Abstract
Anxiety disorders are the most common mental illness in the US affecting 18% of the population. The cause(s) of anxiety disorders is/are not completely clear, and research in the neurobiology of anxiety at the molecular level is still rather limited. Although mounting clinical and pre-clinical evidence now indicates that oxidative stress may be a major component of anxiety pathology, whether oxidative stress is the cause or consequence remains elusive. Studies conducted over the past few years suggest that anxiety disorders may be characterized by lowered antioxidant defenses and increased oxidative damage to proteins, lipids and nucleic acids. In particular, oxidative modifications to proteins have actually been proposed as a potential factor in the onset and progression of several psychiatric disorders, including anxiety and depressive disorders. Oxidized proteins are normally degraded by the Proteasome proteolytic complex in the cell cytoplasm, nucleus, and endoplasmic reticulum. The Lon protease performs a similar protective function inside mitochondria. Impairment of the Proteasome and/or the Lon protease results in the accumulation of toxic oxidized proteins in the brain, which can cause severe neuronal trauma. Recent evidence points to possible proteolytic dysfunction and accumulation of damaged, oxidized proteins as factors that may determine the appearance and severity of psychotic symptoms in mood disorders. Thus, critical interactions between oxidative stress, Proteasome, and the Lon protease may provide keys to the molecular mechanisms involved in emotional regulation, and may also be of great help in designing and screening novel anxiolytics and antidepressants.
Keywords: Anxiety Disorder, Psychiatric Disorders, Antioxidants, Nrf2, Oxidative Stress, Proteasome, Lon protease, Inflammation
Introduction
1. How Oxidative Stress May be Associated with the Genesis of Anxiety
Traditionally, oxidative stress is defined as an imbalance between pro-oxidants and antioxidants, in favor of a pro-oxidant state [1, 2]. It is very clear that high concentrations of oxidants can damage cell components, including proteins, lipids and nucleic acids [3]. Such damage leads to severe physiological distress with impairment of normal cell functions, or even cell death [1] (Fig. 1). The brain represents only 2% of total body weight for human beings, but accounts for around 20% of our total oxygen consumption. Despite the high reliance on oxygen metabolism, the Central Nervous System (CNS) is especially vulnerable to oxidative stress due to several factors: 1) The limited regenerative capacity of neural cells, because adult neurons are post-mitotic cells that do not replicate [4]; 2) Intrinsic metabolic and structural characteristics of neurons make them more sensitive to oxidation compared to cells in other organs [5]; 3) Neuronal membranes are rich in polyunsaturated fatty acids, that make the brain more vulnerable to lipid oxidation[6]; 4) Several neurotransmitters, such as dopamine, norepinephrine, and serotonin easily undergo autoxidation [7]; 5) Brain microglia when activated inappropriately, can produce massive amounts of reactive oxygen and nitrogen species [8, 9]; and 6) CNS antioxidant defenses are relatively modest in comparison with those of other tissues. In particular, the CNS contains rather low levels of both glutathione peroxidase and catalase [5, 10].
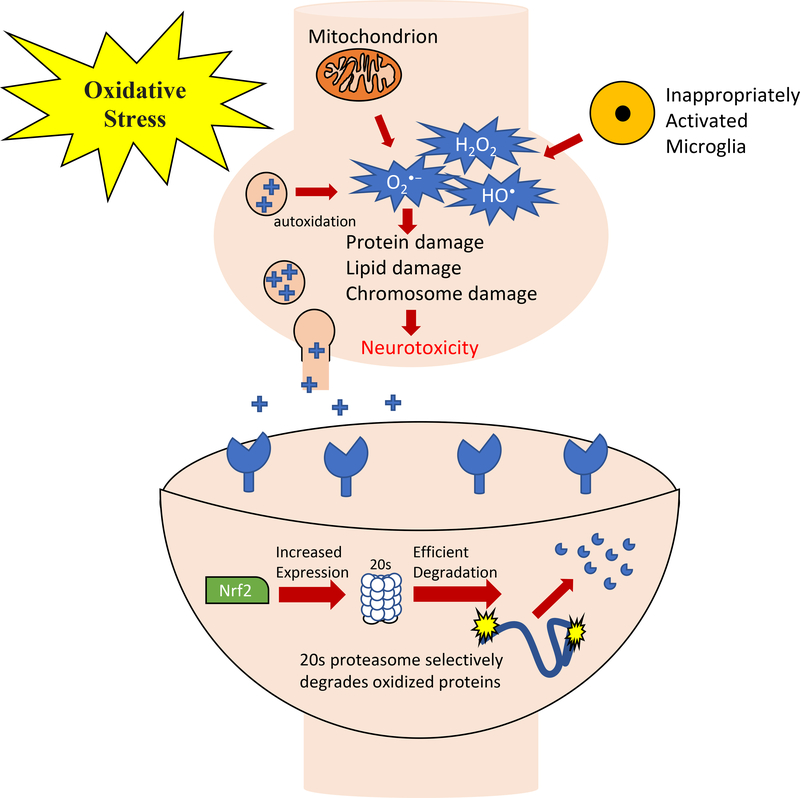
Figure 1. Neurotoxic effects of oxidative stress.
Neurotoxicity may occur through an elevation of superoxide anion (O2•−) by mitochondria, by autoxidation of neurotransmitters such as Dopamine (DA), or by inappropriately activated microglia, any or all of which may result in increased H2O2 levels, increased formation of the highly reactive hydroxyl radical (HO•) and superoxide (O2•−). Phospholipids and proteins, sugars, RNA and DNA are all susceptible to damage by HO• as are cell membranes, and both nuclear and mitochondrial chromosomes. These factors closely link hypotheses involving mitochondrial dysfunction, neuro-inflammation, oxidative stress, and the essential role of Nrf2 in protein degradation, through activation of the 20S Proteasome that selectively degrades oxidized proteins, and modulating macrophage activation in response to neuro-inflammation.
Current evidence connects oxidative stress to several psychiatric disorders, including anxiety and major depression, although the mechanism(s) and pathway(s) involved are not fully understood[11–13] (Fig. 2). Glutamate excitotoxicity is a major effector that causes oxidative stress in the CNS, and autoxidation of neurotransmitters can also generate reactive oxygen species such as the superoxide anion radical (O2•-)[14]. Therefore, oxidative stress could be a primary cause of neuropsychiatric disorders in some instances, or merely a downstream consequence in other cases.
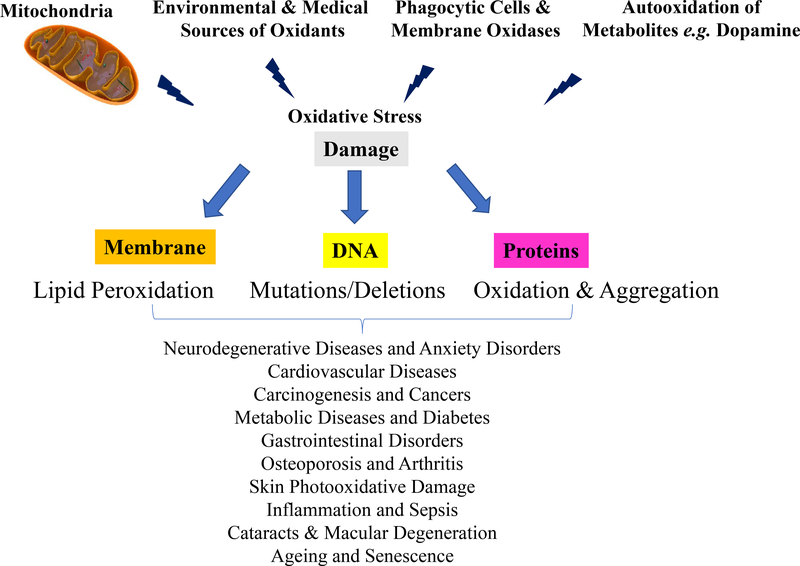
Figure 2. Damaging Effects of Oxidative Stress on Cell Structures and its Relation to Disease initiation/Progression, Ageing, and Senescence.
Oxidative stress arising (largely) from O2•− and H2O2 generated by mitochondria; by environmental and medical sources; by phagocytes such as astrocytes, glia, neutrophils, macrophages, monocytes, etc.; and by autoxidation of metabolites such as dopamine can damage cell structures. Phospholipids, both soluble and membrane-bound proteins, and nuclear and mitochondrial DNA, are easily damaged by oxidation which can lead to subsequent cellular malfunction, tissue dysfunction, and even organ failure. Ultimately, such molecular damage is thought to contribute to the initiation and/or progression of many age-related disorders and diseases that are among the major causes of morbidity and mortality in ageing populations (some of the major ones are shown at the bottom of the figure), and in the very processes of aging and senescence.
Several studies have reported that inhibition of the Proteasome may play a causal role in the neurotoxicity associated with oxidative stress, under conditions that may even be sufficient to induce neural death[15]. The Proteasome is the major proteolytic enzyme responsible for maintaining protein homeostasis (proteostasis) in the cell cytoplasm, nucleus, and endoplasmic reticulum, and plays a particularly important role in dealing with oxidatively damaged proteins [16–22] This functional understanding of the relationship between oxidative stress and anxiety disorder may pave the way for the discovery of novel targets for the treatment of neuropsychiatric disorders [23, 24].
In this review we examine some of the recent discoveries that link oxidative stress with anxiety disorder, particularly focusing on abnormalities at the molecular, mitochondrial, and immunological levels that may be associated with the onset and progression of neuropathology of anxiety, and how the Proteasome may be essential to understanding the neurobiology of anxiety disorder.
1.1. Oxidative Stress and the CNS -
Oxidative stress is an inevitable result of life in an oxygen-rich environment [25] Generation of reactive oxygen species is an aspect of aerobic life and the origin of a complex antioxidant system, synthesized by all known aerobic organisms [26] Since oxidative damage still occurs, however, aerobic organisms have also evolved complex damage removal and repair systems, [27, 28] as well as complex mechanisms to transiently elevate their defense and repair ‘armories’ via processes such as adaptive homeostasis [29–31].
1.1.1. Source of Free Radicals -
In normal mitochondria, oxygen is reduced to water by the cytochrome c oxidase complex (Complex IV) in four consecutive one-electron steps, because molecular oxygen possesses a triplet state configuration (Fig. 3). Release of partially reduced oxygen intermediates does not occur during this process because of the high binding affinity of cytochrome c [27]. However, some 1–2% of the molecular oxygen consumed during normal physiological respiration is converted into O2•- by electron ‘leakage’ to oxygen at other sites in the mitochondrial electron transport chain, such as complexes I and III [7, 27]. The O2•- may also be formed by other cellular sources, such as xanthine oxidase, membrane-bound NADPH oxidases, cytochrome P450 in the endoplasmic reticulum, and flavin oxidases inside peroxisomes [32].
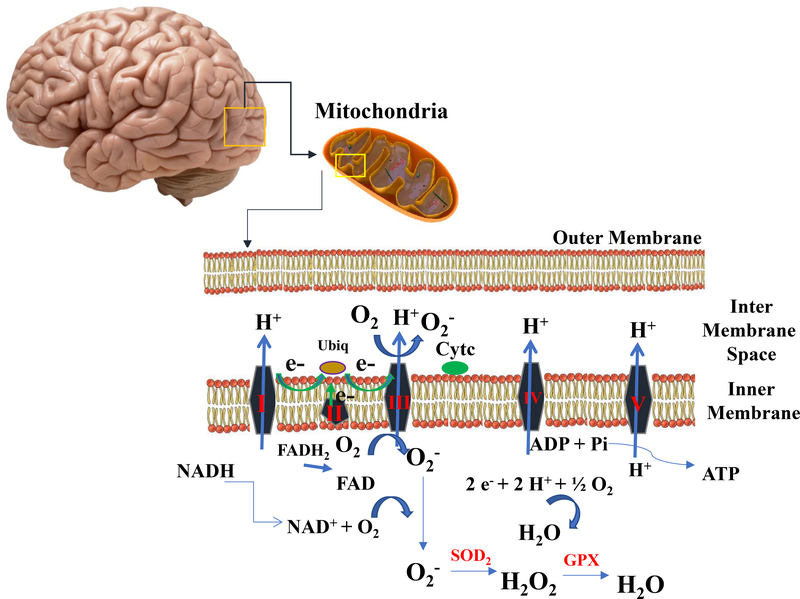
Figure 3. Formation and Neutralization of Reactive Oxygen Species in the Mitochondrial Electron Transport Chain (ETS).
The Krebs cycle is a series of enzymatic reactions that provide electrons (from pyruvate via acetyl CoA) to the ETS in the form of NADH and FADH2. These electrons then undergo vectorial transport along the ETS, generating an electrochemical energy gradient by which ADP can be phosphorylated to ATP at complex V. In order to maintain electron flow (and ATP generation) electrons must ultimately be ‘removed’ from the ETS, and this is accomplished at Complex IV (cytochrome oxidase) where the electrons reduce oxygen to water in four consecutive (but concerted) one-electron steps. Although the whole process is really rather efficient, some 1–2% of the molecular oxygen consumed during normal physiological respiration is reduced in one-electron side reactions (mostly at Complex ‘s I and III) into the superoxide anion radical, O2•- (also commonly just called ‘superoxide’). The O2•- so generated is almost immediately dismutated to hydrogen peroxide (H2O2) by superoxide dismutase (SOD) and, H2O2 can then be removed by the enzyme, glutathione peroxidase (GPX).
Intracellular superoxide dismutase (SOD) in its two forms (Cu-Zn-SOD localized in the cytosol and Mn-SOD localized in the mitochondrial matrix) is responsible for the dismutation of O2•- to hydrogen peroxide (H2O2) [27]. The H2O2 generated by Mn-SOD can be removed by glutathione peroxidase in the mitochondria or, if it diffuses into the cytosol, H2O2 can be removed by cytosolic antioxidants, such as catalase (CAT), and peroxiredoxins (Prx), that act in conjunction with SOD. Although it is often cited as an important cellular antioxidant enzyme, catalase in mostly found within peroxisomes; therefore, although catalase is certainly an important antioxidant enzyme for peroxisomes, its antioxidant value to other parts of the cell is less clear. [33]
Under conditions in which mitochondrial O2•− generation is significantly increased, however, or when antioxidant systems are depleted, H2O2 may accumulate and lead to a state of mitochondrial oxidative stress. In this situation, H2O2 may react with mitochondrial Fe2+, resulting in the formation of the highly oxidizing hydroxyl radical (HO•), via the Fenton reaction[7]. This can cause severe damage to cell structures including membrane phospholipids, proteins, and both nuclear and mitochondrial DNA, and also cause inactivation of membrane receptors, enzymes and ion channels [34]. Under such conditions, changes may occur in neurotransmitter systems and brain activity, leading to a series of cognitive and behavioral alterations commonly observed in several psychiatric disorders [35, 36]. However, the identification of microenvironments affected by oxidative stress and specifically associated with discrete mental disorders has proven to be a challenging task. In vivo molecular imaging techniques, combined with positron emission tomography (PET) have been successfully used to elucidate neuropathological brain changes and their relation to schizophrenia [37]. These techniques may also help to elucidate pathophysiological brain changes associated with specific anxiety disorders in the future.
1.2. Neurobiology of Anxiety Disorder and Oxidative Stress –
Normal levels of anxiety can have a great adaptive value, developing the alert signal that triggers behavioral, physiological, and cognitive changes, that allow us to deal with novel situations or threats [38]. Nevertheless, the persistence of a high alert state, without corresponding risk circumstances, may cause an individual to develop maladaptive responses to real stress, with the manifestation of syndromes such as generalized anxiety disorder, panic disorder, agoraphobia, other phobias, and social anxiety disorders [39]. Similarly, the ability of the body to maintain homeostasis in response to stressors, allostasis, has great benefit, however when severity or frequency of stress is too great the response systems become overloaded, which leads to pathophysiology or allostatic load/overload [40]. This illustrates the necessity of health-promoting behaviors and practices that can help maintain allostasis over the long term to avoid allostatic load.
1.2.1. Allostatic Load/Overload leads to Neuropathology –
When experiencing an approaching threat, or in anticipation of a threatening experience, the hypothalamic-pituitary-adrenal (HPA) axis stimulates a response mediated by glucocorticoids (also known as stress hormones) to increase the organism’s fitness to cope with the threat [41, 42]. As a protective acute-phase response system this is highly effective and efficient in maintaining allostasis. However, the long-term maintenance of such a defensive state requires an additional physiological cost that can overburden the system and lead to allostatic load/overload. Increasing evidence indicates that hypersecretion of glucocorticoids and dysregulation of glucocorticoid receptor function is involved in the pathogenesis of anxiety disorders [43]. Postmortem studies have revealed that oxidative damage to limbic structures is responsible for modulation of anxiety behavior [44]. For example, the HPA axis is activated during stress responses, and can induce significant hippocampal cellular oxidation [45]. In parallel, clinical evidence shows that patients with panic disorder and obsessive–compulsive disorder, have elevated levels of oxidized compounds in their peripheral blood, red blood cells, mononuclear cells, urine and cerebrospinal fluid [46]. Elevated glucocorticoid levels are associated with an increase in oxidant production and therefore elevated oxidative damage [47], and should, therefore, be considered a possible mediator between oxidative stress and anxiety disorders.
Glucocorticoids may also directly affect mitochondrial metabolism and may regulate mitochondrial bioenergetics in rat liver mitochondria [48, 49]. Recent studies have revealed that glucocorticoids modulate mitochondrial calcium homeostasis and the generation of oxidants. [50]. Translocation of glucocorticoids into mitochondria via the glucocorticoid receptor can modulate mitochondrial gene expression [51, 52]. Although the mechanism of this regulation remains unclear, recent studies indicate that release of cytochrome C and calcium from mitochondria is altered when rat brain cells are treated with corticosterone. Regulation of mitochondrial function by corticosterone appears to correlate with neuroprotection; that is, treatment with low doses of corticosterone had a neuroprotective effect, whereas treatment with high doses of corticosterone was toxic to cortical neurons [53, 54]. These results may ultimately contribute to a better understanding of the mechanisms by which glucocorticoids and stress regulate cellular plasticity and maintain allostasis [40], and to the future development of improved therapeutics.
1.2.2. Animal Models link Oxidative Stress and Neuropathology –
Animal studies have been quite useful in clarifying the role of oxidative stress in anxiety-like behaviors (e.g. model mammal systems such as mice and rats) [55–59]. Hovatta et al. (2010), were the first to demonstrate a link between expression of antioxidant defense system genes in the brain, and anxiety-like behaviors in six different mice strains [13]. Berry et al. (2008) showed that deletion of the p66Shc gene, responsible for the regulation of certain reactive oxygen species, results in lower levels of oxidative stress, and reduced anxiety-like behavior in mice evaluated with the elevated plus-maze test [60]. Desrumaux et al. (2010) demonstrated that decreased vitamin E levels and increased levels of central oxidative stress markers, such as cholesterol oxides and cellular peroxides results in anxiogenic behavior in the mice [61].
Several studies have reported a role for oxidative stress in anxiety-like-behaviors in rodents [62–65]. For example, Souza et al (2007) showed that a highly palatable diet (enriched with sucrose) increases protein oxidation in the frontal cortex and appeared to induce anxiety-like behavior in rats [66]. Furthermore, inhibition of GSH synthesis by administration of L-buthionine-(S,R) sulfoximine directly into the mouse hippocampus induced an anxiety-like behavior [67]. In sum, several studies in animal models suggest that genetic or pharmacologic alterations of the redox balance produce behavioral changes related to anxiety disorders. Treatment with antioxidants appears to prevent many of these effects [68–71].
Finally, the evidence presented above might situate glucocorticoid and mitochondrial oxidative interactions as the “Rosetta stone” with which to translate stress hormone effects into psychiatric disorders, mediated by the oxidation of key elements at neurons, astrocytes, or even glial cells. Since many anxiety disorders are an inappropriate cognitive response to a non-threatening environment (though there are notable exceptions), the mechanisms that contribute to the physiopathology of anxiety disorder are important to clarify fully.
2. Oxidative Stress, Antioxidants and Selective Serotonin Reuptake Inhibitors (SSRIs)
2.1. Neurotransmitters and SSRIs –
Predominantly, studies about anxiety disorder have been focused on the regulatory systems, including gamma-aminobutyric (GABA) acidergic and serotoninergic systems [72, 73]. GABA is the principal inhibitory neurotransmitter of the CNS, and is crucial for maintaining homeostasis by counterbalancing the neuronal excitability that characterizes anxiety disorder [74].
When first introduced benzodiazepines, which are selective agonists for the gamma-aminobutyric acid–A receptor (GABA–A), were initially considered to be first-line treatments for anxiety because of their tolerability and rapid mode of action. However, benzodiazepines carry the risk of dependence, sedation and tolerance [75]. A Posteriori evidence from numerous preclinical and clinical studies, suggesting that dysfunction in serotonergic neurotransmission could have a role in the pathophysiology of anxiety disorder, culminated in the classical serotonin hypothesis of anxiety [73]. Subsequently, studies with agents that targeted particular molecular systems, such as the selective serotonin reuptake inhibitors and the serotonin and noradrenaline reuptake inhibitors, constituted another important step as treatments for anxiety disorder [76]. Despite advantages to the introduction of the monoaminergic modulator, the treatment needs several weeks before a therapeutic effect can be observed and the efficacy and duration of relief have not actually improved in most cases [77].
Given the enormous contribution of anxiety disorders to the burden of human disease, it is key to optimize their prevention and treatment. At the moment, more focused development of medications with selective mechanisms of action, followed by rigorous clinical trials to quantify their efficacy and safety are sorely needed [78]. A number of studies have suggested that an imbalance between oxidative stress and antioxidant defenses may be associated with the development of neuropsychiatric disorders, such anxiety and depression [79–82].
2.2. Antioxidant Effects on Pathophysiology –
Oxidative stress is increased in anxiety disorder, and some have suggested that antioxidant therapy may be useful as a treatment, alongside classical medications [83]. Various forms of antioxidants, such as Vitamins E and C, creatine, and CoQ10 have been tested for their neuroprotective potential [83]. Vitamin E deficiency can affect the mitochondrial permeability transition pore and lead to dopaminergic neurotoxicity[84]. On the other hand, administering vitamin E reportedly ameliorated oxidative stress induced by iron accumulation in the mouse brain [85]. A neuroprotective role for CoQ10 was also seen in cultured human dopaminergic neurons where iron-induced cellular damage, mediated by pro oxidants, was attenuated[86]. Similarly, vitamin C ameliorated energy depletion and apoptosis, caused by glutamate-induced excitation, in the hippocampus of developing rat brains [87]. Dietary creatine supplementation was able increase brain creatine concentrations in Huntington’s disease transgenic mice to wildtype levels and resulted in neuroprotective effects [88]. Antioxidant therapies appear to be receiving increasing attention in clinical neurology, and a number of large randomized controlled trials have been initiated.
Interestingly, antioxidant effects of conventional antidepressants have also been reported in several studies [89, 90]. For example, studies have shown that modulation of serotonin may also affect the levels of oxidants in the brain [91]. Battal et al[91] suggested that modulation of serotonin levels by fluoxetine, a selective serotonin reuptake inhibitor, decreases the levels of oxidative stress and is also accompanied by a reduction in anxiety levels as measured by the elevated plus-maze test. The same study also reported that fluoxetine caused an increase in antioxidant enzyme capacity, as measured by increased catalase and glutathione-S-transferase activities in rat hippocampus [91]. Further work indicated that chronic administration of fluoxetine was capable of significantly decreasing oxidative damage in the cerebral cortex and hippocampus of the stressed animals [92]. A similar effect has been observed following administration of several antioxidants used in clinical trials of neurodegenerative diseases [93, 94], and psychiatric disorders such as depression and anxiety disorders [95–97]. A recent systematic review performed by Cipriani et al., revealed that there is variability in the efficacy of antidepressants that may be rooted in the different mechanisms of action[98]. How antioxidant effects factor into drug efficacy would be an interesting research question that would greatly inform the clinical administration of these antidepressents.
The mechanism(s) of action of antioxidants at the CNS is/are not well elucidated, however, one popular hypothesis suggests that antioxidants may exert their antidepressant effects similarly to conventional antidepressants, by increasing the availability of serotonin and noradrenaline in the synaptic cleft [99, 100]. It has also been noted that some polyphenols have antioxidant properties that may underlie the anxiolytic-like effects that several of them produce in in rodents [101]. Thus, there is significant evidence to suggest the relevance of pharmacological interventions focusing on cellular oxidation as a promising strategy for auxiliary, or possibly even primary, treatment of anxiety disorder.
3. Oxidative stress, Inflammation and Microglia
Several clinical studies have reported that neuroinflammation plays a role in the pathogenesis of neurological disorders, such as anxiety, depression and neurodegenerative diseases [102]. Microglia are macrophages that represent the primary immune cells of the CNS, and they play an important role in initiating and mediating neuroinflammation [103]. Furthermore, microglia are involved in the modulation of various neurological functions, such as immunological, neurochemical, neuroendocrine, and behavioral activities [104]. In fact, it has been demonstrated that microglia participate in neurogenesis [105], neuronal transmission [106], and neuronal plasticity [107].
Abnormal activation of microglia may produce high levels of inflammatory molecules such as tumor necrosis factor-α, interleukin-1β, and Nuclear Factor Kappa B (NF-κB) [108]. The release of proinflammatory cytokines can contribute to the development of neuropsychiatric disorders, such as depression and anxiety disorder [109], although the cellular mechanisms responsible for initiating these processes during the stress response remain poorly understood. An important and detrimental consequence of increased cytokine production is the increased generation of reactive oxygen species (that can cause tissue damage and even cell death) such as O2•- whose main source in microglia is NADPH oxidase[110]. The NADPH oxidase complex is also a major source of intracellular pro-oxidant generation in both macrophages and neutrophils. Neutrophils are involved in host-defense responses by oxidation of crucial cellular signaling proteins. The pro-oxidants act as both signaling molecules and mediators of inflammation [111]. The increased microglial cell numbers associated with neuroinflammatory states have recently been found to depend on changes induced by different psychogenic stressors including increased glucocorticoids levels [112]. Several researchers have suggested that an acute stress situation could cause the high levels of inflammation seen in anxiety, so it is possible that dysregulation in cytokine signaling could lead to anxiety disorder and cognitive dysfunction [113–115].
The results of pharmacological strategies to suppress abnormal microglial activity support the involvement of these cells in the development of disease-induced neuroinflammation and behavioral alterations [116]. For example, Fluoxetine, a selective serotonin reuptake inhibitor, affords robust neuroprotection in the post-ischemic brain through its anti-inflammatory effect [117]. Recently, fluoxetine has demonstrated several beneficial effects on ischemic stroke patients [118] as well as several animal models of stroke [119]. Similarly, Propranolol, a β-adrenergic receptor antagonist was capable of attenuating anxiety-like behaviors induced by brain proinflammatory profile (infiltration of peripheral macrophages into the brain and microglial activation) [120]. These results suggest that modulation of microglial proinflammatory profiles could be responsible for anxiolytic effects.
4. Interactions Between Nrf2, Proteasome, and Anxiety Disorder.
As discussed above, the mechanisms involved in changing microglial activity from beneficial to chronic detrimental neuroinflammation are not always clear. However, it is known that there is an increase in oxidant production and several studies have shown that the nuclear factor erythroid 2 related factor 2 (Nrf2), guardian of redox homeostasis, has an essential role in modulating macrophage activation in response to neuroinflammation [121–123]. Innamorato et al., showed that Nrf2 knockout mice were hypersensitive to the neuroinflammation induced by lipopolysaccharides (LPS) [124]. The chronic intraperitoneal administration of LPS induced the increase of several markers of inflammation, such as IL-6 and TNF-alpha [121].
4.1. Nrf2 and Neuropathology –
Nrf2 plays a key role in neuronal resistance to oxidative stress and glutamate-induced excitotoxicity [125]. Various studies show that Nrf2 has neuroprotective effects against oxidative damage injury following cerebral ischemia/reperfusion in rats [126, 127]. Furthermore, Nrf2 deficiency may affect the psychological behavior and neurotransmitter systems in mice, such as reduced mobility in swimming tests, possibly by increasing dopaminergic and serotonergic neurotransmitters[128]. The pre-activation of Nrf2 by electrophilic agents protects cells, partially through enhanced H2O2 scavenging by the glutathione/glutathione peroxidase system, and the detoxification of reactive quinones by NAD(P)H:quinone oxidoreductase 1 [129]. Still, excessive extracellular dopamine itself can be an endogenous signal to activate Nrf2-dependent neuroprotective pathways [129]. Studies with primary cell cultures have also revealed that excessive dopamine release can act as an endogenous Nrf2-inducing signal [130].
Recently, a clinical study reported increased activation of Nrf2 in peripheral blood mononuclear cells of patients with depression, which indicates a pro-oxidative state [131]. In fact, the chronic fluoxetine treatment suggested that fluoxetine-induced neuroprotection may operate via an unexpected mechanism involving 5-HT (serotonin receptor) and a serotonin transporter blockade with Nrf2 signaling. However, the contribution to CNS function remains to be elucidated [132]. It is actually not clear which pathway connects Nrf2 regulation and brain damage in anxiety disorder. Considering the regulation of antioxidant defenses through the Nrf2 pathway, this factor has emerged as a promising approach for neuroprotection, and it is possible that Nrf2 may also play an important role in the regulation of brain inflammation via interactions with NF-κB. Nrf2 clearly has vital functions in various physiological and pathological stresses, and it has been implicated as a causative factor in the pathophysiology of many psychiatric disorders, including anxiety disorder [133].
4.2. Nrf2 and Proteasome Activation –
As previously mentioned, Nrf2 is an important component of the responses to oxidative stress by binding to electrophile responsive element, also called antioxidant response element (EpRE/ARE), sites on numerous target genes. Such binding promotes the synthesis of several antioxidant enzymes, as well as enzymes that are responsible for the repair and/or removal of oxidized cell components that allow restoration of normal cell function [134] (Fig. 1). One such enzyme is the Proteasome proteolytic complex that is comprised of multiple protein subunits, encoded by multiple different genes. Proteasomes are crucial proteolytic enzymes in eukaryotes that are the primary guardians of proteostasis in the cell cytoplasm, nucleus, and endoplasmic reticulum.[135] The 26S Proteasome recognizes and degrades poly-ubiquitinylated proteins (ubiquitin-tagged for degradation by a series of ubiquitin E1 activating, E2 conjugating, and E3 ligating enzymes) in a process that requires multiple steps of ATP hydrolysis. The 26S Proteasome, along with ubiquitin and the E1, E2, and E3 enzymes are collectively referred to as the Ubiquitin-Proteasome System (or UPS). During periods of homeostasis, cells primarily rely on the 26S Proteasome to maintain proteostasis. The 26S Proteasome is formed from the addition of a 19S subunit to each of the α rings of the 20S Proteasome, in an ATP-dependent manner[136]. Under oxidative stress conditions, however, the 26S Proteasome is transiently ‘dismantled or disasembeled’ by Ecm29 and HSP70 [137–139] and the resulting 20S Proteasome (+/− 11S activator) and the Immunoproteasome (+/− 11S activator) are primarily responsible for degrading oxidatively damaged proteins in a process that does not utilize ATP or ubiquitin [16–22].
Under homeostatic conditions, Nrf2 is under tight regulation by the UPS [18, 140]. In the absence of stress, Nrf2 is bound to Keap1which also has a Cul3 E3 ubiquitin ligase attached to the complex. Thus, under normal, non-stressful, conditions, the Keep1-Cul3 complex promotes the ubiquitinylation of Nrf2 and its consequent degradation by the 26S Proteasome, thus keeping the cellular levels of Nrf2 very low. When oxidative stress occurs, however, and the 26S Proteasome is disassembled by Ecm29 and HSP70, Nrf2 can no longer be degraded and its levels rapidly rise [137–139]. Nrf2 also undergoes phosphorylation at multiple sites [141] and is then able to translocate into the cell nucleus where it binds to EpRE sequences (also called ARE sequences) of target genes to activate their increased transcription[142].
4.3. Proteasome Dysfunction and Neuropathology –
Dysfunction of Proteasomes, or other components of the UPS, may be related to deficits in the clearance of misfolded cytoplasmic, nuclear, and ER proteins, leading to intracellular protein aggregation, cytotoxicity, and cell death [143, 144]; such dysfunction(s) is/are also associated with several neurological diseases, including Alzheimer’s disease, Amyotrophic Lateral Sclerosis (ALS), Parkinson’s disease, and Huntington’s disease [145]. The Proteasome has been shown to lose its effectiveness and responsiveness under chronic, repeated, or sever stress regimes [30, 146]. Similarly, dysfunction of the Lon protease in mitochondria (which protects mitochondrial integrity by selectively removing oxidized intramitochondrial proteins) has been associated with a number of neurological disorders [147–150]. Recently, Gragnoli et al., [151] reported that PSMD9, a protein subunit of the 26S Proteasome complex, could potentially contribute to generalized anxiety disorder, but the underlying cause(s) of these changes is/are not known. However PSMD9 does seem to be associated with a significant clinical response to desipramine, a drug used for generalized anxiety treatment [151].
Proteasome dysfunction occurs with aging. With age, the activity and inducibility of the Protease decreases [152]. With the loss of Proteasome activity, negative oxidative outcomes likely become more prevalent and may magnify the negative outcome of anxiety disorders. Similarly, anxiety is also associated with accelerated aging (CITATION). The age-related dysfunction of the Proteasome potentially contributes to the neuropathology associated anxiety dependent accelerated aging.
A number of animal studies have shown increased levels of protein degradation in limbic structures, such as the frontal cortex and hippocampus, in anxiety-like rat models [57, 153–155] and it is well known that oxidative stress can cause significant protein modification and damage. Better understanding of the relationships between protein damage and protein turnover in anxiety disorders may help to provide new therapeutic targets for the development of future drugs.
5. Conclusions
Although there is evidence supporting the involvement of oxidative stress in the pathophysiology of anxiety disorders, the discussion is still open as to whether oxidative stress is the cause or consequence of anxiety. However, the relationship between oxidative stress and anxiety disorders seem more evident in disorders associated with inflammation. In these cases, dysregulation of physiological pathways results in oxidative stress that leads to neuroinflammation and the subsequent manifestation of an anxiety disorder. Further evidence of this link is the fact that effective anxiolytics, such as Fluoxetine decrease inflammation to exert their antianxiety effects. Factors such as neurodevelopment, epigenetic modulation, the neuroendocrine system, the immune system, and the effects of exposure to oxidants, appear to compose the repertoire of elements involved in neuropsychiatric pathogenesis. Animal models of anxiety have produced bidirectional data, and provided important clues to help understand the potential molecular mechanisms associated with, or directly involved in, anxiety-related disorders. In particular, the Nrf2–Proteasome-signaling pathway may provide valuable insights for therapeutic interventions capable of restoring Nrf2-mediated redox homeostasis. Nevertheless, research in neurobiology of anxiety at a molecular level suggests antioxidant therapy for anxiety disorders in humans may be premature, while other reports in the literature suggest a more optimistic potential for antioxidant therapy. The combined use of antioxidants and classic anxiolytics may actually be promising for anxiety-like disorders, but further research is clearly needed to test and validate these new therapies.
Whether oxidative stress is the cause or consequence of anxiety disorders is complex and likely depends on a complex interaction between the specific anxiety disorder, environment, and individual physiology. However, recent focus on Nrf2 and the Proteasome offer promising new avenues for maintaining allostasis and avoiding allostatic overload associated with anxiety disorders. In contrast to antioxidant therapies that limit oxidative load on biological systems, Nrf2-Proteasome focused therapies would aim to maintain the body’s innate defense systems. Clinical applications focused on reinforcing the body’s existing oxidative defense mechanisms, such as Nrf2 and the Proteasome, in conjunction with existing therapies may increase the efficacy of treatments for anxiety disorders.
Acknowledgments
6. Funding and Disclosure
AdGF was supported by a postdoctoral fellowship from the Conselho Nacional de Desenvolvimento Cientifico e Technologico (CNPq) of the Ministry of Science, Technology, and Innovation of Brazil. KJAD was supported by grant # ES003598 from the National Institute of Environmental Health Sciences of the US National Institutes of Health, and by grant # AG 052374 from the National Institute on Aging of the US National Institutes of Health. The authors declare that no competing financial interests exist.
7. List of abbreviations:
- ALS
Amyotrophic Lateral Sclerosis
- CAT
catalase
- CNS
Central Nervous System
- Cu-Zn-SOD
cytosol mitochondrial superoxide dismutase
- EpRE/ARE
electrophile responsive element/antioxidant response element
- GABA
gamma-aminobutyric acid
- GABAergic
gamma-aminobutyric acidergic
- H2O2
peroxide hydrogen
- HPA
hypothalamic-pituitary-adrenal
- MAOI
monoamine oxidase inhibitors
- Mn-SOD
matrix of mitochondrial superoxide dismutase
- NF-κB
Nuclear Factor Kappa B
- Nrf2
factor erythroid 2 related factor
- O2
anion superoxide
- Prx
peroxiredoxins
- PET
positron emission tomography
- SOD
superoxide dismutase
- TCAs
tricyclic antidepressants
- TNFα
tumor necrosis factor-α
- UPS
Ubiquitin-Proteasome System
- LPS
lipopolysaccharides
- XO
xanthine oxidase
8. References
- 1.Lichtenberg D and Pinchuk I, Oxidative stress, the term and the concept. Biochem Biophys Res Commun, 2015. 461(3): p. 441–4. [DOI] [PubMed] [Google Scholar]
- 2.Sies H, Oxidative stress: oxidants and antioxidants. Exp Physiol, 1997. 82(2): p. 291–5. [DOI] [PubMed] [Google Scholar]
- 3.Tabak O, et al. , Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin Invest Med, 2011. 34(3): p. E163–71. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y and Herrup K, Cell division in the CNS: Protective response or lethal event in post-mitotic neurons? Biochimica Et Biophysica Acta-Molecular Basis of Disease, 2007. 1772(4): p. 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman J, Why Is the Nervous System Vulnerable to Oxidative Stress? Oxidative Stress and Free Radical Damage in Neurology, 2011: p. 19–27. [Google Scholar]
- 6.Mason JW, et al. , Marked lability in urinary cortisol levels in subgroups of combat veterans with posttraumatic stress disorder during an intensive exposure treatment program. Psychosom Med, 2002. 64(2): p. 238–46. [DOI] [PubMed] [Google Scholar]
- 7.Gunther M, et al. , Neuronal Vulnerability to Oxidative Stress Is Affected by Genetic Polymorphism and Related to Susceptibility to Inflammation in the Central Nervous System. Journal of Neurotrauma, 2016. 33(3): p. A5–A5. [Google Scholar]
- 8.Wilkinson BL and Landreth GE, The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. Journal of Neuroinflammation, 2006. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SH, et al. , Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: Role of microglial NADPH oxidase. Journal of Neuroscience, 2005. 25(16): p. 4082–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peuchen S, et al. , Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Progress in Neurobiology, 1997. 52(4): p. 261–281. [DOI] [PubMed] [Google Scholar]
- 11.Ciobica A, et al. , Effects of serotonin depletion on behavior and neuronal oxidative stress status in rat: relevance for anxiety and affective disorders. Adv Med Sci, 2010. 55(2): p. 289–96. [DOI] [PubMed] [Google Scholar]
- 12.Salim S, et al. , Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res, 2010. 1359: p. 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovatta I, Juhila J, and Donner J, Oxidative stress in anxiety and comorbid disorders. Neurosci Res, 2010. 68(4): p. 261–75. [DOI] [PubMed] [Google Scholar]
- 14.Savolainen KM, et al. , Glutamate-stimulated ROS production in neuronal cultures: interactions with lead and the cholinergic system. Neurotoxicology, 1998. 19(4–5): p. 669–74. [PubMed] [Google Scholar]
- 15.Ding Q and Keller JN, Proteasomes and proteasome inhibition in the central nervous system. Free Radic Biol Med, 2001. 31(5): p. 574–84. [DOI] [PubMed] [Google Scholar]
- 16.Davies KJ, Degradation of oxidized proteins by the 20S proteasome. Biochimie, 2001. 83(3–4): p. 301–10. [DOI] [PubMed] [Google Scholar]
- 17.Pickering AM and Davies KJ, Differential roles of proteasome and immunoproteasome regulators Pa28αβ, Pa28γ and Pa200 in the degradation of oxidized proteins. Arch Biochem Biophys, 2012. 523(2): p. 181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering AM, et al. , Nrf2-dependent Induction of Proteasome and Pa28 alpha beta Regulator Are Required for Adaptation to Oxidative Stress. Journal of Biological Chemistry, 2012. 287(13): p. 10021–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering AM and Davies KJ, Degradation of damaged proteins: the main function of the 20S proteasome. Prog Mol Biol Transl Sci, 2012. 109: p. 227–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickering AM, et al. , A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J Exp Biol, 2013. 216(Pt 4): p. 543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston-Carey HK, Pomatto LC, and Davies KJ, The Immunoproteasome in oxidative stress, aging, and disease. Crit Rev Biochem Mol Biol, 2015. 51(4): p. 268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raynes R, Pomatto LC, and Davies KJ, Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol Aspects Med, 2016. 50: p. 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquini LA, et al. , Lactacystin, a specific inhibitor of the proteasome, induces apoptosis and activates caspase-3 in cultured cerebellar granule cells. J Neurosci Res, 2000. 59(5): p. 601–11. [DOI] [PubMed] [Google Scholar]
- 24.Lopes UG, et al. , p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem, 1997. 272(20): p. 12893–6. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, et al. , Induction of E-coli oh(8)Gua endonuclease by oxidative stress: Its significance in aerobic life. Mutation Research-DNA Repair, 1996. 363(2): p. 115–123. [DOI] [PubMed] [Google Scholar]
- 26.Lobo V, et al. , Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev, 2010. 4(8): p. 118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies KJA, Oxidative stress: The paradox of aerobic life. Free Radicals and Oxidative Stress: Environment, Drugs and Food Additives, 1995(61): p. 1–31. [DOI] [PubMed] [Google Scholar]
- 28.Davies KJ, Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life, 2000. 50(4–5): p. 279–89. [DOI] [PubMed] [Google Scholar]
- 29.Davies KJ, Adaptive homeostasis. Mol Aspects Med, 2016. 49: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raynes R, et al. , Aging and SKN-1-dependent Loss of 20S Proteasome Adaptation to Oxidative Stress in C. elegans. J Gerontol A Biol Sci Med Sci, 2017. 72(2): p. 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pomatto LCD, Tower J, and Davies KJA, Sexual Dimorphism and Aging Differentially Regulate Adaptive Homeostasis. J Gerontol A Biol Sci Med Sci, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harman D, Aging - a Theory Based on Free-Radical and Radiation-Chemistry. Journals of Gerontology, 1956. 11(3): p. 298–300. [DOI] [PubMed] [Google Scholar]
- 33.Walker CL, et al. , Redox Regulation of Homeostasis and Proteostasis in Peroxisomes. in press, Physiological Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bemeur C, Oxidative Stress in the Central Nervous System Complications of Chronic Liver Failure. Studies on Hepatic Disorders, 2015: p. 357–370. [Google Scholar]
- 35.Cattaneo E and Vercelli A, NEUROBIOLOGY OF BRAIN DISORDERS BIOLOGICAL BASIS OF NEUROLOGICAL AND PSYCHIATRIC DISORDERS Introduction. Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders, 2015: p. 205–206. [Google Scholar]
- 36.Ruparelia A and Mobley WC, NEUROBIOLOGY OF BRAIN DISORDERS BIOLOGICAL BASIS OF NEUROLOGICAL AND PSYCHIATRIC DISORDERS Introduction. Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders, 2015: p. 15–17. [Google Scholar]
- 37.Holmes SE, et al. , In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry, 2016. 21(12): p. 1672–1679. [DOI] [PubMed] [Google Scholar]
- 38.Weinberger DR, Anxiety at the frontier of molecular medicine. New England Journal of Medicine, 2001. 344(16): p. 1247–1249. [DOI] [PubMed] [Google Scholar]
- 39.Gross C and Hen R, The developmental origins of anxiety. Nature Reviews Neuroscience, 2004. 5(7): p. 545–552. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS, Allostasis and the Epigenetics of Brain and Body Health Over the Life Course: The Brain on Stress. JAMA Psychiatry, 2017. 74(6): p. 551–552. [DOI] [PubMed] [Google Scholar]
- 41.Bulfin LJ, et al. , Anxiety and hypothalamic-pituitary-adrenal axis responses to psychological stress are attenuated in male rats made lean by large litter rearing. Psychoneuroendocrinology, 2011. 36(7): p. 1080–1091. [DOI] [PubMed] [Google Scholar]
- 42.Grupe DW and Nitschke JB, Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 2013. 14(7): p. 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F, et al. , Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. Journal of Biomedical Research, 2015. 29(3): p. 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gigante AD, et al. , Morphometric post-mortem studies in bipolar disorder: possible association with oxidative stress and apoptosis. Int J Neuropsychopharmacol, 2011. 14(8): p. 1075–89. [DOI] [PubMed] [Google Scholar]
- 45.Chen HJC, et al. , Response of the nitrergic system to activation of the neuroendocrine stress axis. Frontiers in Neuroscience, 2015. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streck EL, et al. , Mitochondria and the central nervous system: searching for a pathophysiological basis of psychiatric disorders. Rev Bras Psiquiatr, 2014. 36(2): p. 156–67. [DOI] [PubMed] [Google Scholar]
- 47.Costantini D, Marasco V, and Moller AP, A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology, 2011. 181(4): p. 447–456. [DOI] [PubMed] [Google Scholar]
- 48.Arvier M, et al. , Adenine nucleotide translocator promotes oxidative phosphorylation and mild uncoupling in mitochondria after dexamethasone treatment. Am J Physiol Endocrinol Metab, 2007. 293(5): p. E1320–4. [DOI] [PubMed] [Google Scholar]
- 49.Roussel D, et al. , Kinetics and control of oxidative phosphorylation in rat liver mitochondria after dexamethasone treatment. Biochem J, 2004. 382(Pt 2): p. 491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasahara E and Inoue M, Cross-talk between HPA-axis-increased glucocorticoids and mitochondrial stress determines immune responses and clinical manifestations of patients with sepsis. Redox Report, 2015. 20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du J, McEwen B, and Manji HK, Glucocorticoid receptors modulate mitochondrial function: A novel mechanism for neuroprotection. Commun Integr Biol, 2009. 2(4): p. 350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartis D, et al. , Intermolecular relations between the glucocorticoid receptor, ZAP-70 kinase, and Hsp-90. Biochemical and Biophysical Research Communications, 2007. 354(1): p. 253–258. [DOI] [PubMed] [Google Scholar]
- 53.Powell SB, Sejnowski TJ, and Behrens MM, Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology, 2012. 62(3): p. 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson AW, et al. , Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proceedings of the National Academy of Sciences of the United States of America, 2013. 110(30): p. 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Filipovic D, et al. , Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive- and anxiety-like symptoms. Brain Struct Funct, 2016. [DOI] [PubMed] [Google Scholar]
- 56.Chanana P and Kumar A, GABA-BZD Receptor Modulating Mechanism of Panax quinquefolius against 72-h Sleep Deprivation Induced Anxiety like Behavior: Possible Roles of Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation. Front Neurosci, 2016. 10: p. 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patki G, et al. , Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res, 2013. 1539: p. 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reckziegel P, et al. , Oxidative stress and anxiety-like symptoms related to withdrawal of passive cigarette smoke in mice: beneficial effects of pecan nut shells extract, a by-product of the nut industry. Ecotoxicol Environ Saf, 2011. 74(6): p. 1770–8. [DOI] [PubMed] [Google Scholar]
- 59.de Oliveira MR, et al. , Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology, 2007. 28(6): p. 1191–9. [DOI] [PubMed] [Google Scholar]
- 60.Berry A, et al. , Deletion of the lifespan determinant p66(Shc) improves performance in a spatial memory task, decreases levels of oxidative stress markers in the hippocampus and increases levels of the neurotrophin BDNF in adult mice. Exp Gerontol, 2008. 43(3): p. 200–8. [DOI] [PubMed] [Google Scholar]
- 61.Desrumaux C, et al. , Plasma phospholipid transfer protein deficiency in mice is associated with a reduced thrombotic response to acute intravascular oxidative stress. Arterioscler Thromb Vasc Biol, 2010. 30(12): p. 2452–7. [DOI] [PubMed] [Google Scholar]
- 62.Vollert C, et al. , Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res, 2011. 224(2): p. 233–40. [DOI] [PubMed] [Google Scholar]
- 63.Kurhe Y, Mahesh R, and Devadoss T, QCM-4, a 5-HT(3) receptor antagonist ameliorates plasma HPA axis hyperactivity, leptin resistance and brain oxidative stress in depression and anxiety-like behavior in obese mice. Biochem Biophys Res Commun, 2015. 456(1): p. 74–9. [DOI] [PubMed] [Google Scholar]
- 64.Kumar A, Kaur G, and Rinwa P, Buspirone along with melatonin attenuates oxidative damage and anxiety-like behavior in a mouse model of immobilization stress. Chin J Nat Med, 2014. 12(8): p. 582–9. [DOI] [PubMed] [Google Scholar]
- 65.Dhingra MS, et al. , Effect of trimethylgallic acid esters against chronic stress-induced anxiety-like behavior and oxidative stress in mice. Pharmacol Rep, 2014. 66(4): p. 606–12. [DOI] [PubMed] [Google Scholar]
- 66.Souza CG, et al. , Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci, 2007. 81(3): p. 198–203. [DOI] [PubMed] [Google Scholar]
- 67.Masood A, et al. , Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther, 2008. 326(2): p. 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagahara N, et al. , Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: a model for human mercaptolactate-cysteine disulfiduria. Scientific Reports, 2013. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patki G, et al. , Grape powder treatment prevents anxiety-like behavior in a rat model of aging. Nutrition Research, 2015. 35(6): p. 504–511. [DOI] [PubMed] [Google Scholar]
- 70.Patki G, et al. , Tempol Treatment Reduces Anxiety-Like Behaviors Induced by Multiple Anxiogenic Drugs in Rats. Plos One, 2015. 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Tarbali S and Khezri S, Protective Effects of Vitamin D3 on Anxiety-like Behavior and the Total Antioxidant Power Following the Local Injection of Lysophosphatidylcholine in the Adult Rat Dorsal Hippocampus. Journal of Neurological Sciences-Turkish, 2015. 32(3): p. 482–493. [Google Scholar]
- 72.Nuss P, Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatric Disease and Treatment, 2015. 11: p. 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farach FJ, et al. , Pharmacological treatment of anxiety disorders: current treatments and future directions. J Anxiety Disord, 2012. 26(8): p. 833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohler H, The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology, 2012. 62(1): p. 42–53. [DOI] [PubMed] [Google Scholar]
- 75.Cassano GB, Baldini Rossi N, and Pini S, Psychopharmacology of anxiety disorders. Dialogues Clin Neurosci, 2002. 4(3): p. 271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiedorowicz JG and Swartz KL, The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract, 2004. 10(4): p. 239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hindmarch I, Beyond the monoamine hypothesis: mechanisms, molecules and methods. European Psychiatry, 2002. 17: p. 294s–299s. [DOI] [PubMed] [Google Scholar]
- 78.Martin EI, et al. , The Neurobiology of Anxiety Disorders: Brain Imaging, Genetics, and Psychoneuroendocrinology. Clinics in Laboratory Medicine, 2010. 30(4): p. 865-+.. [DOI] [PubMed] [Google Scholar]
- 79.Fox AS and Kalin NH, A Translational Neuroscience Approach to Understanding the Development of Social Anxiety Disorder and Its Pathophysiology. American Journal of Psychiatry, 2014. 171(11): p. 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rauch SL, The pathophysiology of anxiety. International Journal of Neuropsychopharmacology, 2004. 7: p. S17–S17. [Google Scholar]
- 81.Strian F, Pathophysiology of Anxiety. Advances and Continuing Education in Medicine, Vol 19 (1995/96), 1995. 19: p. 73–83. [Google Scholar]
- 82.Black CN, et al. , Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol Med, 2017. 47(5): p. 936–948. [DOI] [PubMed] [Google Scholar]
- 83.Jin H, et al. , Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta, 2014. 1842(8): p. 1282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odunze IN, Klaidman LK, and Adams JD, Mptp Toxicity in the Mouse-Brain and Vitamin-E. Neuroscience Letters, 1990. 108(3): p. 346–349. [DOI] [PubMed] [Google Scholar]
- 85.Lan J and Jiang DH, Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. Journal of Neural Transmission, 1997. 104(4–5): p. 469–481. [DOI] [PubMed] [Google Scholar]
- 86.Seidl SE and Potashkin JA, The promise of neuroprotective agents in Parkinson’s disease. Front Neurol, 2011. 2: p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah SA, et al. , Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochem Res, 2015. 40(5): p. 875–84. [DOI] [PubMed] [Google Scholar]
- 88.Dedeoglu A, et al. , Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. J Neurochem, 2003. 85(6): p. 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishimoto T, et al. , Analysis on mechanisms underlying the antidepressant effect of food-derived antioxidant ergothioneine. Journal of Pharmacological Sciences, 2014. 124: p. 83p–83p. [Google Scholar]
- 90.Mahmoudi M, et al. , Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. African Journal of Biotechnology, 2009. 8(24): p. 7170–7175. [Google Scholar]
- 91.Battal D, et al. , Possible role of selective serotonin reuptake inhibitor sertraline on oxidative stress responses. Eur Rev Med Pharmacol Sci, 2014. 18(4): p. 477–84. [PubMed] [Google Scholar]
- 92.da Silva AI, et al. , Effect of fluoxetine treatment on mitochondrial bioenergetics in central and peripheral rat tissues. Appl Physiol Nutr Metab, 2015. 40(6): p. 565–74. [DOI] [PubMed] [Google Scholar]
- 93.Ienco EC, et al. , Oxidative Stress Treatment for Clinical Trials in Neurodegenerative Diseases. Journal of Alzheimers Disease, 2011. 24: p. 111–126. [DOI] [PubMed] [Google Scholar]
- 94.Ramos E, et al. , Upregulation of Antioxidant Enzymes by ASS234, a Multitarget Directed Propargylamine for Alzheimer’s Disease Therapy. Cns Neuroscience & Therapeutics, 2016. 22(9): p. 799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu Y, et al. , Novel Therapeutic Targets in Depression and Anxiety: Antioxidants as a Candidate Treatment. Current Neuropharmacology, 2014. 12(2): p. 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Black C, et al. , Antioxidant uric acid is lower in current major depression and anxiety disorders. Bipolar Disorders, 2016. 18: p. 141–141. [Google Scholar]
- 97.Islam MR, et al. , Clinical Investigation of Serum Trace Elements, Antioxidants and Immunoglobulins in Generalized Anxiety Disorder Patients in Bangladesh. European Psychiatry, 2013. 28. [Google Scholar]
- 98.Cipriani A, et al. , Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet, 2018. 391(10128): p. 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bouayed J, et al. , The Antioxidant Effect of Plums and Polyphenolic Compounds Against H2O2-Induced Oxidative Stress in Mouse Blood Granulocytes. Journal of Medicinal Food, 2009. 12(4): p. 861–868. [DOI] [PubMed] [Google Scholar]
- 100.Bouayed J, Rammal H, and Soulimani R, Oxidative stress and anxiety Relationship and cellular pathways. Oxidative Medicine and Cellular Longevity, 2009. 2(2): p. 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dias GP, et al. , The Role of Dietary Polyphenols on Adult Hippocampal Neurogenesis: Molecular Mechanisms and Behavioural Effects on Depression and Anxiety. Oxidative Medicine and Cellular Longevity, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Skaper SD, Facci L, and Giusti P, Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol Disord Drug Targets, 2014. 13(10): p. 1654–66. [DOI] [PubMed] [Google Scholar]
- 103.Lynch MA, The multifaceted profile of activated microglia. Mol Neurobiol, 2009. 40(2): p. 139–56. [DOI] [PubMed] [Google Scholar]
- 104.Tian L, et al. , Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation, 2012. 9: p. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Butovsky O, et al. , Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci, 2006. 31(1): p. 149–60. [DOI] [PubMed] [Google Scholar]
- 106.Graeber MB and Streit WJ, Microglia: biology and pathology. Acta Neuropathol, 2010. 119(1): p. 89–105. [DOI] [PubMed] [Google Scholar]
- 107.Schafer DP, et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Streit WJ, Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Front Aging Neurosci, 2010. 2: p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Svenungsson E, et al. , Increased levels of proinflammatory cytokines and nitric oxide metabolites in neuropsychiatric lupus erythematosus. Annals of the Rheumatic Diseases, 2001. 60(4): p. 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang WY, et al. , Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med, 2015. 3(10): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Witko-Sarsat V, et al. , Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest, 2000. 80(5): p. 617–53. [DOI] [PubMed] [Google Scholar]
- 112.Brites D and Fernandes A, Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Frontiers in Cellular Neuroscience, 2015. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salim S, Chugh G, and Asghar M, Inflammation in anxiety. Adv Protein Chem Struct Biol, 2012. 88: p. 1–25. [DOI] [PubMed] [Google Scholar]
- 114.Munk PS, et al. , Symptoms of anxiety and depression after percutaneous coronary intervention are associated with decreased heart rate variability, impaired endothelial function and increased inflammation. Int J Cardiol, 2012. 158(1): p. 173–6. [DOI] [PubMed] [Google Scholar]
- 115.Chen J, et al. , Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis-like colon inflammation. Am J Physiol Regul Integr Comp Physiol, 2015. 308(1): p. R18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lisboa SF, et al. , Microglial Cells as a Link between Cannabinoids and the Immune Hypothesis of Psychiatric Disorders. Front Neurol, 2016. 7: p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu D, et al. , Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology, 2011. 61(4): p. 592–9. [DOI] [PubMed] [Google Scholar]
- 118.Chollet F, et al. , Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol, 2011. 10(2): p. 123–30. [DOI] [PubMed] [Google Scholar]
- 119.Ng KL, et al. , Fluoxetine Maintains a State of Heightened Responsiveness to Motor Training Early After Stroke in a Mouse Model. Stroke, 2015. 46(10): p. 2951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wohleb ES, et al. , beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci, 2011. 31(17): p. 6277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Innamorato NG, et al. , The transcription factor Nrf2 is a therapeutic target against brain inflammation. Journal of Immunology, 2008. 181(1): p. 680–689. [DOI] [PubMed] [Google Scholar]
- 122.Onasanwo SA, et al. , Inhibition of neuroinflammation in BV2 microglia by the biflavonoid kolaviron is dependent on the Nrf2/ARE antioxidant protective mechanism. Mol Cell Biochem, 2016. 414(1–2): p. 23–36. [DOI] [PubMed] [Google Scholar]
- 123.Buendia I, et al. , Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther, 2016. 157: p. 84–104. [DOI] [PubMed] [Google Scholar]
- 124.Innamorato NG, et al. , The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol, 2008. 181(1): p. 680–9. [DOI] [PubMed] [Google Scholar]
- 125.Miller DM, et al. , The Nrf2-Are Pathway as a Therapeutic Target in Traumatic Brain Injury: Genetic and Pharmacological Approaches for Neuroprotection. Journal of Neurotrauma, 2014. 31(12): p. A86–A86. [Google Scholar]
- 126.Shih AY, Li P, and Murphy TH, A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. Journal of Neuroscience, 2005. 25(44): p. 10321–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shah ZA, et al. , Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience, 2007. 147(1): p. 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muramatsu H, et al. , Nrf2 deficiency leads to behavioral, neurochemical and transcriptional changes in mice. Genes to Cells, 2013. 18(10): p. 899–908. [DOI] [PubMed] [Google Scholar]
- 129.Duffy S, So A, and Murphy TH, Activation of endogenous antioxidant defenses in neuronal cells prevents free radical-mediated damage. Journal of Neurochemistry, 1998. 71(1): p. 69–77. [DOI] [PubMed] [Google Scholar]
- 130.Shih AY, Erb H, and Murphy TH, Dopamine activates Nrf2-regulated neuroprotective pathways in astrocytes and meningeal cells. J Neurochem, 2007. 101(1): p. 109–19. [DOI] [PubMed] [Google Scholar]
- 131.Lukic I, et al. , Lymphocyte Levels of Redox-Sensitive Transcription Factors and Antioxidative Enzymes as Indicators of Pro-Oxidative State in Depressive Patients. Neuropsychobiology, 2014. 70(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 132.Mendez-David I, et al. , Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neuroscience Letters, 2015. 597: p. 121–126. [DOI] [PubMed] [Google Scholar]
- 133.Sandberg M, et al. , NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology, 2014. 79: p. 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malhotra D, Decline in NRF2-regulated Antioxidants in Chronic Obstructive Pulmonary Disease Lungs Due to Loss of Its Positive Regulator, DJ-1 (vol 178, pg 592, 2008). American Journal of Respiratory and Critical Care Medicine, 2009. 179(7): p. 624–624. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Gong B, et al. , The Ubiquitin-Proteasome System: Potential Therapeutic Targets for Alzheimer’s Disease and Spinal Cord Injury. Frontiers in Molecular Neuroscience, 2016. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baumeister W, et al. , The proteasome: paradigm of a self-compartmentalizing protease. Cell, 1998. 92(3): p. 367–80. [DOI] [PubMed] [Google Scholar]
- 137.Grune T, et al. , HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med, 2011. 51(7): p. 1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reeg S, et al. , The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic Biol Med, 2016. 99: p. 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X, et al. , Regulation of the 26S proteasome complex during oxidative stress. Sci Signal, 2010. 3(151): p. ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jang JW, et al. , Nrf2, a Regulator of the Proteasome, Controls Self-Renewal and Pluripotency in Human Embryonic Stem Cells. Stem Cells, 2014. 32(10): p. 2616–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thimmulappa RK, et al. , Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest, 2006. 116(4): p. 984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jaiswal AK, Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med, 2004. 36(10): p. 1199–207. [DOI] [PubMed] [Google Scholar]
- 143.Demuro A, et al. , Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem, 2005. 280(17): p. 17294–300. [DOI] [PubMed] [Google Scholar]
- 144.Kravtsova-Ivantsiv Y, Cohen S, and Ciechanover A, Modification by single ubiquitin moieties rather than polyubiquitination is sufficient for proteasomal processing of the p105 NF-kappaB precursor. Mol Cell, 2009. 33(4): p. 496–504. [DOI] [PubMed] [Google Scholar]
- 145.Davidson Y, et al. , Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathologica, 2007. 113(5): p. 521–533. [DOI] [PubMed] [Google Scholar]
- 146.Pickering AM, et al. , Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic Biol Med, 2013. 55: p. 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lomeli N, Bota D, and Davies KJA, The role of diminished stress resistance, and defective adaptive homeostasis in age related diseases. in press, Clinical Science. [DOI] [PubMed] [Google Scholar]
- 148.Bota DA and Davies KJ, Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol, 2002. 4(9): p. 674–80. [DOI] [PubMed] [Google Scholar]
- 149.Bota DA and Davies KJ, Mitochondrial Lon protease in human disease and aging: Including an etiologic classification of Lon-related diseases and disorders. Free Radic Biol Med, 2016. 100: p. 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pomatto LCD, et al. , The Mitochondrial Lon Protease Is Required for Age-Specific and Sex-Specific Adaptation to Oxidative Stress. Curr Biol, 2017. 27(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gragnoli C, Proteasome Modulator 9 Gene SNPs, Responsible for Anti-Depressant Response, Are in Linkage With Generalized Anxiety Disorder. Journal of Cellular Physiology, 2014. 229(9): p. 1157–1159. [DOI] [PubMed] [Google Scholar]
- 152.Shringarpure R and Davies KJ, Protein turnover by the proteasome in aging and disease. Free Radic Biol Med, 2002. 32(11): p. 1084–9. [DOI] [PubMed] [Google Scholar]
- 153.Brocardo PS, et al. , Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology, 2012. 62(4): p. 1607–18. [DOI] [PubMed] [Google Scholar]
- 154.Bouayed J, Rammal H, and Soulimani R, Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev, 2009. 2(2): p. 63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rammal H, et al. , Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun, 2008. 22(8): p. 1156–9. [DOI] [PubMed] [Google Scholar]