Abstract
Calsenilin is a calcium ion (Ca2+) binding protein involved in regulating the intracellular concentration of Ca2+, a second messenger that controls multiple cellular signaling pathways. The ryanodine receptor (RyR) amplifies Ca2+ signals entering the cytoplasm by releasing Ca2+ from endoplasmic reticulum (ER) stores, a process termed calcium- induced calcium release (CICR). Here, we describe a novel mechanism, in which calsenilin controls the activity of neuronal RyRs. We show calsenilin colocalized with RyR2 and 3 in the ER of mouse hippocampal and cortical neurons using immunocytochemistry. The underlying protein-protein interaction between calsenilin and the RyR was determined in mouse central nervous system (CNS) neurons using immuno-precipitation studies. The functional relevance of this interaction was assayed with single channel electrophysiology. At low physiological Ca2+ concentrations, calsenilin binding to the cytoplasmic face of neuronal RyRs decreased the RyR’s open probability, while calsenilin increased the open probability at high physiological Ca2+ concentrations. This novel molecular mechanism was studied further at the cellular level, where faster release kinetics of caffeine-induced Ca2+ release were measured in SH-SY5Y neuroblastoma cells overexpressing calsenilin. The interaction between calsenilin and neuronal RyRs reveals a new regulatory mechanism and possibly a novel pharmacological target for the control of Ca2+ release from intracellular stores.
Keywords: Alzheimer’s disease, calcium, calsenilin, cortex, hippocampus, ryanodine receptor, RyR
Introduction
Calcium plays an important role in numerous cell signaling pathways in neuronal cells, some of which include memory consolidation, vesicle release, and apoptosis. Due to the ubiquitous nature of intracellular calcium, vital key pathways are employed to convert subcellularly localized alterations in calcium concentrations into global cellular responses.1 Propagation of intracellular calcium signals throughout the cell is accomplished through calcium release channels such as ryanodine receptors (RyRs) located on intracellular calcium sources, most notably the endoplasmic reticulum.2 Calcium binding proteins communicate changes in calcium levels through conformational changes that effect phosphorylation, protein binding, and the action of target proteins.3 The neuronal calcium sensor family of proteins provide high affinity calcium-binding sites that alter neuronal cell signaling affecting a diverse range of processes, such as memory formation, vesicle release, and neurite outgrowth.4 In addition, a range of proteins mediate uptake of Ca2+ into intracellular stores and extrusion of Ca2+ into the extracellular space, which shapes cytoplasmic calcium signaling. These two intimately connected processes, calcium release and calcium sequestration, work synergistically to shape calcium signaling throughout the cell.
RyRs are major calcium- induced calcium release (CICR) channels located on the endoplasmic reticulum membrane in neurons and most other cell types.5 RyRs are found throughout the nervous system2,6,7 and control multiple processes in neurons through CICR including vesicle release, membrane depolarization, and apoptosis.1 RyRs contain a large N-terminal cytoplasmic domain, which interacts with proteins, small molecules, and signaling molecules modulating CICR mediated by this channel.3,8 Of the three RyRs, RyR1 was originally described in skeletal muscle, RyR2 in heart tissue, and RyR3 in brain tissue, while all three are differentially expressed in most cell types and tissues.1–3 The three types of RyRs differ in their dependence on the cytosolic Ca2+ concentration for activation and inhibition, which combined with their differential distribution, results in tissue-specific calcium release producing cell- and tissue-specific functions.2 Previous studies have identified protein-protein interactions between RyRs and cytoplasmic proteins resulting in a change in RyR ion channel activity and Ca2+ release from the ER.9,10 At the same time, in both in vitro and in vivo disease models of Alzheimer’s disease (AD), increased release of Ca2+ from ryanodine-sensitive stores was observed in diseased cells,11–14 leading to increased apoptosis of neuronal cells15, 16. Similarly, inhibitory control of RyR activity reduced cell damage and cell death in in vitro models of Parkinson’s disease57 and multiple sclerosis58 and in in vivo models of neuroinflammation59 and Huntington’s disease60 resulting in reduced functional impairment59–60.
Here, we investigated the interactions between calsenilin, a calcium-binding protein also known as KChIP3/DREAM, and RyRs, specifically RyR2 and 3 and determined the impact of this interaction on calcium signaling. Calsenilin is a 31kDa protein of the neuronal Ca2+ sensor family17 with four C-terminus EF-hand motifs and a unique N-terminus that is responsible for binding to target molecules18–20 . Calsenilin is found in the human brain21 and is expressed throughout the CNS, with particularly high expression levels in layers V and VI of the cerebral cortex as well as in the granule layers of the cerebellum, olfactory centers and the hippocampus,22–26 and it is involved in synaptic plasticity.27–29 Calsenilin has also been identified in dorsal root ganglia lacking Kv4.3 expression,30 Aβ mechanoreceptors, Aσ sensory receptors and some C-fiber peptidergic nociceptors30 of the spinal cord.
Calsenilin interacts with the C-terminus of presenilin 1 and presenilin 2 to stabilize the proteolytic fragments of both presenilin 1 and presenilin 219 and enhance the enzyme activity of the presenilin-γ-secretase complex.31 Calsenilin alters cellular Ca2+ dynamics through several mechanisms, such as controlling the gating kinetics of Kv4.2 potassium channels,18 interacting with the downstream regulatory element (DRE) sequence and modulating the transcription of the Na+/Ca2+ exchanger NCX3,20, 32 reversing presenilin 1 mediated enhancement of Ca2+ release,33 altering the Ca2+ concentration of endoplasmic reticulum stores,32, 34, 35 and increasing Ca2+ induced apoptosis in both cellular and mouse models of AD.36, 37
Here, we propose that calsenilin acts as a regulator of RyR-mediated CICR that could be used as a potential drug target for controlling calcium dysregulation in neurodegenerative diseases such as AD. Our study shows that calsenilin and brain RyRs, neuronal RyR2 and RyR3, co-localize and interact directly, which results in altered RyR-mediated Ca2+ release at the single channel level. Our functional data from electrophysiology and calcium imaging experiments show that calsenilin alters the Ca2+ release kinetics of RyRs at the single-channel level in isolated microsomes, and of RyR-channel populations in neuroblastoma cells overexpressing calsenilin. Taken together, these results suggest a novel mechanism whereby calsenilin controls RyR2- and RyR3-mediated CICR release in neurons through direct protein-protein interaction.
Materials and Methods
Immunocytochemistry
NeuroPure embryonic day 18 (E18) Sprague Dawley rat cortical cells were prepared following manufacturer’s protocol (cat. #N200200, Genlantis, San Diego, CA). Immunostaining was conducted as described previously.38, 39 Briefly, media was removed and attached cells were fixed for 20 min in 4% PFA (0.01M PBS, pH 7.4) at room temperature (RT). The fixative was removed, followed by three 10 min washes using PBS (0.01 M PBS, pH 7.4). Blocking solution (10% normal donkey serum, 1% BSA, and 0.05% Triton-X 100 in 0.01 M PBS) was added and cells were incubated for 1 hour at RT. After blocking, the cells were incubated with the primary antibodies: DREAM (1:50; sc-9309, Santa Cruz Biotechnologies, Dallas, TX), RyR2 (1:10,000; AB9080, Chemicon Biotechnologies, Temecula, CA), RyR3 (AB9082 (1:5,000; Chemicon Biotechnologies) in incubation buffer (3% normal donkey serum, 1% BSA and 0.05% Triton-X 100 in 0.01 M PBS) overnight at 4 °C. Following primary incubation cells were washed three times for 10 minutes with PBS. Cells were next incubated with secondary antibodies: AlexaFluor 488 (1:2,000; A11055, Life Technologies, Carlsbad, CA) and AlexaFluor 594 (1:2,000; A11062, Life Technologies), and DAPI (1:20,000) for 1 hour at RT. After three washes, coverslips were mounted on slides using Aqua-Polymount (Polysciences, Warrington, PA). Negative controls consisted of the omission of the primary antibody.
Preparation of Mouse Brain Sections
C57BL/6 mice (Jackson Laboratories, Bar Harbor, MA) were euthanized using the IACUC approved method of carbon dioxide overdose. Brains were removed from the cranial cavity and immersion fixed in 4% PFA at 4 °C overnight. The tissue was then sequentially cryoprotected in a graded series of 10%, 20%, and 30% sucrose at 4 °C in PBS. Tissue was removed from final sucrose solution and placed in Peel-A-Way® mold (R-30, Polysciences Inc., Warrington, PA), and incubated at the Tissue-Tek optimal cutting temperature (OCT) embedding medium (Tissue Tek, Torrance CA) for 1hr at 4 oC. The samples were then placed in −80 oC isopentane (Alfa Aesar, Ward Hill, MA) to solidify the OCT compound and snap-freeze the tissue. Molds were sectioned at 30 μm using a Leica model CM3050 S cryostat.
Immunohistochemistry
Immunohistochemistry was performed as stated previously in the immunocytochemistry section with the addition of the following steps. Before blocking, tissue was placed in 1mg/ml sodium borohydride (Sigma Aldrich) three times for 10 minutes.40 After secondary antibody incubation and subsequent washes brain sections were incubated in 25mM CuSO4 for 10 minutes. The sections were subsequently washed three times with PBS for 10 minutes, followed by a 10-min wash in deionized water. Coverslips were applied using Aqua-Polymount.
Confocal Microscopy
All immunostaining and colocalization images were acquired on a Leica SP5 white-light laser scanning confocal microscope (Leica Microsystems, Bannockburn, IL) with a 63x glycerin-immersion objective. Alexa 488 was excited at 490 nm and collected at 505–550 nm. Alexa 594 was excited at 594 nm and collected at 599–650 nm. Immunocytochemistry optical sections (z-stack with 0.13 μm z-step, 1.6 optical slice thickness) and immunohistochemistry optical sections (z-stack with 0.13 μm z-step 1.0 optical slice thickness) were collected (2048 x 2048 pixel images) running Leica Application Suite Advanced Fluorescence v 2.6. To ensure uniformity of labeling, all images were acquired with same the confocal settings (pinhole size, gain, offset, and laser power). To decrease signal bleed through, images were collected in the order of Alexa 488, Alexa 594, and DAPI.
Statistical Analysis of Colocalization
Colocalization and intensity analysis were conducted with MBF ImageJ (McMaster Biophotonics Facility, Hamilton, ON) utilizing the Pearson’s and Mander’s Coefficient.41 The Pearson’s coefficient and Mander’s coefficients were validated using the Costes method plugin for Fiji ImageJ.
Recombinant Protein
Glutathione S-transferase (GST) tagged calsenilin protein was made by transforming bacteria, strain BL21(DE3) pLysS (Novagen, Madison, WI) with a cDNA construct of the full length mouse calsenilin, as described previously.9 Briefly, calsenilin protein expression was induced by 100 μM IPTG (isopropyl β-D-1-thiogalactopyranoside) at 28 °C for 4 h. Bacterial cultures were then pelleted, lysed, and centrifuged at 18,000×g at 4 °C for 20 min. Bacterial protein was extracted by incubation in 50 μl of immobilized GST resin (Novagen, Madison, WI) at 4 °C for 2 h, and washed in PBS. Recombinant protein was eluted with a buffer containing 10 mM reduced glutathione.
Co-immunoprecipitation
Co-immunoprecipitation and Western blotting was conducted as described previously.9, 39 Briefly, ER microsomes (250 μg protein per sample) were prepared from adult mouse whole brains as described previously.9, 10, 42 Samples were incubated alone, with RyR2 antibody (AB9080), or anti-rabbit IgG (2 mg/ml; Jackson Laboratories, Baltimore, PA) with or without recombinant calsenilin protein with a GST tag (combined molecular weight was between 51–55 kDa) under constant shaking at 4 °C for 12 hrs. Following incubation, samples were combined with protein A magnetic beads (EMD Millipore) for 10 minutes at RT, washed three times in PBS with 0.1% Tween 20, and eluted with Western Blot Sample Buffer (10%, glycerol: 10%, β-mercaptoethanol: 1%, bromophenol blue: 0.004%, tris (hydroxymethyl) aminomethane (Tris)-HCl: 0.5 M, pH 6.8) at 80 °C for 10 min.
Western Blotting
Solubilized proteins and molecular weight markers (HiMark Pre-stained Protein Standard, Thermo Fisher Scientific, Waltham, MA) were separated on a 3–8% NuPAGE® Novex® Midi Tris-acetate gel (Life Technologies) and transferred to Amersham Hybond-ECL blotting paper (0.45μm, GE Healthcare). Blots were incubated for 12–16 hrs at 4 °C with the calsenilin antibody (05-756) (1:500; Upstate, Lake Placid) and a RyR2 antibody (MA3–916) (1:100; Thermo Fisher Scientific). Blots were incubated with a horseradish peroxidase (HRP)-linked secondary antibody (31430) (1:5000; Thermo Fisher Scientific) at RT for 1 hour. Membranes were developed using SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific) and imaged on the Syngene G: BOX using Syngene GeneSnap acquisition software.
Single Channel Electrophysiology
Electrophysiology was conducted as previously stated [17, 18, 32]. Microsomes were added to the cis compartment of the bilayer chamber (Warner Instruments, Hamden, CT), where they were fused to a lipid bilayer formed with a 3:1 mixture of dried phosphatidylethanolamine/phosphatidylserine lipids (Avanti Polar Lipids, Alabaster, AL) on a 150 μm diameter aperture using hyperosmotic conditions (KCl solution of ~300–500 mM).The microsome incorporation was monitored by the appearance of channel activity and followed by perfusion of the cis chambers with 10 volumes of cis (106 mM Tris–OH,220 mM HEPES, pH 7.35, ~320 mOsm) buffer. RyR mediated single channel currents were activated by addition of Ca2+ into the cis chamber at fixed levels with rationed amounts of CaCl2/EGTA calculated using MaxChelator software (Stanford University). Recombinant calsenilin protein was added to the cis side of the chamber. The RyR blocker Ruthenium Red (10–25 μM) was routinely added at the end of the experiment to verify the identity of the RyRs. Experiments were performed at RT with single-channel currents recorded at a holding potential of 0mV (trans chamber grounded) using a BC-525 amplifier (Warner Instruments), filtered at 500 Hz and digitized at 5 kHz using pCLAMP™10 software (Molecular Devices, Sunnyvale, CA). pCLAMP™10 was used to conduct offline current trace filtering (320 Hz), to determine dwell time, to provide a amplitude histogram and to calculate open-channel probability calculations.
Single-Channel Electrophysiology Statistics
All recordings were normalized to control values and reported as percentage of controls. GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA) was used for all analysis and data presentation. Nonlinear curve fitting was performed using the Levenberg–Marquardt algorithm and tested for goodness of fit using the chi squared algorithm. A one-way ANOVA with a Dunnett’s post hoc test was used to test significance (Mean ± SEM; p< 0.05*, p< 0.01**, p<0.001***).
Transfection
Calsenilin cDNA was prepared from total RNA using SuperScript™ (Invitrogen) II Reverse Transcriptase (RT) (Thermo Fisher Scientific) and poly T primers, as described previously.9 SH-SY5Y neuroblastoma cells (ATCC, Manassas, VA) were co-transfected per manufacturer’s instruction using a Lonza 4D-nucleofector transfection unit (Lonza, Walkersville, MD). Briefly, cells were suspended in Lonza transfection media with 2 μg of calsenilin pcDNA3.1 (+) Zeo mammalian expression vector and 0.5 μg of tdTomato (reporter protein) pcDNA3.1 (+) Zeo mammalian expression vector. Transfected cells were then plated at 25,000 cells per laminin/poly-d lysine coverslips (BD Biocoat, Bedford, MA) using SH-SY5Y media (10% fetal bovine serum (PAA, Piscataway, NJ), 1:200 penicillin/streptomycin, 50% Ham’s F-12 media, 50% eagle minimum essential media (Lonza, Walkersville, MD) and maintained at 37 ºC, 5% CO2, 95% O2 for 36–48 hours.
Calcium Imaging
Media from mock- or calsenilin-transfected SH-SY5Y cells were removed and washed in extracellular solution buffer (ECS) (137 mM NaCl, 5mM KCl, 1 mM Na2HPO4, 1 mM MgSO4, 10 mM HEPES, 22 mM D-(+)-glucose, and 1.8 mM CaCl2 ; pH 7.35 at 37 °C); then, cells were incubated in 2 μM fura-2 AM (Invitrogen) in ECS for 30 minutes. Coverslips were washed with ECS containing 55 mM KCl to empty and subsequently refill their intracellular Ca2+ stores; they were then washed two more times with ECS. Coverslips were assembled to the imaging platform and continually perfused at 2 ml/minute with ECS at a constant temperature of 37 °C. Perfusion protocols were the same for all experiments and controlled through MetaFluor sequential journals. The perfusion protocol was as follows: 1 minute of ECS to establish a baseline, 3 minutes of 30 mM caffeine in ECS to stimulate the RyRs, and 3 minutes of ECS to return the response to baseline. Regions of Interest (ROIs) were selected following Ca2+ imaging to adjust for drift. Background ROI’s (N = 4 for each coverslip) were selected using a region of the optical field not occupied by cells.
Calcium Imaging Statistical Analysis
GraphPad Prism 5 was used for all analysis and data presentation. The calculation for the area under the curve (AUC) was defined as follows: the beginning of the curve was the time point where the Ca2+ value rose above 5% of baseline, and the ending of the curve was defined by the last point in the curve that was above 5% of the baseline. Both maximum value and width of response were calculated from this curve. Linear regression was done to calculate the slope values of each response using the max value and the first point 5% above the baseline. All values were averaged and statistically analyzed using the student’s t-test (Mean ± SEM, p < 0.05,* p< 0.01,** p < 0.001***).
Results
Calsenilin and RyR are co-localized and have a direct protein-protein interaction in neuronal tissue
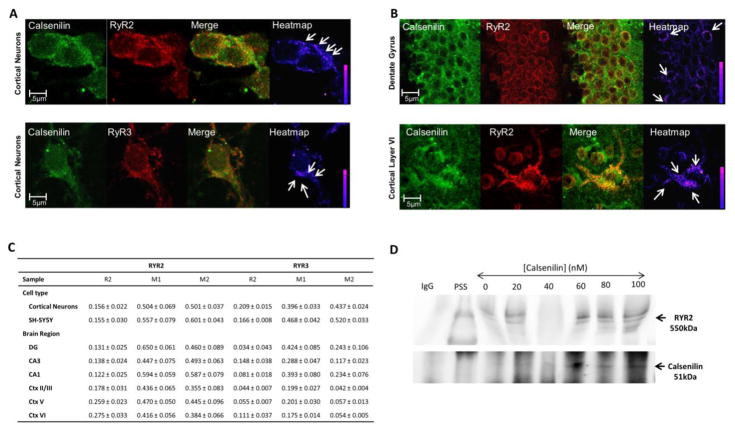
Figure 1 shows typical calsenilin and RyR2 or RyR3 (Figure 1A) staining patterns in primary cultured cortical neurons and RyR2 in the dentate gyrus and cortical layer VI of a 6-week-old C57 mouse brain (Figure 1B). Co-localization between the two proteins is highest in the perinuclear region of the cells for both RyR2 and RyR3. This staining pattern is indicative of ER Ca2+ release channels in neuronal tissue.43–45 Figure 1C shows this pattern of co-localization, which appears for RyR2 and calsenilin in all cortical neurons, hippocampal and cortical layers tested. Co-localization of RyR3 and calsenilin in both the hippocampus and the cortex is region-specific with higher co-localization seen in the CA3 region of the hippocampus and cortical layer VI (Figure 1C). Immunoblotting of proteins captured using an anti-RyR2 antibody (Fig 1D) shows expression of both RyR2 (approximately 550 kDa) and calsenilin recombinant protein with a GST tag (approximately 51 kDa). Substituting the anti-RyR2 antibody with IgG yielded no bands for RyR2 or calsenilin (Fig 1D), proving the specificity of the anti-RyR2 antibody. Together these data suggest that calsenilin and RyR2 /RyR3 not only reside in the same subcellular location, but interact.
Figure 1. Calsenilin and RyR are co-localized and have a direct protein-protein interaction in neuronal tissue.
Calsenilin (green) and A) RyR2 or RyR3 (red) immunoreactivity in E18 primary cultured cortical neurons B) RyR2 (red) expression in the dentate gyrus and cortical layer VI show a perinuclear staining pattern in regions adjacent to the nucleus indicative of ER staining (Merge) and a high degree of punctate co-localization in the perinuclear region (+PDM) as indicated by arrows and LUT in the last panel. C) Co-localization measurements between calsenilin and RyR2 or RyR3 for neuronal cell types (E18 primary cortical neurons and SH-SY5Y neuroblastoma cells) and 6-week-old C57BL/6 mouse brain sections (dentate gyrus, CA3, CA1, cortical layers II/III, V, VI). The Rr = Pearson’s coefficient and M1 = Mander’s coefficient describe the amount of calsenilin co-localized with the two RyR subtypes, whereas the M2 = Mander’s coefficient describes the amount of RyR2 or RyR3 co-localized with calsenilin. Statistical significance represents the SEM for each replicate; co-localization was tested with the Costes method to ensure true co-localization. D) Western blot shows the presence of calsenilin in the brain-derived ER microsomes incubated with calsenilin protein and subjected to co-immunoprecipitation separation using the RyR2 antibody; the IgG control is shown in lane “IgG”, and molecular weight standards are shown in lane “PSS”. Abbreviations: RyR, ryanodine receptor; DG, Dentate Gyrus; CA1 or CA3, Cornu Ammonis 1 or 3; Ctx, Cortex layer; IgG, Immunoglobulin G; MW, molecular weight; PSS, pre-stained standard.
Calsenilin Alters RyR Single Channel Ca2+ Release in Single Channel Electrophysiological Experiments
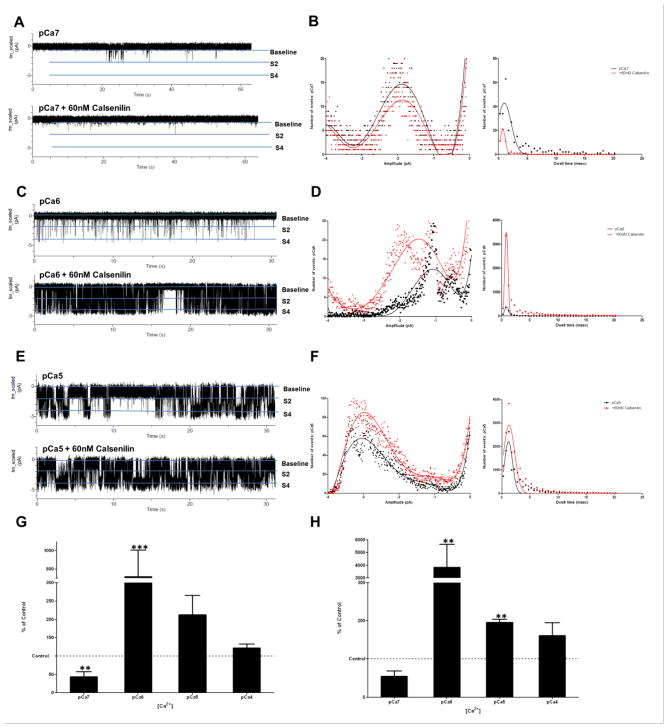
Figure 2 shows typical recordings of RyR activity at physiological intracellular calcium ion concentrations of 100 nM (pCa7; Figure 2A), 1 μM (pCa6; Figure 2C), and 10 μM (pCa5; Figure 2E) before and after calsenilin addition. The pCa7 amplitude histogram (Figure 2B) shows that calsenilin reduces the number of times the channel opens and decreases the duration of each opening. The decrease in the number and duration of channel openings translates to a significant decrease in the open probability of the subconductance state ( 2 pA) of the RyR (Figure 2G). This data suggests that at a resting cytosolic Ca2+ concentration, calsenilin decreases Ca2+ release from RyRs. Conversely, at higher Ca2+ concentrations, calsenilin has the opposite effect on RyR single-channel kinetics. After the addition of calsenilin (Figure 2C and 2D), RyR activity increases, characterized by an increase in the number of times the channel opens and the duration of each opening (Figure 2D). The changes in RyR activity after the addition of calsenilin at 1 μM Ca2+ translates to a significant increase in the open probability of the channel at the subconductance ( 2pA; Figure 2G) and full conductance state ( 4 pA; Figure 2H). Similarly, the activity of the RyR at 10 μM Ca2+ after the addition of calsenilin shows increases in frequency and duration of openings (Figure 2E–H) , translating into a significant increase of the full conductance state ( 4 pA; Figure 2H). At 100 μM Ca2+, the addition of calsenilin did not result in significantly different RyR channel activity (data not shown). The data indicates that calsenilin affects the Ca2+ release kinetics of the RyR in a Ca2+ concentration dependent fashion. Calsenilin causes a sharpening of the CICR of RyRs by significantly decreasing the activity of the receptor in a normally low-activity state (100 nM Ca2+) and increasing receptor activity at high activity states (1 μM and 10 μM, respectively).
Figure 2. Calsenilin causes calcium concentration-dependent alterations in the biophysical Ca2+ release from single-brain RyRs.
Representative planar lipid bilayer 60 sec traces (A, C and E) at varying physiological calcium concentrations in the presence (bottom trace) and absence (top trace) of calsenilin, and respective histograms (B, D and F) for amplitude (pA, left) and dwell time (msec, right). Graphs G and H show the open probability of the RyR in the presence of 60 nM calsenilin for io= −2 pA and − 4 pA, respectively, expressed as the percentage of the respective controls recorded in the absence of calsenilin at pCa4-7 (one-way ANOVA with Dunnett’s post-test; values are represented as mean +/−SEM; ***, p < 0.001 and **, p < 0.01; n = 3–8).
Calsenilin Overexpression in SHSY-5Y Neuroblastoma Cells
Alters Caffeine-Induced Ca2+ Release
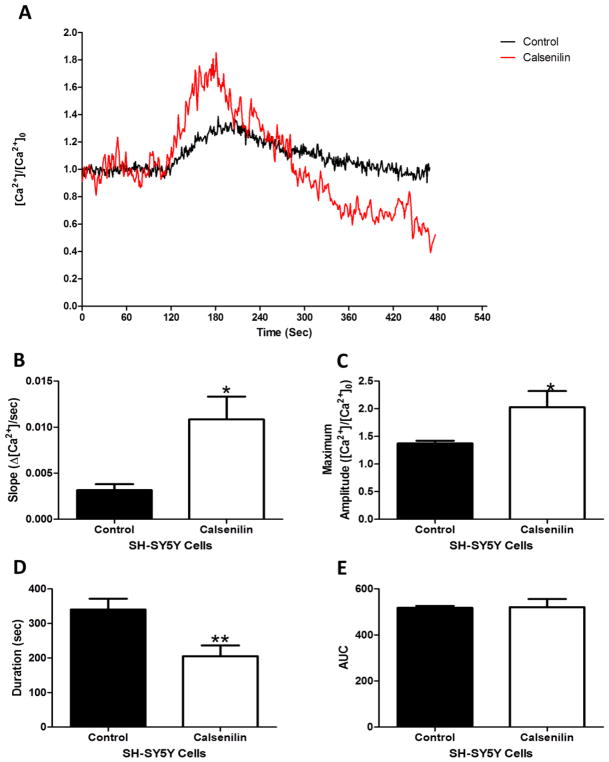
Figure 3 shows a typical caffeine-induced Ca2+ response in SHSY-5Y control cells (black line) and calsenilin overexpressing cells (red line). Calsenilin overexpression increases the release rate of Ca2+ from RyR sensitive stores compared to control cells. Calculation of the slope of Ca2+ release for both control and calsenilin overexpressing cells shows a significant increase in the rate of Ca2+ release (Figure 3B). The increased calcium release rate corresponds to a significant decrease in the duration of Ca2+ release of calsenilin overexpressing cells as compared to control cells calculated by the width of Ca2+ release (Fig 3D). The maximum amplitude of caffeine-induced Ca2+ release is significantly increased in calsenilin overexpressing cells compared to control cells (Fig 3C). Interestingly, a calculation of the area under the curve, which is a relative measure of the amount of Ca2+ released, shows no difference between control cells and calsenilin overexpressing cells (Fig 3E). Together these data suggest that calsenilin modulates CICR kinetics of the RyR.
Figure 3. Calsenilin changed the kinetics of caffeine-induced calcium release.
(A) Representative trace showing caffeine-induced calcium release from untransfected (black) and calsenilin-transfected (red) SH-SY5Y cells, and (B) the respective kinetic analysis of slope, (C) maximum amplitude, (D) duration, and E) area under the curve (E). Significance parameters were obtained using Student’s t-test. Values represented as Mean SEM *p < 0.05, **p < 0.01, n = 3–8.
Discussion
We have shown that calsenilin and RyR2 and RyR3 co-localize in the endoplasmic reticulum of mouse hippocampal and cortical neurons (Figure 1A–C), and have a probable protein-protein interaction between calsenilin and RyR2 in microsomes derived from brain tissue (Figure 1D). We found that calsenilin causes calcium ion concentration-dependent alterations in the Ca2+ release from single brain RyRs (Figure 2) and alters the kinetics of caffeine-induced calcium release from intracellular stores in a whole cell paradigm (Figure 3). The modulation of single channel RyRs by calsenilin decreased channel activity at lower 100-nM calcium concentrations (Figure 2A, B, G and H), and increased channel activity at 1 μM and 10 μM calcium concentrations (Figure 2C–F, G and H). In a whole cell paradigm, calsenilin modulation of multiple RyRs caused faster and higher amplitude caffeine-induced calcium release in SHSY-5Y cells overexpressing calsenilin (Figure 3). The alteration in calcium release dynamics from RyRs can have profound effects on the functioning of neurons.
Transient increases in calcium concentration in neurons can lead to opposite responses based on magnitude, timing, and duration of the elevated calcium release. For example, the consolidation, or lack of consolidation, of memories through long-term potentiation (LTP) and long-term depression (LTD) is a function of firing patterns in pre- and post- synaptic neurons.46 The magnitude and timing of calcium signals in neurites decides whether a signal received by a post synaptic neuron will lead to LTP or LTD. LTP is a function of large rapid calcium transients, while LTD is a function of slow longer duration calcium transients.47 These two phenomena are profoundly affected by release from intracellular calcium stores such as the RyRs and inositol triphosphate receptors (IP3Rs).48 Further, neuronal calcium sensors that affect the magnitude of calcium signals in neurons can change the polarization of growth cones, branching of neurites, and rate of elongation.47 The novel interaction between calsenilin and RyRs describes a modulatory mechanism that can alter duration, timing, and magnitude of calcium transients in neurons.
Alterations in calcium-induced calcium signaling in neurons is implicated in several neurodegenerative diseases (Parkinson’s disease57; multiple sclerosis58; neuroinflammation59; Huntington’s disease60; reviewed in 49). The long-standing calcium hypothesis of AD states that alterations in calcium homeostasis occur before the clinical symptoms of AD, but lead to the slow progressive decline of memory and cognition in sporadic AD (reviewed in 50). Further, altered RyR calcium release in the dendrites of pre-symptomatic 3xTg-AD mice51 and young AD mice52 shows RyR-mediated release affects synaptic plasticity in hippocampal and cortical neurons. Calsenilin is expressed in hippocampal and cortical dendrites and is involved in synaptic plasticity.27–29 Mellström et al. found dendritic changes in the hippocampal neurons of a transgenic mouse model expressing the daDREAM mutant, suggesting calsenilin is involved in structural plasticity in the hippocampus.28 In the present study, we have shown that calsenilin and RyR2 have a high degree of co-localization in proximal dendritic branch points (Fig 1B) indicating that this association occurs in more distal dendritic branches. Dysregulation of calcium homeostasis leads to an increase in resting calcium concentrations seen in both the soma53 and dendrites54 of neurons affected by AD, as well as in neurons of in vivo models of neuroinflammation59 and Huntington’s disease60.
The association of calsenilin with RyRs increases calcium release at higher calcium levels and leads to faster and higher amplitude calcium transients in cells overexpressing calsenilin. The increase in calsenilin55 and RyR56 expression in AD, as well as increased resting calcium concentration in AD53–54, in vivo models of neuroinflammation59 and Huntington’s disease60 taken together with the increase in RyR activity in the presence of calsenilin and high calcium concentrations indicates a role for this interaction in the etiology of AD, and potentially other neurodegenerative diseases, such as Parkinson’s disease57, multiple sclerosis58, neuroinflammation59 and Huntington’s disease60.
From this data, we propose that calsenilin has an important modulatory role in calcium release from RyRs by which calsenilin acts as a filter on calcium-induced calcium release. By silencing the receptor at low physiological calcium concentrations and increasing the activity at higher physiological calcium concentrations, calsenilin sharpens calcium release from RyRs mimicking ion signals seen in neuronal cells. This novel interaction could be exploited pharmacologically to reduce calcium levels in neurodegenerative disorders by displacing calsenilin from the RyR. The exact binding sites and calcium dependence of this interaction must be explored to further utilize this potential tool for the alleviation of neuronal dysfunction in neurodegeneration.
Acknowledgments
Research reported in this publication was supported in part by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss JL, Hui H, Burgoyne RD. Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell Mol Neurobiol. 2010;30:1283–1292. doi: 10.1007/s10571-010-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johenning FW, et al. Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992;312:229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- 7.Lai FA, et al. Expression of a cardiac Ca(2+)-release channel isoform in mammalian brain. Biochem J. 1992;288( Pt 2):553–564. doi: 10.1042/bj2880553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Petegem F. Ryanodine receptors: structure and function. J Biol Chem. 2012;287:31624–31632. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44:507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer's disease. J Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelliher M, et al. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer's disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92:499–513. doi: 10.1016/s0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 13.Stutzmann GE, et al. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Ann N Y Acad Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- 14.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, et al. PS2 mutation increases neuronal cell vulnerability to neurotoxicants through activation of caspase-3 by enhancing of ryanodine receptor-mediated calcium release. FASEB J. 2006;20:151–153. doi: 10.1096/fj.05-4017fje;1. [DOI] [PubMed] [Google Scholar]
- 16.Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CM. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol Dis. 2006;23:669–678. doi: 10.1016/j.nbd.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- 18.An WF, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 19.Buxbaum JD, et al. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998;4:1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 20.Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 21.Buxbaum JD, et al. Genomic structure, expression pattern, and chromosomal localization of the human calsenilin gene: no association between an exonic polymorphism and Alzheimer's disease. Neurosci Lett. 2000;294:135–138. doi: 10.1016/s0304-3940(00)01553-6. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi NF, et al. Biochemical and immunocytochemical characterization of calsenilin in mouse brain. Neuroscience. 2002;114:247–263. doi: 10.1016/s0306-4522(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 23.Duncan CE, Schofield PR, Weickert CS. K(v) channel interacting protein 3 expression and regulation by haloperidol in midbrain dopaminergic neurons. Brain Res. 2009;1304:1–13. doi: 10.1016/j.brainres.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Hammond PI, Craig TA, Kumar R, Brimijoin S. Regional and cellular distribution of DREAM in adult rat brain consistent with multiple sensory processing roles. Brain Res Mol Brain Res. 2003;111:104–110. doi: 10.1016/s0169-328x(02)00693-9. [DOI] [PubMed] [Google Scholar]
- 25.Spreafico F, Barski JJ, Farina C, Meyer M. Mouse DREAM/calsenilin/KChIP3: gene structure, coding potential, and expression. Mol Cell Neurosci. 2001;17:1–16. doi: 10.1006/mcne.2000.0913. [DOI] [PubMed] [Google Scholar]
- 26.Wang WC, Cheng CF, Tsaur ML. Immunohistochemical localization of DPP10 in rat brain supports the existence of a Kv4/KChIP/DPPL ternary complex in neurons. The Journal of comparative neurology. 2015;523:608–628. doi: 10.1002/cne.23698. [DOI] [PubMed] [Google Scholar]
- 27.Johnston D, et al. Active dendrites, potassium channels and synaptic plasticity. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellström B, et al. Specific cytoarchitectureal changes in hippocampal subareas in daDREAM mice. Molecular brain. 2016;9:22. doi: 10.1186/s13041-016-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Aguilera A, Sanchez-Alonso JL, Vicente-Torres MA, Colino A. A novel short-term plasticity of intrinsic excitability in the hippocampal CA1 pyramidal cells. The Journal of physiology. 2014;592:2845–2864. doi: 10.1113/jphysiol.2014.273185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng CF, et al. Coexpression of auxiliary subunits KChIP and DPPL in potassium channel Kv4-positive nociceptors and pain-modulating spinal interneurons. The Journal of comparative neurology. 2016;524:846–873. doi: 10.1002/cne.23876. [DOI] [PubMed] [Google Scholar]
- 31.Jo DG, Jang J, Kim BJ, Lundkvist J, Jung YK. Overexpression of calsenilin enhances gamma-secretase activity. Neurosci Lett. 2005;378:59–64. doi: 10.1016/j.neulet.2004.12.078. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Villafuertes R, et al. Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J Neurosci. 2005;25:10822–10830. doi: 10.1523/JNEUROSCI.3912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leissring MA, et al. Calsenilin reverses presenilin-mediated enhancement of calcium signaling. Proc Natl Acad Sci U S A. 2000;97:8590–8593. doi: 10.1073/pnas.97.15.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lilliehook C, et al. Calsenilin enhances apoptosis by altering endoplasmic reticulum calcium signaling. Molecular and cellular neurosciences. 2002;19:552–559. doi: 10.1006/mcne.2001.1096. [DOI] [PubMed] [Google Scholar]
- 35.Fedrizzi L, Lim D, Carafoli E, Brini M. Interplay of the Ca2+-binding protein DREAM with presenilin in neuronal Ca2+ signaling. J Biol Chem. 2008;283:27494–27503. doi: 10.1074/jbc.M804152200. [DOI] [PubMed] [Google Scholar]
- 36.Jo DG, et al. Pro-apoptotic function of calsenilin/DREAM/KChIP3. FASEB J. 2001;15:589–591. doi: 10.1096/fj.00-0541fje. [DOI] [PubMed] [Google Scholar]
- 37.Jo DG, et al. Induction of pro-apoptotic calsenilin/DREAM/KChIP3 in Alzheimer's disease and cultured neurons after amyloid-beta exposure. J Neurochem. 2004;88:604–611. doi: 10.1111/j.1471-4159.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 38.Rybalchenko V, et al. The unliganded long isoform of estrogen receptor beta stimulates brain ryanodine receptor single channel activity alongside with cytosolic Ca2+ J Recept Signal Transduct Res. 2009;29:326–341. doi: 10.3109/10799890903295168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan RS, Hwang SY, Koulen P. Differential inositol 1,4,5-trisphosphate receptor signaling in a neuronal cell line. Int J Biochem Cell Biol. 2007;39:1852–1862. doi: 10.1016/j.biocel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Beisker W, Dolbeare F, Gray JW. An improved immunocytochemical procedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry. 1987;8:235–239. doi: 10.1002/cyto.990080218. [DOI] [PubMed] [Google Scholar]
- 41.Collin T. McMaster Biophotonics Facility. McMaster University; Hamilton, ON: [Google Scholar]
- 42.Hwang SY, et al. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium. 2003;34:177–184. doi: 10.1016/s0143-4160(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 43.Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci U S A. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp AH, et al. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seymour-Laurent KJ, Barish ME. Inositol 1,4,5-trisphosphate and ryanodine receptor distributions and patterns of acetylcholine- and caffeine-induced calcium release in cultured mouse hippocampal neurons. J Neurosci. 1995;15:2592–2608. doi: 10.1523/JNEUROSCI.15-04-02592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nature reviews Neuroscience. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci. 2012;13:157–168. doi: 10.1038/nrn3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan RS, et al. Control of intracellular calcium signaling as a neuroprotective strategy. Molecules. 2010;15:1168–1195. doi: 10.3390/molecules15031168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berridge MJ. Calcium signalling and Alzheimer's disease. Neurochem Res. 2011;36:1149–1156. doi: 10.1007/s11064-010-0371-4. [DOI] [PubMed] [Google Scholar]
- 51.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer's disease mice. J Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez JR, et al. Increased intraneuronal resting [Ca2+] in adult Alzheimer's disease mice. J Neurochem. 2008;105:262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 54.Kuchibhotla KV, et al. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin JK, et al. Expression of calsenilin in neurons and astrocytes in the Alzheimer's disease brain. Neuroreport. 2005;16:451–455. doi: 10.1097/00001756-200504040-00007. [DOI] [PubMed] [Google Scholar]
- 56.Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1–42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J Biol Chem. 2006;281:38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 57.Huang L, Xue Y, Feng D, Yang R, Nie T, Zhu G, Tao K, Gao G, Yang Q. Blockade of RyRs in the ER Attenuates 6-OHDA-Induced Calcium Overload, Cellular Hypo-Excitability and Apoptosis in Dopaminergic Neurons. Front Cell Neurosci. 2017 Mar 3;11:52. doi: 10.3389/fncel.2017.00052. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiz A, Matute C, Alberdi E. Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis. 2010 Jul 15;1:e54. doi: 10.1038/cddis.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopp SC, Royer SE, D'Angelo M, Kaercher RM, Fisher DA, Wenk GL. Differential neuroprotective and anti-inflammatory effects of L-type voltage dependent calcium channel and ryanodine receptor antagonists in the substantia nigra and locus coeruleus. J Neuroimmune Pharmacol. 2015 Mar;10(1):35–44. doi: 10.1007/s11481-014-9568-7. Epub 2014 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raymond LA. Striatal synaptic dysfunction and altered calcium regulation in Huntington disease. Biochem Biophys Res Commun. 2017 Feb 19;483(4):1051–1062. doi: 10.1016/j.bbrc.2016.07.058. Epub 2016 Jul 14. [DOI] [PubMed] [Google Scholar]