Abstract
Honey is becoming accepted as a reputable and effective therapeutic agent by practitioners of conventional medicine and by the general public. It has many biological activities and has been effectively used in the treatment of many diseases, e.g. gastrointestinal diseases, skin diseases, cancer, heart diseases, and neurological degeneration. Honey is an excellent source of energy containing mainly carbohydrates and water, as well as, small amounts of organic acids, vitamins, minerals, flavonoids, and enzymes. As a natural product with a relatively high price, honey has been for a long time a target for adulteration. The authenticity of honey is of great importance from commercial and health aspects. The study of the physical and chemical properties of honey has been increasingly applied as a certification process for the purpose of qualification of honey samples. The current work focusses on studying the authenticity of various types of honey sold in Riyadh market (24 samples). For this purpose, physical properties (pH, hydroxylmethylfurfural HMF, and pollen test) were measured. Besides, sugar composition was evaluated using Fehling test and an HPLC method. Elemental analysis was carried out using inductively coupled plasma (ICP). In addition, the presence of drug additives was assessed by means of GC–MS. The obtained results were compared with the Saudi Arabian standards, Codex Alimentarius Commission (2001), and harmonized methods of the international honey commission.
Keywords: Honey, Adulteration, HPLC, GC–MS, ICP, Saudi market
1. Introduction
Honey is the natural sweet substance produced by honey bees from the nectar of plants or from secretions of living parts of plants. It could also be produced from excretions of plant-sucking insects on the living parts of plants which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store, and leave in the honey comb to ripen and mature (CAC, 2001).
Honey is an excellent source of energy containing approximately 80 g/100 g carbohydrates (35 g/100 g glucose, 40 g/100 g fructose, and 5 g/100 g sucrose) and 20 g/100 g water which is quantitatively the second most important component of honey. Its content is critical since it affects the storage of honey. In addition, honey contains more than 180 substances (Rodriguez et al., 2004), including organic acids such as acetic acid and gluconic acid. Despite being minor constituents, they are responsible for the acidity of honey and contribute largely to its characteristic taste. Vitamins and minerals are present in a very small quantity, particularly iron and copper which are responsible for the redox properties of honey and potassium, being the most abundant. Honey also contains trace amounts of niacin, calcium, copper, riboflavin, iron, magnesium, and zinc (Sampath Kumar et al., 2010). Dark honey is the richest in minerals among all honey types. The main enzymes in honey are invertase, diastase and glucose oxidaze. Among honey constituents are also amino acids, 5-hydroxymethylfurfural (HMF), and phenolic compounds. Flavonoids present in honey are comprised of flavanones, flavones, and flavonols while phenolic acids are substituted cinnamic acids and benzoic acids. These compounds are the main contributors for color, taste, and aroma of honey (Bertoncelj et al., 2007, Al et al., 2009) (Ferreira et al., 2009, Meda et al., 2005, Karabagias et al., 2016).
Honey is becoming accepted as a reputable and effective therapeutic agent by practitioners of conventional medicine and by the general public. This has been a consequence of there being an increasing awareness of the good clinical results that are obtained and of there being rational explanations for its therapeutic actions (Sampath Kumar et al., 2010). Honey possesses various biological activities like wound healing, anti-inflammatory, antimicrobial activities, as well as in the treatment of gastrointestinal disorders, skin diseases, cancer, heart diseases, and neurological degeneration (Subrahmanyam, 1991, Ladas et al., 1995, Tonks, 2003, Nasir et al., 2010, Hussein et al., 2011, Bogdanov and Martin, 2002).
As a natural product with a relatively high price, honey has been for a long time a target for adulteration (Bogdanov and Martin, 2002). The authenticity of honey is of great importance both from commercial and health aspects. Detection of adulteration in honey is difficult because of the large natural variability of honey due to differences in plant species, maturity, environment, processing, and storage techniques (Sivakesava and Irudayaraj, 2002). Honey adulteration can occur by the addition of foreign substances such as, molasses, starch solution, glucose, sucrose, water, and inverted sugar, or by a change in the physiochemical parameters (El-Bialee and Sorour, 2011). The following sweeteners have been used for honey adulteration: acid inverted sugar syrups, corn syrups, syrups of natural origin such as maple, cane sugar, beet sugar and molasses. On the other hand, the use of excessive heat for pasteurization and liquefaction might have adverse effects on honey quality, e.g. loss of volatile compounds and reduction of enzyme activity (Nasir et al., 2010). Harvesting of honey with high humidity or subsequent addition of water to honey can result in honey fermentation and spoilage (Bogdanov and Martin, 2002). Adulterated honey loses some of the nutritional and medicinal benefits, compared with pure honey, and may vary greatly with respect to its physicochemical properties, e.g. refractive index (R.I), moisture content, total soluble solids, density, specific weight, capillary action, surface tension, and pH value. Pure honey has Newtonian behavior, while adulterated honey exhibited non-Newtonian pseudo-plastic behavior which was fitted well to Power law model. The study of the physical and chemical properties of honey has increased in recent years because these parameters are important for the certification process that determines honey quality (Ur-Rehman et al., 2008). Different reports were found in the literature dealing with the evaluation of the physical and/or chemical characteristics of honey samples all over the world (Karabagias et al., 2016, Aghamirlou et al., 2015, Al et al., 2009, Bertoncelj et al., 2007 Meda et al., 2005, Nurul Syazana et al., 2013, Siddiqui et al., 2017, Bahaffi and Al-lihaibi, 2005).
However, unfortunately, only a few reports have been conducted in Saudi Arabia dealing with studying the quality of honey despite the popularity of multiple nutrition and therapeutic uses among the Saudi population (Alqarni et al., 2014, Alqarni et al., 2016, Mesallam and El-Shaarawy, 1987, Osman et al., 2007, Ahamed et al., 2017).
This study aims at identifying the authenticity and investigating the safety of representing various types of honey products sold in Riyadh market (24 samples). For this purpose, physicochemical properties, pH, HMF, and pollen test were performed. Sugar composition was also evaluated by means of Fehling test and a high-performance liquid chromatography (HPLC) technique. In addition, elemental analysis was performed by ICP. The presence of drug additives was also tested by means of GC–MS. All results were assessed based on Saudi Arabian standards (SSA102/1978), Codex Alimentarius Commission (CAC, 2001), and harmonized methods of the international honey commission (ICH, 2002; Bogdanov, 2009) (Table 1).
Table 1.
Physical and chemical specifications of honey compositions.
| Honey composition | Specifications |
|---|---|
| Hydroxylmethylfurfural (HMF) | Not more than 60 mg/kg |
| Total reducing sugar | Not less than 60% |
| Fructose | 27–44.3% |
| Glucose | 22–40.7% |
| Sucrose | Not more than 5% |
| Fructose/Glucose ratio | Not less than 0.95% |
| Heavy metals and other additives (arsenic, lead, mercury, pesticide, etc) | Absent or not exceed maximum levels allowed |
| pH | 3.24–6.1 |
| Pollen grains | Present |
2. Materials and methods
2.1. Honey samples
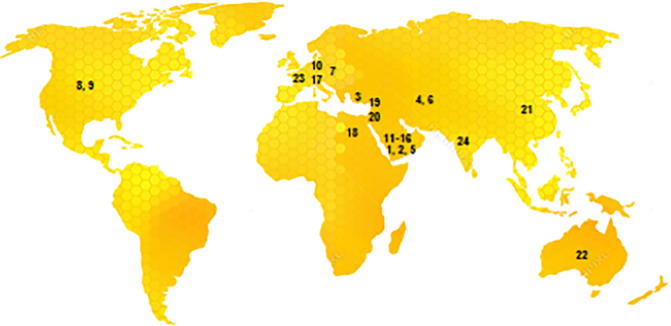
Twenty-four samples were collected from different sources of Riyadh market as arranged in Table 2. The tested samples were of different origin and from different countries all over the world (Fig. 1).
Table 2.
Sources of the studied honey samples.
| Sample number | Sample source |
|---|---|
| 1–7 | Street, imported, unprocessed natural (raw) |
| 4, 8, 9, 10, 17, 18, 19, 20, 21, 22, 23, and 24 | Supermarket, imported, processed honey (branded) |
| 11, 12, 13 and 14 | Supermarket, Local, processed honey (branded) |
| 15 and 16 | Unprocessed honey (raw), local |
Fig. 1.
Distribution of the tested honey samples (1–24) all over the world based on the country of origin as labelled on the samples.
2.2. Chemicals and reagents
D(+) Glucose, D(+)-Saccharose (Sucrose), D(+) Fructose, glycerine, gelatin, fuchsin (pollen stain), resorcinol, copper sulphate pentahydrate (CuSO4·5H2O), potassium tartrate (C4H4NaO5), methylene blue, aluminum cream (K2SO4AL2(SO4). 24H2O) were purchased from Sigma-Aldrich (Steinheim, Germany). Acetic anhydride, sulphuric acid, dichloromethane, isopropanol, hydrochloric acid (HCl), sodium hydroxide (NaOH) and ether were obtained from Supelco (Bellefonte, Pennsylvania, USA).
Arsenic (As), cadmium (Cd), lead (Pb), mercury (Hg), nitric acid (65%), and hydrogen peroxide (30%) were of Spec pure grade (Merck, Darmstadt, Germany). The reference slides of pollen were collected directly from the plants in the study area.
2.3. Instrumentation and experimental conditions
2.3.1. pH measurements
The pH of the samples was measured potentiometrically at 20 °C using a pH-meter (HANNA 2211-02 pH/MV/C) bench meter with electrode holder.
2.3.2. HPLC analysis
HPLC analysis was performed using SCHIMADZU high performance liquid chromatography (HPLC) instrument (Japan) equipped with a diode array detector (DAD) and data handling system comprised of Dell personal computer and SCHIMADZU class-Vp software. Separation was carried out using Ammonia (NH2) USP L8 analytical column (25 cm × 4.6 mm, 5 µm i.d.). Chromatographic analysis was performed using a mobile phase comprised of a mixture of distilled water: acetonitrile (20:80, v/v) and an injection volume of 5 μL with a flow rate of 1 mL/min. Detection was performed at the wavelength of 195 nm. Temperature was kept at 35 °C during the whole run.
2.3.3. Elemental analysis
Elemental analysis was carried out using inductively coupled plasma ICP (ICP ACTIVA S), HORIBA JOVYN, with data handling system comprised of Dell personal computer and Activa analyst 5.4.2 software. Samples were prepared using Microwave acid digestion apparatus, Milestone Ethos Start Lab Station with internal temperature sensor, 640-260 terminal with Easy Control software installed and HPR1000/10S high pressure segmented rotor. Analysis of arsenic As and mercury Hg elements was performed using the hydride generator ICP.
2.3.4. Gas chromatography/mass spectrometry (GC/MS)
GC/MS analysis was done by SCHIMADZU GC/MS-QP2010 GC/MS instrument (SCHIMADZU Corporation, Kyoto, Japan) with data handling system comprised of Dell personal computer and SCHIMADZU GC–MS Real Time Analysis software. The column used was fused silica capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness) DB-5 (5% phenyl, 95% dimethyl polysiloxane), (J &W Scientific, Rancho Cordopa, CA, USA). The following temperature program was applied: 50 °C for 5 min followed by temperature increase from 50 °C till 320 °C (rate 6 °C/min), then the temperature was kept constant at 320 °C for 5 min (a total run time of 55 min). The injector was used in the split injection mode with split ratio of 1:25. Helium was used as a carrier gas and the flow rate of 1.5 mL/min was applied.
2.4. Analytical procedures
2.4.1. Physical properties
2.4.1.1. Detection of HMF
The presence of HMF in honey samples was detected by Fiehe's Test (Bogdanov, 2009) which depends on the formation of sherry red color upon the reaction of HMF with resorcinol. The following procedure was carried out: 5 mL of ether was added to equal amount of honey sample and then was shaken well. After transferring the reaction mixture into a porcelain dish, the ether was evaporated to dryness then resorcinol powder was added with drops of concentrated hydrochloric acid.
2.4.1.2. pH measurement
The pH of honey samples was measured as referenced (SSA102/1978; Bogdanov, 2009). Aqueous solutions of honey were prepared by dissolving 1 g of honey in 10 mL distilled water (10% w/v).
2.4.1.3. Pollen test
The Pollen test was performed as mentioned in reference (Song et al., 2012) as follows: 10 gm of each honey sample was dissolved in 20 mL of warm water (40 °C). The solution was centrifuged for 10 min at 2500 r/min, the supernatant solution was decanted, and the sediments were collected into a conical tube and were than treated with the acetolysis mixture (acetic anhydride: conc. sulphuric acid, 9:1 v/v) for approximately 30 min at room temperature. After treatment with the acetolysis mixture, the sediments were rinsed with distilled water, centrifuged for 5 min at 2500 r/min, spread on glass slides, and dried on a warm plate. A drop of warmed fuchsin stained jelly was added by using a glass rod with a cover slip placed gently on top to avoid trapping of air bubbles. The slides were then left on a hot plate for 10 min and were then examined under the microscope (Leica DM2500 light microscope). Pollen types were identified by comparison with reference slides.
2.4.2. Chemical properties
2.4.2.1. Determination of sugar content
Two methods were used to measure and evaluate glucose, fructose, and sucrose levels.
2.4.2.1.1. Fehling test
For the determination of total sugars, Fehling test (SSA102/1978; Bogdanov, 2009) was carried out in order to calculate the percentage of total reducing sugar in the honey samples. The procedure was performed as follows: 1 g of honey sample was diluted to 100 mL with distilled water. Fehling reagent was prepared by boiling 5 mL of each of Fehling A (7 g CuSO4·5H2O dissolved in 100 mL of distilled water with 2 drops of dilute sulfuric acid) and Fehling B (35 g of potassium tartrate and 10 g of NaOH in 100 mL of distilled water) gently with 150 mL of distilled water over a small flame. The prepared Fehling reagent was titrated against the diluted honey sample, being used as the titrant, till the blue color of Fehling fades to almost 90% of its intensity after which 2 drops of methylene blue indicator were added. Titration was continued till the observation of a clear red precipitate. A second titration was carried out by adding all honey sugar solution consumed in the previous titration, less 1 mL. The mixture of honey solution and Fehling solutions were brought to boiling. After 1–2 min, titration was continued until the end point was reached. The reading from the second titration was recorded and the percentage of total reducing sugars was calculated.
Accordingly, the percentage of total reducing sugar was determined by the equation
where the first procedure has been used:
where the second procedure has been used:
Where C, gram invert sugar per 100 g honey
W1, weight (g) of honey sample according to first procedure
W2, weight (g) of honey sample according to second procedure
Y1, volume (mL) of diluted honey solution consumed in the determination
carried out according to first procedure
Y2, volume (mL) of diluted honey solution consumed in the determination
carried out according to second procedure
2.4.2.1.2. HPLC method
HPLC determination of the sugar content was performed, as per the published method (Bogdanov, 2009) using the HPLC instrument, described at “the instrumentation and experimental conditions”, Section 2.2. Standard solutions of fructose (2 g%), glucose (2 g%), and sucrose (0.5 g%) were prepared in distilled water. The working sugar mixture solution was prepared by transferring 1 mL of each of standard solution of the three sugars to 10 mL volumetric flask and then the final volume was completed with distilled water. Sample preparation was carried out from the corresponding honey sample by dissolving 2 g of sample in 20 mL distilled water. An injection volume of 5 μL of each of the prepared standards and sample solutions were chromatographed using the HPLC conditions mentioned in Section 2.2.
Identification of sugars in the analyzed honey samples was made possible by comparing the resulting chromatograms with those obtained from standard sugar mixture, as prepared above. Peak identification was performed through retention time matching, along with spiking with reference standard of a particular sugar to assess the identification. Also, the use of DAD is a significant tool for assessing peak purity by comparing the absorption spectra and calculating peak purity. Moreover, quantitative determination of each sugar was accomplished by relating the peak area obtained for a particular sugar in the examined samples to that of standard sugar solutions.
2.4.2.2. Toxic element analysis
ICP method was used to analyze the toxic elements in the investigated samples. Standard solutions of As, Cd, Pb, and Hg (1000 ppm) were prepared in deionized water. Suitable dilutions from each element were made in water to get standard solutions of working concentrations 50, 200, and 500 ppb. For sample preparation, microwave acid digestion apparatus was used. Honey samples (up to 0.5 g) were separately digested in TFM vessels with a mixture of nitric acid (65%) and hydrogen peroxide (30%). The detailed procedure was described by Milestone Srl Company [www.milestonesrl.com-rev 04-09]. Analysis of suspected elements (As, Cd, Pb, and Hg) in honey samples was performed by comparing the response obtained for each element with the analytical response obtained from a set of standards of these particular elements, previously used to construct the concentration – based calibration graphs.
2.4.2.3. Detection of organic additives and foreign substances
GC/MS was used to identify the presence of organic additives and foreign substances using the above mentioned GC/MS instrumentation and chromatographic condition, as described at Section 2.2. All conditions were selected based on the reference method (Nurul Syazana et al., 2013). Honey Samples were extracted with dichloromethane/isopropanol. The extract was evaporated to dryness, reconstituted in 200 μL with the same solvent, and then analyzed under the conditions mentioned at Section 2.2. The identification of the mass spectra of tested substances was carried out in reference to NIST library. The resulting mass spectra were compared with those published in the library. In addition, interpretation of the mass spectral fragmentation pattern was helpful for the identification purpose.
3. Results and discussion
3.1. Physical properties
3.1.1. HMF detection
Determination of HMF in honey samples is a reflection of the freshness of the samples (Bogdanov, 2010). HMF is a deposition product of fructose. Increased HMF levels are associated with prolonged storage at high temperatures or over heating of honey samples. The acceptance level of HMF in honey samples is different among countries, being larger with hot tropical countries. Thus based on the Saudi Arabia standards (SSA102/1978), HMF level should not exceed 60 mg/kg. All investigated samples gave faint pink color for Fiehe's test indicating that all samples were within the required specifications of Codex Alimentarius Standard (CAC, 2001). Therefore, no deterioration was detected for all tested 24 samples.
3.1.2. pH measurement
Generally, honey is mildly acidic with an average pH of 3.9. This acidity is due to the minor acid content of honey, mainly amino acids and organic acids that are responsible for the characteristic taste of honey. It is also important to mention that honey from tropical countries is generally characterized by lower acidity. This is due to the water content of these samples resulting in increased fermentation with a further decrease in the pH value. Relatively more acidic values (pH < 3.24) indicate improper storage or impure samples.
All tested samples had pH values within the accepted range (3.24–6.1) with the exception of samples 2 and 4 which exceeded the specified range of natural honey stated by The Codex Alimentarius Standard (CAC, 2001) (Table 1). Thus, these two samples, presenting 8% of total samples examined, were considered not fully authentic as shown in Fig. 2.
Fig. 2.
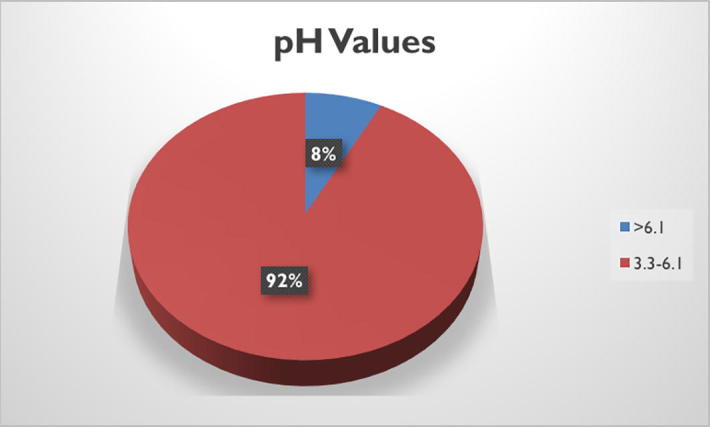
pH measurements for the 24 honey samples.
3.1.3. Pollen content
Examination of the honey samples revealed that only sample 8 had no pollen content and according to The Codex Alimentarius (CAC, 2001), no pollen should be removed because pollen is an essential tool in the analysis of honey. Species of pollen are generally used to indicate the floral nectar sources utilized by bees to produce honey. Fig. 3 and Table 3 show the bushes and pollen found under the microscope for the tested honey samples.
Fig. 3.
Bushes and pollen seen under the microscope for the most dominant pollen found in the study samples where, (A) Cichorium, (B) Ocimum, (C) Basilicum Propolis, and (D) Malva.
Table 3.
Microscopical examination of pollen grains present in honey samples.
| Sample number | Type of pollen grains |
|---|---|
| 1 | Cichorium, Ocimum, and Malva |
| 2 | Ocimum and Basilicum Propolis |
| 3 | Ocimum |
| 4 | Cichorium, Melissa and Ocimum |
| 5 | Ocimum |
| 6 | Malva |
| 7 | Cichorium, Melissa, Basilicum Propolis and Ocimum |
| 8 | Absent |
| 9 | Cichorium and Basilicum Propolis |
| 10 | Ocimum and Basilicum Propolis |
| 11 | Cichorium, and Basilicum Propolis |
| 12 | Cichorium, and Ocimum |
| 13 | Basilicum Propolis and Ocimum |
| 14 | Cichorium, and Ocimum |
| 15 | Cichorium, Hyacinthus Bacilicum Propolis and Ocimum |
| 16 | Ocimum |
| 17 | Cichorium, Syzygium, Basilicum Propolis and Ocimum |
| 18 | Cichorium, and Ocimum |
| 19 | Ocimum and Basilicum Propolis |
| 20 | Cichorium and Basilicum Propolis |
| 21 | Cichorium, and Ocimum |
| 22 | Cichorium, Basilicum Propolis and Ocimum |
| 23 | Cichorium, Basilicum Propolis and Ocimum |
| 24 | Basilicum Propolis and Sunflower |
3.2. Chemical properties
3.2.1. Determination of sugar content
Total content of reducing sugar cannot distinguish pure from adulterated honey samples. For this purpose, other quality measurements should be carried out (e.g. Glucose, fructose, sucrose content, and fructose/glucose ratio). The content of reducing sugar depends upon different factors (e.g. storage time and even time of collection of honey). The sugar content of honey is mainly fructose (∼38% w/v), glucose (∼31%), and to a lesser amount sucrose (∼1%). Fructose is the sugar responsible for the sweetness of honey while glucose content depends upon the source of nectar.
Although determination of the individual sugar content of either monosaccharides (glucose, fructose) or disaccharides (sucrose) is essential, significant qualification of honey samples should be accomplished by further determination of fructose to glucose ratio (Rybak-Chmielewska. 2007).
The problem of honey adulteration with syrups (e.g. maple syrup, sugar cane syrup) should be detected as recommended by the reference organization (CAC, 2001; SSA102/1978; Bogdanov, 2009). Based on a previous report (Rybak-Chmielewska, 2007, El Sohaimy et al., 2015), determination of each of the glucose content, fructose content, sucrose content, and fructose to glucose ratio, are among the most important trails used to distinguish syrup from honey. Another important problem in honey production is the crystallization of sugars in the comb cells. The crystallization problem could be detected by measuring glucose to fructose ratio since crystallization is accompanied with lower content of fructose and higher content of glucose (Rybak-Chmielewska, 2007). It is also noteworthy to mention that sucrose content in the honey sample is a significant parameter of quality assessment. Generally, the low content of sucrose in pure honey is due to the breakdown of the sucrose by the action of the invertase enzymes present in honey. Honey adulteration by the addition of sucrose syrups or over-feeding of bees with sucrose is very common. Thus, adulterated or over-heated honey samples are characterized by higher sucrose content (>8%) (Rybak-Chmielewska, 2007, El Sohaimy et al., 2015, Siddiqui et al., 2017).
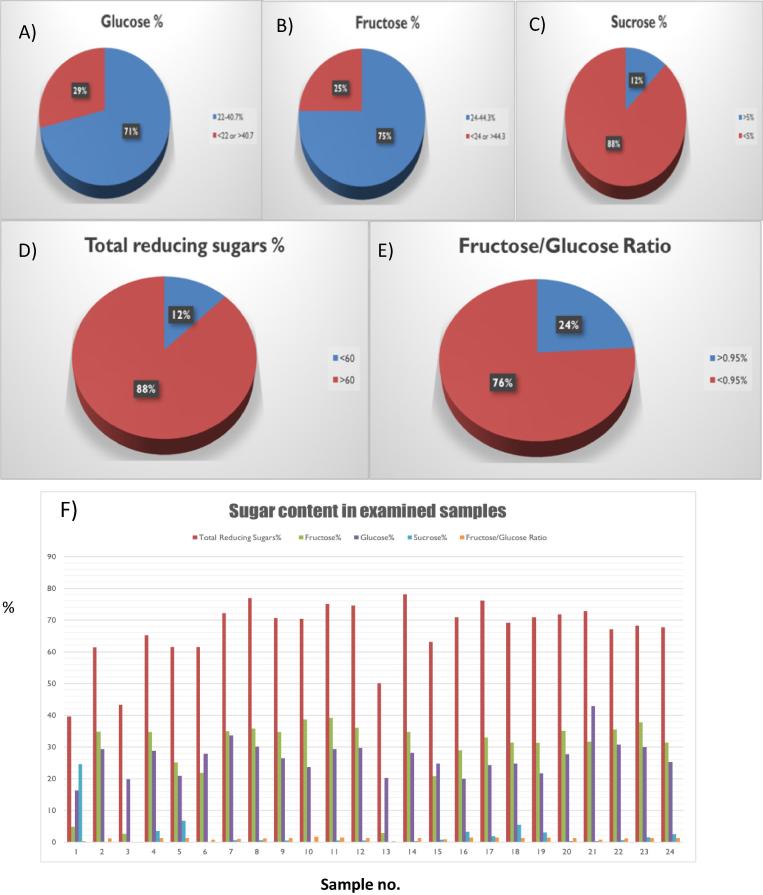
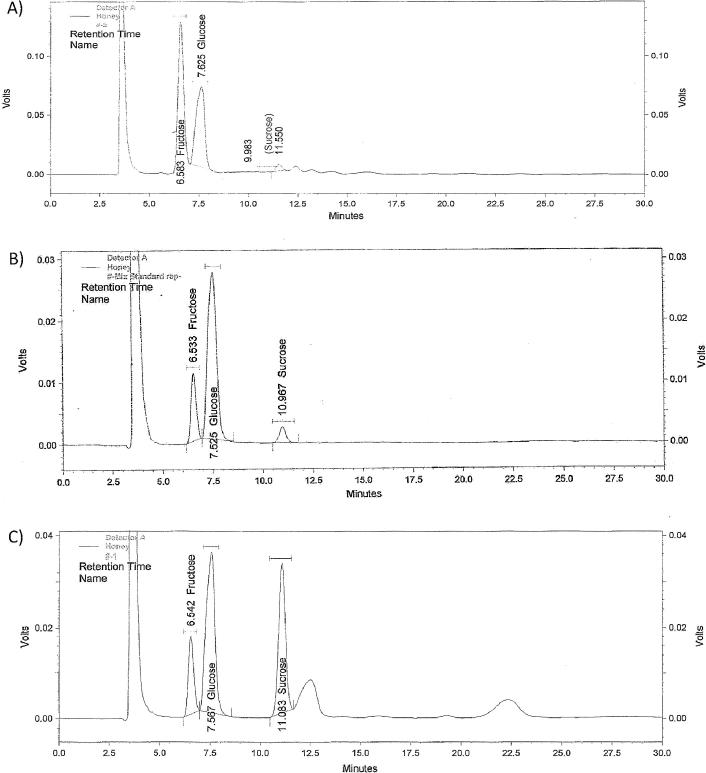
The percentage of the total sugar in the tested samples were calculated by Fehling‘s test and HPLC carbohydrates profile. Table 4 gives a detailed report of the results of sugar testing for all honey samples (1–24). It was found that the following samples: (1, 3, 5, 6, 13, 15, 16, 18, 19 and 21) had failed one or more of the sugar tests based on the specifications of natural honey as listed in Table 1, indicating their adulteration. On the other hand, the rest of the samples gave results within the required specifications, so they were considered natural honey. Fig. 4 shows a summary of the percentage of samples within/out of the specification limit for each sugar test. Fig. 5 shows representative HPLC chromatograms of a standard sugar mixture, along with those of samples 1 and 3.
Table 4.
Results of total sugar calculated by Fehling’s test and HPLC carbohydrates profile.
| Sample No. | Total reducing sugars% | Fructose% | Glucose% | Sucrose% | Fructose/Glucose ratio |
|---|---|---|---|---|---|
| 1 | 39.60 | 4.839 | 16.265 | 24.607 | 0.297 |
| 2 | 61.39 | 34.843 | 29.272 | 0 | 1.190 |
| 3 | 43.24 | 2.636 | 19.777 | 0 | 0.133 |
| 4 | 65.26 | 34.625 | 28.813 | 3.550 | 1.201 |
| 5 | 61.55 | 25.026 | 20.891 | 6.589 | 1.197 |
| 6 | 61.54 | 21.814 | 27.889 | 0 | 0.782 |
| 7 | 72.25 | 34.882 | 33.672 | 0.572 | 1.035 |
| 8 | 76.97 | 35.681 | 30.055 | 0.662 | 1.187 |
| 9 | 70.60 | 34.679 | 26.377 | 0.434 | 1.314 |
| 10 | 70.39 | 38.635 | 23.596 | 0 | 1.637 |
| 11 | 75.10 | 39.143 | 29.278 | 0.420 | 1.336 |
| 12 | 74.51 | 35.997 | 29.745 | 0.418 | 1.210 |
| 13 | 50.04 | 2.847 | 20.272 | 0 | 0.140 |
| 14 | 78.13 | 34.634 | 28.069 | 0.400 | 1.233 |
| 15 | 63.13 | 20.774 | 24.636 | 0.783 | 0.843 |
| 16 | 70.83 | 28.880 | 19.933 | 3.241 | 1.448 |
| 17 | 76.12 | 32.970 | 24.242 | 1.728 | 1.360 |
| 18 | 69.20 | 31.411 | 24.727 | 5.521 | 1.270 |
| 19 | 70.83 | 31.201 | 21.606 | 3.025 | 1.444 |
| 20 | 71.79 | 35.096 | 27.655 | 0.295 | 1.269 |
| 21 | 72.86 | 31.6 | 42.84 | 0.336 | 0.74 |
| 22 | 67.10 | 35.532 | 30.770 | 0.571 | 1.154 |
| 23 | 68.28 | 37.698 | 29.955 | 1.554 | 1.258 |
| 24 | 67.78 | 31.422 | 25.166 | 2.499 | 1.248 |
Fig. 4.
Summary of the results of sugar analysis in tested honey samples (1–24),% of samples within/out of the specified limits, (A) Glucose%, (B) Fructose%, (C) Sucrose%, (D) Total reducing sugar%, (E) Fructose/glucose ratio, and (F) Detailed sugar content in all examined samples.
Fig. 5.
Typical HPLC chromatograms of (A) standard mixture of fructose (0.2 g%), glucose (0.2 g%), and sucrose (0.05 g%), (B) prepared solution of sample 1, and (C) prepared solution of sample 2.
3.2.2. Elemental analysis
Elements are minor constituents of honey. The kind of these elements in honey is related to the type of raw floral materials, i.e., the nectar, the pollen, and the honey dew which are collected by bees. Metal concentrations in different honey types depend largely on the elemental composition of flowers, with regard to their botanical and geographical origin. These metals may also come from external sources such as industrial smelter pollution, industrial unit emissions, and improper procedures during honey processing and maintenance stages. Also, the metals in honey may originate from agrochemicals such as organic mercury, cadmium-containing fertilizers, and arsenic-based pesticides (Aghamirlou et al., 2015). Therefore, determination of metals in honey samples assists in quality evaluation.
The viscous and sugary nature of honey makes it a difficult substance for quantitative trace elemental analysis. A microwave-assisted digestion protocol was implemented in this study for the analysis of the four toxic contaminants (As, Cd, Pb, and Hg) by ICP (Thermo Fisher, 2016).
Results of elemental analysis of the honey samples are given in Table 4. It was noticed that only sample 19 had lead contamination with concentration exceeding the maximum limits allowed in honey according to Prevention of Food Adulteration (PFA) (Aghamirlou et al., 2015). Accordingly, this sample was not suitable for human use. The other samples were considered safe for human use due to no/trace amounts of the different heavy metals examined (arsenic, lead, cadmium and mercury). Table 4 summarizes the results of the elemental analysis for all honey samples.
3.2.3. Organic compounds and drug additives
It was indicated that the most encountered organic compounds found in honey samples belong to different chemical classes namely, oxygenated compounds (aldehyde, ketones, esters, alcohols, and carboxylic acids), hydrocarbons (aliphatic, aromatic, and cyclic), in addition to heterocyclic compounds (pyrol, quinoline, and furan derivatives). The presence of a particular type of organic compound in tested honey samples depends mainly on the enzymatic process in bees, as well as the type of flora in the nearby area. Furan derivatives are produced as a result of sugar degradation due to improper or prolonged storage at high temperatures. The presence of HMF is another indication of a breakdown of sugar as described at Section 2.2, due to improper or prolonged storage at elevated temperature. Moreover, honey adulteration with invert sugars could be reflected on the excessive high value of HMF (>150 mg/kg).
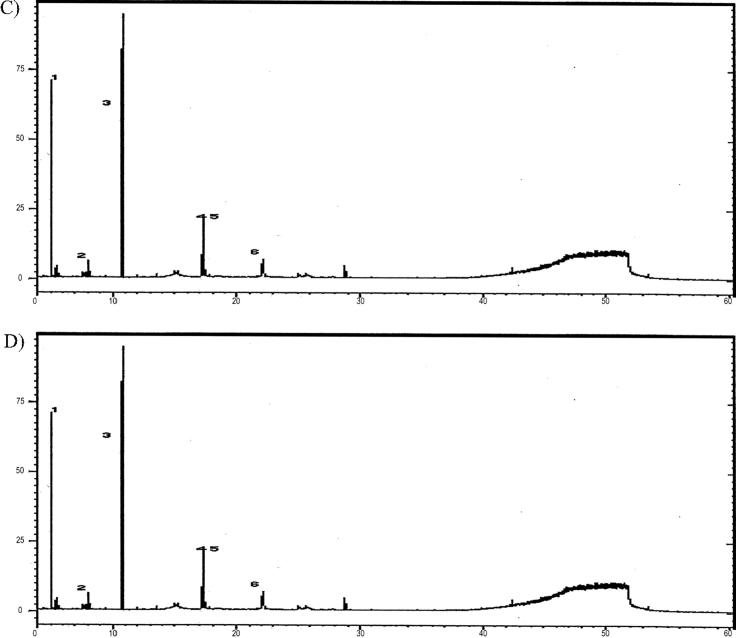
Testing the drug additives in honey samples were carried out in this study using GC–MS as mentioned at Section 2.2. Honey samples were free from drug additives except sample 3 which contained testosterone decanoate, a male sex hormone and an anabolic-androgenic steroid hormone. It is the most popular testosterone on the market present as a main constituent in athletic body builder food supplements to build muscles, burn fat, and enhance energy. However, it has many side effects and health hazards particularly with its illegal use without physician supervision. Among the most predominant testosterone-related toxicities are, salt and water retention, hepatic toxicity, atherosclerosis, aggressive depression, testicular atrophy and infertility, and my even result in sudden death (Applied Pharmacology-Book). The source of testosterone in this particular sample (sample 3) may be due to contamination with residual testosterone during processing or as a residue from male bees inside the hive (see Table 5).
Table 5.
Results of the elemental analysis for the honey samples by ppm.
| No. of sample | Results of the elemental analysis for the honey samples by ppm |
|||
|---|---|---|---|---|
| As | Pb | Cd | Hg | |
| 19 | Nl | 2.722 ppm | Nl | 0.0032 ppm |
| Rest of samples | Not more than 1.1 ppm | Not more than 2.5 ppm | Not more than 1.5 ppm | Not more than 1 ppm |
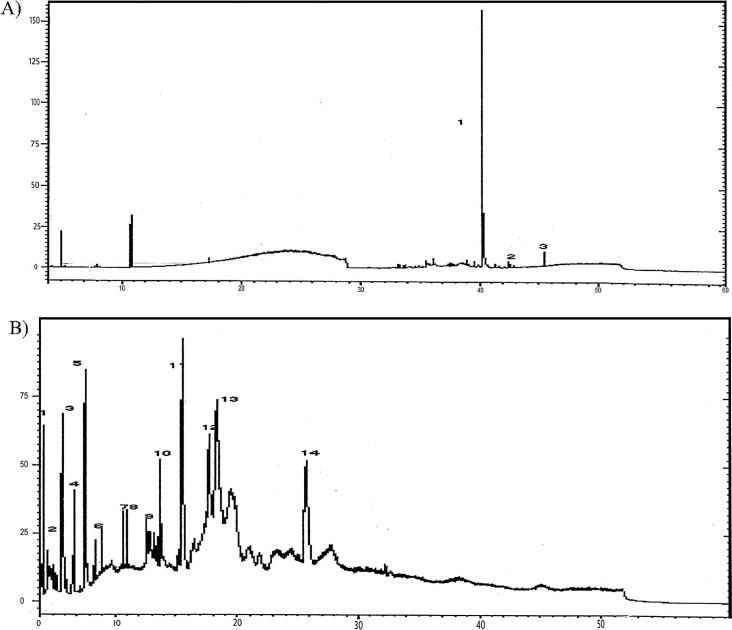
GC/MS profiling was recorded for all samples. A list of the organic compounds found in the tested samples was given in Table 6. Fig. 6 shows the GC–MS chromatograms of some representative samples. The results revealed that the same organic compounds were detected in samples 2 and 4. On the hand, sample 1 had different organic substances than those found in samples 2 and 4. In this respect, it is noteworthy to mention the feasibility of using GC–MS for the detection of different organic compounds available in the tested honey samples.
Table 6.
Additives detection test by GC/MS.
| Sample No. | Organic compounds |
|---|---|
| 1 | Triphrnylphosphine oxide, 2-(octadecyloxy)ethanol, 2,5-dihydroxyacetophenone |
| 2, 4 | Oxalic acid, furfural, 2-furanmenthol, propanoic acid, furanone, 1,2-cyclopentanedione, methyl propylthiophane, diethoxy-pyrroline, oxopentanoic acid, 3-pyridinol, hexanoic acid, 4-mercaptophenol, triacetin, trimehoxy phenol |
| 3 | Threonine, glyceraldehyde, arabinose, diethoxy-propane, dipropoxy-propane, maltol, 3- butylthiolane, phenol, 4-mercaptophenol, benzeneacetic acid, sucrose, dibutyl phthalate, testosterone decanoate |
| 5–24 | Propyl ester propanoic acid, diethoxy propane, dipropoxy propane, carbamic acid phenyl ester, 5-hydroxymethylfurancarboxaldehyde, sucrose |
Fig. 6.
GC chromatograms of tested samples, (A) sample 1, (B) sample 2, (C) sample 3, and (D) sample 5. The numbers on the peaks refer to the compounds identified in Table 5 in the same order.
3.3. Comparison of the proposed study with previous reports
Quality assessment of honey samples has gained much attention. Literature review revealed that many reports were concerned with evaluating the physical properties and/or performing chemical profiling of the investigated samples. One study conducted in Romania focused on measuring the physico-chemical properties (moisture, color, ash, and sugars content), besides the total flavonoids, phenolic content, and antioxidant activity honey samples (Al et al., 2009). In Slovenia, honey samples were investigated for their total phenolic content and antioxidant properties (Bertoncelj et al., 2007). Other studies used GC/MS to screen the volatile constituents of Tualang honey (Nurul Syazana et al., 2013) or Turkish honey (Bahaffi and Al-lihaibi, 2005). In a Greek study, the antioxidant properties and color intensity of unifloral honey were determined (Karabagias et al., 2016). Determination of the total phenolic and flavonoid contents in Burkina Fasan honey was also conducted (Meda et al., 2005). In addition, NMR was used as a fingerprinting technique to compare different honey samples (Siddiqui et al., 2017). Heavy metals were also previously determined in honey samples by ICP (Aghamirlou et al., 2015).
In Saudi Arabia, different studies have been conducted to qualify honey samples either by measuring only physicochemical properties (Ahamed et al., 2017, Mesallam and El-Shaarawy, 1987), physical properties and mineral content (Alqarni et al., 2014, Osman et al., 2007), or physical properties and phenolic content (Alqarni et al., 2016). Compared with the previous studies conducted in Saudi Arabia, our proposed study was concerned with performing a wide screening of honey samples available in the Saudi market. The proposed study is considered advantageous over the previous local studies since it focused on measuring the physicochemical properties (pH, hydroxylmethylfurfural HMF, and pollen test), as well as sugar profiling (by HPLC), elemental analysis (by ICP), and testing for the presence of drug additives (by GC–MS).
4. Conclusion
Despite the popularity of multiple nutrition and therapeutic uses of honey among the Saudi population, only a few reports have been conducted in Saudi Arabia dealing with studying the quality of honey. This is extremely important since the authenticity of honey is of great importance both from commercial and health aspects. In this work, 24 honey samples collected from Riyadh market were tested according to the Saudi Arabian standards (SSA102/1978), Codex Alimentarius Commission (CAC, 2001), and harmonized methods of the international honey commission (ICH, 2002). Physicochemical examination of all samples was performed for quality assessment. The physical properties tested include HMF, pH measurement, as well as pollen detection. The chemical testing included sugar composition, elemental analysis, and detection of drug additives. Detailed sugar profiling was carried out using HPLC analysis, as well as Fehling test for the determination of total sugar content. Elemental Analysis was performed using ICP, while detection of drug additives was carried out using GC–MS. The results revealed the importance of carrying out routine analyses testing for authentication of honey samples available in the Saudi market since some of the investigated samples failed to meet the specified criteria and are thus considered not safe for human use.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aghamirlou H.M., Khadem M., Rahmani A., Sadeghian M., Mahvi A.H., Akbarzadeh A., Nazmara S. Heavy metals determination in honey samples using inductively coupled plasma-optical emission spectrometry. J. Environ. Health Sci. Eng. 2015;13:39. doi: 10.1186/s40201-015-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed Mohammed E.M., Alfifi A., Aalmudawi A., Alfaifi M.Y., SeragEldin I.E., Al-bushnaq H.A. Some physiochemical properties of Acacia honey from different altitudes of the Asir Region in Southern Saudi Arabia. Czech J. Food Sci. 2017;35(4):321–327. [Google Scholar]
- Al M., Daniel D., Moise A., Bobis O., Laslo L., Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112(4):863–867. [Google Scholar]
- Alqarni A., Owayss A., Mahmoud A., Hannan M. Mineral content and physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014;18(5):618–625. [Google Scholar]
- Alqarni A., Owayss A., Mahmoud A. Physicochemical characteristics, total phenols and pigments of national and international honeys in Saudi Arabia. Arabian J. Chem. 2016;9(1):114–120. [Google Scholar]
- Bahaffi S.O., Al-lihaibi S.S. Determination of organic compounds in local honey by gas chromatography-mass spectrometer. Comm. Fac. Sci. Univ. Ank. Series B. 2005;51(2):1–12. [Google Scholar]
- BertoncelJ J., Dobersek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105(2):822–828. [Google Scholar]
- Bogdanov S., Martin P. Honey authenticity: a review. Mitt. Lebensm. Hyg. 2002;93:232–254. [Google Scholar]
- Bogdanov, S., 2009. Harmonised Methods of the International Honey Commission, http://www.ihc-platform.net/ihcmethods2009.pdf (accessed 12-2017).
- Bogdanov S. Nutritional and functional properties of honey. Vopr Pitan. 2010;79(6):4–13. [PubMed] [Google Scholar]
- Codex Alimentarius Commission, 1981. Revised Codex Standard for Honey Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001), Codex Standard, vol. 12, pp. 1–7.
- E-Book. Applied Pharmacology. In: Bardal, Stan, Waechter, Jason, Martin, Doug (Eds.), pp. 168. https://books.google.com.sa/books (Chapter 14).
- El-Bialee N., Sorour M. Effect of adulteration on honey properties. Int. J. Appl. 2011;1(6):122–132. [Google Scholar]
- El Sohaimy S.A., Masry S.H.D., Shehata M.G. Physicochemical characteristics of honey from different origins. Ann. Agric Sci. 2015;60(2):279–287. [Google Scholar]
- Ferreira I., Aires E., Barreira J., Estevinho L. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114(4):1438–1443. [Google Scholar]
- Hussein S., Yusoff K., Makpol S., Yusof Y. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules. 2011;16(9):6378–6395. doi: 10.3390/molecules16086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabagias I.K., Dimitriou1 E., Kontakos S., Kontominas M.G. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur. Food Res. Technol. 2016;242:1201–1210. [Google Scholar]
- Ladas S.D., Haritos D.N., Raptis S.A. Honey may have a laxative effect on normal subjects because of incomplete fructose absorption. Am. J. Clin. Nutri. 1995;62(6):1212–1215. doi: 10.1093/ajcn/62.6.1212. [DOI] [PubMed] [Google Scholar]
- Meda A., Lamien C., Romito M., Millogo J., Nacoulma O. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91(3):571–577. [Google Scholar]
- Mesallam A., El-Shaarawy M. Quality attributes of honey in Saudi Arabia. Food Chem. 1987;25(1):1–11. [Google Scholar]
- Nasir N., Halim A., Singh K., Dorai A., Haneef M. Antibacterial properties of tualang honey and its effect in burn wound management: a comparative study. BMC Complement. Alternat. Med. 2010;10(31) doi: 10.1186/1472-6882-10-31. http://www.biomedcentral.com/1472-6882/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurul Syazana M.S., Gan S.H., Halim A.S., Shah N.S., Gan S.H., Sukari H.A. Analysis of volatile compounds of Malaysian Tualang (Koompassia Excelsa) honey using gas chromatography mass spectrometry. Afr. J. Tradit. Complement. Altern. Med. 2013;10(2):180–188. doi: 10.4314/ajtcam.v10i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman K.A., Al-Doghairi M.A., Al-Rehiayani S., Helal M.I. Mineral contents and physicochemical properties of natural honey produced in Al-Qassim region, Saudi Arabia. J. Food, Agric. Environ. 2007;5(3/4):142–146. [Google Scholar]
- Rodriguez G.O., Ferrer B.S., Ferrer A., Rodriguez B. Characterization of honey produced in Venezuela. Food Chem. 2004;84:499–502. [Google Scholar]
- Rybak-Chmielewska H. high performance liquid chromatography (HPLC) study of sugar composition in some kinds of natural honey and winter stores processed by bees from starch syrup. J. Apicultural Sci. 2007;51(1):23–37. [Google Scholar]
- Sampath Kumar K.P., Bhowmik Debjit, Chiranjib Biswajit, Chandira M.R. Medicinal uses and health benefits of honey: an overview. J. Chem. Pharm. Res. 2010;2(1):385–395. [Google Scholar]
- Sivakesava S., Irudayaraj J. Classification of simple and complex sugar adulterants in honey by mid-infrared spectroscopy. Int. J. Food Sci. Technol. 2002;37(4):351–360. [Google Scholar]
- Siddiqui A.J., Syed Ghulam Musharraf S.M., Choudhary M.I., ur- Rahman A. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017;217:687–698. doi: 10.1016/j.foodchem.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam M. Topical application of honey in treatment of burns. Brit. J. Surgery. 1991;78(4):497–498. doi: 10.1002/bjs.1800780435. [DOI] [PubMed] [Google Scholar]
- Song X.Y., Yao Y.F., Yang W.D. Pollen analysis of natural honeys from the central region of Shanxi, North China. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermo Fisher Scientific. The Analysis of Trace Elements in Honey by Flame and Graphite Furnace Atomic Absorption Spectrometry Rebecca Price, Cambridge, UK. https://tools.thermofisher.com/content/sfs/brochures/AN-43060-Analysis-Trace-Elements-Honey-Flame-Graphite-Furnance-AAS.pdf (accessed 24/12/2017).
- Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21(5):242–247. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Ur-Rehman S., Farooq Khan Z., Maqbool T. Physical and spectroscopic characterization of Pakistani honey. Cien. Inv. Agr. 2008;35(2):199–204. [Google Scholar]