Abstract
Objective
This retrospective study was conducted to investigate the relationship between the superior sagittal sinus (SSS) to bone flap distance and clinical outcome in patients with traumatic brain injury (TBI) who underwent decompressive craniectomy (DC).
Methods
A retrospective review of medical records identified 255 adult patients who underwent DC with hematoma removal to treat TBI at our hospital from 2016 through 2017; of these, 68 patients met the inclusion criteria and underwent unilateral DC. The nearest SSS to bone flap distances were measured on postoperative brain computed tomography images, and patients were divided into groups A (distance ≥20 mm) and B (distance <20 mm). The estimated blood loss (EBL) and operation time were evaluated using anesthesia records, and the time spent in an intensive care unit (ICU) was obtained by chart review. The clinical outcome was rated using the extended Glasgow Outcome Scale (GOS-E) at 3 and 6 months postoperatively.
Results
The male to female ratio was 15:2 and the mean subject age was 55.12 years (range, 18–79 years). The mean EBL and operation times were significantly different between groups A and B (EBL: 655.26 vs. 1803.33 mL, p<0.001; operation time: 125.92 vs. 144.83 min, p<0.001). The time spent in the ICU and GOS-E scores did not differ significantly between the groups.
Conclusion
We recommend that when DC is indicated due to TBI, an SSS to bone flap distance of at least 20 mm should be maintained, considering the EBL, operation time, and other outcomes.
Keywords: Brain injuries, traumatic; Decompressive craniectomy; Superior sagittal sinus
Introduction
Traumatic brain injury (TBI) is defined as an acute injury to the head caused by blunt or penetrating trauma or by acceleration/deceleration forces, but not by degenerative, or congenital problems.1,5) The major principles involved in managing severe TBI are control of intracranial pressure (ICP) and ensuring adequate cerebral perfusion pressure (CPP).19,32) In patients with severe TBI, cerebral autoregulation ceases to function because of pathologic ICP increases that may compromise CPP and lead to neurological deterioration and fatal brain herniations.23) Medical treatments aimed at achieving ICP control include head elevation, intubation for normocarbic ventilation, sedation, hyperosmolar therapy mannitol or hypertonic saline, induced hypocapnia, hypothermia, and by barbiturate induced metabolic suppression.23,24) Surgical treatments involving ventricular cerebrospinal fluid drainage and decompressive craniectomy (DC) are also effective at achieving ICP control.25,26)
DC, which is performed worldwide to treat severe TBI, involves removing part of the skull to allow the brain to swell for ICP control. However, the efficacy of the procedure in terms of improving patient outcome remains controversial.5,9,27) Nevertheless, DC is still widely used as a last resort in patients with uncontrollable ICP. Several retrospective and prospective studies have suggested that DC effectively reduces ICP and improves prognosis in patients with refractory intracranial hypertension after TBI.5,10,12,22) However, indications for DC remain difficult to define for surgeons in emergency setting, as surgical techniques remain a controversial issue in the literature.6,15)
Classic technical recommendations for DC stress are that bone cutting should be performed in the frontotemporoparietal region to reach the base of the frontal bone and spare calvarium 20 mm from midline, to prevent injury to bridging veins and additional bleeding.28,29) Wagner et al.29) suggested that a diameter of greater than 12 cm is desirable, after observing that doubling of the diameter from 6 to 12 cm resulted in an increase in decompressive volume from 9 to 86 cc. However, no definitive standard surgical technique is available for DC and surgeons use various methods to control ICP on a case by case basis. Thus, in view of the above, we retrospectively investigated the relationship between superior sagittal sinus (SSS) to bone flap distance and clinical outcomes.
Materials and Methods
Study population
This retrospective study investigated 68 patients treated during a 24-month period from January 2016 to December 2017 at the trauma center of Pusan National University Hospital. These 68 patients were selected from 255 TBI patients that underwent DC with acute subdural hematoma removal by either of two surgeons. Patients over 16 years of age with mild to severe TBI, a high or mixed density lesion (<25 cc), and progressive deterioration of neurological status (a Glasgow Coma Scale [GCS] motor score decrease of 2 points or blunt papillary response) within 24 hours of injury were included. However, we excluded those with a serious extracranial comorbidity and an Abbreviated injury scale scores of >3, those taking antiplatelets or anticoagulants with a bleeding tendency (low platelet count <80,000, prolonged international normalized ratio >1.5, activated prothrombin time >60 sec), those with intense brain injuries, such as, multiple skull fractures, a bilateral lesion, or penetrating brain injury, with bilateral mydriasis of critically endangered status and a definite surgical contraindication, and those that received a unilateral large frontotemporoparietal craniectomy of anteroposterior (AP) with a maximum diameter of <12 cm.
Surgical procedure
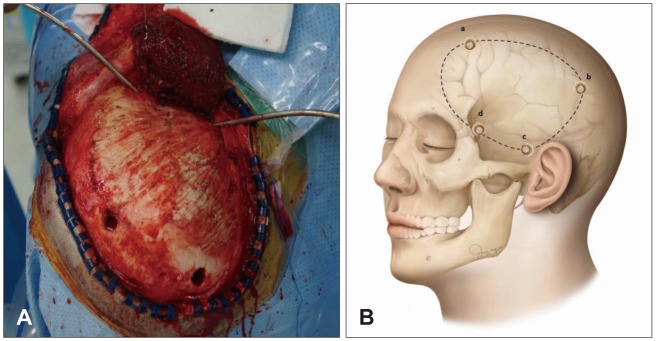
Different DC methods have been applied for decompression of refractory intracranial hypertension in TBI patients. Types of DC were as follows: 1) subtemporal decompression; 2) circular decompression; 3) fronto or temporoparietal DC; 4) large frontotemporoparietal DC; 5) hemispheric craniectomy; and 6) bifrontal DC.7) In this study, all patients received unilateral large frontotemporoparietal craniectomy (Figure 1) of at least 12 cm AP maximum diameter in accordance with the classic technical recommendations. Simultaneously, all patients received stellate type duraplasty with artificial dura mater to maximize brain expansion after bone removal. Medical care was sustained and included rehabilitation after surgery.
FIGURE 1. In the operative field view (A) and illustration (B) showing unilateral frontotemporoparietal craniectomy: frontal area 2 cm in front of the coronal suture and close to the skin incision (a), parietal area just posterior to the parietal bone and close to the skin incision (b), temporal squama (c), key hole area behide the zygomatic arch of the frontal bone (d).
Definitions of variables
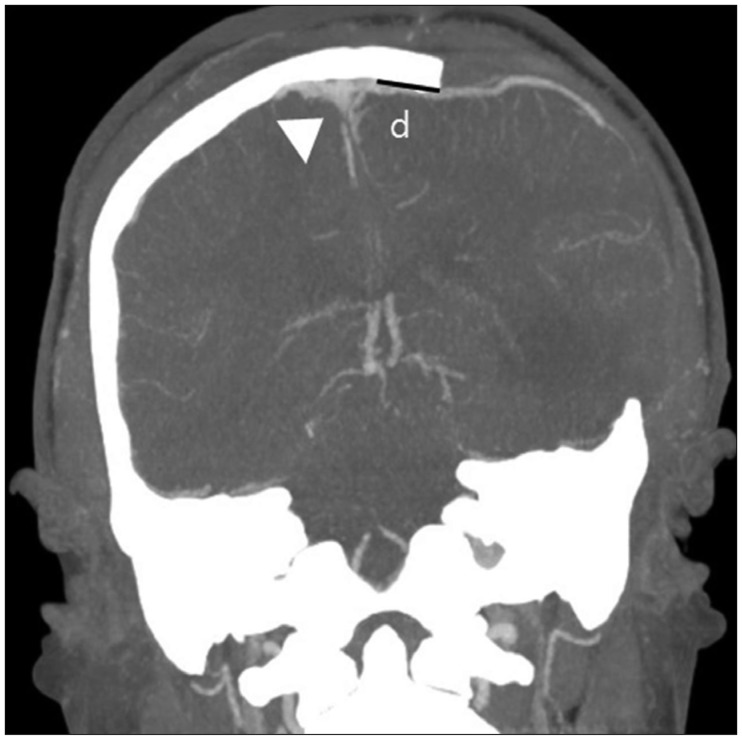
We measured the nearest SSS margin to bone flap distances using postoperative brain computed tomography (CT) images (Figure 2) rather than skull X-ray images for accuracy. Measuring the distance from sagittal suture to bone flap by skull X-ray may result in an overestimation of distance. Therefore, patients were divided into two groups based on SSS to bone flap distance using a 20 mm cut-off in accordance with classic technical recommendations for DC, group A ≥20 mm and group B <20 mm. Estimated blood loss (EBL) and operation times were obtained from anesthesia records. Anesthesiologists determined EBL by blood soaked gauzes, suction bottles fluid volumes, and transfusion volumes. Times spent in the intensive care unit (ICU) were obtained by chart review, and clinical outcomes were rated using the extended Glasgow Outcome Scale (GOS-E, 8-point scale, ranging from death at 0 points to good recovery at 7–8 points) at 3 and 6 months.
FIGURE 2. Measure of the nearest distance from superior sagittal sinus (SSS) to decompressive bone flap using postoperative coronal brain computed tomography for more accuracy. SSS (white arrow head). D: distance from SSS margin to bone flap.
Statistical analysis
Statistical analyses were supported by the Department of Biostatistics, Clinical Trial Center, Biomedical Research Institute, Pusan National University Hospital. To compare the characteristics of participants with respect to SSS to bone flap distance, continuous variables were analyzed using either the independent t-test and reported as means and standard deviations. Categorical variables were analyzed using Fisher's exact test or χ2 test and reported as percentages. All analyses was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA), and p-values less than 0.05 were considered statistically significant.
Results
Relationship between patients and SSS to bone flap distance
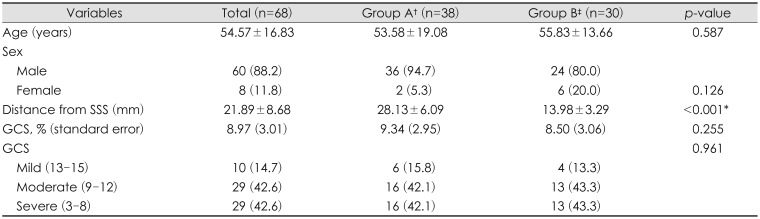
Baseline characteristics of the 68 (60 men and 8 women, mean age of 54.57 years) study subjects and SSS to bone flap distances are summarized in Table 1. The sample size of each group was 38 in group A and 30 in group B. The male/female ratios were 18:1 and 4:1, and the means ages were 53.58 and 55.83 years, respectively. The mean SSS to bone flap distances in groups A and B were 28.13±6.09 mm and 13.98±3.29 mm, respectively. The TBI severity in both groups of patients, as determined using GCS scores, was classified as mild to severe, and no significant intergroup difference was evident.
TABLE 1. Characteristics according to distance from superior sagittal sinus.
The data is presented as the mean± standard error or number (%). †≥20 mm, ‡<20 mm. SSS: superior sagittal sinus, GCS: Glasgow Coma Scale
Outcomes
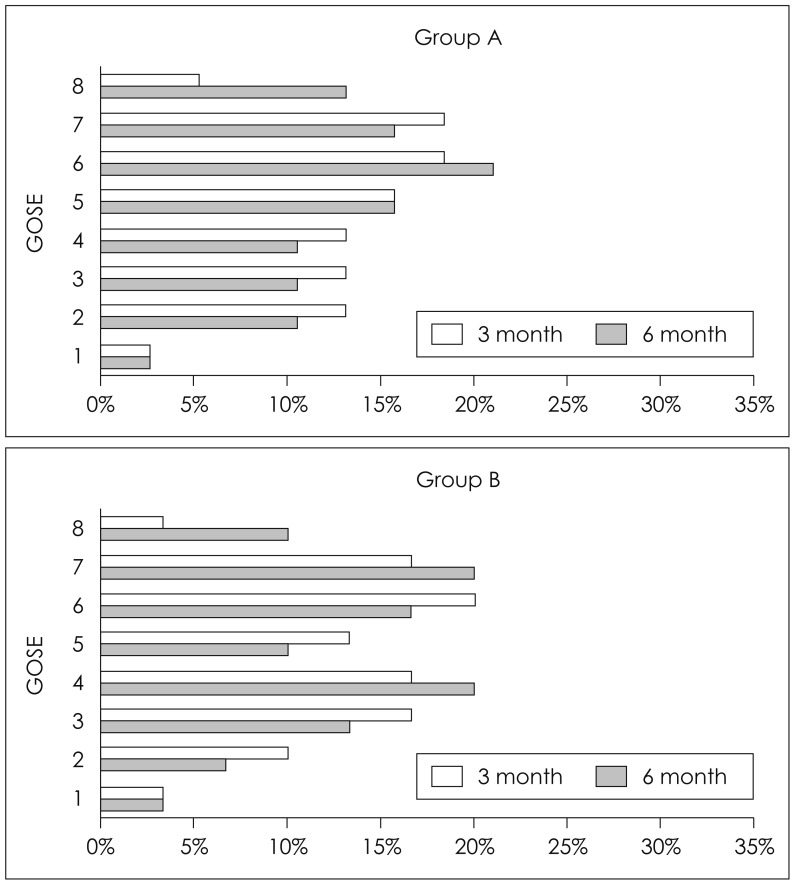
Table 2 summarizes perioperative outcomes by SSS to bone flap distance. Mean EBL and operation times were significantly lower in group A (mean EBL 655.26±208.85 mL in group A and 1803.33±1083.25 mL in group B, and mean operation times 125.92±29.78 min in group A and 144.83±20.99 min in group B). Table 3 summarizes clinical outcomes by SSS to bone flap distance. The mean ICU stay was shorter for group A (16.67±10.56 days vs. 19.45±10.51 days), but not significantly. In addition, the mean GOS-E scores at 3 and 6 month follow-up visits (Figure 3) were not significantly different.
TABLE 2. Perioperative outcomes according to distance from superior sagittal sinus.
The data is presented as the mean±standard error. †≥20 mm, ‡<20 mm. EBL: estimated blood loss
TABLE 3. Clinical outcomes according to distance from superior sagittal sinus.
The data is presented as the mean±standard error. *≥20 mm, †<20 mm. ICU: intensive care unit, GOS-E: extended Glasgow Outcome Scale
FIGURE 3. Distributions of extended Glasgow Outcome Scale (GOS-E) scores at 3 and 6 months.
Discussion
DC is a neurosurgical technique in which a portion of the skull is removed to reduce ICP. The rationale for this procedure is based on the Monro-Kellie theory that expanding physical space to accommodate confined edematous brain tissue after brain injury will reduce ICP.4,18) Many surgical techniques to control ICP after TBI have been studied. DC involves making a standard trauma flap skin incision with the goal of exposing margins anteriorly to the superior border of the orbital roof while avoiding entry into the frontal sinus, posteriorly to at least 2 cm posterior to the externa meatus, medially to a point 2 cm lateral to midline to maintain a SSS to bone flap distance of ≥20 mm, and inferiorly to the floor of the middle cranial fossa.1,28) The temporalis muscle is reflected anteriorly and can be resected if necessary.33) Burr holes are placed in the keyhole, the zygoma root and as preferred along the planned craniotomy route. A high-speed drill is used for the craniotomy. The lesser wing of the sphenoid is fractured and removed to the superior orbital fissure to provide sufficient decompression to prevent uncal herniation. The bone flap can be stored in abdominal subcutaneous fat or in situ using the hinge craniectomy method or it can be cryopreserved.11) Dural edges can be tagged up to the skull to minimize epidural hematoma formation.31) Dura can be opened different ways, but are typically is opened in a crescent or stellate fashion in the present study. Dura closure is not mandatory at this point and can either be left open, with mild approximation of dural leaflets or replaced with dural substitute.3,8)
Many factors influence the postoperative outcomes of TBI patients. The most powerful independent prognostic variables identified to date are; age, GCS motor score, pupil response, and CT characteristics, including the Marshall CT classification and the presence of traumatic subarachnoid hemorrhage. Other important prognostic factors include PT, hypotension, hypoxia, the eye and verbal components of the GCS and glucose, platelet, and hemoglobin level.16) In the present study, we investigated the relationship between SSS to bone flap distance and clinical outcomes, as well as EBL, operation times, ICU stay and GOS-E scores at 3 and 6 months postoperatively. We found that proximity between SSS and bone flap substantially increased EBL and prolonged operation times because of bridging veins compromise. Intraoperative bleeding obviously affects hemodynamic stability, extends operation anesthesia times, and increases the risk of wound infection, which indicates surgical intervention closer to the SSS to bone flap distance is associated with poorer clinical courses.2,13,14,20) To achieve better clinical outcomes in TBI, two requirements must be met. First, accurate, delicate illustrations of the surgical skin flap incision line and accurate patient positioning are needed, and due consideration should be given to the effects of gravity and skin retraction, which cause the incision line to be displaced toward the SSS. Second, anatomical SSS variations and the fracture line should be determined cautiously before surgery. In particular, a SSS that begins posterior to the foramen cecum in the frontal bone and courses backwards along the superior margin of falx cerebri, then widens near the internal occipital protuberance is referred to as the confluence of the sinuses. Surgeons should be aware that SSS variations sometimes occur during embryonic development.17) Many studies showed that a large craniectomy results in a significant decrease in ICP or the increase in CPP and GOS-E.21,30,32) Obviously, it is likely that decompressive effects depend on the volume gained by the craniectomy. However, we advise operators not to deviate from preoperative plans to create a larger bone flap and to strive to maintain a SSS to a bone flap distance of at least 20 mm intraoperatively. Because, if the AP diameter of bone flap is more than 12 cm, it is expected to be sufficient decompressed without closing to SSS. Also the possibility of complication will be increased as craniotomy in close proximity to SSS.
The current study has several limitations. First, it is inherently limited by its retrospective, single center design, and the relatively small number of patients included. Second, the method of EBLs calculation used depended on anesthesiologist experience and was somewhat subjective. Third, there was a lack of correlation between bone flap size and ICP/CPP, which are important factors of clinical prognosis in TBI. The study was also limited in not classifying acute brain injuries such as underlying cerebral contusions or diffuse axonal injury, which can significantly affect GOS-E. Nevertheless, the study has several strengths. 1) It is the first study to investigate relationships between SSS to bone flap distance during unilateral DC and clinical outcomes; 2) Since operations were performed by either of two neurosurgeons, operator-associated factors are unlikely to have affected outcomes; 3) It provides surgeons the opportunity to reflect on the non-uniformity of SSS to bone flap distances.
We suggest further larger-scale investigations be conducted with a multicenter, a randomized study design and extended follow-up that includes adjustment for potential confounders.
Conclusion
The present study confirms that smaller SSS to bone flap distances increase operation times and EBL because bridging veins are compromised. Although no significant differences were observed between the GOS-E scores of the two study groups, ICU stays were slightly longer in the group with a SSS to bone flap distance of <20 mm. We recommed that in cases of TBI, preoperative radiological images be cautiously evaluated. Moreover, if decompression is indicated a minimum SSS to bone flap distance of 20 mm should be maintained for better outcomes.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Adewumi D, Colohan A. Decompressive craniectomy: Surgical indications, clinical considerations and rationale. In: Agrawal A, editor. Brain injury: Pathogenesis, monitoring, recovery and management. Rijeka, HR: InTech; 2012. pp. 475–486. [Google Scholar]
- 2.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgicalsite infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–937. 937.e931–937.e932. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Burger R, Duncker D, Uzma N, Rohde V. Decompressive craniotomy: durotomy instead of duroplasty to reduce prolonged ICP elevation. Acta Neurochir Suppl. 2008;102:93–97. doi: 10.1007/978-3-211-85578-2_19. [DOI] [PubMed] [Google Scholar]
- 4.Citerio G, Andrews PJ. Refractory elevated intracranial pressure: intensivist's role in solving the dilemma of decompressive craniectomy. Intensive Care Med. 2007;33:45–48. doi: 10.1007/s00134-006-0381-5. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 6.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling indications and results. J Neurosurg. 1999;90:187–196. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Wen L. Technical considerations in decompressive craniectomy in the treatment of traumatic brain injury. Int J Med Sci. 2010;7:385–390. doi: 10.7150/ijms.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YH, Lee TC, Chen WF, Wang YM. Safety of the nonabsorbable dural substitute in decompressive craniectomy for severe traumatic brain injury. J Trauma. 2011;71:533–537. doi: 10.1097/TA.0b013e318203208a. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson PJ, Corteen E, Czosnyka M, Mendelow AD, Menon DK, Mitchell P, et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study ( www. RESCUEicp.com) Acta Neurochir Suppl. 2006;96:17–20. doi: 10.1007/3-211-30714-1_4. [DOI] [PubMed] [Google Scholar]
- 10.Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623–628. doi: 10.1089/neu.2005.22.623. [DOI] [PubMed] [Google Scholar]
- 11.Ko K, Segan S. In situ hinge craniectomy. Neurosurgery. 2007;60:255–258. doi: 10.1227/01.NEU.0000255380.64969.81. [DOI] [PubMed] [Google Scholar]
- 12.Kolias AG, Adams H, Timofeev I, Czosnyka M, Corteen EA, Pickard JD, et al. Decompressive craniectomy following traumatic brain injury: developing the evidence base. Br J Neurosurg. 2016;30:246–250. doi: 10.3109/02688697.2016.1159655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989;141:243–247. doi: 10.1016/s0022-5347(17)40731-2. [DOI] [PubMed] [Google Scholar]
- 14.Mor E, Jennings L, Gonwa TA, Holman MJ, Gibbs J, Solomon H, et al. The impact of operative bleeding on outcome in transplantation of the liver. Surg Gynecol Obstet. 1993;176:219–227. [PubMed] [Google Scholar]
- 15.Mori K, Nakao Y, Yamamoto T, Maeda M. Early external decompressive craniectomy with duroplasty improves functional recovery in patients with massive hemispheric embolic infarction: timing and indication of decompressive surgery for malignant cerebral infarction. Surg Neurol. 2004;62:420–429. doi: 10.1016/j.surneu.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 17.Özen OA, Turamanlar O, Kırpıko O, Songur A, Eser O. Superior sagittal sinus bifurcation variation. Eur J Gen Med. 2013;10:56–58. [Google Scholar]
- 18.Quinn TM, Taylor JJ, Magarik JA, Vought E, Kindy MS, Ellegala DB. Decompressive craniectomy: technical note. Acta Neurol Scand. 2011;123:239–244. doi: 10.1111/j.1600-0404.2010.01397.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949–962. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 20.Sisk AL, Hammer WB, Shelton DW, Joy ED., Jr Complications following removal of impacted third molars the role of the experience of the surgeon. J Oral Maxillofac Surg. 1986;44:855–859. doi: 10.1016/0278-2391(86)90221-1. [DOI] [PubMed] [Google Scholar]
- 21.Skoglund TS, Eriksson-Ritzén C, Jensen C, Rydenhag B. Aspects on decompressive craniectomy in patients with traumatic head injuries. J Neurotrauma. 2006;23:1502–1509. doi: 10.1089/neu.2006.23.1502. [DOI] [PubMed] [Google Scholar]
- 22.Stiefel MF, Heuer GG, Smith MJ, Bloom S, Maloney-Wilensky E, Gracias VH, et al. Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg. 2004;101:241–247. doi: 10.3171/jns.2004.101.2.0241. [DOI] [PubMed] [Google Scholar]
- 23.Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370:2121–2130. doi: 10.1056/NEJMra1208708. [DOI] [PubMed] [Google Scholar]
- 24.Stocchetti N, Rossi S, Buzzi F, Mattioli C, Paparella A, Colombo A. Intracranial hypertension in head injury: management and results. Intensive Care Med. 1999;25:371–376. doi: 10.1007/s001340050860. [DOI] [PubMed] [Google Scholar]
- 25.Timofeev I, Czosnyka M, Nortje J, Smielewski P, Kirkpatrick P, Gupta A, et al. Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg. 2008;108:66–73. doi: 10.3171/JNS/2008/108/01/0066. [DOI] [PubMed] [Google Scholar]
- 26.Timofeev I, Dahyot-Fizelier C, Keong N, Nortje J, Al-Rawi PG, Czosnyka M, et al. Ventriculostomy for control of raised ICP in acute traumatic brain injury. Acta Neurochir Suppl. 2008;102:99–104. doi: 10.1007/978-3-211-85578-2_20. [DOI] [PubMed] [Google Scholar]
- 27.Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 28.Valença MM, Martins C, da Silva JC, Mendonça CMF, Ambrosi PB, Andrade-Valença LPA. An innovative technique of decompressive craniectomy for acute ischemic stroke. In: Balestrino M, editor. Advances in the treatment of ischemic stroke. Rijeka, HR: InTech; 2012. pp. 227–246. [Google Scholar]
- 29.Wagner S, Schnippering H, Aschoff A, Koziol JA, Schwab S, Steiner T. Suboptimum hemicraniectomy as a cause of additional cerebral lesions in patients with malignant infarction of the middle cerebral artery. J Neurosurg. 2001;94:693–696. doi: 10.3171/jns.2001.94.5.0693. [DOI] [PubMed] [Google Scholar]
- 30.Wang YS, Wang Y, Shi XW, Zhang JD, Ma YY. Size of bone flap and bone window area may impact the outcome of decompressive craniectomy using standard bone flap. Eur Rev Med Pharmacol Sci. 2016;20:3679–3682. [PubMed] [Google Scholar]
- 31.Winston KR. Efficacy of dural tenting sutures. J Neurosurg. 1999;91:180–184. doi: 10.3171/jns.1999.91.2.0180. [DOI] [PubMed] [Google Scholar]
- 32.Wirtz CR, Steiner T, Aschoff A, Schwab S, Schnippering H, Steiner HH, et al. Hemicraniectomy with dural augmentation in medically uncontrollable hemispheric infarction. Neurosurg Focus. 1997;2:E3. doi: 10.3171/foc.1997.2.5.7. [DOI] [PubMed] [Google Scholar]
- 33.Yu SH, Kim BC, Choi JY, Lee JI, Cho WH, Choi HJ. Addition of resection of temporal muscle and fascia in decompressive craniectomy in the treatment of traumatic brain injury. Korean J Neurotrauma. 2016;12:84–88. doi: 10.13004/kjnt.2016.12.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]