Abstract
The objective of this research was to investigate the effect of polymer length on the in vitro characteristics of thymoquinone-melatonin (TQ-MLT) when loaded into our previously prepared targeted drug delivery system (TDDS). Our system constructed from silica nanoparticles (NPs) and modified with diamine polymer (D4000), carboxymethyl-β-cyclodextrin (CM-β-CD) and folic acid (FA), respectively. In this study, three other different lengths of polymers (D230, D400 and D2000) were used and compared to D4000. The surface modification was characterized using fourier transform infrared spectroscopy (FTIR) and the mean particle size as well as polydispersity (PD) was measured using dynamic light scattering (DLS). The results, in general, showed that the release rate increases as the polymer length decreases. Also, shorter polymers showed an obvious burst release of most of the drug within the first hour. On the other hand, longer polymers exhibited a more sustained release in a pulsatile manner, with two moderate drug burst patterns occurred within the first and the last few hours. The in vitro cell viability assay showed that the percentage of cell toxicity toward HeLa cells increases with increasing the polymer length.
Keywords: Silica nanoparticles, Diamine polymers, Targeting, In vitro, Toxicity
1. Introduction
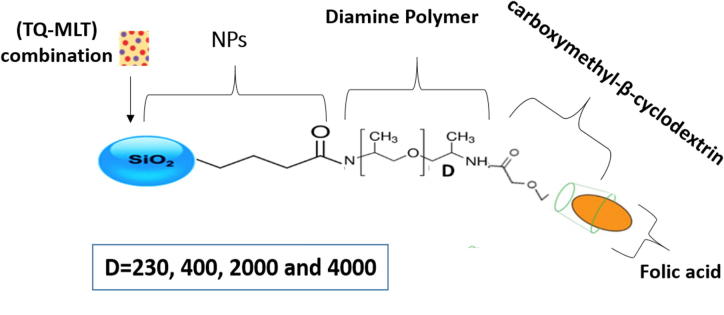
Targeted drug delivery is a promising tool that has been used to ensure the accumulation of drugs at desired target in therapeutic concentration and restrict their access to other parts, thus minimizing their toxic effects (Patwekar et al., 2014, Baea and Parkb, 2012). The anticancer agents, for example, can be targeted toward cancerous cells using different strategies based on NPs modified with folic acid (FA) ligands (Liang et al., 2017, Khattabi et al., 2017, Song et al., 2013). Such strategies lead to minimum distribution of the agents at the normal cells and a higher and effective concentration at the targeted cancerous cells. This is due to the fact that many cancerous cells produce folate receptors in a high amount and FA ligands have a good affinity to these receptors (Low et al., 2008, Salazar and Ratnam, 2007). We have recently prepared a targeted drug delivery system (TDDS) consisting of silica NPs, loaded with a combination of anticancer agents made of (TQ-MLT), then modified with a long diamine polymer (poly (propyleneglycol)bis(2-aminopropyletherdiamine), D4000), carboxymethyl-β-cyclodextrin (CM-β-CD) and folic acid (FA) ligands, respectively (Khattabi et al., 2017). The efficacy and the in vitro characteristics of this system, which is related to its unique surface modification and its high encapsulation efficiency (more than 80%), have been well investigated by us (Khattabi et al., 2017). In this work, however, we have prepared three similar systems but with three different lengths of diamine polymers (D230, D400 and D2000) and compared to our original system with D4000, in order to study the effect of polymer length on its in vitro characteristics.
Inorganic nanomatetials, such as silica NPs, exhibit interesting properties related to their nano-scale dimensions that allow their use as targeted drug delivery systems (TDDSs) for cancer treatment (Achilleos and Vamvakaki, 2010). However, these attractive properties may be lost due to the agglomeration of the NPs which arises from their high surface-to-volume ratio (Achilleos and Vamvakaki, 2010, Bagwe et al., 2006). It has been speculated that certain compounds such as cyclodextrins (CDs), when conjugated to the surface of NPs, can minimize their agglomeration by improving their steric hindrance (Su et al., 2014, Gidwani and Vyas, 2015). This property of CD has been exploited to hold FA ligands, via host-guest interaction, as reported in literatures (Khattabi et al., 2017). Regarding our prepared TDDS, we have already investigated the role of CD on both the agglomeration and the in vitro characteristics of this system (Khattabi and Alqdeimat, 2018).
To overcome the problem of agglomeration, the surface of different inorganic NPs can also be functionalized with polymer chains (Gann and Yan, 2008), either chemically (through covalent bonding) or physically (by physical adsorption) (Achilleos and Vamvakaki, 2010, Gann and Yan, 2008, Tang and Cheng, 2013). This polymer modification leads to formation of NPs that are more sterically stabilized than particles modified with shorter alkyl chains (Achilleos and Vamvakaki, 2010).
Polymers have also other advantages such as improving the intracellular uptake of the NPs and preparing systems which are affected by different external stimuli such as temperature, pH and ionic strength (Huck and Wilhelm, 2003). Moreover, polymers influence NPs’ behavior and properties such as their solubility, biocompatibility and mechanical stability (Gann and Yan, 2008).
Different types of polymers have been conjugated on the surface of NPs and used as crosslinkers. They may have two terminal chemical groups capable of binding to groups on both the NPs and other molecules (Cai et al., 2006, Jun et al., 2005). Herein, the diamine polymers, which were used as cross linkers, have two terminal amine groups that can be conjugated via covalent permanent bonds with both the carboxylic acid functionalized silica NPs and the (CM-β-CD).
To our knowledge, the effect of polymer length on the in vitro characteristics of drug delivery systems (DDSs) based on polymers conjugated to NPs has not been thoroughly studied and thus more research is needed to be done. Our present study, therefore, aims to investigate how the in vitro drug release rate and the toxicity of our previously prepared and novel DDS, toward cancerous cells, will be affected and controlled by the length of the diamine polymer. Understanding the relation will be a guide to predict and choose a suitable polymer length with specific and desired properties.
2. Materials and methods
2.1. Materials
FITC-labeled propylcarboxylic acid functionalized silica NPs (particle size 200 nm, pore diameter 4 nm), melatonin (98%), thymoquinone (99%), the four different lengths of poly (propyleneglycol)bis(2-aminopropyletherdiamine, D230, D400, D2000 and D4000) and carboxymethyl-B-cyclodextrin sodium salts (CM-β-CD) were purchased from Sigma Aldrich. Phosphate Buffered Saline (PBS, PH = 7.4) and folic acid (purity > 98%) were obtained from Bioworld. All reagents and materials used for cell culture experiment were obtained from Sigma Aldrich and used without any modification.
2.2. Preparation of drug loaded FA-CM-β-CD aminated silica NPs
The drug loading step with (TQ-MLT) was performed at the beginning, before the surface modification, with an encapsulation efficiency (% EE) of about 84%, as already demonstrated by us (Khattabi et al., 2017). The loaded NPs were then equally divided into four different sets which were treated similarly except for the length of the polymer (D230, D400, D2000 and D4000), as shown in Scheme 1. The procedures for drug loading and synthesis of FA-CM-β-CD aminated silica NPs with diamine polymer (D4000) were presented in our previous work (Khattabi et al., 2017). The same procedures were performed in this work for all sets but with different polymer lengths.
Scheme 1.
Surface modification of silica NPs with diamine polymers (D230, D400, D2000 and D4000), CM-β-CD and folic acid to form (FA-CM-β-CD aminated silica NPs).
2.3. FTIR and DLS analysis of the modified silica NPs
The FTIR spectra of the four types of the modified as well as pure silica NPs were collected within IR range (500–4000 cm−1) using WQF-521 fourier transform infrared spectrophotometer. The DLS technique was used to measure the mean particle size (Z-average) and the polydispersity of diluted suspensions, (4.5 × 10−3 mg/ml) of NPs in ethanol, using (Zetasizernano series, Malvern U.K). The settings of the instrument were adjusted to have a refractive index (RI) = 1.362 for ethanol and 1.48 for silica NPs and viscosity = 1.20 cp. The suspensions were first sonicated for few minutes and the measurements were performed in triplicates with 6 runs and the average was then calculated.
2.4. In vitro drug release study
Similar amounts (0.015 g) of each of the four drug loaded and modified NPs were mixed, separately, with 2 ml PBS (pH = 7.4) in a dialysis bag (SnakeSkin Dialysis Tubing, 22 mm × 35 feet dry diameter). Each sample was then added to a beaker containing 23 ml of PBS and shaken at 37 °C. Aliquots of 1.5 ml were removed at different time intervals (1, 2, 5, 24, 26, 28 and 30 h) and replaced each time with 1.5 ml of fresh PBS. The absorbance of the released amount of drug in the supernatant was measured via UV–Vis spectroscopy using (Uv/vis spectroscopy spuv-19) at λmax specific for both TQ and MLT (256 nm and 276 nm, respectively). The experiments were performed in triplicate and the cumulative drug release represent the average of these three experiments.
2.5. Antiproliferative (cell viability) assay
HeLa Cells were grown in 96-well tissue culture plates (100 μl/well) at a concentration of about 15,000 cells/well using complete tissue culture medium. The media were removed after 24 h and the attached cells were treated in triplicates with 200 μl of 0.05 mg/ml of five different samples of suspended NPs as well as free drugs combination and incubated for 24 h. The amount of free drugs was equivalent to the amount encapsulated into NPs. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was then performed to measure the cell viability. From each well, 100 μl of culture media was removed and 10 μl of thiazolyl blue tetrazolium solution was added and the plates were then kept in the incubator for 3 h. The reaction was then stopped by adding 100 μl/well of MTT solubilization solution and incubated for another one hour. Absorbance was measured at 550 nm by microplate reader and the cell viability (% survival) was calculated and used to get the percentage of cell toxicity.
3. Results and discussions
3.1. FTIR and DLS analysis
To study the effect of polymer length on the in vitro characteristics of our previously prepared TDDS, called FA-CM-β-CD aminated silica NPs, four samples of NPs were modified and treated similarly with different polymer lengths (D230, D400, D2000 and D4000) (Scheme 1). The successful attachment of diamine polymer (D4000), CM-β-CD and FA was previously confirmed by us using FTIR (Khattabi et al., 2017). Since the main difference between the samples in this work is the length of the polymer, we have focused on the peaks specific for this polymer. As we have found before, as well as here, The FTIR spectra of the NPs, after their modification with the damine polymer, showed small distinct peaks at about 2900–2950 cm−1 compared to unmodified NPs. These peaks are most likely due to N-H stretching of the amide bond which are shifted here toward lower frequency as a result of the interaction between such modified NPs. As shown in Fig. 1, the peaks at 2900 cm−1 became more intense and a broader peak appeared at about 3300 cm−1 as the polymer length increased. The later peak might appear due to increase in the number of functional groups which lead to form more bonds like hydrogen bonds.
Fig. 1.

FTIR spectra of (a) pure (propylcarboxylic acid functionalized silica NPs) and NPs modified with diamine polymers (b) (D230), (c) (D400), (d) (D2000) and (e) (D4000).
This observation was more obvious for D2000 and D4000 compared to D230 and D400 since the difference in the molecular weight is more significant.
In addition to the FTIR, the attachment of diamine polymers was characterized using DLS to measure the mean particle size and the polydispersity of the NPs. As shown in Table 1, the size increased as the polymers’ length increased indicating their successful conjugation.
Table 1.
Characterization of the modified NPs with diamine polymers using DLS.
| Diamine polymer | Mean particle size (nm) and polydispersity (PD) |
|---|---|
| Pure Silica NPs | ∼200 nm |
| D230 | ∼236 nm, ∼0.23 |
| D400 | ∼272 nm, ∼0.31 |
| D2000 | ∼356 nm, ∼0.47 |
| D4000 | ∼550 nm, ∼0.55 |
3.2. In vitro drug release study
In order to study the effect of polymer length on the release rate of the drugs and to compare the release profiles of the four NPs modified with the diamine polymers (D230, D400, D2000 and D4000), the percentage of cumulative drug release was calculated at different time intervals (after 1, 2, 5, 24, 26, 28 and 30 h).
In general, the release rate increased as the polymer length decreased, as shown in Fig. 2, which means the rate was in the order D230 > D400 > D2000 > D4000.
Fig. 2.
In vitro drug release of NPs modified with diamine polymers (D230, D400, D2000 and D4000) in PBS (pH 7.4).
The samples were loaded with the same amount of the same drug combination then modified similarly except for the length of the polymer. Thus, the observed difference in the release rate is due mainly to the difference in the polymer length.
It has been shown that the drug release rate from tablet matrix systems was affected by polymer length in which a slower rate was observed with increasing the length (Emeje et al., 2006, Ofoefule and Chukwu, 2001). Even though matrix systems are totally different DDSs compared to NPs, it appears that in our current work the longer the polymer attached to the NPs, the slower the rate will be, as well. According to this, it will be generally accepted to conclude that longer polymers, conjugated to NPs, provide a thick barrier (shield) to both the water and drugs diffusion into and out the NPs, respectively, which is not the case with shorter polymers. As a result, a slower rate of drug release will be observed for these polymers compared to shorter ones.
It was also obvious that NPs with diamine polymers D230 and D400 provided a massive burst release, in which about 80% and 70% of drug was respectively released within the first hour. On the other hand, D2000 and D4000 exhibited a more sustained release in a pulsatile manner, with two moderate drug burst of about 20% of the drug within the first 5 h and after 24 h. This is presumably due to the fact that some drug molecules, which were encapsulated in a high percentage, were adsorbed onto these long polymer chains during preparation steps. In other words, these long polymers were more efficient in hindering the diffusion of water into NPs compared to both D230 and D400, but the higher amount of adsorbed drug resulted in a first drug burst seen within the first five hours. On the other hand, the second burst seen within the last few hours for long polymers might be due to the drug encapsulated in the core of the NPs.
3.3. In vitro cell viability assay
For cell viability assay, five different suspensions (0.05 mg/ml) of silica NPs as well as free drug combination, were incubated with HeLa cells for 24 h under the same conditions. In general, as shown in Fig. 3, the percentage of cell toxicity increased as the polymer length increased (D4000 > D2000 > D400 ∼ D230) and the free drug effect is slightly greater than D230 and D400. Since efficient NPs internalization into HeLa cells takes only few hours (Gratton et al., 2008), then it is likely that in the case of D230 and D400 all the drug was released within the first hour (as shown in Fig. 2) i.e. before the NPs can enter the cells. The toxicity effect is therefore due mainly to the free drug, which is spontaneously released, and not to its encapsulation into NPs. More importantly, the targeting effect of the NPs was almost lost, because the NPs entering the cells would not release any more drug after 1 h (Fig. 3). This is in agreement with the results of the toxicity effect of the free drug seen here as well as in our previous work (Khattabi et al., 2017). The slightly smaller toxicity effect of D230 and D400 compared to the free drug is most likely due to the fact that only 80% and 70% of the drug is released from D230 and D400 NPs and the remaining drug encapsulated in the NPs cannot exhibit any toxic effect to the cells. On the other hand, the greater toxicity effect observed with both D2000 and D4000 is probably due to their slower drug release. As the NPs gradually entered HeLa cells, some drug was released from the NPs inside the cells. In this case, the FA-targeting might be more crucial to deliver the NPs and release the drug inside the cells. It can be concluded that, if the drug release from the DDS is too fast, as for D230 and D400, then such system wouldn’t have a time to function as an efficient carrier for the drug. In contrary, the slower release achieved with D2000 and D4000 allows the DDS to be internalized into the cells and then release the drug there. Another factor, which should be considered, is the effect of polymer length on the extent of cellular uptake. It has been speculated that, from similar studies, long polymers associate with and capture folate receptors much better than short polymers (Shiokawa et al., 2005., Ukino et al., 2008). However, to further confirm this, the percentage of cellular internalization of these NPs needs to be visualized using microscopy techniques, as an extension of this work, in the near future.
Fig. 3.
The percentage of cell toxicity of silica NPs, modified with four different lengths of diamine polymers, as well as pure NPs and free drugs, toward HeLa cells after incubation for 24 h.
Finding that drug distribution depends on the carrier itself rather than the physicochemical properties of the drug (Barratt, 2000), different NPs have been constructed to gain different properties and release characteristics to enhance their drug delivery or encapsulation efficiencies (Singh, 2009, Barratt, 2000). From this point of view, we have focused on the effect of polymer length on such properties. Depending on our results, we believe that it will be more straightforward to establish TDDSs of anticancer agents with desired proprieties such as release rate patterns and toxicity effects or to predict these properties based on the polymer length used.
Different findings have also demonstrated that NPs therapeutics’ efficacy and toxicity in vivo are determined and affected by the NPs’ drug release profiles (Sethi et al., 2014), thus this work deserves a further investigation through in vivo studies, as well, in the future.
4. Conclusions
In summary, we have examined the effect of polymer length on the in vitro characteristics of our previously prepared and investigated TDDS called FA-CM-β-CD aminated silica NPs. Four different lengths of poly (propyleneglycol)bis(2-aminopropyletherdiamine) (D230, D400, D2000 and D4000) were examined here. Using a combination of anticancer agents made of (TQ-MLT) as drug models, we found that the in vitro drug release rate increases as the polymer length decreases mainly within the first few hours. In addition, shorter polymers exhibited a massive burst release, in which most of the drug was released within the first hour. On the other hand, longer polymers showed a sustained release in a pulsatile manner, with two moderate drug burst patterns occurred within the first 5 h and between 24 and 30 h. The results obtained from MTT assay using HeLa cells, showed that the cell toxicity increases with increasing the polymer length. These results confirmed that the targeting effect achieved by longer polymers is more crucial than shorter polymers with respect to their toxicity effect and release rate. However, these findings deserve more work to be done in the future to evaluate the polymers’ effects through in vivo studies and to evaluate the effect of polymer length on the extent of cellular uptake.
Author contributions
Areen Khattabi presented the ideas and the experiments and wrote the manuscript, Wamidh Talib designed the experiment of the antiproliferative assay, Diala Alqdeimat conducted the rest of the experiments.
Conflicts of interest
The authors report no conflict of interest.
Acknowledgments
This project has been financially supported by Applied Science Private University, Amman, Jordan (Grant No. DRGS-2015-2016-35).
Footnotes
Peer review under responsibility of King Saud University.
References
- Achilleos D.S., Vamvakaki M. End-grafted polymer chains onto inorganic nano-objects. Mater. (Basel). 2010;3:1981–2026. [Google Scholar]
- Baea You Han, Parkb Kinam. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control Release. 2012;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwe R.P., Hilliard L.R., Tan W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22:4357–4362. doi: 10.1021/la052797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt G.M. Therapeutic applications of colloidal drug carriers. Pharm. Sci Tech Today. 2000;3:163–171. doi: 10.1016/s1461-5347(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Cai W., Shin D.W., Chen K., Gheysens O., Cao Q., Wang S.X., Gambhir S.S. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Letters. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- Emeje M.O., Kunle O.O., Ofoefule S.I. Effect of the molecular size of carboxymethylcellulose and some polymers on the sustained release of theophylline from a hydrophilic matrix. Acta Pharm. 2006;56:325–335. [PubMed] [Google Scholar]
- Gann John P., Yan Mingdi. A versatile method for grafting polymers on nanoparticles. Langmuir. 2008;24:5319–5323. doi: 10.1021/la7029592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidwani B., Vyas A. A Comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/198268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton Stephanie E.A., Ropp Patricia A., Pohlhaus Patrick D., Christopher Luft J., Madden Victoria J., Napier Mary E., DeSimone Joseph M. The effect of particle design on cellular internalization pathways. PNAS. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck M.K., Wilhelm T.S. Hyperbranched polyglycidol on Si/SiO2 surfaces via surface-initiated polymerization. Macromolecules. 2003;36:5088–5093. [Google Scholar]
- Jun Y., Huh Y.M., Choi J.S., Lee J.H., Song H.T., Kim S., Yoon S., Kim S.K., Shin J.S., Suh J.S. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005;127 doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- Khattabi A.M., Alqdeimat D.A. The effect of cyclodextrin on both the agglomeration and the in vitro characteristics of drug loaded and targeted silica nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018;305:012008. [Google Scholar]
- Khattabi A.M., Talib W.H., Alqdeimat D.A. A targeted drug delivery system of anti-cancer agents based on folic acid-cyclodextrin-long polymer functionalized silica nanoparticles. J. Drug Deliv. Sci. Technol. 2017;41:367–374. [Google Scholar]
- Li Tang, Jianjun Cheng. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8:290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Xuhua, Fan Jun, Zhao Yanyan, Cheng Min, Wang Xuejun, Jin Ruyi, Sun T.A. A targeted drug delivery system based on folic acid-functionalized upconversion luminescent nanoparticles. J. Biomater. Appl. 2017;31:1247–1256. doi: 10.1177/0885328217701289. [DOI] [PubMed] [Google Scholar]
- Low P.S., Henne W.A., Doorneweerd D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- Ofoefule S.I., Chukwu A. Effects of polyethylene 4000 and sodium lauryl sulphate on the release of hydrochlorothiaze embedded in the dika fat matrix. Acta Pharm. 2001;51:233–239. [Google Scholar]
- Patwekar S., Gattani S., Giri R., Bade A., Sangewar B. ISSN 2278–4357 Review on Nanoparticles Used in Cosmetics and dermal products. World J. Pharm. Pharm. Sci. 2014;3:1407–1421. [Google Scholar]
- Salazar M.D.A., Ratnam M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007 doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- Sethi Manish, Sukumar Rohit, Karvea Shrirang, Wernera Michael E., Wang E.C., Mooreb Dominic T., Kowalczyka Sonya R., Zhang Liangfang, Wang A.Z. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale. 2014;6:2321–2327. doi: 10.1039/c3nr05961h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa T., Hattori Y., Kawano K., Ohguchi Y., Kawakami H., Toma T., Maitani Y. Effect of polyethylene glycol linker chain length of folate- linked microemulsions loading aclacinomycin a on targeting ability and antitumor effect in vitro and in vivo. Clin. Cancer Res. 2005;11:2018–2025. doi: 10.1158/1078-0432.CCR-04-1129. [DOI] [PubMed] [Google Scholar]
- Singh J.W.L.R. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Su C., Cui W., Zhu B., Liu L., Chen Z., Zhao L. Folic acid-chitosan conjugated nanoparticles for improving tumor-targeted drug delivery. Biomed Res. Int. 2013;2013 doi: 10.1155/2013/723158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Li H., Shi Y., Wang G., Liu L., Zhao L., Su R. Carboxymethyl-β-cyclodextrin conjugated nanoparticles facilitate therapy for folate receptor-positive tumor with the mediation of folic acid. Int. J. Pharm. 2014;474:202–211. doi: 10.1016/j.ijpharm.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Ukino O., Kumi K., Yoshiyuki H., Yoshie M. Selective delivery of folate-PEG-linked, nanoemulsion-loaded aclacinomycin A to KB nasopharyngeal cells and xenograft: Effect of chain length and amount of folate-PEG linker. J Drug Target. 2008;16:660–667. doi: 10.1080/10611860802201464. [DOI] [PubMed] [Google Scholar]