Abstract
Pulmonary parasitic infestations are a worldwide problem associated with significant morbidity and socioeconomic impact. They are known to have varied clinical presentations and radiological appearances. Prevention of parasite transmission and medical treatment of cases form the two pillars of control of these diseases. The role of surgery is limited to the diagnosis and definitive treatment of the minority of pulmonary parasitic afflictions, most notably hydatidosis. Despite surgery being established as the treatment of choice in pulmonary hydatid cysts (PHCs) for over half a century, variations and unresolved controversies persist regarding the best surgical technique. Complications brought on by cyst rupture, multiplicity and multi-organ involvement add complexity to treatment decisions. The development of video-assisted thoracoscopic surgery (VATS) brings the promise of reduced peri-operative morbidity but is yet to be universally accepted as a safe technique. In this review, we endeavor to discuss the common pulmonary infestations focusing on the current trends and controversies surrounding surgery for PHC.
Keywords: Echinococcosis, pulmonary parasitic infestations, surgical management
Introduction
Pulmonary parasitic infestations, though endemic to certain areas, are a worldwide problem. Improvement in socio-economic conditions with concomitant improvement in hygiene has resulted in the decline in incidences in developed countries in the latter half of the last century. However, increasing migration and the rising incidence of immunosuppression will likely ensure doctors practising outside endemic areas will be required to deal with these problems with increasing frequency (1). Wider use of screening techniques for lung cancer will invariably bring to medical attention large numbers of pulmonary nodules and infiltrates. Pulmonary infestations and their radiological manifestations will regularly need to be considered in their differential diagnosis, especially in endemic areas.
Clinically significant pulmonary involvement occurs due to a range of protozoal and helminthic infestations. They may affect a range of thoracic sites from the tracheobronchial tree, pulmonary parenchyma, and pleural space to the chest wall. They can have wide ranging and non-specific clinical manifestations leading to diagnostic and therapeutic dilemmas and often confusion with malignancy (2). Though a vast majority of these ailments are treated medically or are self-remitting, the role of surgery is paramount in the diagnosis of some and treatment of others, most prominently pulmonary hydatid cysts (PHC). Despite growing experience in the surgical management of these problems, controversies and ambiguities in different aspects of their management persist. A prime example is the acceptance of video-assisted thoracoscopic surgery (VATS) as a viable and safe technique in these operations. In this review, we outline the scope of this problem, tackle some associated polemics and discuss their management.
Pulmonary parasitic infestations—a brief overview
Pulmonary manifestations are seen in a myriad of parasitic infestations (3,4). They can have a wide variety of clinical presentations and diverse radiological appearances. A brief description of the common human parasitic (helminthic and protozoal) infestations which can have pulmonary involvement, their mechanism of transmission, pulmonary symptoms and radiological findings are summarised in Table 1.
Table 1. Common parasites with pulmonary manifestations.
| Disease | Organism | Route of entry | Pulmonary manifestations | Radiological findings | Treatment/role of surgery |
|---|---|---|---|---|---|
| Nematodes | |||||
| Ascariasis | Ascaris lumbricoides | Faeco-oral route | Larval ascariasis causes Löffler’s syndrome (wheezing, pulmonary infiltrations, and eosinophilia) (5); can cause alveolar inflammation, necrosis, and haemorrhage | Solitary pulmonary nodules can develop if the larva dies causing granulomatous inflammation (6); lobar collapse can be caused by adult worm in children | Mebendazole and albendazole; no role for surgery |
| Ancylostomiasis (Hookworm Disease) | Ancylostoma duodenale; Necator americanus |
Via the skin | Patients usually present with transient eosinophilic pneumonia (Löffler’s syndrome) (7); larval migration may also cause alveolar haemorrhage (8) | CT scan may demonstrate transient, migratory, patchy alveolar infiltrates (9) | Mebendazole and albendazole; no role for surgery |
| Strongyloidiasis | Strongyloides stercoralis | Via the skin | Common pulmonary symptoms include wheezing, hoarseness, dyspnoea, and haemoptysis (10) | Focal or bilateral interstitial infiltrates; pleural effusions are present in 40% of patients, and lung abscess is found in 15% (11); diffuse alveolar haemorrhage in patients with disseminated strongyloidiasis | Oral ivermectin; no role for surgery |
| Syngamosis | Mammomonogamus genus | Ingestion of food or water containing eggs/larvae | Cough, lobar/sub lobar collapse | Bronchoscopic removal of adult worms | |
| Dirofilariasis | Dirofilaria immitis | Mosquito-borne | Most patients are asymptomatic; some patients (about 5%) may develop cough, haemoptysis, chest pain, fever, dyspnoea, and mild eosinophilia (12) | Solitary pulmonary nodule (usually peripheral/pleural-based); the nodule may be FGD avid causing confusion with malignancy (13,14); calcification can occur in 10% of these nodules; CT scan may show a branch of the pulmonary artery entering the nodule | No specific treatment required; surgical excision may be required for the diagnosis |
| Tropical pulmonary eosinophilia | Brugia malayi and Wuchereria bancrofti | Mosquito-borne | Asthma-like symptoms due to the strong antigenicity triggered by the microfilariae. | Reticulonodular opacities, predominantly in the middle and the lower lung zones miliary mottling, predominant hila with increased vascular markings at the bases (15); chest CT scanning may demonstrate bronchiectasis, air trapping, calcification, and mediastinal lymphadenopathy; the chronic phase of TPE may lead to progressive and irreversible pulmonary fibrosis (16) | The standard treatment is diethylcarbamazine (DEC); no role for surgery |
| Toxocariasis | Toxocara canis; Toxocara cati | Faeco-oral route | Hypersensitivity response to the migrating larvae; visceral larva migrans can present with fever, cough, wheezing, seizures and anaemia | Pulmonary infiltrates with hilar and mediastinal lymphadenopathy; bilateral pleural effusion can occur (17); noncavitating pulmonary nodules have also been reported (18) | DEC usually in combination with corticosteroids |
| Trichinella infection | Trichinella spiralis | Foodborne disease from undercooked pork | Dyspnoea—caused by parasitic invasion of the diaphragm and the accessory respiratory muscles | Pulmonary infiltrates | Mebendazole, along with analgesics and corticosteroids |
| Trematodes | |||||
| Schistosomiasis | Five schistosomes species cause disease in humans: Haematobium, Mansoni, Japonicum, Intercalatum, and Mekongi (8) | through the skin from contact with fresh water containing Schistosomal cercaria | In acute schistosomiasis, patients present with dyspnoea, wheezing, dry cough (19); pulmonary involvement can occur as a result of the systemic migration of parasitic eggs from the portal system. The eggs trigger an inflammatory response that leads to pulmonary arterial hypertension (PAH) and subsequent development of corpulmonale in 2% to 6% of patients (20) | Chest radiographs and CT scanning may show a diffuse reticulonodular pattern or ground-glass opacities | Acute schistosomiasis is treated with praziquantel |
| Paragonimiasis | Paragonimus species, including westernmani | Ingestion of the metacercaria from undercooked crustaceans | Typically acute symptoms include fever, chest pain, and chronic cough with haemoptysis (21); pleural effusion and pneumothorax may be the due to the migration of the juvenile worms through the pleura | Patchy infiltrates, nodular opacities, pleural effusion, and fluid-filled cysts with ring shadows on chest X-rays (2); CT scans may reveal a band-like opacity abutting the visceral pleura (worm migration tracks), bronchial wall thickening, and centrilobular nodules | Praziquantel and triclabendazole are the treatments of choice; no role for surgery |
| Cestodes | |||||
| Echinococcosis | Echinococcus granulosus and multilocularis | Ingestion of food contaminated with faeces, containing parasite egg (2) | Discussed in text | Discussed in text | Cystic hydatidosis is the only infestation that needs surgical treatment |
| Mesomycetozoea | |||||
| Rhinosporidiosis | Rhinosporidium seeberi | Airway | Lesions can involve the tracheobronchial tree, leading to partial or complete airway obstruction | CT imaging may demonstrate endobronchial lesions | Dapsone is the only medication to arrest the maturation of the sporangia (22); follow-up bronchoscopy is recommended to monitor signs of recurrence |
| Protozoal parasites | |||||
| Amoebiasis | Entamoeba histolytica | Local extension from the amoebic liver abscess | Patients usually present with fever, right-upper-quadrant abdominal pain, and cough; sterile pleural effusion, lung abscess, hepatobronchial fistula, empyema, and pyopneumothorax have also been reported (23) | Metronidazole is the treatment of choice for invasive amoebiasis | |
| Leishmaniasis | Leishmania Donovani | Blood borne | Usually in patients who have undergone lung transplants (24) | Pulmonary manifestations include pneumonitis, pleural effusion, and mediastinal lymphadenopathy (2) | Treatments of choice include pentavalent antimonials and liposomal amphotericin B; miltefosine can be used as an oral agent against visceral leishmaniasis (25) |
In a significant proportion of these infestations the pulmonary symptoms arise because the worms either in their larval [ascariasis (5), hookworm (7), strongyloidiasis (10)] or adult form [Dirofilaria (26), microfilaria (16)] travel through the lungs during their life cycle. This migration through the lungs results in a hypersensitivity reaction with the patient presenting with transient eosinophilic pneumonia (Löffler’s syndrome—wheezing, pulmonary infiltrations, and eosinophilia). Few worms (mammomonogamus species) actually complete their life cycle in the human pulmonary system with the central airway being the site of infestation. They can cause bronchial obstruction and relentless cough (27). In echinococcosis, human disease is characterised by formation of cysts in different organs including the lung.
The role of surgery in the management of the majority of pulmonary parasitic infestations is very limited. Other than for purpose of obtaining a surgical biopsy of pulmonary nodules caused by some of the parasites (e.g., dirofilariasis) and bronchoscopic removal of obstructing adult worms (syngamosis), surgery is mainly confined to the treatment of PHC. Further discussions in this review will therefore be centred on PHC and their surgical management.
PHCs (echinococcosis)
Echinococcosis or hydatid disease is caused by larvae (metacestode stage) of the tapeworm Echinococcus which is a cestode of the Taeniidae family. Although three other species of this worm are known, human disease is mainly caused by E. granulosus. This species causes cystic hydatid cyst. E. multilocularis, causes alveolar echinococcosis, occurs in colder areas and is associated with animals in wild ecosystems, especially foxes. E. vogeli and E. oligarthrus are rare species and cause polycystic echinococcosis.
Epidemiology
Echinococcosis has a world-wide distribution (28). More than one million people are thought to be affected with echinococcosis at any given time. It forms a significant public health problem in many areas including central and South America, South-western Europe, the Middle East, North Africa, sub-Saharan Africa, Russia and surrounding countries and China. A review of available literature on worldwide frequency of echinococcosis showed the prevalence to be 1–7% in community based studies and 0–32 cases per 100,000 in hospital based studies (29).
Life cycle of Echinococcus granulosus and structure of hydatid cyst
For the hydatid tapeworm, humans are accidental hosts and do not actually play a role in the biological cycle. The definitive hosts are dogs (and other canines) and varieties of warm-blooded vertebrates like sheep, goats, cattle, horses and pigs are the intermediate hosts. The adult worm which inhabits the small intestine of the definitive host is usually 2–7 mm long. They attach to the intestinal mucosa and have proglottids containing numerous eggs. The eggs are passed out in the faeces of the definitive host and stick to either the animal’s fur or the grass. The intermediate hosts ingest the eggs while grazing on the contaminated grass. The embryos hatch in the small intestine of the intermediate host. They enter the portal circulation via the intestinal wall and then travel to the visceral capillary bed; most commonly the liver and lungs. Here, they develop into metacestodes and grow into a cyst filled with fluid. The interior of the cyst is filled with hundreds to thousands of protoscolices; each of which has the potential to develop into an adult worm when ingested by a canine definitive host. Once in the intestine of the definitive host, the development into a mature worm to complete the life cycle takes 4–7 weeks (30,31). Humans are infected by either ingesting uncooked food contaminated by dog faeces or by direct contact with dogs. Humans represent a dead end for the life cycle of the parasite and human to human transmission does not occur (30,31).
The fully developed cysts are composed of three layers. The pericyst, or outer layer, is composed of inflamed fibrous tissue derived from the host; the ectocyst is an acellular laminated membrane; and the innermost layer, or endocyst, is the germinative layer of the parasite and gives rise to brood capsules (secondary cysts), which bud internally. Protoscolices are produced within the brood capsules. The fluid, which is antigenic, may contain debris, hooklets and scolices. These are referred to as hydatid sand. Daughter cysts may develop directly from the endocyst, resulting in multicystic structures (32).
Clinical presentation and diagnosis of PHC
Clinical presentation of PHC is diverse and does not present a constant clinical pattern. This often poses diagnostic difficulties. Clinical manifestations vary widely depending on the site, size and status (intact or ruptured) of the cyst. Cough and chest pain are the commonest symptoms. Intact cysts are frequently incidental findings or present with cough, dyspnoea or chest pain. Symptoms are usually secondary to cyst rupture which can be contained (rupture of endocyst contained by pericyst) or communicating (the contents of the cyst escape into the tracheo-bronchial tree or the pleural cavity) (33). Cysts in the middle lobe and lingula have been found in a study to have greater rupture rates (34). Rupture of the cyst into a bronchus, may present with expectoration of cystic contents, productive cough, repetitive haemoptysis, fever or even rarely anaphylactic shock (35). Pieces of cyst membrane may be expectorated (hydatoptysis). Spontaneous healing is theoretically possible if one expectorates the whole cyst membrane. Some rarer potential clinical effects of hydatid infection include immune complex-mediated disease, glomerulonephritis leading to nephrotic syndrome, and secondary amyloidosis (36,37). Ruptured cysts may become infected with bacteria or saprophytic or invasive fungi (38,39). Hydatid disease has been reported to cause recurrent acute pulmonary embolism (40).
Radiology forms the principal diagnostic tool and hydatid disease should come high on the list of differential diagnosis in patients with cystic lung lesion especially in endemic areas and in presence of appropriate history. Serological tests, though described, are usually restricted to use in case confirmation, especially among patients with atypical lung lesion on account of their low sensitivity and incomplete specificity (41). Serology may also be of use for patient follow-up as an indicator of relapse or recurrence (42). The use of synthetic peptides as antigens is thought to provide more reliability (41). The synthetic peptide p176, corresponding to the N-terminal extreme of the subunit of antigen B (AgB8/1), has shown promising performances for diagnosis of hydatid disease. Patients with complicated (ruptured) or multiple cysts are more likely to have positive serum antibody reactions (41).
Chest X-ray and computed tomography (CT) are the usual imaging modalities used. An uncomplicated PHC appears as a well-defined homogenous radio-opacity on a chest X-ray. The lower lobes are the most common location in the lungs (in 60% of cases). These cysts can be multiple in 30% of cases and bilateral in 20% (43). Calcification is very rare. On CT scan, uncomplicated PHCs appear as well-circumscribed fluid attenuation lesions with homogenous content and smooth, hyper dense walls. Unlike hydatid cysts of the liver, daughter cyst formation are rare in lung hydatids (43,44). Bronchial rupture and subsequent drainage of the cyst fluid can lead to a number of X-ray and CT scan appearances or signs (Figure 1). A detailed discussion of these radiological features is beyond the scope of this review but the reader is directed to a recent review on this topic by Garg et al. (45).
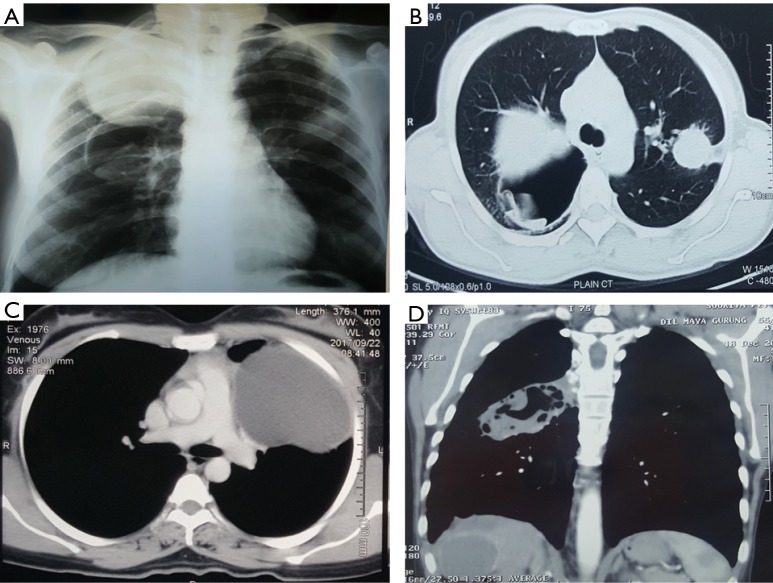
Figure 1.
Radiological appearances of pulmonary hydatid cysts. (A) X-ray chest showing multiple and bilateral cysts; two in right lung (one intact and one ruptured) and single intact left lung cyst; (B) CT scan of the same patient; (C) CT scan showing a giant hydatid cyst in the left upper lobe; (D) Multi-organ involvement—CT scan demonstrating a lung and liver hydatid cysts. CT, computed tomography.
Bronchoscopy is not routinely required in patients with PHCs with a typical clinico-radiological features. However, fiber-optic bronchoscopy may be helpful in clinching the diagnosis in patients with atypical clinical and radiological features (46,47). Discovery of endobronchial whitish yellow or white gelatinous membrane on bronchoscopy is typical.
Treatment of PHCs
Medical therapy
Treatment of PHC is primarily surgical but pharmacotherapy does have a role in selected cases. PHCs have been demonstrated to be more sensitive to chemotherapy than liver cysts (48,49). Medical therapy of PHC includes benzimidazoles group of drugs, namely mebendazole or albendazole. Chemotherapy is considered suitable for smaller cysts (<5 cm), patients with contraindication for surgery: poor surgical risk, refusal for surgery and multi-organ disease, multiple cysts, recurrent cysts, and patients with intraoperative spillage of hydatid fluid (50,51). Albendazole has better bioavailability and is more effective in lower doses than mebendazole and is now the drug of choice (52). The usual recommended dosage is 10–15 mg/kg/day (53). Although the optimal duration of pharmacotherapy in pulmonary hydatidosis is not known, it is usually given for 3–6 months. The convention in the past was to give albendazole in 1 month courses interrupted by 14-day intermissions in order to avoid hepatotoxicity (54). More recently, continuous therapy has been demonstrated to be more efficacious than cyclic therapy with no increase in adverse effects (53,55).
Small, multiple cysts, younger cysts which have thin walls, and cysts without daughter cysts are thought to show the most favourable responses with pharmacotherapy (56-58). When effective, at 2 months, the cyst becomes smaller and fibrous and within 3–6 months, all of the empty cysts become fibrotic. Most of the lung cysts disappear by 5–14 months after treatment (59). Albendazole treatment in the pre-operative setting has been demonstrated to weaken the walls of pulmonary cysts and might cause their rupture, especially in larger cysts (60). Also, it is known that despite a high concentration of albendazole in the serum and cyst fluid, the cyst can continue to be viable. The protoscolices maintain their viability in dead cysts (61). Therefore the role of routine pre-operative albendazole to prevent post-operative recurrences is reserved for hydatid cysts that have ruptured preoperatively (60,62).
Recurrence rates of PHC without postoperative antihelminthic therapy have been reported to be high (63). Therefore, postoperatively, all patients should receive albendazole (10 mg/kg per day) for 6 months to prevent recurrence of the disease (62).
Surgical treatment
The principles of surgical treatment of hydatid cyst include: complete evacuation of the cyst with removal of the endocyst; avoidance of contamination and spillage; meticulous closure of bronchial openings; management of the residual cavity (64); and maximal preservation of pulmonary parenchyma (64). Enucleation (Barrett’s technique) with or without capitonnage is the classical operation performed (65,66). However, multiple factors influence the operation performed including whether the cyst is: intact or ruptured; single or multiple; unilateral or bilateral; associated with liver dome cyst; and associated with destruction of lung parenchyma (54). In the ensuing paragraphs, we shall discuss some of the pertinent issues and controversies surrounding surgical techniques and procedures for PHCs.
Technique of enucleation
Classically, once the cyst has been located and the lobe containing the cyst adequately mobilized, the surrounding area is packed with packs soaked in a scolicidal agent (povidone iodine or hypertonic saline). The pericyst (seen as a fibrous whitish layer over the cyst dome) or the lung parenchyma over the cyst (when the cyst has not reached the lung surface) is then incised with a blade till the ectocyst pouts through. Once this happens, the pericyst is cut in a cruciate fashion to create an opening large enough for the cyst to extrude. The anaesthetist is now asked to apply positive pressure ventilation to the ipsilateral lung. This manoeuvre should cause the cyst to be expelled intact from the cavity. The cyst is then collected in a kidney dish. The cavity is cleaned; meticulous search is conducted for bronchial openings. These bronchial openings are then closed individually using non-absorbable (polypropylene) stitches (Figure 2).
Figure 2.
Enucleation of a pulmonary hydatid cyst. (A) Isolation of the cyst containing lobe with povidone iodine soaked packs; cruciate incision on the pericyst; (B) delivery of the intact cyst by positive pressure ventilation on the ipsilateral lung; (C) intact cyst after delivery; (D) bronchial opening seen after cyst delivery.
Capitonnage aims to obliterate the residual cavity in an attempt to avoid prolonged post-operative air leak and abscess formation. It involves applying a series of purse string sutures on the cyst wall starting from its base till the cavity is completely occluded (67). Some consider this procedure potentially lung parenchyma disfiguring. There is concern of capitonnage sutures leading to infection and laceration of the pulmonary tissue especially in infected and complicated cysts and as such controversy persists as to the need for capitonnage. While several authors looking at both uncomplicated and complicated hydatid cysts have shown no difference in rates of post-operative air leak and infection with or without capitonnage (68-70), there have been multiple more recent reports of increased complication rates (prolonged air leaks, abscess formation) associated with the practice of not performing capitonnage (71-73). The practice at the authors’ unit has been to meticulously look for and close all bronchial openings and then drain the cyst adequately through a dependent cystotomy. We do not routinely perform capitonnage when performing an enucleation via a thoracotomy. We have not experienced excessive leak and/or infection rates. In our experience, many factors including size (larger cysts would be more difficult to close) and location (capitonnage may be better for cysts that cannot be drained effectively), pre-existing infection and parenchymal destruction determine the need for capitonnage and therefore should be considered on an individual basis.
Percutaneous aspiration, instillation of scolicidal agents and reaspiration of contents (PAIR) is now considered an effective procedure for hepatic hydatid cysts of Gharbi types I–III (74). The experience of this procedure in PHCs has been inadequate and available literature has been limited to individual case reports (75) or small case series (76,77). Although success in individual cases have been reported, results have largely been disappointing (77).
Thoracotomy has been the traditional approach to PHC surgery. However, due to the purported benefits of lesser pain and quicker recovery after VATS, it has been increasingly applied to this surgery. Concerns however remain regarding the ability to adhere to the basic principles of PHC surgery via VATS. For instance, because the cyst has to be punctured and drained before cystotomy can be done via VATS; there is consequent potential for pleural contamination and even anaphylaxis. Consequently, some authors have in the past advised that the thoracoscopic approach be used only in dead cysts (78). Also, there is apprehension about the ability to identify and close bronchial fistulae adequately. Available literature on VATS for hydatid cyst remains limited and the numbers involved have typically been low. To the best of our knowledge, there have not been prospective randomised trials to compare VATS with thoracotomy for PHC. Experience with VATS does not appear uniform. Several reports have shown less postoperative pain, better cosmetic result, shorter surgical time, lower drainage volume, and shorter time to drain removal (79,80). We have previously demonstrated feasibility and safety of VATS in both intact and ruptured cysts (81). However, in a comparison between 10 paediatric patients who were operated via VATS versus 18 patients operated via a thoracotomy, Dokumcu et al. recorded higher rates of residual bronchial fistula, prolonged air leak, pneumothorax, and localized air cyst and consequently a higher median hospital stay in the thoracoscopy group (82). The perceived higher rates of these complications are arguably due to the greater difficulty at VATS to identify all open bronchioles and adequately close them. For example, in the series presented by Dokumcu et al., the fistulae were successfully identified and closed only in 4/8 cases of VATS group but 14/18 of the thoracotomy group. This may be a function of surgical experience with the procedure and may improve with the learning curve. Further, the initial fears of increased recurrences due to potential spillage or incomplete removal at VATS have not materialised (79). At present, available literature suggests VATS approach is viable, reasonably safe and can be applied without increase in surgical morbidity as long as the basic principles of PHC surgery are adhered to. Better definitions of the indications and contraindications to the VATS approach are awaited.
Parenchymal preservation is one of the principles of PHC surgery. However, cyst rupture can lead to infection leading to abscess formation and parenchymal destruction. This may necessitate lung resection in the form of wide wedge resection or even lobectomy (83). Another indication for anatomic resection is a ruptured or complicated hydatid cyst that cannot be differentiated convincingly from lung cancer or aspergilloma (84). Some large cysts may also compel a lobectomy when lesser resection may preserve minimal healthy parenchyma. However, reported experiences with these large cysts (>10 cm) suggest that parenchyma preservation may be achieved with acceptable morbidity (85).
Bilateral multiple lung hydatid cysts are not uncommon and may be dealt with via staged thoracotomies or median sternotomy (86). When using the staged approach, the complicated or largest uncomplicated cyst is tackled first. Concurrent right lung and dome of liver cysts may be dealt with simultaneously via a thoracotomy and phrenicotomy (54).
Hydatid cysts complicated by rupture either into the bronchi or the pleural space often present a treatment challenge. Besides often posing a diagnostic dilemma, they tend to cause significant pleural thickening and parenchymal destruction; therefore, increasing need for decortications (empyema) or resectional surgery (87). They are also associated with increased peri-operative complications and longer hospital stays (87).
Cysts above 10 cm in size are considered “giant hydatid cysts”. Younger patients, perhaps on account of their lung tissues being more elastic and also their delayed presentation, are more likely to have large cysts (88,89). This group is more likely to be symptomatic at presentation, to rupture and to need an anatomic resection compared to smaller cysts. However, the vast majority of these large cysts can be treated successfully with the same surgical principles as for smaller cysts. They do not necessarily entail higher complication rates (89).
Bilio-bronchial fistulae are rare complications which arise as a result of intra-thoracic rupture of liver dome hydatid cysts. Repeated pneumonias, bilioptysis, and dyspnoea are common symptoms. The surgical approach is usually via a thoracotomy, although thoraco-abdominal incision has been described in one study to provide better access (90,91). The principles however remain the same and comprise: evacuation of the intrahepatic cysts; obliteration of the cyst space; freeing the adherent lung; and dissection and closure of the fistula (90). Pulmonary parenchymal destruction may necessitate resection.
Recurrence after surgery for PHCs is a significant problem with incidences varying between 4.6–22% (92-94). Spillage of scolices containing cyst contents during surgery is cited as the primary aetiology of recurrence. This however would explain pleural dissemination but not isolated parenchymal cysts which have been the commoner form of recurrence reported. Re-infestation or subsequent growth of small cysts missed at the time of the first operation are alternate explanations (95). Treatment of recurrent PHCs remains surgical if not contraindicated by patient co-morbidities or advanced age. Re-operations can be significantly more difficult and associated with increased morbidity (94). Prevention of recurrence necessitates a number of pre-emptive efforts including: avoidance of operative spillage and dissemination; adequate post-surgical albendazole treatment; and patient education regarding prevention of re-infestation.
In conclusion, parasitic infestations of the lung are a significant public health problem occurring worldwide among both immunocompetent and immunocompromised patients (1). Hydatid disease alone causes significant socio-economic impact and healthcare burden often greatest amongst the poorest communities (29,96). Future efforts to decrease the incidence and impact of this preventable ailment mandate public health initiatives directed at preventing transmission of the parasites. This must involve increasing hand hygiene education amongst poorer and less educated communities, education regarding proper washing of fruits and vegetables before consumption, control of stray dog population and also control of home slaughter of sheep and goats including eradication of the feeding of offal to dogs.
While the role of surgery in treatment of most pulmonary infestations is limited, it does play a definitive curative role in PHCs. Variations persist in the surgical approach and methodology usually based on individual or institutional experiences and practices; but there exists no doubt that surgery is the treatment of choice in most PHCs. Moving forwards, VATS will likely gain greater acceptance and help decrease post-operative morbidity if applied to the appropriate patients and basic principles of hydatid cyst surgery are maintained despite a minimally invasive approach.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kunst H, Mack D, Kon OM, et al. Parasitic infections of the lung : a guide for the respiratory physician. Thorax 2011;66:528-36. 10.1136/thx.2009.132217 [DOI] [PubMed] [Google Scholar]
- 2.Vijayan VK, Kilani T. Emerging and established parasitic lung infestations. Infect Dis Clin North Am 2010;24:579-602. 10.1016/j.idc.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 3.Cheepsattayakorn A, Cheepsattayakorn R. Parasitic Pneumonia and Lung Involvement. Biomed Res Int 2014;2014:874021. 10.1155/2014/874021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khemasuwan D, Farver CF, Mehta AC. Parasites of the Air Passages. Chest 2014;145:883-95. 10.1378/chest.13-2072 [DOI] [PubMed] [Google Scholar]
- 5.Gelpi AP, Mustatafa A. Ascaris pneumonia. Am J Med 1968;44:377-89. 10.1016/0002-9343(68)90109-5 [DOI] [PubMed] [Google Scholar]
- 6.Osborne DP, Brown RB, Dimmette RM. Solitary pulmonary nodule due to Ascaris lumbrocoides. Dis Chest 1961;40:308-10. 10.1378/chest.40.3.308 [DOI] [PubMed] [Google Scholar]
- 7.Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med 2004;351:799-807. 10.1056/NEJMra032492 [DOI] [PubMed] [Google Scholar]
- 8.Sarinas PS, Chitkara RK. Ascariasis and hookworm. Semin Respir Infect 1997;12:130-7. [PubMed] [Google Scholar]
- 9.Kuzucu A. Parasitic diseases of the respiratory tract. Curr Opin Pulm Med 2006;12:212-21. 10.1097/01.mcp.0000219271.80804.9e [DOI] [PubMed] [Google Scholar]
- 10.Keiser PB, Nutman TB. Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev 2004;17:208-17. 10.1128/CMR.17.1.208-217.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodring JH, Halfhill H, 2nd, Berger R, et al. Clinical and imaging features of pulmonary strongyloidiasis. South Med J 1996;89:10-9. 10.1097/00007611-199601000-00002 [DOI] [PubMed] [Google Scholar]
- 12.Milanez de Campos JR, Barbas CS, Filomeno LT, et al. Human pulmonary dirofi lariasis: analysis of 24 cases from São Paulo, Brazil. Chest 1997;112:729-33. 10.1378/chest.112.3.729 [DOI] [PubMed] [Google Scholar]
- 13.Oshiro Y, Murayama S, Sunagawa U, et al. Pulmonary dirofilariasis: computed tomography findings and correlation with pathologic features. J Comput Assist Tomogr 2004;28:796-800. 10.1097/00004728-200411000-00011 [DOI] [PubMed] [Google Scholar]
- 14.Moore W, Franceschi D. PET findings in pulmonary dirofilariasis. J Thorac Imaging 2005;20:305-6. 10.1097/01.rti.0000181524.95015.ea [DOI] [PubMed] [Google Scholar]
- 15.Savani DM, Sharma OP. Eosinophilic lung disease in the tropics. Clin Chest Med 2002;23:377-96. 10.1016/S0272-5231(01)00012-0 [DOI] [PubMed] [Google Scholar]
- 16.Boggild AK, Keystone JS, Kain K. Tropical pulmonary eosinophilia: a case series in a setting of nonendemicity. Clin Infect Dis 2004;39:1123-8. 10.1086/423964 [DOI] [PubMed] [Google Scholar]
- 17.Figueiredo SD, Taddei JA, Menezes JJ, et al. Clinicalepidemiological study of toxocariasis in a pediatric population. J Pediatr (Rio J) 2005;81:126-32. 10.2223/1317 [DOI] [PubMed] [Google Scholar]
- 18.Sane AC, Barber BA. Pulmonary nodules due to Toxocara canis infection in an immunocompetent adult. South Med J 1997;90:78-9. 10.1097/00007611-199701000-00019 [DOI] [PubMed] [Google Scholar]
- 19.Bottieau E, Clerinx J, de Vega MR, et al. Imported Katayama fever: clinical and biological features at presentation and during treatment. J Infect 2006;52:339-45. 10.1016/j.jinf.2005.07.022 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz E. Pulmonary schistosomiasis. Clin Chest Med 2002;23:433-43. 10.1016/S0272-5231(01)00013-2 [DOI] [PubMed] [Google Scholar]
- 21.Vélez ID, Ortega JE, Velásquez LE. Paragonimiasis: a view from Columbia. Clin Chest Med 2002;23:421-31. 10.1016/S0272-5231(02)00003-5 [DOI] [PubMed] [Google Scholar]
- 22.Job A, Venkateswaran S, Mathan M, et al. Medical therapy of rhinosporidiosis with dapsone. J Laryngol Otol 1993;107:809-12. 10.1017/S002221510012448X [DOI] [PubMed] [Google Scholar]
- 23.Martínez S, Restrepo CS, Carrillo JA, et al. Thoracic manifestations of tropical parasitic infections: a pictorial review. Radiographics 2005;25:135-55. 10.1148/rg.251045043 [DOI] [PubMed] [Google Scholar]
- 24.Morales P, Torres JJ, Salavert M, et al. Visceral leishmaniasis in lung transplantation. Transplant Proc 2003;35:2001-3. 10.1016/S0041-1345(03)00664-X [DOI] [PubMed] [Google Scholar]
- 25.Croft SL, Engel J. Miltefosine–discovery of the antileishmanial activity of phospholipid derivatives. Trans R Soc Trop Med Hyg 2006;100:S4-8. 10.1016/j.trstmh.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 26.Theis JH. Public health aspects of dirofilariasis in the United States. Vet Parasitol 2005;133:157-80. 10.1016/j.vetpar.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 27.Severo LC, Conci LM, Camargo JJ, et al. Syngamosis: two new Brazilian cases and evidence of a possible pulmonary cycle. Trans R Soc Trop Med Hyg 1988;82:467-8. 10.1016/0035-9203(88)90165-4 [DOI] [PubMed] [Google Scholar]
- 28.Eckert J. WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. World Organization for animal health and World Health Organization, 2001.
- 29.Budke CM, Carabin H, Ndimubanzi PC, et al. A Systematic Review of the Literature on Cystic Echinococcosis Frequency Worldwide and Its Associated Clinical Manifestations. Am J Trop Med Hyg 2013;88:1011-27. 10.4269/ajtmh.12-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am 1996;25:655-89. 10.1016/S0889-8553(05)70268-5 [DOI] [PubMed] [Google Scholar]
- 31.Kammerer WS, Schantz PM. Echinococcal disease. Infect Dis Clin North Am 1993;7:605-18. [PubMed] [Google Scholar]
- 32.Golzari SEJ, Sokouti M. Pericyst: The outermost layer of hydatid cyst. World J Gastroenterol 2014;20:1377-8. 10.3748/wjg.v20.i5.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewall DB, McCorkell SJ. Rupture of echinococcal cysts: diagnosis, classification, and clinical implications. AJR Am J Roentgenol 1986;146:391-4. 10.2214/ajr.146.2.391 [DOI] [PubMed] [Google Scholar]
- 34.Onal O, Demir OF. The relation between the location and the perforation rate of lung hydatid cysts in children. Asian J Surg 2018;41:422-6. 10.1016/j.asjsur.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 35.Solak H, Ceran S, Ozpinar C, et al. Lung hydatid cyst rupture and its surgery. Indian J Med Sci 1994;48:155-7. [PubMed] [Google Scholar]
- 36.Gelman R, Brook G, Green J, et al. Minimal change glomerulonephritis associated with hydatid disease. Clin Nephrol 2000;53:152-5. [PubMed] [Google Scholar]
- 37.Ali-Khan Z, Rausch RL. Demonstration of amyloid and immune complex deposits in renal and hepatic parenchyma of Alaskan alveolar hydatid disease patients. Ann Trop Med Parasitol 1987;81:381-92. 10.1080/00034983.1987.11812136 [DOI] [PubMed] [Google Scholar]
- 38.Kini U. Invasive mycosis of a pulmonary hydatid cyst in a non-immunocompromised host. J Trop Med Hyg 1995;98:404-6. [PubMed] [Google Scholar]
- 39.Date A, Zachariah N. Saprophytic mycosis with pulmonary echinococcosis. J Trop Med Hyg 1995;98:416-8. [PubMed] [Google Scholar]
- 40.Lioulias A, Kotoulas C, Kokotsakis J. Acute pulmonary embolism due to multiple hydatid cysts. Eur J Cardiothorac Surg 2001;20:197-9. 10.1016/S1010-7940(01)00734-5 [DOI] [PubMed] [Google Scholar]
- 41.Santivañez SJ, Arias P, Portocarrero M, et al. Serological Diagnosis of Lung Cystic Hydatid Disease Using the Synthetic p176 Peptide. Clin Vaccine Immunol 2012;19:944-7. 10.1128/CVI.05540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortona E, Riganò R, Margutti P, et al. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol 2000;22:553-9. 10.1046/j.1365-3024.2000.00336.x [DOI] [PubMed] [Google Scholar]
- 43.Jerray M, Benzarti M, Garrouche A, et al. Hydatid disease of the lungs. Study of 386 cases. Am Rev Respir Dis 1992;146:185-9. 10.1164/ajrccm/146.1.185 [DOI] [PubMed] [Google Scholar]
- 44.Lewall DB. Hydatid disease: biology, pathology, imaging and classification. Clin Radiol 1998;53:863-74. 10.1016/S0009-9260(98)80212-2 [DOI] [PubMed] [Google Scholar]
- 45.Garg MK, Sharma M, Gulati A, et al. Imaging in pulmonary hydatid cysts. World J Radiol 2016;8:581-7. 10.4329/wjr.v8.i6.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saygi A, Oztek I, Güder M, et al. Value of fibreoptic bronchoscopy in the diagnosis of complicated pulmonary unilocular cystic hydatidosis. Eur Respir J 1997;10:811-4. [PubMed] [Google Scholar]
- 47.Yilmaz A, Tuncer LY, Damadoglu E, et al. Pulmonary hydatid disease diagnosed by bronchoscopy: A report of three cases. Respirology 2009;14:141-3. 10.1111/j.1440-1843.2008.01390.x [DOI] [PubMed] [Google Scholar]
- 48.Keshmiri M, Baharvahdat H, Fattahi SH, et al. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg 2001;95:190-4. 10.1016/S0035-9203(01)90162-2 [DOI] [PubMed] [Google Scholar]
- 49.Keshmiri M, Baharvahdat H, Fattahi SH, et al. A placebo controlled study of albendazole in the treatment of pulmonary echinococcosis. Eur Respir J 1999;14:503-7. 10.1034/j.1399-3003.1999.14c05.x [DOI] [PubMed] [Google Scholar]
- 50.Morar R, Feldman C. Pulmonary echinococcosis. Eur Respir J 2003;21:1069-77. 10.1183/09031936.03.00108403 [DOI] [PubMed] [Google Scholar]
- 51.Dakak M, Genç O, Gürkök S, et al. Surgical treatment for pulmonary hydatidosis (a review of 422 cases). J R Coll Surg Edinb 2002;47:689-92. [PubMed] [Google Scholar]
- 52.Anadol D, Ozçelik U, Kiper N, et al. Treatment of hydatid disease. Paediatr Drugs 2001;3:123-35. 10.2165/00128072-200103020-00005 [DOI] [PubMed] [Google Scholar]
- 53.Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ 1996;74:231-42. [PMC free article] [PubMed] [Google Scholar]
- 54.Halezeroglu S, Okur E. Surgical management for hydatid disease. Thorac Surg Clin 2012;22:375-85. 10.1016/j.thorsurg.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 55.De Rosa F, Lastilla MG, Franchi C. Advances of medical treatment of human hydatidosis. Recenti Prog Med 1996;87:346-52. [PubMed] [Google Scholar]
- 56.Schantz PM, Van den Bossche H, Eickert J. Chemotherapy for larval echinococcosis in animals and humans: Report of a workshop. Z Parasitenkd 1982;67:5-26. 10.1007/BF00929509 [DOI] [PubMed] [Google Scholar]
- 57.Messaritakis J, Psychou P, Nicolaidou P, et al. High mebendazole doses in pulmonary and hepatic hydatid disease. Arch Dis Child 1991;66:532-3. 10.1136/adc.66.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anadol D, Göçmen A, Kiper N. Hydatid disease in childhood: A retrospective analysis of 376 cases. Pediatr Pulmonol 1998;26:190-6. [DOI] [PubMed] [Google Scholar]
- 59.Todorov T, Vutova K, Donev S, et al. The types and timing of the degenerative changes seen in the cysts during and after benzimidazole treatment of cystic echinococcosis. Ann Trop Med Parasitol 2005;99:649-59. 10.1179/136485905X65125 [DOI] [PubMed] [Google Scholar]
- 60.Usluer O, Kaya SO, Samancilar O, et al. The effect of preoperative albendazole treatment on the cuticular membranes of pulmonary hydatid cysts: should it be administered preoperatively? Kardiochir Torakochirurgia Pol 2014;11:26-9. 10.5114/kitp.2014.41926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saimot AG, Meulemans A, Cremieux AC, et al. Albendazole as a potential treatment for human hydatidosis. Lancet 1983;2:652-6. 10.1016/S0140-6736(83)92533-3 [DOI] [PubMed] [Google Scholar]
- 62.Nabi MS, Waseem T. Pulmonary hydatid disease : What is the optimal surgical strategy ? Int J Surg 2010;8:612-6. 10.1016/j.ijsu.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 63.Tor M, Atasalihi A, Altuntas N, et al. Review of cases with cystic hydatid lung disease in a tertiary referral hospital located in an endemic region: a 10 years’ experience. Respiration 2000;67:539-42. 10.1159/000067470 [DOI] [PubMed] [Google Scholar]
- 64.Kurul IC, Topcu S, Altinok T, et al. One-stage operation for hydatid disease of lung and liver: Principles of treatment. J Thorac Cardiovasc Surg 2002;124:1212-5. 10.1067/mtc.2002.127314 [DOI] [PubMed] [Google Scholar]
- 65.Barrett NR. Removal of simple univesicular pulmonary hydatid cyst. Lancet 1949;2:234. 10.1016/S0140-6736(49)91241-6 [DOI] [PubMed] [Google Scholar]
- 66.Lichter I. Surgery of pulmonary hydatid cyst- the Barrett technique. Thorax 1972;27:529-34. 10.1136/thx.27.5.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dor J, Reboud E, de Cutolli JP. Capitonnage in the surgical treatment of pulmonary hydatid cyst. J Chir (Paris) 1951;67:113-24. [PubMed] [Google Scholar]
- 68.Erdogan A, Ayten A, Demircan A. Methods of surgical therapy in pulmonary hydatid disease: is capitonnage advantageous? ANZ J Surg 2005;75:992-6. 10.1111/j.1445-2197.2005.03594.x [DOI] [PubMed] [Google Scholar]
- 69.Eren MN, Balci AE, Eren S. Non-capitonnage method for surgical treatment of lung hydatid cysts. Asian Cardiovasc Thorac Ann 2005;13:20-3. 10.1177/021849230501300105 [DOI] [PubMed] [Google Scholar]
- 70.Turna A, Yilmaz MA, Haciibrahimoğlu G, et al. Surgical treatment of pulmonary hydatid cysts: is capitonnage necessary? Ann Thorac Surg 2002;74:191-5. 10.1016/S0003-4975(02)03643-3 [DOI] [PubMed] [Google Scholar]
- 71.Nabi MS, Waseem T, Tarif N, et al. Pulmonary hydatid disease : Is capitonnage mandatory following cystotomy? Int J Surg 2010;8:373-6. 10.1016/j.ijsu.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 72.Sokouti M, Golzari SE, Aghdam BA. Surgery of uncomplicated pulmonary hydatid cysts: capitonnage or uncapitonnage? Int J Surg 2011;9:221-4. 10.1016/j.ijsu.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 73.Bilgin M, Oguzkaya F, Akçali Y. Is capitonnage unnecessary in the surgery of intact pulmonary hydatic cyst? ANZ J Surg 2004;74:40-2. 10.1046/j.1445-1433.2003.02684.x [DOI] [PubMed] [Google Scholar]
- 74.Rajesh R, Dalip DS, Anupam J, et al. Effectiveness of Puncture-Aspiration-Injection-Reaspiration in the Treatment of Hepatic Hydatid Cysts. Iran J Radiol 2013;10:68-73. 10.5812/iranjradiol.7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lakshmanan PH, Musthafa AM, Suraj K, et al. Pleuropulmonary hydatid disease treated with thoracoscopic instillation of hypertonic saline. Lung India 2008;25:34-7. 10.4103/0970-2113.44138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gabal AM, Khawaja FI, Mohammad GA. Modified PAIR technique for percutaneous treatment of high-risk hydatid cysts. Cardiovasc Intervent Radiol 2005;28:200-8. 10.1007/PL00021047 [DOI] [PubMed] [Google Scholar]
- 77.Rai SP, Panda BN, Ganguly D, et al. Pulmonary Hydatid: Diagnosis and Response to Hypertonic Saline Irrigation and Albendazole. Med J Armed Forces India 2005;61:9-12. 10.1016/S0377-1237(05)80109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paterson HS, Blyth DF. Thoracoscopic evacuation of dead hydatid cyst. J Thorac Cardiovasc Surg 1996;111:1280-1. 10.1016/S0022-5223(96)70231-3 [DOI] [PubMed] [Google Scholar]
- 79.Alpay L, Lacin T, Ocakcioglu I, et al. Is Video-Assisted Thoracoscopic Surgery Adequate in Treatment of Pulmonary Hydatidosis? Ann Thorac Surg 2015;100:258-62. 10.1016/j.athoracsur.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 80.Findikcioglu A, Karadayi S, Kilic D, et al. Video-assisted thoracoscopic surgery to treat hydatid disease of the thorax in adults: is it feasible? J Laparoendosc Adv Surg Tech A 2012;22:882-5. 10.1089/lap.2012.0272 [DOI] [PubMed] [Google Scholar]
- 81.Thapa B, Sapkota R, Sayami P. Surgery For Pulmonary Hydatid Cyst: Our Experience With Thoracoscopy. JSSN 2013;16:79-82. [Google Scholar]
- 82.Dokumcu Z, Arslan S, Divarci E, et al. Thoracoscopic Treatment of Pulmonary Hydatid Cysts May Have a High Morbidity Risk in Children : Retrospective Analysis. Eurasian J Med 2017;49:172-7. 10.5152/eurasianjmed.2017.17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasquez JC, Montesinos E, Peralta J, et al. Need for lung resection in patients with intact or ruptured hydatid cysts. Thorac Cardiovasc Surg 2009;57:295-302. 10.1055/s-0029-1185604 [DOI] [PubMed] [Google Scholar]
- 84.Kilic D, Findikcioglu A, Bilen A, et al. Management of complicated hydatid cyst of the thorax. ANZ J Surg 2007;77:752-7. 10.1111/j.1445-2197.2007.04217.x [DOI] [PubMed] [Google Scholar]
- 85.Dakak M, Caylak H, Kavakli K, et al. Parenchyma-saving surgical treatment of giant pulmonary hydatid cysts. Thorac Cardiovasc Surg 2009;57:165-8. 10.1055/s-2008-1039210 [DOI] [PubMed] [Google Scholar]
- 86.Petrov DB, Terzinacheva PP, Djambazov VI, et al. Surgical treatment of bilateral hydatid disease of the lung. Eur J Cardiothorac Surg 2001;19:918-23. 10.1016/S1010-7940(01)00693-5 [DOI] [PubMed] [Google Scholar]
- 87.Kuzucu A, Soysal O, Ozgel M, et al. Complicated Hydatid Cysts of the Lung : Clinical and therapeutic issues. Ann Thorac Surg 2004;77:1200-4. 10.1016/j.athoracsur.2003.09.046 [DOI] [PubMed] [Google Scholar]
- 88.Halezeroglu S, Celik M, Uysal A, et al. Giant hydatid cysts of the lung. J Thorac Cardiovasc Surg 1997;113:712-7. 10.1016/S0022-5223(97)70228-9 [DOI] [PubMed] [Google Scholar]
- 89.Usluer O, Ceylan KC, Kaya S, et al. Surgical Management of Pulmonary Hydatid Cysts. Tex Heart Inst J 2010;37:429-34. [PMC free article] [PubMed] [Google Scholar]
- 90.Gerazounis M, Athanassiadi K, Metaxas E, et al. Bronchobiliary fistulae due to echinococcosis. Eur J Cardiothorac Surg 2002;22:306-8. 10.1016/S1010-7940(02)00257-9 [DOI] [PubMed] [Google Scholar]
- 91.Tocchi A, Mazzoni G, Miccini M, et al. Treatment of hydatid bronchobiliary fistulas: 30 years of experience. Liver Int 2007;27:209-14. 10.1111/j.1478-3231.2007.01435.x [DOI] [PubMed] [Google Scholar]
- 92.Little JM, Hollands MJ, Ekberg H. Recurrence of hydatid disease. World J Surg 1988;12:700-4. 10.1007/BF01655892 [DOI] [PubMed] [Google Scholar]
- 93.Prousalidis J, Kosmidis C, Anthimidis G, et al. Postoperative recurrence of cystic hydatidosis. Can J Surg 2012;55:15-20. 10.1503/cjs.013010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Velasco-Tirado V, Romero-alegría Á, Belhassen-garcía M, et al. Recurrence of cystic echinococcosis in an endemic area : a retrospective study. BMC Infect Dis 2017;17:455. 10.1186/s12879-017-2556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahmodlou R, Sepehrvand N. Recurrence Rate of Pulmonary Hydatidosis After Surgery: Reply. World J Surg 2014;38:267-8. 10.1007/s00268-013-2181-8 [DOI] [PubMed] [Google Scholar]
- 96.Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis 2006;12:296-303. 10.3201/eid1202.050499 [DOI] [PMC free article] [PubMed] [Google Scholar]