, 2006; Van, Coen, & Aziz, 2007), and are involved in visceral sensory (pain) and movement regulation. Precuneus and MPFC are important elements of DMN (Buckner, Andrews-Hanna, & Schacter, 2008; Mantini & Vanduffel, 2013). Our previous findings regarding brain areas involved in altered regional homogeneity (ReHo) in CD patients during resting-state is consistent with these results (Bao et al. 2016).
Although the inflammation is relatively stable in patients with CD in remission, long-term chronic inflammation may have an impact on functional activities of brain owing to the relapsing-remitting disease course. In CD patients, intestinal inflammation / pain signals are transmitted through the brain–gut axis to the brain, and may lead to alterations in multiple functional brain networks (Bonaz & Bernstein, 2013). Differences in spontaneous fluctuations in insula, cingulate cortex, hippocampus, PFC and other brain areas associated with visceral activity and pain regulation may suggest different neural network activities involved in processing visceral sensory and pain in these patients. Indeed, more than a half of all CD patients present with abdominal pain/abdominal distension due to long-term chronic inflammation of the intestines, despite being in remission. In our previous study, we found different brain activities between CD patients with and without abdominal pain (Bao et al., 2016), which suggests that chronic abdominal pain in patients in remission may affect functional activities of brain.
In addition, the spontaneous fluctuations in DMN were also different in CD patients. Precuneus and MPFC are all component of DMN. Spontaneous fluctuations were lower in anterior DMN (MPFC), and higher in posterior DMN (precuneus), which suggests lower coupling between the anterior and posterior DMN in patients with CD. The anterior DMN is mainly involved in monitoring one’s own state, predicting nociceptive stimulus, and emotional assessment or integration (Blakemore, 2008; Wiech et al., 2005), while the posterior DMN is mainly associated with self-introspection and episodic memory processing (Cavanna & Trimble, 2006; Margulies et al., 2009). These results suggested that relevant DMN functions, monitoring of the external environment and spontaneous cognition processing (Buckner et al., 2008; Mantini & Vanduffel, 2013), were different in CD patients.
Previously, we found lower gray matter volume in MPFC, but higher gray matter volume in precuneus in patients with CD. We speculated that the different gray matter volume in CD patients may be the structural basis of abnormal spontaneous activities.
Differences in hippocampus-related functional connectivity between CD patients and HCs
Our study demonstrated different spontaneous functional activities in hippocampal cortex, which is consistent with findings of previous neuroimaging studies which showed abnormal gray matter structure and function of hippocampus in these patients (Agostini et al. 2013; Bao et al. 2016; Bao et al. 2016; Bao et al. 2015). The hippocampus plays an important role in neuroimmunological regulation by affecting cellular and humoral immunity through the hypothalamic-pituitary-adrenal (HPA) axis and neurohumoral pathways (Lathe, 2001). Further, the hippocampus can also interact with the vagus nerve to regulate the function of immune cells in the intestinal wall through the release of acetylcholine, and, thereby, be involved in neural regulation of intestinal inflammation. Studies in chemically induced models of CD intestinal inflammation have shown increased CNS excitability and behavioral changes, which may be related to hippocampal microglia activation, increased expressions of TNF-α, nitric oxide synthase (iNOS), and nitrite content (Heydarpour et al. 2016; Riazi et al. 2008), enhanced glutamatergic synaptic transmission and plasticity, and reduced neurogenesis (Riazi et al., 2015; Zonis et al., 2015). Therefore, we speculate that intestinal immune dysfunction / inflammation may affect the structure and function of hippocampal cortex in patients with CD.
Whole-brain seed-based FC analysis using bilateral hippocampus as seed regions showed lower functional connectivity between hippocampus and brain structures of the limbic system. These brain regions are may be mainly involved in the regulation of visceral functions (including visceral sensation and pain). The result is consistent with the previous findings.
The limbic system is considered to be a higher center for regulation of visceral functions. In particular, visceral functional activity is regulated by hippocampus and other brain regions of the limbic system (Drevets, Price, & Furey, 2008; Jones, Dilley, Drossman, & Crowell, 2006). Previous results have confirmed different spontaneous activities in networks related to visceral sensory and pain in CD patients. As a key brain region involved in neuroimmune regulation, the hippocampus, together with other brain structures of the limbic systems, regulates visceral sensation and movement (Drevets et al., 2008; Jones et al., 2006). The finding of lower functional connectivity between hippocampus and other brain structures of the limbic system may suggest reduced ability of the limbic system in regulating visceral sensation and pain network.
Limitations and future directions
This study has several limitations. First, this is a cross-sectional study, which makes it impossible to determine a potential causal relationship between abnormal brain activity and the occurrence of disease. Second, immunosuppressive therapy may be a confounding factor that could not be controlled in this study. This is probably a common limitation for many fMRI studies (Mikdashi, 2016).
In conclusion, this study found differences in spontaneous fluctuations of activities of multiple brain regions in patients with CD in remission. Since the combined functions of these brain regions involve visceral sensation, pain processing, and DMN, the observed different brain activity in CD patients may reflect their abnormal functions in visceral sensation and pain, external environment monitoring, and cognition processing. In addition, functional connectivity between hippocampus and visceral sensory and pain network was also lower. These results may provide a better understanding of the pathophysiological mechanisms of CD.
Acknowledgments
Many thanks to Dr. Lili Ma at Zhongshan Hospital of Fudan University for endoscopy examination and scoring. This work was funded by the National Key Basic Research Program of China (973 program), No. 2009CB522900, 2015CB554501; the Program for Outstanding Medical Academic Leader, No. 80; the Program of Shanghai Academic Research Leader, No. 17XD1403400, the National Natural Science Foundation of China, No. 81471738 and the National Institutes of Health grant (P20GM103472).
Footnotes
Compliance with ethical standards
Conflicts of interest: The authors disclose no conflicts.
Research involving human participants: All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from every participant included in the study.
References
- Agostini A, Benuzzi F, Filippini N, Bertani A, Scarcelli A, Farinelli V et al. (2013). New insights into the brain involvement in patients with Crohn′s disease: a voxel-based morphometry study. Neurogastroenterology & Motility, 25(2), 147–e82. [DOI] [PubMed] [Google Scholar]
- Al OY & Aziz Q (2014). The brain-gut axis in health and disease. Advances in Experimental Medicine & Biology, 817, 135–153. [DOI] [PubMed] [Google Scholar]
- Bao CH, Liu P, Liu HR, Jin XM, Calhoun VD, Wu LY et al. (2016). Different brain responses to electro-acupuncture and moxibustion treatment in patients with Crohn’s disease. Scientific Reports, 18(6), 36636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao CH, Liu P, Liu HR, Wu LY, Jin XM Wang. SY et al. (2016). Differences in regional homogeneity between patients with Crohn’s disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain, 157(5),1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao CH, Liu P, Liu HR, Wu LY, Shi Y, Chen WF et al. (2015). Alterations in Brain Grey Matter Structures in Patients With Crohn’s Disease and Their Correlation With Psychological Distress. Journal of Crohn S & Colitis, 9(7), 532–540. [DOI] [PubMed] [Google Scholar]
- Best WR, Becktel JM & Singleton JW (1979). Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology, 77(4 Pt 2), 843–846. [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. [DOI] [PubMed] [Google Scholar]
- Bonaz BL & Bernstein CN (2013). Brain-gut interactions in inflammatory bowel disease. Gastroenterology, 144(1), 36–49. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Cavanna AE & Trimble MR (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt3), 564–583. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y & Yu-Feng Z (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 14(4), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL & Furey ML (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function, 213(1–2), 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM et al. (2008). The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America, 105(10), 4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711. [DOI] [PubMed] [Google Scholar]
- Heydarpour P, Rahimian R, Fakhfouri G, Khoshkish S, Fakhraei N, Salehi-Sadaghiani M et al. (2016). Behavioral despair associated with a mouse model of Crohn’s disease: Role of nitric oxide pathway. Progress in neuro-psychopharmacology & biological psychiatry, 4(64), 131–141. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A et al. (1994). Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn′s Relapse Prevention Trial Study Group. Gastroenterology, 106(2), 287–296. [DOI] [PubMed] [Google Scholar]
- Jones MP, Dilley JB Drossman D & Crowell MD (2006). Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterology & Motility, 18(2), 91–103. [DOI] [PubMed] [Google Scholar]
- Lathe R (2001). Hormones and the hippocampus. Journal of Endocrinology, 169(2), 205. [DOI] [PubMed] [Google Scholar]
- Li F, He N, Li Y, Chen L, Huang X, Lui S et al. (2014). Intrinsic brain abnormalities in attention deficit hyperactivity disorder: a resting-state functional MR imaging study. Radiology, 272(2), 514–523. [DOI] [PubMed] [Google Scholar]
- Li F, Lui S, Yao L, Hu J, Lv P, Huang X et al. (2016). Longitudinal Changes in Resting-State Cerebral Activity in Patients with First-Episode Schizophrenia: A 1-Year Follow-up Functional MR Imaging Study. Radiology, 279(3), 867–875. [DOI] [PubMed] [Google Scholar]
- Ma X, Li S, Tian J, Jiang G, Wen H, Wang T et al. (2015). Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: A resting-state fMRI study. Clinical Neurophysiology, 126(6), 1190–1197. [DOI] [PubMed] [Google Scholar]
- Mantini D & Vanduffel W (2013). Emerging roles of the brain’s default network. Neuroscientist A Review Journal Bringing Neurobiology Neurology & Psychiatry, 19(1), 76–87. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB et al. (2009). Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD & Craig ADB (2006). Neuroimaging of the Brain-Gut Axis: From Basic Understanding to Treatment of Functional GI Disorders. Gastroenterology, 131(6), 1925–1942. [DOI] [PubMed] [Google Scholar]
- Mikdashi JA (2016). Altered functional neuronal activity in neuropsychiatric lupus: A systematic review of the fMRI investigations. Semin Arthritis Rheum, 45(4), 455–462. [DOI] [PubMed] [Google Scholar]
- Ng SC, Tang W, Ching JY, Wong M, Chow C, Hui M, et al AJ. (2013). Incidence and Phenotype of Inflammatory Bowel Disease Based on Results From the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology, 145(1), 158–165.e2. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA & Pittman QJ (2015). Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 35(12), 4942–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kuzmiski JB, Ho W Sharkey KA & Pittman QJ (2008). Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 17151–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ et al. (2011). REST: A Toolkit for Resting-State Functional Magnetic Resonance Imaging Data Processing. Plos One, 6(9), e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Mehandru S, Colombel JF & Peyrin-Biroulet L (2016). Crohn’s disease. Lancet, 67(27), 822–824. [DOI] [PubMed] [Google Scholar]
- Van Hees S, Mcmahon K, Angwin A, De Zubicaray G, Read S & Copland DA (2014). A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post‐stroke aphasia. Human Brain Mapping, 35(8), 3919–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van OL, Coen SJ & Aziz Q (2007). Functional brain imaging of gastrointestinal sensation in health and disease. World Journal of Gastroenterology , 13(25), 3438–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W, De Man JG, Pelckmans PA & De Winter BY (2014). Neuroanatomy of lower gastrointestinal pain disorders. World Journal of Gastroenterology, 20(4), 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ShyhYuh., Chao HsiangTai., Tu ChengHao, et al. (2016). Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain,157(1),92–102. [DOI] [PubMed] [Google Scholar]
- Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J et al. (2005). Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage, 27(1), 59–69. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development, 29(2), 83–91. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu X, Wang X, Zhu X, Zhong M, Yi J et al. (2014). First-Episode Medication-Naive Major Depressive Disorder Is Associated with Altered Resting Brain Function in the Affective Network. Plos One, 9(1), e85241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Liu P, Wang J, Wen H, Zhu M, Zhao R et al. (2013). Fractional amplitude of low-frequency fluctuation changes in functional dyspepsia: A resting-state fMRI study. Magnetic Resonance Imaging, 31(6), 996–1000. [DOI] [PubMed] [Google Scholar]
- Zigmond AS & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. [DOI] [PubMed] [Google Scholar]
- Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS et al. (2015). Chronic intestinal inflammation alters hippocampal neurogenesis. Journal of Neuroinflammation, 12, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]