Abstract
Background
Errors in medication use are a patient safety concern globally, with different regions reporting differing error rates, causes of errors and proposed solutions. The objectives of this review were to identify, summarise, review and evaluate published studies on medication errors, drug related problems and adverse drug events in the Gulf Cooperation Council (GCC) countries.
Methods
A systematic review was carried out using six databases, searching for literature published between January 1990 and August 2016. Research articles focussing on medication errors, drug related problems or adverse drug events within different healthcare settings in the GCC were included.
Results
Of 2094 records screened, 54 studies met our inclusion criteria. Kuwait was the only GCC country with no studies included. Prescribing errors were reported to be as high as 91% of a sample of primary care prescriptions analysed in one study. Of drug-related admissions evaluated in the emergency department the most common reason was patient non-compliance. In the inpatient care setting, a study of review of patient charts and medication orders identified prescribing errors in 7% of medication orders, another reported prescribing errors present in 56% of medication orders. The majority of drug related problems identified in inpatient paediatric wards were judged to be preventable. Adverse drug events were reported to occur in 8.5–16.9 per 100 admissions with up to 30% judged preventable, with occurrence being highest in the intensive care unit. Dosing errors were common in inpatient, outpatient and primary care settings. Omission of the administered dose as well as omission of prescribed medication at medication reconciliation were common. Studies of pharmacists’ interventions in clinical practice reported a varying level of acceptance, ranging from 53% to 98% of pharmacists’ recommendations.
Conclusions
Studies of medication errors, drug related problems and adverse drug events are increasing in the GCC. However, variation in methods, definitions and denominators preclude calculation of an overall error rate. Research with more robust methodologies and longer follow up periods is now required.
Keywords: Medication error, Adverse drug event, Drug related problem, Medication safety, Gulf Cooperation Council
1. Background
The use of medication is perhaps the most common intervention in medical practice. Medication use occurs in many different settings and involves different health care practitioners, as well as patients and their carers (Franklin and Tully, 2016). There are different types of problems associated with medication use, some are preventable events, some are not, and some result in injury and some do not. Medication errors (ME), are a global health care concern with the majority of research published from developed countries such as the United States of America and Europe (Morimoto et al., 2010) and (Jha et al., 2010) and much less information on the incidence and types of errors within the Middle East and the GCC in particular.
The GCC comprises six countries: Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates (UAE). These countries are listed by the United Nations World Bank as high income countries (World Bank, 2017). Total expenditure on health as a percentage of the country’s gross domestic product ranged from 2.2% to 5% in 2014 (World Health Organization, 2014). The GCC countries’ health systems are also comparable and are more similar to each other than to other countries within the Eastern Mediterranean Region or other Middle Eastern countries.
A previous systematic review of MEs across the whole of the Middle East concluded that ‘the main factor contributing to MEs […] is poor knowledge of medicines by both doctors (prescribers) and nurses (administering drugs).’ (Alsulami et al., 2013). Publications specifically from the GCC region report ME as an issue within both primary care and the inpatient setting (Al Khaja et al., 2005, Al Khaja et al., 2007, Al-Dhawailie, 2011). However, ME terminology used among the studies is different, as are the different types of medication safety aspects studied. The concept of “medication safety” potentially encompasses a wide range of areas (Ackroyd-Stolarz et al., 2006, McLeod, 2016). We therefore aimed to conduct an updated systematic review of medication safety research from the GCC countries in order to describe the breadth of problems associated with medication use. This will enable a more complete representation of what has been explored in this region regarding medication errors and helps identify gaps in the literature and focusses on preventable harm relating to medication use in clinical practice. Our objectives were to summarise and evaluate quantitative as well as qualitative evidence published on MEs, drug related problems (DRPs) and adverse drug events (ADEs) within the GCC region and to make recommendations for addressing any gaps in the literature identified.
2. Methods
2.1. Data sources and search strategy
The following electronic databases were searched for the period 1 January 1990 to 31 August 2016: CINAHL Plus, EMBASE, International Pharmaceutical Abstracts, PubMed (MEDLINE), ScienceDirect, and Web of Science. Bibliographies of relevant publications were also hand searched.
The search strategy was tailored to each database and medical search headings (MeSH terms) were also utilised for PubMed (MEDLINE). One author (JAS) screened the titles and abstracts of all 3115 records identified, for relevance and to determine if the complete text should be retrieved for comparing against inclusion/exclusion criteria and potential inclusion to the review. A random 10% sample was then screened by a second reviewer NAS to assess reliability. Cohen’s kappa (K) value (Cohen, 1960) was calculated to be 0.568; according to Landis and Koch (1977) this is interpreted as indicating moderate agreement. Any differences in opinion about the relevance of the papers were resolved by discussion. For final study selection, the full text was assessed by JAS; any uncertainty was referred to NAS. Any cases of disagreement were referred to BDF, with further clarification and consultation undertaken by JP and HA as needed. Details of the protocol were registered with the PROSPERO international prospective register of systematic reviews (reference CRD42016038733).
2.2. Study selection
2.2.1. Inclusion criteria
Research articles focussing on medication errors in the GCC countries published in English or Arabic were included. Both qualitative and quantitative studies of all study designs were included. Relevant conference abstracts were also included given the anticipated paucity of published full text research articles within the GCC. Studies on prescribing, dispensing, administration and monitoring errors in inpatient, hospital outpatient and primary care settings whether hospital or community pharmacies were included. Also included for review were studies examining administration errors by patients/caregivers in their own homes as well as original descriptive research on medication reconciliation errors and medication history errors (errors in the process of documenting the medications the patient is taking or used to take). According to the definitions utilised by the Saudi Arabian Ministry of Health the term ME was defined as: “Any preventable event that may cause or lead to inappropriate use or patient harm while the medication is in the control of the health care professional, patient or consumer” (Saudi Arabian Ministry of Health, 2012). Studies assessing DRPs and ADEs, (both preventable and non-preventable) potentially inappropriate medications (PIMs) were also included. Lastly, studies of pharmacists’ interventions to reduce MEs, ADEs or DRPs were also reviewed for inclusion. Studies meeting the review inclusion criteria were included in the analysis.
2.2.2. Exclusion criteria
Letters, opinion pieces, editorials and case reports were excluded. Studies focussing on expected side effects occurring with the proper use of a medication were also excluded, where a side effect was defined as: “An expected, well known reaction that results in little or no change in patient management”(Ninno and Ninno, 2000). Research concerned with blood/blood products, parenteral and enteral nutrition was excluded. Systematic reviews that had studies involving the GCC countries among their review of studies were not included in the review but used as a potential source to identify further relevant studies for inclusion. Finally, articles associated with attitudes, perceptions, or views on clinical services were excluded.
2.2.3. Process of data extraction
Search results were exported to Endnote X7 (Thomson Reuters, Times Square New York, NY, USA).
Duplicates were removed. Article titles and abstracts were initially screened for relevance to the systematic review inclusion and exclusion criteria, followed by full text retrieval analysis. Any ambiguities were discussed with BDF and NAS. Further clarification and consultation was undertaken by JP and HA if needed. Data from included studies was extracted on to a data collection sheet developed for this purpose. The extracted data comprised of country, setting and data collection duration, study design, definitions used for study outcomes, the medication safety aspect analysed method of error identification, and reported results. The extraction form was completed by JAS and reviewed by NAS and BDF. Any discrepancies were resolved by discussion with the remaining authors.
2.2.4. Quality assessment
Quality assessment of the reviewed included studies was carried out by JAS and NAS with any remaining uncertainties directed to and resolved by BDF. The quality assessment was carried out as part of the analysis of the included studies; study quality was based on specific aspects relating to medication safety research (e.g. methods used for identifying medication errors). The studies were therefore reviewed according to 15 criteria (Appendix B) adapted from previous studies (Allan and Barker, 1990, Ghaleb et al., 2006, Alsulaimi et al., 2013, McLeod and Barber, 2013). The first thirteen criteria are relevant to all medication error study types, while the remaining two apply only to studies of administration error. Any criteria not applicable to the study design were classified as ‘not applicable’ For the purpose of this study it was decided to assess the quality of the conference abstracts by adapting same criteria as for full text articles, as there are no is universally accepted criteria to evaluate conference abstracts.
3. Results
3.1. Search results
The search yielded a total of 3115 hits; an additional two were identified via search of bibliographies to give a total of 3117. After duplicate removal, the number of records remaining was 2094. All studies identified were in English, no studies in Arabic were identified. Following initial screening, 294 records were assessed as being potentially relevant. Following full text screening, 54 studies were identified for inclusion: 42 full papers and 12 conference abstracts (Fig. 1).
Fig. 1.
Prisma® flow chart.
The geographical distribution of the studies (Fig. 2) reveals that the majority of the studies were carried out in Saudi Arabia. None of the included studies were conducted in Kuwait.
Fig. 2.
Distribution of studies according to country.
3.2. Quality assessment
Quality of the studies varied (Appendix B). Many studies did not specify a definition for ME, and/or the categories of ME included. Criteria 14 and 15 were only applicable to three studies, one abstract by Aljamal (2012) and two full text studies, by Almazrou et al. (2015) and by Sadat-Ali et al. (2010). Most studies adequately described the setting and study objectives. Only six of the 54 studies adequately described sample size calculations. The majority of the conference abstracts did not meet quality assessment criteria such as the inclusion of sample size calculations, validity and reliability, study limitations or details of ethical approval, most likely due to their limited word count.
3.3. Description of included studies
Studies were classified within five main categories, with studies of ME further categorised into six subcategories (Fig. 3).
Fig. 3.
Study types included:
The following is a summary of the characteristics of the included studies; more details are given in Appendix D. It was noticed that drug classes commonly studied were antibiotics (Al Khaja et al., 2006, Alanazi et al., 2015) and drug classes used inappropriately were benzodiazepines and tri-cyclic antidepressants (Al Omar et al., 2013).
3.3.1. Studies describing medication errors
3.3.1.1. Studies describing prescribing errors
Seventeen studies described prescribing errors. Seven were cross-sectional audits of prescriptions (Al Khaja et al., 2006, Al Khaja et al., 2007, Al Khaja et al., 2005, Al Khaja et al., 2008; Khoja et al., 2011, Al-Hussein, 2008, Albarrak et al., 2014); all studied handwritten prescriptions except for Albarrak et al. (2014) who included both handwritten and electronic prescriptions. Five were retrospective audits (Al Khaja et al., 2012, Altebenaui et al., 2015, Al Shahaibi et al., 2012, Irshaid et al., 2005, Aseeri, 2013), as well as three retrospective analyses of patient charts (Mahmoud et al., 2016, Al-Jeraisy et al., 2011, Youssef et al., 2015) and two cross-sectional chart reviews (Alanazi et al., 2015, Al-Dhawailie, 2011). Data collection periods ranged from two weeks to three years. Common prescribing error definitions were those of Dean et al., 2000, Neville et al., 1989. Appendix C gives more information on the definitions.
Ten studies were conducted within a primary care or outpatient care setting while the remaining four related to the hospital setting. Two studies assessed prescriptions from inpatient and outpatient setting. Within outpatient and primary care settings, the prevalence and types of prescribing errors have been described for infants as well as adults. In a study analysing prescriptions issued for infants (Al Khaja et al., 2007) approximately 91% of prescriptions contained an error. In another study by Al Khaja et al. (2006), approximately one fifth of infants were prescribed antibiotics at subtherapeutic doses. For adults, studies report varying results, ranging from approximately 7% as reported by Al Khaja et al. (2005), to 18% of prescriptions containing errors (Khoja et al. 2011). Al Khaja et al. (2012) revealed approximately one quarter of prescriptions ordered by two-thirds of primary care physicians had errors. Other studies reported up to 50% of prescriptions missing at least one item of information (Altebenaui et al. 2015). Furthermore up to 88% of prescriptions written by junior doctors were identified to contain major errors of omission and commission or errors of integration (Al Khaja et al., 2008). Errors of integration or knowledge-based errors in prescribing were defined in the study to include potential drug-drug interactions or drug allergies, which may reflect a failure of the prescriber to integrate information about the patient or drug history (Al Khaja et al., 2008). An Omani study by Al Shahaibi et al. (2012) found that different kinds of omission error were evident in up to 72% of prescriptions. While Irshaid et al. (2005) reported, from their analysis of prescriptions, that physicians' handwriting was illegible in approximately 64% of prescriptions. In 2015, Alanazi, Aljeraisy, Salam reported that the prevalence of inappropriate antibiotic prescriptions with at least one or more types of error was significantly higher among paediatric patients compared to adult patients. In 2008 Al-Hussein carried out a study in which prescriptions were checked for compliance with 14 components of local guidelines; 87% of prescriptions did not meet these requirements (Al-Hussein, 2008).
Regarding prescribing errors within an inpatient setting, following analysis of inpatient handwritten medication charts and medication orders, Mahmoud et al. (2016), reported that the incidence of prescribing errors was 3.6 (95% CI, 3.3–3.9) per 100 prescriptions, 33.9 (95% CI, 31.5–36.6) per 100 admissions and 76.5 (95% CI, 70.9–82.3) per 1000 patient days. Al-Dhawailie (2011) reported approximately 7% of medication orders had prescribing errors. Aljeraisy et al. (2011) reported that in a tertiary paediatric inpatient setting in Saudi Arabia, the overall error rate was 56 per 100 medication orders (95% CI: 54.2%, 57.8%). Dosing errors were most prevalent error type in all three studies. In a Saudi Arabian study by Youssef et al., (2015) on types of contraindicated medications, approximately 14% of the contraindicated medications that resulted in a computerised prescribing system alert were still administered to patients with renal insufficiency by the ordering physician.
Two studies assessed prescribing errors from more than one hospital area. In 2014 Albarrak et al. compared handwritten and electronic prescriptions in primary care and outpatient surgery setting and inpatient respectively, for legibility, completeness and medication errors. A statistically significant difference was identified (P < 0.001) between handwritten and e-prescriptions in omitted dose and omitted routes of administration. One study described the use of a tool to decrease problems associated with medication use, retrospectively reviewing prescriptions from outpatient, inpatient and emergency department settings (Aseeri, 2013). This study reported the outcome of introducing an antibiotic dosing standardisation policy and its reduction of prescribing errors. Physicians were vigilant in documenting patient weight on prescriptions after the implementation of standardized dosing policy, and dosing errors identified on prescriptions reduced from approximately 34% of prescriptions pre-implementation phase to approximately 5% of prescriptions analysed after the implementation phase, a statistically significant reduction.
3.3.1.2. Studies describing administration errors
Two studies were identified, both observational in nature, where observation of medication administration was the method of data collection. One was carried out in an outpatient pharmacy waiting area, and the other in the inpatient setting. In the outpatient setting, Almazrou et al. (2015), revealed that 58% of mothers (patient carers’) measured an accurate dose of paracetamol using an oral syringe versus 50% of mothers using a dropper and 51% using a dosing cup. Dosing accuracy for each type of instrument was influenced by the mothers’ education status.
Aljamal (2012) observed nurses during medication administration in the inpatient setting. A total of 169 medication administration errors were observed of 2112 opportunities for error, representing an error rate of 8%. The most common errors were wrong time, dose omission, and wrong dose.
3.3.1.3. Studies assessing errors in medication history and medication reconciliation errors
There were several methods used by the five studies of medication reconciliation or medication history errors. Pharmacists screened the patient chart and performed interviews to identify discrepancies at admission or at discharge, triage or transfer between inpatient wards; and then compared with patient medical record or patient discharge medication list (Abu Yassin et al., 2011, Aljadhey and Al-Rashoud, 2013a, Al Anany et al., 2012, Rehmani, 2011). Another study developed a medication reconciliation (MR) form as a tool to detect medication discrepancies (Sonallah et al. 2014). Discrepancies found in patients medication lists at triage, admission or discharge ranged from 18% up to approximately 77% of patient cases interviewed (Aljadhey and Al-Rashoud, 2013) and Sonallah et al., (2014). The most common types of unintended medication discrepancies were medication omissions, followed by errors in dosages.
3.3.1.4. Studies assessing potentially inappropriate medications (PIM) use
Two studies, both from Saudi Arabia, assessed PIMs in elderly. Al-Omar et al. (2013) reported that approximately 44% of the patients had filled a prescription for at least one PIM in the outpatient setting, and Al Odhayani et al. (2016) reported that approximately 53% of the elderly participants attending appointments in the outpatient clinics or as part of home health care programme were using one or more PIMs. Harm caused by these PIMs was not assessed.
3.3.1.5. Studies assessing more than one type of medication error
‘Overall, eight studies reported more than one type of medication error. Two studies were retrospective in designs. The first, a retrospective study by Dibbi et al. (2006) reviewed medical records for adult hospitalised patients for 2 years and reported that the most common type of error was wrong strength (concentration). The second, a retrospective study by Alakhali et al. (2014) reviewed prescriptions from the outpatient setting for two months and identified 1850 opportunities for error and 201 (10.9%) prescribing, dispensing and administration errors.
Five studies assessed more than one type of medication errors using incident reports. The following studies all took place in large tertiary care hospitals in Saudi Arabia. Incidents were reported at a rate of 5.8 per 1000 patient days as stated by Arabi et al. (2012). Medication errors made up approximately 7% of all incident reports from the hospital and approximately 13% of all incident reports from the ICU. In a study by Alshaikh et al. (2013), the medication error rate over the 1-year study period was 0.4% (949 medication errors for 240,000 prescriptions), approximately 2% of the medication errors were categorised as resulting in any harm to the patient. Medication errors were reported to have originated predominantly at the prescribing stage of the medication process. In the third study, all medication error incident reports collected in the two year period were analysed (Sadat-Ali et al., 2010), and 38 medication errors reported from 23,597 admissions, giving a medication error reporting rate of 0.15% per admission. The fourth study was specific to a neonatal intensive care unit (Hemida et al., 2011), estimating an incidence of one report involving medication error per 250 admissions, with antibiotics most commonly involved. The last study (Al-Khani et al., 2014) determined 10% of prescribing errors included in the hospital reporting system were identified by pharmacists to be prescribing errors involving the wrong drug.
The remaining study by Elnour et al. (2007) reported the impact of the structured educational programme for inpatient nursing staff on the usage of MedSafe tool, a medication error reporting program launched in all inpatient nursing stations. Results indicated an increase in the number of ME reported to the Med Safe Tool after the structured program, the types of errors most often reported were monitoring errors and dosage errors.
3.3.2. Studies of DRPs
Five studies assessed DRPs. All five differentiated preventable and non-preventable DRPs. The DRP definition and classification of the Pharmaceutical Care Network in Europe (PCNE 2006, and 2010) was used by two studies (Rashed et al., 2012, Al Hamid et al., 2016). Three studies (Al-Olah and Al Thiab, 2008, Al-Arifi, 2014, Alghamdy et al., 2015) defined DRPs according to the definition of Strand et al. (1990). The different DRP categories identified across all five studies included adverse reactions, drug choice problems, dosing problems, and interactions. Pharmacists’ clinical interventions on identification of DRPs were at the prescriber level, patient/caregiver level, and the drug level.
Al-Olah and Al Thiab (2008) reported that the most common definite DRP-related admission to hospital was due to failure to receive medications accounting for approximately 47% of all DRPs, followed by adverse drug reactions approximately 25% of all identified DRPs respectively. Al-Arifi, 2014 reported approximately 19% of patients presented to the emergency department due to DRPs and approximately 93% of these patients needed hospital admission. The most common DRPs were adverse drug reactions (ADRs) and patients’ non-adherence. Alghamdy et al. (2015) reported approximately 5% of admissions were due to DRPs, 70% of which were preventable. Rashed et al. (2012) assessed attendance of paediatric patients to the emergency department and DRP incidence was reported as approximately 29%; the majority were judged preventable. Al Hamid et al. (2016), randomly selected 150 patient medical records from all admissions for patients over 18 years of age and identified 94 MRPs, of which 67% were definite (actual as defined by PCNE 2010, based on personal communication Alhamid, October 2017), while 33% were probable (potential as defined by PCNE 2010). Major risk factors associated with MRPs were polypharmacy and patient non-adherence.
3.3.3. Studies assessing adverse drug events
Three studies assessed ADE. A study from UAE, by Al-Tajir and Kelly (2005), compared two methods to detect ADEs. The first method of data collection for ADEs was limited to spontaneous reporting. For the second arm of the study active monitoring for ADEs took place. It was concluded that the incidence of ADEs detected through surveillance (active monitoring) was significantly higher than for ADEs reported spontaneously for both inpatients and outpatients. About 56% of ADEs identified by both methods combined were judged definite or probable and, of these, approximately 14% were consistently judged preventable.
In Saudi Arabia, Aljadhey et al. (2013) determined the incidence of in-hospital ADEs and assessed their severity and preventability in an academic tertiary hospital. Incidents were identified through a combination of medical record review by study pharmacists and voluntary reports from other health care professionals. The incidence of ADEs was 8.5 per 100 admissions. Incidences of preventable and non-preventable ADEs were 2.6 and 6 per 100 admissions respectively. In a more recent study, Aljadhey et al. (2016) determined the incidence of in-hospital ADEs and assessed their severity and preventability in four hospitals in Saudi Arabia. Incidents were identified as described in the study above (Aljadhey et al. 2013). Authors used a variety of ages to differentiate adults from children. The incidence of ADEs per 100 admissions was 6.1 and the incidence of potential ADEs was 16.9 per 100 admissions where a potential ADE was defined as an error that carried a risk of causing injury related to the use of a medication but harm did not occur, either because of specific circumstances or because the error was intercepted (Morimoto et al. 2004).
3.3.4. Studies of pharmacists’ interventions
Nine studies focused on pharmacists’ interventions, these had a variety of designs and interventions were aimed to address assessment/reduction of medication errors or problems associated with medication use. The interventions included pharmacists’ documentation of interventions on prescriptions on the inpatient ward, or in primary care clinics; pharmacist performing medication use reviews pharmacist counselling to patients, pharmacist education to physicians. The first was a conference abstract (Rahman et al., 1994) in which authors documented interventions on prescriptions with an intervention rate (error rate) was 1.3%. Al-Rashdi et al., 2010, Al Rahbi et al., 2014 both studies from Oman. Hooper et al. (2009), was a study from Qatar. These three studies documented pharmacists’ interventions on prescriptions and reported rates of interventions acceptance ranging from 53% up to approximately 98% of all suggested interventions. Al-Ghamdi et al. (2012), reported benefits of comprehensive pharmacist counselling. There was a significant difference in the occurrence of ADEs between the control group (no pharmacist counselling) and intervention group. In the control group, 61% of ADEs were judged preventable, and 39% were judged to be serious. Al-Jazairi et al. (2008) studied the participation of a clinical pharmacist in an ICU setting, reporting that the medical team accepted approximately 95% of the interventions. The main DRPs were: no drug prescribed for the medical condition, inappropriate dosing regimen and no indication for drug use. Mitwally et al. (2015) in Qatar reported a 52% reduction in prescribing errors after the prescribing physicians attended educational sessions prepared by the clinical pharmacy team. Rashed et al. (2012) investigated DRPs in hospitalised children and 258 DRP were identified for 186 children. The median number of DRPs per patient was one. Dosing problems were the most common, followed by drug choice problems and ADRs. Regarding the interventions, approximately 43% of all interventions were at drug level, approximately 40% of interventions at prescriber level and approximately 10% of interventions were done at patient/caregiver level. Kheir et al. (2014) reported the results of their exploratory study of conducting interviews as part of medication use reviews within a primary health care facility in Qatar. The most commonly encountered DRPs were non-adherence and adverse drug reactions.
3.3.5. Studies on perceptions of health care practitioners towards medication errors
Three studies considered this topic; all were conducted in Saudi Arabia. Al-Rowibah et al. (2013) reported the findings on the impact of computerised physician order entry (CPOE): 72% of physicians agreed that CPOE helped them to decrease ADEs, but 55% reported that it created new types of errors. Al-Arifi, 2014 reported the results of a validated questionnaire to community pharmacists on dispensing errors. The majority of respondents indicated that the risk of dispensing errors was increasing and most of them were aware of dispensing errors. Al Anazi and Al-Jeraisy (2015) used a survey to collect information on healthcare professionals’ perceptions towards contributing factors of medication error (ME) occurrence. Some of the underlying factors of MEs reported were interruptions while writing the order, lack of clarity of physicians order, and no double-checking of the doses.
4. Discussion
4.1. Key findings
Fifty-four studies were identified that met our inclusion criteria; the majority were conducted in Saudi Arabia. No articles from Kuwait met inclusion criteria while only two were from the UAE. Notably, Kuwait is the only one of the six that is not a member of the Uppsala Monitoring Centre which is part of the WHO Programme for International Drug Monitoring (WHO Collaborating Centre for International Drug Monitoring, 2017). Saudi Arabia is the largest and has the highest population of the GCC countries, all of which are factors that may account for the larger number of studies from this country. None of the included studies had a qualitative study design, and it may therefore be important to supplement quantitative studies with qualitative research to help understanding of the causes of medication errors and other problems, as well as identifying barriers and facilitators to addressing them.
4.2. Interpretation
Only one study was related to the community pharmacy setting (Al-Arifi ,2014). The studies concerning incident reporting were all in tertiary care hospitals in large cities of Saudi Arabia, with only one study educating staff on usage of a medication error reporting tool in Qatar. It is important to encourage a culture of ‘no blame’ across the GCC, and attempts to report and therefore analyse incidents in smaller healthcare institutions would help identify unique issues faced by the practitioners in these establishments. There was a lack of medication safety studies in health care settings of rural areas. Few studies had a multi-site setting described, which would make generalisation of results more difficult. In addition, studies identified errors and provided recommendations but there were few follow up studies of strategies to address them. Multi-disciplinary research is a concept that could be integrated within curricula of medical schools, pharmacy schools and allied health sciences for enhanced collaboration in focusing on medication safety. Lack of standardised terminology related to ME studies has been reported internationally (Lisby et al., 2010) and the studies included in this review also used a variety of definitions to determine study outcomes as shown in Appendix C. Even among younger patients or elderly patients, there is a variety of definitions used as to what comprises a medication error or what comprises a drug related problem, even from within the same country. This is in agreement with (Alsulaimi et al., 2013).
4.3. Recommendations
These findings highlight the need to attempt to standardise GCC terminology related to ME and ADRs and create a unified platform to establish patient safety. It must also be kept in mind that in studies of voluntary reporting, the hospital culture and environment must be considered. Furthermore these reported numbers are an underrepresentation of reality as data from rural areas has not been reported, perhaps incentive scheme for reporting in rural health areas should be explored. Patient interactions, factors which would affect their adherence are worth exploring, various studies reviewed identified that patient non-compliance was a factor in emergency department use, and in interventional studies, only 10% of interventions were recorded on the patient level. Understanding of whether there are specific reasons and identifying of issues existing with patients in the GCC is a step closer to developing practical solutions.
In recent years, advances in technology has played a big part in evolution of medication prescribing and administration; research evaluating the use of such technology should be encouraged and supported. The majority of studies were descriptive (n = 43) while only 11 were experimental in nature i.e. evaluating some kind of intervention.
5. Strengths and limitations
The strengths of this review are that it is the first systematic review focussing on ME research within GCC; due to expected paucity of studies, conference abstracts were reviewed as well as full text journal articles, and thus the search was very comprehensive. While it was difficult to assess the quality of the included abstracts, we used the same criteria as for the full text articles.
Limitations are that no quality threshold was in place and so full text articles as well as abstracts were included if they met inclusion criteria without regard to assessment of their quality. This was done to ensure all potential studies related to ME were included; while it allows us to present a comprehensive picture of research within the GCC it does mean that some included studies were of a higher quality than others. Due to the lack of standardisation, whether in terminology used or type of data collected, or age groups defined as ‘adult’ or defined as ‘child’ it was not possible to conduct any form of meta-analysis on the studies identified. A kappa value was not calculated for the quality assessment but the authors ensured that every article included was assessed by two reviewers and any differences were discussed and resolved. Also, the degree or the magnitude of harm caused by improper medication use was not assessed consistently in studies and could not be reported.
6. Conclusions
This systematic literature review highlights several findings. The first finding is that the literature on ME in the GCC is very diverse, with a wide range of definitions, denominators and measurement approaches. This also meant it was not possible to calculate an overall incidence of medication error. Future studies could be improved to provide wider impact and a clearer rationale. Some suggestions are to enhance coordination between healthcare colleges in the region, and to strengthen research methodologies to increase validity of results and allow them to be placed in context, for example increase follow up studies. Other suggestions are to place more emphasis on research of medication errors in the community pharmacy setting. Increased understanding of patient behavior and medicine management is also justified. Medication error research is generally increasing in the region, several unique issues are noteworthy to be explored for a better understanding of errors occurring and solutions to overcome them.
Funding statement
Bryony Dean Franklin is supported by the National Institute for Health Research Imperial Translational Research Centre (NIHR Imperial PSTRC) and affiliated with the NIHR Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, Public Health England or the Department of Health.
Conflict of Interest:
Bryony Dean Franklin is supervising a PhD student who is part funded by an electronic prescribing vendor.
Acknowledgement
Acknowledgement to Mansour Adam Mahmoud for his assistance during the full text article review process, and to Medication Safety Research Chair, Vice Deanship of Research Chairs.
Footnotes
Peer review under responsibility of King Saud University.
Appendix A. Search terms used and databases searched
| Search term (terms) | Number of hits |
|---|---|
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR “Saudi Arabia” OR “United Arab Emirates” AND “Patient Safety” OR “Medication Safety” OR “Medication Reconciliation” OR Pharmacovigilance |
CINAHL = 62 Embase = 740 IPA = 24 PubMed = 654 Science Direct = 356 Web of Science = 79 |
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR “Saudi Arabia” OR “United Arab Emirates” AND “Adverse drug reaction” OR “Adverse Drug Event” |
CINAHL = 14 Embase = 343 IPA = 12 PubMed = 45 Science Direct = 160 Web of Science = 20 |
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR “Saudi Arabia” OR “United Arab Emirates” AND “medication error” OR “Prescribing error” OR “prescribing mistake” |
CINAHL = 1 Embase = 139 IPA = 4 PubMed = 13 Science Direct = 92 Web of Science = 11 |
| G.C.C “OR Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates AND “dispensing error” OR “dispensing mistake” |
CINAHL = 0 Embase = 1 IPA = 1 PubMed = 1 Science Direct = 9 Web of Science = 1 |
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR “Saudi Arabia” OR United Arab Emirates“ AND ”Medication administration error“ OR ”medication administration mistake“ |
CINAHL = 0 Embase = 1 IPA = 0 PubMed = 1 Science Direct = 2 Web of Science = 1 |
| “ G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates) AND “medication error” OR “drug error” OR “drug mistake” | CINAHL = 1 Embase = 139 IPA = 3 PubMed = 15 Science Direct = 76 Web of Science = 10 |
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR “Saudi Arabia” OR “United Arab Emirates” AND “transcription error” OR “transcription mistake” |
CINAHL = 0 Embase = 1 IPA = 0 PubMed = 0 Science Direct = 17 Web of Science = 0 |
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR “Saudi Arabia” OR United Arab Emirates AND “monitoring error” OR “monitoring mistake” |
CINAHL = 0 Embase = 0 IPA = 0 PubMed = 0 Science Direct = 2 Web of Science = 0 |
| “G.C.C” OR “Gulf Cooperation Council” OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates AND “wrong drug” OR “wrong drug error” OR “wrong drug mistake” |
CINAHL = 0 Embase = 6 IPA = 1 PubMed = 4 Science Direct = 12 Web of Science = 2 |
| G.C.C“ OR ”Gulf Cooperation Council“ OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates AND ”dosage error“ OR ”wrong dose“ OR ”dosage mistake“ |
CINAHL = 0 Embase = 5 IPA = 2 PubMed = 4 Science Direct = 14 Web of Science = 2 |
| G.C.C“ OR ”Gulf Cooperation Council“ OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates AND Cause of medication error |
CINAHL = 0 Embase = 1 IPA = 0 PubMed = 4 Science Direct = 5 Web of Science = 0 |
| “G.C.C“ OR ”Gulf Cooperation Council“ OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates AND Preventable drug related problem |
CINAHL = 0 Embase = 0 IPA = 0 PubMed = 2 Science Direct = 1 Web of Science = 0 |
| “G.C.C“ OR ”Gulf Cooperation Council“ OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR United Arab Emirates AND Risk factors for medication error |
CINAHL = 0 Embase = 0 IPA = 0 PubMed = 3 Science Direct = 0 Web of Science = 0 |
| DataBase | Total Number of hits from each database |
|---|---|
| CINAHL | 78 |
| EMBASE | 1376 |
| IPA | 47 |
| PubMed | 742 |
| Science Direct | 746 |
| Web of Science | 126 |
| Total = 3115 | |
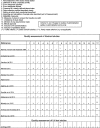
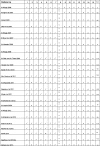
Appendix B. Quality assessment of included studies
The numbers across the top of the table relate to the criteria which were used to assess quality of studies. The first twelve studies are published as conference abstracts, the remaining 42 are full text journal articles.
 |
 |
 |
Appendix C. Definitions used for outcomes by studies included in the review
| Term | Definition | Definition reference | Used by |
|---|---|---|---|
| Medication error | Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in control of healthcare professional, patient, or consumer | NCCMERP (1995, 2005, 2008, 2012) | Al Khaja et al., 2005, Elnour et al., 2007, Hooper et al., 2009, Khoja et al., 2011, Arabi et al., 2012, Alshaikh et al., 2013, Alakhali et al., 2014, Mahmoud et al., 2016, Alghamdy (2015), Aljadhey et al. (2012)a |
| A failure in the treatment process that leads to, or has the potential to lead to, harm to the patient | Ferner and Aronson (2006) | Al Khaja et al. (2012) | |
| A medication error was defined as an error in the medication process: ordering, transcription, dispensing, and administration, and discharge summaries | Bates et al. (1995)a | Almazrou et al., 2015, Aljadhey et al., 2016 | |
| A medication error was defined as an error in the medication process: ordering, transcription, dispensing, and administration, and discharge summaries. Errors included wrong as well as missing actions | Lisby et al. (2010) | Alakhali et al. (2014) | |
| Medication errors were defined as errors in drug ordering, transcribing, dispensing, administering or monitoring | Kaushal et al. (2001) | Aljeraisy et al. (2011) | |
| Medication errors were defined as any preventable error in the medication administration process starting from prescribing and including preparing, dispensing, administering, monitoring the patient for effect, and transcribing (eg medication administration record (MAR) | Miller et al. (2007) | Aljeraisy et al. (2011) | |
| A dose of medication that deviates from the physician’s order as written in the patient’s chart or from standard hospital policy and procedures | American Society of Health system pharmacists ASHP (1982) | Sadat-Ali et al. (2010) | |
| Prescription error | Prescription error categories:
|
Shaughnessy and Nickel (1989) | Khoja et al. (2011), Al Khaja et al. (2005), Al Khaja et al. (2007) |
| Prescribing error | A clinically meaningful prescribing error occurs when, as a result of a prescribing decision or prescription writing process, there is an unintentional significant
|
Dean et al. (2000) | Al Khaja et al., 2012, Al-Dhawailie, 2011 |
| Prescribing errors identified were dealt with in one of two ways: (1) if medication orders were ambiguous but the pharmacist could determine the medication intended, he or she would endorse the drug chart accordingly; (2) if the pharmacist was not certain of the medication intended or if the error concerned more fundamental errors in the choice of drug or dose, the prescriber would be contacted to resolve the issue | Dean et al., 2002a, Dean et al., 2002b | Al-Dhawailie (2011) | |
| Prescribing errors identified were dealt with in one of two ways: (1) if medication orders were ambiguous but the pharmacist could determine the medication intended, he or she would endorse the drug chart accordingly; (2) if the pharmacist was not certain of the medication intended or if the error concerned more fundamental errors in the choice of drug or dose, the prescriber would be contacted to resolve the issue | Dean et al. (2002b) | Al-Dhawailie (2011) | |
| Classification of prescribing errors as type A, Type B, Type C, Type D | Neville et al. (1989) | Khoja et al. (2011), Al-Hussein, 2008, Altebenaui et al., 2015) | |
| Prescribing errors may be defined as an incorrect drug selection for a patient | ASHP (1993) | Elnour et al. (2007) | |
| Dispensing error | Dispensing errors are mistakes made by staff when distributing medications to nursing units or directly to patients in an ambulatory-care pharmacy | Allan and Barker, 1990, Flynn et al., 2002 | Elnour et al. (2007) |
| Dispensing errors are defined as any inconsistencies or deviations from the prescription order such as dispensing the incorrect drug, dose, dosage form, wrong quantity, inappropriate incorrect or inadequate labelling, confusing or inappropriate preparation, packaging, or storage of medication prior to dispensing | Szeinbach et al. (2007) | Al-Arifi (2014) | |
| Administration error | A drug administration error is error or omission or commission that occurs in the administration stage when the medication has to be given by a nurse, the patient himself or herself, or a caregiver | Flynn et al. (2002) | Elnour et al. (2007) |
| Administration error | An opportunity for error is defined as any drug prescribed, any unordered or omitted drug, and any dose given and any dose omitted | Definitions utilised but not referenced | Aljamal (2012) |
| Medication reconciliation errors | Discrepancies were classified as omissions (not ordering a medication used by a patient prior to admission); commission (adding a medication not used prior to admission); or wrong dose, frequency, or route of administration | Definitions utilised but not referenced | Abu Yassin et al. (2011) |
| Drug related problem | A drug related problem is an undesirable patient experience that involves drug therapy and that actually or potentially interferes with a desired patient outcome | Strand et al. (1990) | Al-Olah and Al Thiab, 2008, Al-Arifi, 2014, Alghamdy et al., 2015 |
| An event or circumstance involving drug therapy that actually or potentially interferes with the desired health outcome | Pharmaceutical care network Europe (PCNE) (1999, 2008, 2010, 2012) | Hooper et al., 2009, Rashed et al., 2012), Rashed et al. (2013), Kheir et al. (2014), Alghamdy et al. (2015), Al Hamid et al. (2016) | |
| Adverse drug event (ADE) | ADEs are any noxious, unintended, and undesired reaction that occurs because of a drug in doses normally used in man for prophylaxis, diagnosis, or therapy of disease or for the modification of physiologic function, or that is caused by drug interactions, allergic drug reactions and medication errors | WHO (1999) | Al-Tajir and Kelly (2005) |
| An adverse drug event is an injury caused by a medication, which include both adverse drug reactions (an effect which is noxious and unintended, and which occurs at doses used in man for prophylaxis, diagnosis or therapy) as well as harmful effects arising from errors at any stage including ordering, transcribing, dispensing, administering, or monitoring of a drug | Bates et al. (1995b), Jha et al. (1998), Gandhi et al. (2000) | Aljadhey et al. (2013)a, Aljadhey et al. (2016) | |
| An ADE was defined as an injury due to a medication, including both adverse drug reactions and injuries caused by medication errors | Nebeker et al. (2004) | Al-Ghamdi et al. (2012) | |
| Potential adverse drug event | A potential adverse drug event is a medication error with the potential to cause an injury but which does not actually cause any injury, either because of specific circumstances, chance, or because the error is intercepted and corrected | Morimoto et al. (2004) | Aljadhey et al. (2013)a, Aljadhey et al. (2016) |
| Non preventable adverse drug reaction | Non preventable adverse drug reactions, also known as adverse drug reactions, are defined by the WHO as ‘a response to a drug which is noxious and unintended, and which occurs at doses used in man for prophylaxis, diagnosis, or therapy of disease, or for the modifications of physiological function | WHO (2014) | Aljadhey et al. (2016) |
| Potentially Inappropriate medications | Potentially inappropriate medications are medications in which the risks of use outweigh the benefits | Rancourt et al. (2004) | Al-Omar et al. (2013) |
| Beer’s criteria-Potentially inappropriate medication list as updated by Fick and colleagues in 2003 | Fick et al. (2003) | Al-Omar et al. (2013) | |
| American Geriatrics Society Beers Criteria Update Expert Panel (2012) | Al Odhayani et al. (2016) | ||
Appendix D. Data extracted from included studies
| Reference | Country | Design | Definitions used for study outcomes | Method of error identification | Medication safety aspect analysed | Reported results |
|---|---|---|---|---|---|---|
| ||||||
| Al Khaja et al. (2005) | Bahrain | Retrospective clinical prescription review | A medication error is defined as (NCCMERP, 2012) | Audit of prescriptions to screen for errors (for a two week period) | Identification of prescribing errors and their determinants in a primary care setting | 77,511 prescriptions audited, 5959 (approximately 8%) were identified to contain errors. The 5959 prescriptions contained 16,901 medications, of which 13,630 (approximately 85%) were with errors. Major errors of omission associated with topical preparations were significantly higher than those with systemic preparations. However, prescriptions with systemic preparations had a higher rate of commission errors |
| Irshaid et al. (2005) | Saudi Arabia | Retrospective analysis of prescriptions | None used | Prescriptions were analysed for the essential elements to be included in the prescription order and the data recorded using a coding key. The information within the prescription was judged “unclear” if one word was not written clearly and “unreadable” if none of the three investigators present during the screening session could read it | Prescriptions written by physicians were screened for the stated essential elements | Three thousand seven hundred ninety-six prescriptions analysed, approx. seven percent of the total prescriptions written during that period. The name and signature of the prescriber was included in approx. 83% and 82% of prescriptions respectively. The handwriting of the prescriber was not clear in approx. 64% of the prescriptions. The strength of the medications was included in approximately 26% of the prescriptions |
| Al Khaja et al. (2006) | Bahrain | Cross- sectional prescription review | Major errors of omission, major errors of commission were defined as per Al Khaja et al. (2005). The British national formulary (#37; 2002) was referred to for the dose ranges and paediatric doses were calculated as per Insley, 1996 | Prescriptions issued to infants were analysed for prescribing pattern and prescribing errors | Nationwide study evaluated the prescribing profile and prescribing errors of antimicrobials in infants, in primary care | Of 2282 dispensed prescriptions, 543 included an antimicrobial agent. Of these: 119 were prescribed at subtherapeutic doses 28 at supratherapeutic doses. The remainder 394 at therapeutic doses Major errors of omission from prescription involved duration of therapy, dosage form. Errors of commission commonly included dosing frequency and dosage of antimicrobials |
| Al Khaja et al. (2007) | Bahrain | Retrospective clinical prescription review | Definitions were as per Al Khaja et al. (2005) | Prescriptions issued for infants were collected and reviewed over a 2-week period from 20 health centres | The trends in drug utilisation including off-label drug prescribing and the prevalence, and the type of medication-related prescribing errors | In 2282 dispensed prescriptions a total of 5745 medications were included. 2066 (approximately 91% of the prescriptions) were identified to contain major errors of omission, commission, and errors of integration. Errors of omission accounted for approximately 54%, and included length of therapy/quantity and dosage form. Errors of commission accounted for approximately 44%, commonly incorrect dosing frequency and incorrect dose/strength. Prescribing of an off-label drug was observed in approximately 16% of cases |
| Alkhaja et al. (2008) | Bahrain | Prospective collection of prescriptions for two consecutive cohorts | Prescribing errors were defined according to the definitions of: | All prescriptions issued by 12 final year residents in May 2004 and 14 final year residents in 2005 were collected. Prescriptions were screened by one author of this study, and subsequently audited by another author | The percentage of omission commission and integration related errors in prescriptions written by final year residents were calculated | A total of 2692 prescriptions were collected. Eighty-eight percent were identified to include major errors of omission. Dosage form and length of treatment were frequent omissions. As for errors of commission, dosing frequency was the most common incorrectly stated component |
| Al-Hussein (2008) | Saudi Arabia | Cross-sectional audit of prescriptions | Nonconformities were classified according to the component of prescribing process involved… patient, provider, prescribing, drug/dispensing or others (as described by study author). A second classification was used according to that of Neville et al. (1989) |
Prescriptions were collected during audits done fortnightly by sampling random selection of 30 prescriptions, for a total of 330. Information about each prescription was entered in a database by the pharmacists and based on yes-no answers to status of compliance to the indicators, an automated decision was made on conformity | To explore the degree of prescription conformity to the prescribing guidelines at primary care. This is in order to develop and incorporate a systematic process in prescription errors in primary care and provide the health care providers with feedback | Approximately 13% of prescriptions fully conformed to the given guidelines, while the remainder (87%) did not conform. Less than 1% of the inconsistencies were potentially harmful to the patient, approx. 77% had possible negative effect on the pharmacist’s work. Patient information was deficient in approx. 17% of cases |
| Khoja et al. (2011) | Saudi Arabia | Cross-sectional audit of prescriptions prescribed or dispensed over one full working day | Defined prescription errors as per US Pharmacopeia, 1995 Utilised the classification of Neville et al. for system for classifying prescription errors to classify errors as type A, B, C or D Neville et al. (1989) |
Samples of prescriptions were analysed to obtain evidence about the nature and extent of errors. Prescriptions containing errors were allocated an error classification following a discussion between one investigator and pharmacists involved | Prescriptions errors and comparison between private and public sectors | Public clinics – 1182 prescriptions (2463 prescribed drugs). Private clinics – 1200 prescriptions (2836 prescribed drugs) = Total (5299) drugs. Prescribing errors were found on 990 (approximately 19%). Both type B and type C errors were more common in public than private PHC centres. Type D were more frequently found on private than public clinic prescriptions |
| Al-Dhawailie (2011) | Saudi Arabia | Not stated | The sited medication errors were categorized according to the definition of Dean et al., 2002a, Dean et al., 2002b | The inpatient medication charts and hand written orders were identified and rectified by ward and practicing pharmacists within inpatient pharmacy services. Data were collected and evaluated. The causes of problem were identified and dealt with in one of two ways | To detect the incidence of prescribing errors for hospitalized patient, to evaluate the clinical impact of pharmacist intervention on the detection of these errors | Of 1580 prescriptions, 113 (approx. 7%) were detected to contain prescribing errors and intervened by the clinical pharmacists. The errors of wrong strength and wrong administration frequency of the prescribed drug were the most errors reported (approx. 35%, and 23%, respectively). Other errors as wrong patient/ drug or wrong dose were also encountered. The prescribing errors encountered were of varying severity. Multiple factors were identified: lack of training for medical students about this during undergraduate studies, work load, stress and ineffective communication between healthcare professionals |
| Al-Jeraisy et al. (2011) | Saudi Arabia | Retrospective cohort study | A prescription error was defined per (Kaushal et al. (2001) as well as (Miller et al. (2007) and Lesar et al. (2006), Abushaiqa et al. (2007) and Kohn et al. (2000) | Physical inspection of physician medication and reviews of patients files | Determine incidence and types of medication prescription errors, and identify some potential risk factors in a paediatric inpatient tertiary care setting | Out of 2380 orders examined in the five week period, error rate was 56 per 100 medication orders (CI: 54.2%, 57.8%) Dose errors accounted for approx. 40% of these errors, while incorrect dose errors approx. 21%. The errors occurred more frequently with intravenous route of administration and one third occurred in paediatric intensive care unit |
| Al Khaja et al. (2012) | Bahrain | A retrospective, nationwide audit of prescriptions | Medication error has been defined according to the definitions of (Ferner and Aronson (2006) A prescribing error has been defined according to the definitions of (Dean et al., 2000) |
The eligible prescriptions were carefully audited by the first author and then independently reviewed by second and third authors. Discrepancies were resolved by discussion | This study was carried out to identify the frequency and nature of medication prescribing errors the medication prescribing errors pertaining to cardiovascular/antidiabetic medications in prescriptions written by primary care physicians | Two thousand, seven hundred and seventy-three prescriptions were analysed. Approximately 26% of prescriptions had medication prescribing errors. No significant differences with respect to overall errors were evident in prescriptions ordered by the family physicians and general practitioners. Prescribing errors commonly involved lipid lowering medications, β-blockers, high dose metformin and high dose glibenclamide |
| Al Shahaibi et al. (2012) | Oman | Observational, retrospective | None stated | Retrospective analysis of 900 prescriptions from four different hospitals. Each prescription was checked five times, once for the superscription errors, then second inscription, next for the subscription errors, followed by legal errors, and last for reviewing it all | To evaluate and analyse the handwritten outpatient prescriptions and associated error of omissions | Nine hundred handwritten outpatient prescriptions were analysed; a total of 1471 drugs were prescribed. Most common type of superscription error of omission was found to be age and gender. The most common inscription omissions were of dosage form and strength/dose of drug. Subscriptions omission were omission of prescriber’s signature, while date of dispensing medication was omitted in 100% of prescriptions |
| Aseeri (2013) | Saudi Arabia | Retrospective cohort study. | A dosing error was defined as per McPhillips et al. (2005). A dosing error was defined as the presence of an antibiotic dose that was 110% or more of the maximum recommended daily dose or below 90% of the minimum recommended daily dose | A retrospective cohort study of 300 randomly collected, physician-prescribed antibiotic order sheets was performed over a 2-week period within different settings in the tertiary hospital (inpatient unit, ambulatory care clinic, emergency department) 300 prescriptions collected in pre implementation phase and post implementation phase 300 sheets were collected |
To compare the rate of dosing errors for antibiotic orders in paediatric patients before and after the implementation of a standard dosing table. It is for oral or parenteral antibiotics with pre-calculated dosage for different weight ranges | Physician compliance with the antibiotic dosing standardization policy after implementation was 62%. The dosing standardization policy reduced the rate of dosing errors from approx. 34% to approx. 5% (P = 0.0001), and weight documentation on the antibiotic prescriptions improved from approx. 65% to approx. 85% (P = 0.0001) |
| Albarrak et al. (2014) | Saudi Arabia | Prospective study | None stated | Handwritten prescriptions were received from three outpatient departments whereas electronic prescriptions were collected from the paediatric ward. The handwritten prescriptions were evaluated for completeness and legibility by two pharmacists. The comparison between handwritten and electronic prescription errors was assessed based on the validated checklist adopted from previous studies. Delgado et al. (2007), Bobb et al. (2004), Al-Jeraisy et al. (2011). The prescriptions were evaluated for legibility by two pharmacists according to a three point legibility scoring Likert scale similar to the study Mendonca et al. (2010) | To assess the legibility and completeness of handwritten prescriptions and compare with electronic prescription system for medication errors | Three hundred ninety-eight prescriptions (199 handwritten, 199 electronic prescriptions) were assessed. Seventy-one (approx. 36%) of handwritten and 5 (approx. 3%) of electronic prescriptions were identified to contain errors. A significant statistical difference (P < 0.001) was observed concerning omitted dose and omitted route of administration. The rate of medication prescription completeness in handwritten prescriptions approximately ranged from 88% to 91% from the three different clinical units. In handwritten prescriptions there were drug interactions evident but in the electronic prescriptions drug interactions were not reported |
| Altebenaui et al. (2015) | Saudi Arabia | Not stated | As per Neville’s classification (1989). Prescription errors were classified as major (potentially life threatening), minor (non-life threatening) or trivial | Retrospective cross sectional analysis of physician prescriptions that were issued over a one month period. One thousand prescriptions were randomly selected for review | Identifying the types and frequency of prescription errors from different departments, outpatient and emergency room (ER). | Patient file numbers and medication dosages were missing in more than 20 and 40%, of reviewed prescriptions, respectively. At least 30% of the reviewed prescriptions were deemed to have had illegible handwriting. Non-life threatening items including age, physician signature and stamp, date, sex diagnosis were missing in more than 50%. Weight was missing from all transcripts. Prescriptions written by ER physicians had more missing items compared to those wrote by outpatients clinics (P = 0.01) |
| Youssef et al. (2015) | Saudi Arabia | Retrospective study | To calculate renal function the modification of diet in renal disease (MDRD) formula was used, and Cockgroft Gault, as per National Institute of Diabetes and Digestive Kidney Diseases (2014) | Detailed prescriptions were abstracted from the electronic medical record. Examination of the data was performed for medications that are renally cleared and/or potentially nephrotoxic. These medications were then categorized according to the CDSS internal database into two types, as a contraindicated medication OR not a contraindicated medication | Determination of various types of contraindicated medications that are administered to patients with renal insufficiency by physicians who override alerts provided by the Computerized Decision Support Systems (CDSS) | Out of the 314 prescriptions that were renally cleared and/or potentially nephrotoxic, 44 (14%) were for contraindicated medications. The contraindicated medications ordered were limited to: aspirin, gliclazide, nitrofurantoin; and spironolactone |
| Alanazi et al. (2015) | Saudi Arabia | Cross-sectional | Not stated | Reviewing charts and prescriptions of patients complaining of infections. The prevalence of an inappropriate antibiotic prescription was accounted as a physician order with at least one type or more of errors divided by total number of prescriptions and multiplied by 100 | Study purpose was to assess the prevalence and predictors of antibiotic-related prescription errors among patients admitted to an emergency centre at a tertiary health care facility | Adults (>15 years) were approx. 61%, whereas paediatrics (<15 years) were approx. 39%. Majority of patients were not screened for antibiotic allergies (approx. 92%). Three main antibiotic categories were prescribed in both age groups: penicillin, cephalosporin, and macrolide. The prevalence of inappropriate antibiotic prescriptions with at least one or more types of errors was approx. 46%, which was significantly higher among paediatrics compared to adults (P = 0.001). Physicians tend to prescribe antibiotics with higher than the recommended dosages and/or frequencies |
| Mahmoud et al. (2016) | Saudi Arabia | Retrospective chart review chart study | (National Coordination Council for Medication Errors Reporting and Prevention NCC MERP) index (2005) | Four month retrospective chart review chart study. The severity of prescribing errors was determined by two independent reviewers | Aim was to determine the incidence of prescribing errors using a validated definition. The main study outcomes were the percentage of medication orders and hospital admissions with prescribing errors; and the types of prescribing errors | Six hundred ninety-one prescribing errors were in 2033 patient files. The incidence of prescribing errors was 3.6 (95% CI, 3.3–3.9) per 100 prescriptions. Per 100 admissions the prescribing error incidence was 33.9 (95% CI, 31.5–36.6) and 76.5 (95% CI, 70.9–82.3) per 1000 patients days. The most common prescribing error type was dosing errors, while antibiotics were the most common drug class involved with prescribing errors |
| ||||||
| Aljamal (2012) | Saudi Arabia | A cross-sectional prospective observational study | An opportunity for error is defined as any drug prescribed, any unordered or omitted drug, and any dose given and any dose omitted. Disguised method was used. Definitions utilised but not referenced | Medication administration error was calculated by dividing actual errors by the total number of opportunities for errors. Disguised method was used. The nurses were accompanied during medication administration. These medications were then registered and compared with eligible prescriptions in the medication chart | The objective of this study was to assess the frequency, type, and potential clinical consequences of medication administration errors in a tertiary hospital | A total of 169 medication administration errors were observed out of 2112 opportunities for error, representing an error rate of eight percent. Five types of errors were detected including dose omission (35%), wrong dose (5%), wrong drug (2%), wrong technique (1%) and wrong time (57%). Majority of errors did not cause harm and six errors were prevented before reaching patients |
| Almazrou et al. (2015) | Saudi Arabia | A cross-sectional observational study | A medication error was defined as per Bates et al. (1995) | Interviews of the mothers were performed by pharmacy students The mothers were required to demonstrate how to measure 5 mL of paracetamol syrup using a cup and a syringe and 1 mL of paracetamol syrup using a dropper while being observed, dosing errors were evaluated visually |
A cross-sectional study in which mothers were observed as they used a set of commonly available dosing devices which are a dosing cup, syringe, and dropper | Of 575 participants these measured an accurate dose of paracetamol using: an oral dosing syringe 334 (58%) dropper-286 (50%) dosing cup. As for errors, participants measured more than the intended dose with the dosing cup and less than the intended dose with the dropper |
| ||||||
| Rehmani (2001) | Saudi Arabia | Prospective cross-sectional survey | None Specified | Nurses recorded a medication list during triage in the electronic medical record (EMR). This home medication list was not placed in the emergency department chart. Records were then reviewed by a physician. The research generated home medication list was compared to the standard medication list and the number of omissions, duplications, and dosing errors was determined | Evaluate the accuracy of medication history taking in emergency department triage | Two thousand one hundred seventy adults completed the survey (88% of patients approached). Discrepancies in medication lists obtained during triage were documented in (52%) of patients. The dosing or frequency error was (62.0%). Discontinued medications were included, additional medications were omitted, and patients reported taking a non-prescription medication not listed in the electronic medical record |
| Abu Yassin et al. (2011) | Saudi Arabia | A prospective observational study | Definition for discrepancies utilised but not referenced. Discrepancies were classified as omissions (not ordering a medication used by a patient prior to admission); commission (adding a medication not used prior to admission); or wrong dose, frequency, or route of administration | A pharmacist screened the patient’s chart and reviewed recent lab results and interviewed patients to acquire comprehensive medication history. All information obtained from patients was compared with medications recorded by the physician upon the patients’ admissions to the hospital | To investigate the role of pharmacists in identifying discrepancies in medication history at admission to hospitals | Sixty patients were interviewed, taking a total of 564 medications. At least one discrepancy was found in 37% of patients, and the most common discrepancies observed were omissions of medications and dosage errors |
| Al Anany et al. (2012) | Qatar | Not stated but from the description it is a cross sectional interventional study | None stated | Clinical pharmacists conducted interviews with the patient or caregiver in the first 24 h of admission or transfer to review their medications. The collected data were then compared with the current medication list prescribed after admission or transfer. The interventions were done through a medication reconciliation process on specially prepared form | To highlight the impact of medication reconciliation conducted by clinical pharmacists on reducing adverse drug events during admission and transfer by identifying different types of interventions | For the 52 patients interviewed, the total number of medications reconciled was 263. Of these, 93 medications (35%) required the intervention of clinical pharmacists. Omission was the most common type of error, followed by wrong doses and medications with no indication |
| Aljadhey et al. (2013)b | Saudi Arabia | Observational Cross-sectional | None stated | Discrepancies (number and type) were recorded in a data collection sheet. Then the discharge counselling pharmacist conducted medication reconciliation by comparing the discharge medication list with the best possible medication history provided by hospital pharmacy records | To identify the discrepancies number and type upon conducting discharge reconciliation | One-hundred and seventy-three patients were screened and 568 discrepancies were identified in 121 patients, with a mean of 4.7 ± 2.8 per patient. Eighteen percent of patients presented with at least one unintentional discrepancy, which were omission, commission, changed frequency, duplication and wrong duration |
| Sonallah et al. (2014) | Qatar | Retrospective, descriptive and post-interventional study | None stated | A standardized medication reconciliation form was developed and used by clinical pharmacists as a tool to detect the number and types of medication discrepancies and document clinical pharmacist interventions | This study was conducted to evaluate the medication reconciliation (MR) process as a newly initiated service by clinical pharmacists. | Two hundred thirty-two forms were collected and 1640 medications were reconciled. One hundred and seventy-eight cases (approximately 77%) had medication discrepancies upon hospital admission, Most of the discrepancies were due to medication omissions, incorrect dosages, and different medications. Clinical pharmacists’ interventions were carried out in 150 cases (approx. 65%) |
| ||||||
| Al-Omar (2013) | Saudi Arabia | A retrospective review of prescription records | Potentially inappropriate medication (PIM) defined as the definition of: Rancourt et al. (2004) Beers criteria (as updated by Fick et al. (2003) were used to identify the PIMs |
The source of the data was outpatient pharmacy prescription records at Riyadh Military Hospital (RMH) for 2002, 2003 and 2004. Data were re-coded, new variables were created and the total cost of medications was calculated | To explore the prevalence of (PIM) use in the elderly, to identify the trends and patterns of prescribing such medication, and to calculate the associated direct medication cost of such practice in Saudi hospital | A total of 20 521 PIM were identified. The prevalence of PIM for 2002, 2003 and 2004 was approx. 3%, 2% and 2%, respectively. A total of approx. 43% of the patients had filled a prescription of one PIM, the remainder had filled a prescription with 2 or more PIM. Digoxin accounted for approx. 24% of these PIM. Other medications involved were cardiovascular drugs, iron supplements, and laxatives. The total direct cost that was associated with inappropriate prescribing was 518 314 Saudi Riyals (United States $138 217, where one US dollar = 3.75 Saudi Riyal) |
| AlOdhayani et al. (2016) | Saudi Arabia | Retrospective | The study participants were elderly, as defined by the World Health Organisation (WHO, 2011) Beers crtieria (Fick et al., 2003) was used to determine the number of PIMs |
Data were collected from patients’ medical electronic and non-electronic records, and from the main hospital laboratory framework. The number of PIMs was determined by using Beers criteria 2012 and a review of the literature | This study aimed to establish the extent of inappropriate drug prescription for and use by elderly patients | Of the 798 included patients; 419 were using one or more PIMs. The most common PIM was a high dose of ferrous sulphate, in about 33% of the participants compared to the rest of the group (P < 0.001). Analgesics, opioids, antispasmodics and muscle relaxants, were frequently prescribed |
| ||||||
| Dibbi et al. (2006) | Saudi Arabia | A retrospective review of patient medical records | None stated | Retrospective review of medical records for adult hospitalised patients 18 month period | The study focused on types, causes, contributing factors, frequency of medication errors and patients outcome | Two thousand six hundred twenty-seven medical records were reviewed and 3963 errors were identified. One thousand five hundred fifty-nine files contain one error, 800 files with 2 errors and 268 with 3 or more errors. The most common was wrong strength (confusion between microgram and milligram). Other errors included wrong route of administration, wrong dosage form and wrong dose which included over, under and extra doses. Medication errors were possible to be one of the related factors among 26 deaths Causes of error cited- Human factors as miscommunication (including misinterpretation of order and written miscommunication) |
| Elnour et al. (2007) | United Arab Emirates | Prospective interventional study | A medication error is defined by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP 2005) Prescribing errors defined as per American Society for Hospital Pharmacists ASHP (1993) Dispensing errors defined as per Allan and Barker (1990) and as per Flynn et al. (2002) Drug administration error defined as per Flynn et al. (2002) |
A systematic random sample of the inpatient nursing staff completed a structured program consisting of pre/post self-reported questionnaire on a new medication safety program (Med Safe Tool) for medication error reporting The generated medication errors reported were edited by the clinical pharmacists, and root cause analysis was performed. The research clinical pharmacists reviewed and assessed the accuracy of each true medication error incident report |
Demonstrates the benefits of implementing a computerized medication safety program (Med Safe Tool) with regard to reporting all types of medication errors | The number of medication errors reported to the Med Safe Tool before the program (n = 41) versus (n = 57) after the structured program. There were 9 types of medication errors (Most errors occurred during the medication administration stage]. Most of the medication errors pertain to the outcome category and severity code B, as per NCC MERP 2005 |
| Sadat-Ali et al. (2010) | Saudi Arabia | Retrospective | The definition of medication error was of Health System Pharmacists (ASHP, 1982) | The incident reports during two year period were collected and analysed for pertinent data. The medical charts were evaluated | The prevalence and characteristics of medication errors reported | Twenty-three thousand and nine hundred fifty-seven patients admitted and 38 medication errors reported. Most common errors: missed medication, expired medication, wrong time of medications There were three adverse events where the patients had extended hospital stay. No patients died or experienced permanent harm |
| Hemida, et al. (2011) | Saudi Arabia | Observational study | None specified | All incident reports that were voluntarily reported from the neonatal intensive care unit were reviewed for medication errors From these reports, the incidence and nature of medication errors was estimated | To study the nature of medication errors of neonates admitted to level III neonatal intensive care unit (NICU) | There were 66 incident reports involving medication errors with estimated incidence of one per 250 admissions. Most prevalent type was dispensing error (91%). Nurses were involved more commonly than pharmacists and physicians. The most common type of MEs for nurses: delay/not giving; pharmacists: delay /not dispensing and physicians; incomplete prescriptions. The most common medications involved were antibiotics and total parenteral nutrition |
| Arabi et al. (2012) | Saudi Arabia | Descriptive study of paper based incident reports | Medication error as per National Coordination Council for Medication Errors Reporting and Prevention NCC MERP) index (2008) | Evaluated submitted incident reports from all hospital areas including the intensive care unit for one year period | To examine the rates and categories of incident reports, both hospital-wide and in the intensive care unit (ICU) | There were 38,171 hospital admissions. Total of 3041 incident reports from all hospital areas, yielding a rate of 5.8 per 1000 patient days. Medication errors accounted for approximately 7% and 13% of all incident reports from the hospital and the ICU respectively |
| Alshaikh et al. (2013) | Saudi Arabia | Cross sectional review of occurrence/variant reports related to medication errors | (National Coordination Council for Medication Errors Reporting and Prevention NCC MERP) index (2008) | All occurrence/variant reports related to medication errors were documented on a hospital web-based medication error form that was designed to capture information on all aspects. Medication error reports were reviewed and reported at quarterly intervals over a 1-year period | The objective of the current study was to explore the rate of reporting medication errors and factors associated with the root causes of these errors in a large tertiary teaching hospital | The medication error rate over the 1-year study period was 0.4% (949 medication errors for 240,000 prescriptions). During this period, approx. 1.5% of the errors were categorized as resulting in any harm to the patient (all category E). Medication errors were reported predominantly at the prescribing stage (approx. 89%). Illegible or unclear handwriting (17%) was a reported cause of error |
| Al-Khani et al. (2014) | Saudi Arabia | Retrospective study including incorrect drug error reports | Medication prescribing error was defined as per Dean et al. (2000) | The study was a review of incorrect drug error reports for 21 month period. Reports were reviewed by two pharmacists to ensure accuracy of data classification | The objective was to explore factors that help pharmacists identify and thus prevent harm from incorrect drug prescribing errors in an ambulatory care setting. | During the specified period 2073 prescribing errors were reported in the hospital safety reporting system. Incorrect drug prescribing errors occurred at a rate of 10% (203 reports). Factors that allowed the pharmacist to identify incorrect drug prescribing errors before dispensing the medication include- reviewing the mandatory electronic prescription indication field, reviewing the patient medication history |
| Alakhali et al. (2014) | Saudi Arabia | Retrospective prescription review | A medication error was defined as per National Coordinating Council for Medication Error and Prevention (NCCMERP) 2005, and as per Lisby et al. (2010) | Retrospective study reviewing all the prescriptions for two months | To detect the medication errors in the different stages of medication use process such as prescribing, transcription, dispensing and administration | Total 1850 opportunities for errors registered. Prescribing errors were 10% of these errors, dispensing errors and administration errors both approx. 0.5% No transcription errors were observed |
| ||||||
| Al-Olah and Al Thiab (2008) | Saudi Arabia | Prospective observational study | A drug-related problem (DRP) was defined as per Hepler and Strand (1990). | On a daily basis, the investigators collected data on a data collection sheet for all emergency department (ED) admissions during the previous 24 h | To identify and evaluate admissions due to DRPs through the ED | Of 557 patients admitted through the ED, 82 were admissions due to DRP (approx. 15%). Fifty-three were definite, 29 were probable. The most common definite DRP admission was due to failure to receive medications followed by adverse drug reactions and drug overdose |
| Rashed et al. (2012) | Saudi Arabia | A prospective cohort study | DRP were defined as per the (PCNE) Pharmaceutical Care Network Europe, 2008) | DRPs were identified by a researcher reviewing the medical records of children attending the ED during a three month period | DRPs incidence in children attending an ED was calculated, preventability was determined and severity assessed | The results from KSA arm of the study: Total Patients (n = 143) Fifty-two DRPs identified; most common types were dosing problems followed by drug choice problems. Fifty-one of the 52 DRPs identified were preventable; and approx. 77% of minor severity |
| Al-Arifi et al. (2014) | Saudi Arabia | Prospective cohort observational study | Drug related problems (DRP) were defined according to the (Strand et al., 1990 | Information was taken by one of the authors from the patient file and/or patient interviewing using the specially designed data collection sheet | Aims were:
|
Random selection of 300 patients presenting to emergency department of which, 56 (approx. 19%) were presented to ED due to DRPs. The most common DRPs was due to adverse drug reactions (approx. 30%) and patients’ non-compliance (approx. 30%), followed by untreated indication then drug interactions; supratherapeutic and subtherapeutic dose. It was noted that adverse drug reaction incidence was almost double in female patients than male (11:6) |
| Alghamdy et al. (2015) | Saudi Arabia | Retrospective review of medical records of selected emergency department admissions | Definitions of DRP: (Strand et al., 1990) While medication error is defined as (Lisby et al., 2010) and (NCCMERP 2012) |
Files of suspected cases of DRPs reporting to ED in the 12 month period were scrutinized. Suspicion arose from the hospital record system based on Diagnosis Code Numbers (ICD-9-CM, Professional 2010) and from triggers, such as some drugs, laboratory tests, and signs and symptoms pointing to DRPs | To estimate prevalence of admissions as a result of DRPs at the emergency department (ED) | Of 5574 admissions, 253 were DRPs. They were categorised as: non-compliance to treatment (approx. 44%), overdose toxicity and side effects of drugs (approx. 20%), drug-interactions (approx. 12%), accidental and suicidal drug ingestions (approx. 10%), drug allergy (4%), Over 60% of DRPs were preventable and approx. 4% of patients died |
| Al Hamid et al. (2016) | Saudi Arabia | Retrospective medical record review | Medicine-related problem (MRP) is defined as per Pharmaceutical Care Network Europe (PCNE 2010) | A data collection tool was developed based on the Pharmaceutical Care Network Europe (PCNE) classification tool (PCNE 2010). The tool was used to extract data from each medical record | The aims of this study were to:
|
Out of 150 medical records reviewed, 94 medicine related problems were identified of which approx. 67% resulted in hospitalisations. Commonly encountered medicine related problems were treatment effectiveness and adverse drug reactions, accounting for approx. 98%. Polypharmacy was a major risk factor associated with medicine related problems. Insulin was implicated in approx. 47% of MRPs while oral antidiabetic agents. Approximately 34% of the MRPs were related to cardiovascular medicines, including antihypertensive (i.e., ACEIs, CCBs), anticoagulants (aspirin), antiarrhythmic (beta blockers and digoxin), and antihyperlipidemics (statins) |
| ||||||
| Al-Tajir and Kelly (2005) | United Arab Emirates | Prospective cohort study | Definition for ADE according to WHO (WHO, 1999) | The incidence of ADE was detected through spontaneous reporting the first and last quarter of the year 2003. During the second and third quarters, active monitoring for ADEs took place. ADEs were identified by looking for the documented events and by using an ADE trigger list (Used by Gandhi et al., 2001) ADEs were assessed for causality using the Naranjo algorithm (Naranjo et al., 1981) and for severity and preventability | The incidence of ADEs was calculated and the two different detection methods were compared | The incidence of ADEs detected through surveillance was significantly higher (P < 0.001) than for ADEs reported spontaneously for both inpatients (and outpatients. Most ADEs were judged to be of mild to moderate severity. Approx. 56% of ADEs were judged definite or probable and, of these, approx. 14% were consistently judged preventable. The most prevalent drugs implicated were central nervous system, antiinfective, and cardiovascular agents |
| Aljadhey et al. (2013)a | Saudi Arabia | Prospective cohort study | ADE as per (Jha et al., 1998) as well as Gandhi et al. (2000) A potential ADE as per (Morimoto et al., 2004) Medication error and category classification as per Medication Error Reporting and Prevention (NCC MERP) (2005) were defined as harm from medications |
Incidents were identified through a combination of medical record review by study pharmacists and voluntary reports from other healthcare professionals. Trigger tool was used to guide chart review further | Primary outcomes of this study were the frequency of, ADEs, potential ADEs and medication errors The secondary outcomes were the severity of these events, their preventability and the associated risk factors |
During the study period, there were 977 admissions with 9585 patient-days in the 5 study units. Pharmacists identified 361 incidents in 261 patients during the study period, of which the reviewers accepted 281. Approximately 30% of the accepted incidents were ADEs, judged definitely or probably preventable. Two hundred and twenty-three incidents were classified as medication errors, of which (approximately 59%) had the potential to cause harm. The incidence of ADEs in was 8.5 per 100 admissions. Preventable ADEs most commonly occurred in the ordering stage |
| Aljadhey et al. (2016) | Saudi Arabia | Prospective cohort study | Each incident was defined as an ADE (preventable and non-preventable), potential ADE (PADE) (which was classified as either intercepted or non-intercepted), or a medication error with low risk of causing harm. Used definitions adapted from Bates et al. (1995)a,b and Morimoto et al., 2004, World Health Organisation., 2014. |
Data collected from four hospitals (a teaching hospital, one large and one small government hospital and one private hospital), Incidents were identified through a combination of medical record review by study pharmacists and voluntary reports from other healthcare professionals. Two independent clinicians were provided with a study manual guide to independently review the incidents and decide on inclusion of incidents and further classify them as ADEs, PADEs or medication errors with low risk of causing harm. They were then able to assess severity and preventability. This was a methodology developed by the Brigham and Women’s Hospital’s Centre for Patient Safety Research and Practice Bates et al. (1995)a | Objective was to estimate the incidence and risk factors associated with ADEs and determine their severity and preventability. Primary outcomes were incidence of ADEs, PADEs and medication errors with low risk of causing harm. Secondary outcomes were severity of events, their preventability, and associated risk factors | Complete data for 3985 patients were analysed. One thousand six hundred seventy-six cases of ADEs, PADEs, and medication errors were identified. Physicians reviewed and accepted 1531 (approx. 91%). They were classified as: Approx. 40% medication errors with low risk of harm, approx. 44% PADEs, and approx. 16% ADEs. Of the ADEs, approx. 35% were deemed preventable. “Errors resulting from preventable ADEs were most common at the prescribing stage followed by the dispensing and administering stages. Most of the preventable ADEs were judged to be serious” |
| ||||||
| Rahman et al. (1994) | Saudi Arabia | Interventional study | Not stated (it’s an abstract) | The pharmacist, after confirmation with the physician, documented the intervention. A computer program was developed using FOXPRO. Interventions made during the one year study period were analysed | Pharmacist intervention, documentation and problem resolution of erroneous physician prescription | The intervention (error rate) was approx. 1%. Approx. 34% were dose related while 67% as minor in terms of severity. Anti-infectives were involved in approx. 21% |
| Al-Jazairi et al. (2008) | Saudi Arabia | Prospective, non-randomised observational study | None Stated | The clinical pharmacist performed daily multi-disciplinary rounds, with documentation of all interventions. At the end of the round the clinical pharmacist completed a data collection form to record each intervention given. A physician verified all interventions for validity and clinical significance | To evaluate the rate, (and clinical significance, acceptance by medical team) of clinical pharmacist’s interventions in a cardiac surgery intensive care setting | The clinical pharmacist made 394 interventions on 600 patients. The medical team accepted 328 interventions (approx. 83%). Main drug related problems and interventions were: no drug prescribed for the medical condition, inappropriate dosing regimen (including dose, rate, frequency, and route), no indication for drug use and inappropriate drug selection. The anticipated outcome of the interventions were targeted enhancing therapeutic outcomes, resolution/prevention of an adverse drug reaction or toxicity and cost saving |
| Hooper et al. (2009) | Qatar | Prospective, Interventional study | 1. Definition for a pharmacy intervention was: Working definition for intervention: ‘any contact made by a pharmacist during the dispensing process with a prescriber or a patient and that was aimed at rationalizing drug prescribing or use’ Medication errors defined as per Flynn and Barker (1999) Drug related problem defined as per PCNE (1999) 3. Considered a prescribing error as any prescribing decision which results, or had the potential to result in, an unintentional significant reduction in the probability of treatment being timely and effective, or an increase in the risk of patient harm |
Pharmacists used online integrated health care software (TrakCare®; InterSystems, Cambridge, MA, USA) to document all interventions made. Each intervention made was communicated to the respective prescriber. All interventions and their outcomes were reviewed later by two members from the research team | Prescribing error interventions documented by pharmacists in four pharmacies in a primary health care service in Qatar | Of 82,800 patients’ prescriptions, 594 patients’ prescriptions were intercepted for suspected errors (approx. 1%) The total number of DRP-related interventions made was 890 interventions Over half of all errors were related to drug choice problems, followed by drug safety problems. Fifty-three percent of all interventions were accepted. Interventions as a result of transcription errors, legality and formulary issues were eliminated from this study through the use of computerised physician order entry (CPOE) |
| Al-Rashdi et al. (2010) | Oman | Prospective interventional study | None stated (it’s an abstract) | Interventions on electronic prescriptions over one-year were evaluated. A standard data collection form was used to capture the relevant data. Clinical relevance was defined as to whether efficacy or toxicity was either improved or reduced. Clinical relevance was based on the judgments of at least two pharmacists | To evaluate the number and types of pharmacists’ interventions of electronic prescriptions at a University Hospital. | Out of 186,353 prescriptions, 454,654 items were dispensed and 1123 interventions were recorded. Only 3% of the interventions were administrative (absence of doctor’s signature/ wrong patient’s card) while 97% clinical. The clinical interventions were categorized into drug regimen and drug choice. Approx. 62% of problems associated with drug regimen were related to wrong doses. Interventions improved efficacy and avoided toxicity |
| Al-Ghamdi et al. (2012) | Saudi Arabia. | Prospective, nonrandomised observational study | An ADE was defined as per (Nebeker et al., 2004) ADEs due to medication errors were considered to be preventable, while those caused by adverse drug reactions (without an error) were considered to be non-preventable. The incidences of ADEs after discharge from the hospital were identified using a questionnaire | The intervention pharmacist comprehensively counselled patients about their discharge medications. The control group included similar patients who received routine discharge counselling by nurses. Two weeks after discharge, the same pharmacist called the patients and assessed the frequency of ADEs. Two independent clinicians reviewed each ADEs and judged its severity and preventability | To assess a program involving comprehensive medication counselling provided by pharmacists at the time of discharge. The study outcome was the incidence of patient-reported ADEs after discharge | Two hundred patients were included, 100 in the control group and 100 in the intervention group. Approx. 88% (175/200) patients were successfully contacted two weeks after. ADEs occurred in 2 patients in the intervention group and in 21 patients (23 incidents in 21 patients) in the control group (P < .001). 14 ADEs were judged as preventable, and 9 were judged as serious. Warfarin, insulin, anti-laxatives and iron supplements were some of the agents involved |
| Rashed et al. (2012) | Saudi Arabia | A prospective cohort study | Drug-related problems (DRP) defined as per (PCNE) Pharmaceutical Care Network Europe, (2008) | Adopted the data collection method of intensive chart review, used by Ghaleb et al. (2010) and by Dean et al. (2002). For measurement of the severity of the DRPs used validated scale for medication errors published by Dean and Barber (1999). Data were collected using a modified version of the DRP Registration Form version 5.01 designed by (PCNE) | Of interest was the epidemiology of and potential associated risk factors of drug-related problems in hospitalised children. Once a potential DRP was identified, causes, intervention, and outcome of the intervention were identified and recorded. | Total paediatric patients were 364, from medical ward, neonatal intensive care unit and paediatric intensive care unit Total No. of DRPs 258; most common types identified: Dosing problems made up approx. 71% of the problems, drug choice problems approx. 11%, and adverse drug reactions approx. 6%, with other problem types making up approx. 12% The majority of DRPs were preventable and interventions mostly made at prescriber level |
| Kheir et al. (2014) | Qatar | Cross-sectional, descriptive and exploratory study | The authors adopted the PCNE’s definition of a DRP (PCNE) Pharmaceutical Care Network Europe (2008) | Data generated via semi-private interviews was documented A medication use review form was adapted from the form developed by one of the United Kingdom’s National Health Services (NHS) primary care trusts Cumbria. (NHS, 2011) |
The primary outcome measure for this preliminary study was characterising the drug related problems (DRPs) (types and number) captured by the pharmacists during the medication use reviews | Fifty-two eligible patients were reviewed by six pharmacists A total of 175 DRPs were identified with an average of approx. 3 DRPs per patient The most common DRPs reported were: non-adherence to drug therapy (approx. 31%), need for education and counselling (approx. 23%), and adverse drug reactions (approx. 21%). There was a strong association between the incidence of DRPs and the patients’ age; as the age increases, the number of DRPs consistently increases. As medications increased, the number of identified DRPs increased (P ≤ 0.05) |
| Al Rahbi et al., (2014) | Oman | Systematic Retrospective Study | Not specified and referenced | The interventions filed by pharmacists and assistant pharmacists in outpatient pharmacy department were collected, categorized and analysed after a detailed review | The primary objective was to determine the number and types of medication errors intervened by the dispensing pharmacists at outpatient pharmacy department. The study period was one year | Thirty thousand five hundred sixty-three prescriptions dispensed. The number of interventions collected in this period was 692 interventions, approx. 2% of the prescriptions. Approx. 99% of all interventions were prescribing errors, Ninety-eight percent were accepted by prescribers. Approx. 15% of the interventions were administrative |
| Mitwally et al., (2015) | Qatar | Pre and post-interventional analysis of prescriptions | None stated (abstract) | Random prescriptions were collected for 1 week both prior and after the educational phase. The use of unapproved abbreviations, trade names, and the absence/incorrect patient label was also considered as a prescribing error | Investigate whether physician education had an impact on reducing prescribing errors within inpatient setting. The intervention consisted of the clinical pharmacy team preparing educational sessions discussing prescribing errors. The educational material included real case scenarios and the institution’s prescribing policies | The overall physician attendance for the educational session was 92 from a total of 102 (approx. 90%) A total of 1822 prescriptions were involved in the study, with 948 in the pre sample and 874 in the post sample. The total number of errors within the pre sample was (approx. 20%) in comparison to (approx. 10%) errors for the post sample, an overall reduction of 52% in prescribing errors (P < 0.001) |
| ||||||
| Al-Rowibah and Younis (2013) | Saudi Arabia | Cross-sectional questionnaire | None stated | Not applicable | To determine whether CPOE improves the quality of care by increasing patient safety and decreasing medication errors at study setting | The response rate was 31%, with 93 physicians participating. Up to 88% of the physicians agreed that the use of CPOE improved their performance and 76% reported that the use of CPOE increased their productivity. In addition, 64% reported that it was easy to use. Fifty-five percent reported that it created new types of errors. However, 72% of the physicians agreed that CPOE helped them to decrease adverse drug events and 91% of the physicians agreed that CPOE reduced errors related to hand-written prescriptions |
| Al-Arifi (2014) | Saudi Arabia | A cross sectional survey | Dispensing errors defined as per Szeinbach et al. (2007) | Not applicable | To survey pharmacists’ attitudes toward dispensing errors and factors contributing to these errors in community pharmacy settings | Response rate approx. 82% Seventeen factors were identified as contributing to errors, some are: pharmacist assistant, high workload, lack of time, and similar or confusing drug names. Among the major factors believed to reduce dispensing errors were improving doctors’ handwriting, reducing pharmacist work load, having drug names that are distinctive, privacy when counselling patients, having mechanism for checking dispensing procedure, keeping drug knowledge up to date |
| Al Anazi and Al-Jeraisy (2015) | Saudi Arabia | Cross-sectional study conducted A self-administered paper based surveys was used | Not Stated | Not applicable | The perceptions of healthcare professionals with respect to the underlying factors of medication errors. | Response rate was 82%. The study cohort made up of approx. 42% pharmacists, approx. 31% physicians, and approx. 27% nurses. The perceptions of the professionals on the causes of errors differed on the following: interruptions while writing the order, clarity of physician’s order and knowledge of allergies |
References
- Abushaiqa M., Zaran F., Bach D., Smolarek R., Farber M. Educational interventions to reduce use of unsafe abbreviations. Am J Health-Syst Pharm. 2007;64:1170–1173. doi: 10.2146/ajhp060173. [DOI] [PubMed] [Google Scholar]
- Abu Yassin B., Aljadhey H., Al-Sultan M., Al-Rashed S., Adam M., Bates D. (2011) Accuracy of the medication history at admission to hospital in Saudi Arabia. Saudi Pharm. J. 2011;19:263–267. doi: 10.1016/j.jsps.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackroyd-Stolarz S., Hartnell N., MacKinnon N. Demystifying medication safety: making sense of the terminology. Res Social Adm Pharm. 2006;2:280–289. doi: 10.1016/j.sapharm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Alakhali Ansari, Alavudeen Medication errors at the outpatient pharmacy in a hospital in Aseer region, Kingdom of Saudi Arabia. Eur. J. Clin. Pharm. 2014;16(2):144–146. [Google Scholar]
- Al Anany R., Badran H., Al Tamimi H., El Hamid M., Khudair I., Alabad O. Making the case for early medication reconciliation by clinical pharmacists. Pharmacotherapy. 2012;32(10):e267. [Google Scholar]
- Al Anazi M., Al-Jeraisy M. Perspectives of healthcare professionals on reasons for medication errors: a cross-sectional study. J. Infec. Public Health. 2015;8(4):398. [Google Scholar]
- Alanazi M., Al-Jeraisy M., Salam M. Prevalence and predictors of antibiotic prescription errors in an emergency department, Central Saudi Arabia. Drug, Healthcare Patient Safety. 2015;7:103–111. doi: 10.2147/DHPS.S83770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Arifi M. Community pharmacists’ attitudes toward dispensing errors at community pharmacy setting in Central Saudi Arabia. 2014. Saudi Pharm. J. 2014;22:195–202. doi: 10.1016/j.jsps.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Arifi A., Abu-Hashem H., Al-Meziny M., Said R., Aljadhey H. Emergency department visits and admissions due to drug related problems at Riyadh military hospital (RMH), Saudi Arabia. Saudi Pharm. J. 2014;22(17–25):116. doi: 10.1016/j.jsps.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarrak A., Al Rashidi E., Fatani R. Assessment of legibility and completeness of handwritten and electronic prescriptions. Saudi Pharm. J. 2014;22:522–527. doi: 10.1016/j.jsps.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dhawailie A.A. Inpatient prescribing errors and pharmacist intervention at a teaching hospital in Saudi Arabia. Saudi Pharm. J. 2011;19(3):193–196. doi: 10.1016/j.jsps.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi S., Mahmoud M., Alammari M. The outcome of pharmacist counselling at the time of hospital discharge: an observational nonrandomized study. Ann. Saudi Med. 2012;32(5):492–497. doi: 10.5144/0256-4947.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdy M.S., Randhawa M.A., Al-Wahhas M.H., Al-Jumaan M.A. Admissions for drug-related problems at the emergency department of a University Hospital in the Kingdom of Saudi Arabia. J. Fam. Commun. Med. 2015;22:44–48. doi: 10.4103/2230-8229.149590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hamid A., Aslanpour Z., Aljadhey H., Ghaleb M. Hospitalisation Resulting from Medicine-Related Problems in Adult Patients with Cardiovascular Diseases and Diabetes in the United Kingdom and Saudi Arabia. Int. J. Environ. Res. Public Health. 2016;13(5):479. doi: 10.3390/ijerph13050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hussein F. Prescription non- conformities in primary care settings: how useful are guidelines. J. Fam. Commun. Med. 2008;15(2):51–56. [PMC free article] [PubMed] [Google Scholar]
- Aljadhey H., Al-Rashoud I. Medication discrepancy at discharge in a tertiary hospital in Saudi Arabia. Pharmacoepidemiol Drug Saf. 2013;22(S1):75–76. [Google Scholar]
- Aljadhey H., Mahmoud M., Mayet A., Alshaikh M., Ahmed Y., Murray M.D., Bates D.W. Incidence of adverse drug events in an academic hospital: a prospective cohort study. Int. J. Qual. Health C. 2013;25(6):648–655. doi: 10.1093/intqhc/mzt075. [DOI] [PubMed] [Google Scholar]
- Aljadhey H., Mahmoud M.A., Ahmed Y.R. Incidence of adverse drug events in public and private hospitals in Riyadh, Saudi Arabia: the (ADESA) prospective cohort study. BMJ Open. 2016;6:e010831. doi: 10.1136/bmjopen-2015-010831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljamal M. Medication administration errors at a major hospital in Saudi Arabia. Int. J. Clin. Pharm. 2012;35:876. [Google Scholar]
- Al-Jazairi A., Al-Agil A., Asiri Y. The impact of clinical pharmacist in a cardiac surgery intensive care unit. Saudi Med. J. 2008;29(2):277–281. [PubMed] [Google Scholar]
- Al-Jeraisy M.I., Alanazi M.Q., Abolfotouh M.A. Medication prescribing errors in a pediatric inpatient tertiary care setting in Saudi Arabia. BMC Res. Notes. 2011;4 doi: 10.1186/1756-0500-4-294. 294 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khaja K., Al-Ansari T., Damanhori A., Sequeiraa R. Rational use of antimicrobials in infants in primary care of Bahrain. J. Trop. Pediatr. 2006;52(6):390–393. doi: 10.1093/tropej/fml020. [DOI] [PubMed] [Google Scholar]
- Al Khaja K., Al-Ansari T., Damanhori A., Sequeira R. Evaluation of drug utilization and prescribing errors in infants: a primary care prescription based study. Health Policy (New York) 2007;52(6):350–357. doi: 10.1016/j.healthpol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Al Khaja K., Al-Ansari T., Sequeira R. An evaluation of prescribing errors in primary care in Bahrain. Int. J. Clin. Pharmacol. Ther. 2005;43(6):294–301. doi: 10.5414/cpp43294. [DOI] [PubMed] [Google Scholar]
- Al Khaja K., Sequeira R., Al-Ansari T., Damanhori A. Prescription writing skills of residents in a family practice residency programme in Bahrain. Postgrad Med J. 2008;84:198–204. doi: 10.1136/pgmj.2007.062547. [DOI] [PubMed] [Google Scholar]
- Al Khaja K., Sequeira R., Damanhori A. Medication prescribing errors pertaining to cardiovascular/antidiabetic medications: a prescription audit in primary care. Fundam. Clin. Pharmacol. 2012;26(410–417):118. doi: 10.1111/j.1472-8206.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- Al-Khani S., Moharram A., Aljadhey H. Factors contributing to the identification and prevention of incorrect drug prescribing errors in outpatient setting. Saudi Pharm. J. 2014;22:429–432. doi: 10.1016/j.jsps.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan E.L., Barker K.N. Fundamentals of medication error research. Am. J. Hosp. Pharm. 1990;47:555–571. [PubMed] [Google Scholar]
- Almazrou S., Alsahly H., Alwattar H. Ability of Saudi mothers to appropriately and accurately use dosing devices to administer oral liquid medications to their children. Drug Healthc Patient Saf. 2015;7:1–6. doi: 10.2147/DHPS.S72315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Odhayani A., Tourkmani A., Alshehri M. Potentially inappropriate medications prescribed for elderly patients through family physicians. Saudi J. Biol. Sci. 2016 doi: 10.1016/j.sjbs.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Olah Y.H., Al Thiab K. Admissions through the emergency department due to drug-related problems. Ann. Saudi Med. 2008;28(6):426–429. doi: 10.5144/0256-4947.2008.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Omar H., Al-Sultan M., Abu-Auda H. Prescribing of potentially inappropriate medications among the elderly population in an ambulatory care setting in a Saudi Military hospital: trend and cost. Geriatr. Gerontol. Int. 2013;13:616–621. doi: 10.1111/j.1447-0594.2012.00951.x. [DOI] [PubMed] [Google Scholar]
- Al-Rashdi I.S., Victoria M., Susan S. Analysis of pharmacists’ interventions of electronic prescriptions at Sultan Qaboos University hospital in Oman. Value in Health. 2010;13(7):A425. [Google Scholar]
- Al Rahbi H., Al-Sabri R., Chitme M. Interventions by pharmacists in out-patient pharmaceutical care. Saudi Pharm. J. 2014;22:101–106. doi: 10.1016/j.jsps.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rowibah F., Younis M., Parkash J. The Impact of Computerized Physician Order Entry on Medication Errors and Adverse Drug Events. J. Health Care Finance. 2013;40(1):93–102. [PubMed] [Google Scholar]
- Al Shahaibi N., Al Said L., Kini T., Chitme H. Identifying errors in handwritten outpatient prescriptions in Oman. J. Young Pharm. 2012;4(4):267–272. doi: 10.4103/0975-1483.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaikh M., Mayet A., Aljadhey H. Medication error reporting in a university teaching hospital in Saudi Arabia. J. Patient. Saf. 2013;9:145–149. doi: 10.1097/PTS.0b013e3182845044. [DOI] [PubMed] [Google Scholar]
- Alsulaimi Z., Conroy S., Choonara I. Medication errors in the Middle East countries: a systematic review of the literature. Eur. J. Clin. Pharmacol. 2013;69:995–1008. doi: 10.1007/s00228-012-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tajir G., Kelly W. Epidemiology, comparative methods of detection and preventability of adverse drug events. Ann. Pharmacother. 2005;39:1169–1174. doi: 10.1345/aph.1E559. [DOI] [PubMed] [Google Scholar]
- Altebenaui A., Alrashedi M., Alshammari T. Completeness of medications prescriptions: prescription errors study in hail region (PeSHR) Pharmacoepidemiol. Drug Saf. 2015;24:101. [Google Scholar]
- American Geriatrics Society Beers Criteria Update Expert Panel American Geriatrics Society Updated Beers Criteria for potentially inappropriate medication use in older adults. J. Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y., Alamry A., Al Owais S., Al-Dorzi H. Incident reporting at a tertiary care hospital in Saudi Arabia. J. Patient Saf. 2012;8:81–87. doi: 10.1097/PTS.0b013e31824badb7. [DOI] [PubMed] [Google Scholar]
- Aseeri M. The impact of a pediatric antibiotic standard dosing table on dosing errors. J. Pediatr. Pharmacol. Ther. 2013;18(3):220–226. doi: 10.5863/1551-6776-18.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHP, 1982. Standard definition of a medication error. Am. J. Hosp. Pharm. 1982; 39 321. 120 [PubMed]
- ASHP ASHP guidelines on preventing medication errors in hospitals. Am. J. Hosp. Pharm. 1993;50(2):305–314. [PubMed] [Google Scholar]
- Bates D.W., Cullen D., Laird N. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960;20:37–46. [Google Scholar]
- Dean B.S., Barber N.D. A validated, reliable method of scoring the severity of medication errors. Am. J. Health Syst. Pharm. 1999;56:57–62. doi: 10.1093/ajhp/56.1.57. [DOI] [PubMed] [Google Scholar]
- Dean B., Schachter M., Vincent C., Barber N. Prescribing errors in hospital inpatients: their incidence and clinical significance. Qual. Saf. Health Care. 2002;11:340–344. doi: 10.1136/qhc.11.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B., Schachter M., Vincent C., Barber N. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet. 2002;359:1373–1378. doi: 10.1016/S0140-6736(02)08350-2. [DOI] [PubMed] [Google Scholar]
- Dean B., Barber N., Schachter M. what is a prescribing error? Qual Health Care. 2000;9:232–237. doi: 10.1136/qhc.9.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbi H., Al-Abrashy H., Hussein W. Causes and outcome of medication errors in hospitalised patients. Saudi Med J. 2006;27(10):1489–1492. [PubMed] [Google Scholar]
- Elnour A., Ellahham N., Qassas H. Implementation of medication error reporting through med safe tool: the clinical pharmacists and the inpatient nursing staff collaborative approach. J. Patient Saf. 2007;3(4):177–183. [Google Scholar]
- Ferner R.E., Aronson J.K. Clarification of terminology in medication errors. Drug Saf. 2006;29:1011–1022. doi: 10.2165/00002018-200629110-00001. [DOI] [PubMed] [Google Scholar]
- Fick D., Cooper J., Wade W., Waller J., MacLean R., Beers M. Updating the Beers Criteria for Potentially Inappropriate Medication use in older adults. Results of a US Consensus Panel of Experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- Franklin and Tully, preface In Tully, M., Franklin, B.D. (Eds.) Safety in Medication Use. Boca Ranton, United States, Taylor and Francis Group, pp xiii.
- Flynn E.A., Barker K.N. Medication error research. In: Cohen M.R., editor. Medication Errors. American Pharmaceutical Association; Washington DC: 1999. [Google Scholar]
- Flynn E.A., Barker K.N., Pepper G.A. Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. Am. J. Health Syst. Pharm. 2002;59:436–446. doi: 10.1093/ajhp/59.5.436. [DOI] [PubMed] [Google Scholar]
- Ghaleb M., Barber N., Franklin B., Yeung V. Systematic review of medication errors in pediatric patients. Ann. Pharmacother. 2006;40:1766–1776. doi: 10.1345/aph.1G717. [DOI] [PubMed] [Google Scholar]
- Ghaleb M.A., Barber N., Dean Franklin B., Wong I.C.K. The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch. Dis. Child. 2010;95:113–118. doi: 10.1136/adc.2009.158485. [DOI] [PubMed] [Google Scholar]
- Gandhi T.K., Seger D.L., Bates D.W. Identifying drug safety issues: from research to practice. Int J Qual Health Care. 2000;12:69–76. doi: 10.1093/intqhc/12.1.69. [DOI] [PubMed] [Google Scholar]
- Hemida AlHawawi, Alawwad, Medication errors in a neonatal intensive care unit, Saudi ArabiaA retrospective study. Pediatr. Res. 2011;70:604. 101038/pr.2011.829. [Google Scholar]
- Hepler C.D., Strand L.M. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–543. [PubMed] [Google Scholar]
- Hooper R., Adam A., Kheir N. Pharmacist-documented interventions during the dispensing process in a primary health care facility in Qatar. Drug Healthc Patient Saf. 2009;1:73–80. doi: 10.2147/dhps.s5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insley J. 13th Edition. Arnold; London: 1996. Prescribing. A pediatric vade-mecum. [Google Scholar]
- Irshaid Y.M., Al Homrany M., Hamdi A.A., Adjepon-Yamoah K.K., Mahfouz A.A. Compliance with good practice in prescription writing at outpatient clinics in Saudi Arabia. East Mediterr Health J. 2005;11(5–6):922–928. [PubMed] [Google Scholar]
- Jha A., Kuperman G.J., Teich J.M. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and simulated voluntary report. J Am Med Inform Assoc. 1998;5:305–314. doi: 10.1136/jamia.1998.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A.K., Prasopa-Plaizier N., Larizgoita I., Bates D.W. On behalf of the research priority setting working group of the world alliance for patient safety (2010). Patient safety research an overview of the global evidence. Qual. Saf. Health Care. 2010;19:42–47. doi: 10.1136/qshc.2008.029165. [DOI] [PubMed] [Google Scholar]
- Kaushal R., Bates D.W., Landrigan C., McKenna K.J., Clapp M.D. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- Kheir N., Awaisu A., Sharfi A. Drug-related problems identified by pharmacists conducting medication use reviews at a primary health centre in Qatar. Int. J. Clin. Pharm. 2014;36:702–706. doi: 10.1007/s11096-014-9962-5. [DOI] [PubMed] [Google Scholar]
- Khoja T., Neyaz Y., Qureshi N., Magzoub M. Medication errors in primary care in Riyadh City. Saudi Arabia. East Mediterr. Health J. 2011;17(2):156–159. [PubMed] [Google Scholar]
- Kohn L.T., Corrigan J.M., Donalson M.S., editors. To err is human: building a safer health system. National Academy Press; Washington, DC: 2000. (Vol. 627) [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lisby M., Nielsen L.P., Brock B., Mainz J. How are medication errors defined? A systematic literature review of definitions and characteristics. Int. J. Qual. Health Care. 2010;22(507–518):126. doi: 10.1093/intqhc/mzq059. [DOI] [PubMed] [Google Scholar]
- Mahmoud M.A., Aljadhey H., Hassali M.A. Incidence of prescribing errors in hospitalized patients in Saudi Arabia. Value In Health. 2016;1(9):A1–A318. [Google Scholar]
- McLeod, M.C., Barber, N., Franklin, B.D., 2013. Methodological variations and their effects on reported medication administration error rates. BMJ Qual Saf. Published Online First: [15/01/2013] 10.1136/bmjqs-2012-001330. [DOI] [PubMed]
- McLeod M. Measuring medication errors. In: Tully M., Franklin B.D., editors. Safety in Medication Use. Boca Ranton; United States, Talor and Francis Group: 2016. pp. 61–72. [Google Scholar]
- McPhillips H.A., Stille C.J., Smith D., Hecht J., Pearson J. Potential medication dosing errors in outpatient pediatrics. J Pediatr. 2005;147(6):761–767. doi: 10.1016/j.jpeds.2005.07.043. [DOI] [PubMed] [Google Scholar]
- Mendonca J.M., Lyra D.P., Jr, Rabelo J.S. Analysis and detection of dental prescribing errors at primary health care units in Brazil. Pharm World Sci. 2010;32(1):30–35. doi: 10.1007/s11096-009-9335-7. [DOI] [PubMed] [Google Scholar]
- Miller M., Robinson K., Lubomski L., Rinke M., Pronovost P. Medication errors in paediatric care: a systematic review of epidemiology and an evaluation of evidence supporting reduction strategy recommendations. Qual. Safety in Health Care. 2007;16(2):116–126. doi: 10.1136/qshc.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitwally H., Ibrahim T., Fayez A. (2015) Impact of physicians education on prescribing errors within an inpatient setting. Pharmacotherapy. 2015;35(11) e183. [Google Scholar]
- Morimoto T., Gandhi T.K., Seger A.C. Adverse drug events and medication errors: detection and classification methods. Qual. Saf. Health Care. 2004;13(4):306–314. doi: 10.1136/qshc.2004.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T., Sakuma M., Kunihko M., Kuramoto N. Incidence of adverse drug events and medication errors in Japan: the JADE study. J. Gen. Intern. Med. 2010;26(2):148–153. doi: 10.1007/s11606-010-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I., Roberts E.A. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- National Coordinating Council for Medication Error Reporting and Prevention. NCC MERP (2008). National Coordinating Council for Medication Error Reporting and Council for Medication Error Reporting and Prevention. Available at http://www.nccmerp.org. [DOI] [PubMed]
- NCCMERP (2012). National Coordinating Council for Medication Error Reporting and Prevention, About Medication Errors: What is a Medication Error? Available from: <http://www.nccmerp.org/aboutMedErrors.html>. (Last accessed on 2012 Jan 19).
- Nebeker J.R., Barach P., Samore M.H. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann. Intern. Med. 2004;140(10):795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- Neville R.G., Robertson F., Livingstone S. A classification of prescription errors. J. Royal College Gener. Practi. 1989;39(320):110–112. [PMC free article] [PubMed] [Google Scholar]
- Ninno S.D., Ninno M.A., editors. Pharmacotherapy Self-assessment Program. Kansas City (MO); American College Clinical Pharmacy: 2000. pp. 113–148. [Google Scholar]
- Pharmaceutical Care Network Europe (PCNE), 2008 http:// www.pcne.org/ (accessed January 2012).
- Rahman, S, Farooki, M.Q., Alsalamah S., 1994 Pharmacist intervention in preventing prescribing error in a tertiary care hospital in Saudi Arabia. IPA 51 (Jun) MCS-14.
- Rancourt C., Moisan J., Baillargeon L., Verreault R., Laurin D., Gregoire J.P. Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatrics. 2004;4:9. doi: 10.1186/1471-2318-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed A., Neubert A., Tomlin S., Jackman J. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur. J. Clin. Pharmacol. 2012;68:1657–1666. doi: 10.1007/s00228-012-1302-x. [DOI] [PubMed] [Google Scholar]
- Rashed A., Neubert A., Alhamdan H., Tomlin S., Alazmi A. Drug related problems found in children attending an emergency department in Saudi Arabia and the United Kingdom. Int J Clin Pharm. 2013;35:327–331. doi: 10.1007/s11096-013-9758-z. [DOI] [PubMed] [Google Scholar]
- Rehmani R. Medication history taking in emergency department is unreliable. Ann. Emerg. Med. 2011;58(4S):S251. [Google Scholar]
- Sadat-Ali M., Al-Shafei B., Al-Turki R., Ahmed S., Al-Abbas S., Al-Omran A. Medication administration errors in Eastern Saudi Arabia. Saudi Med. J. 2010;31(11):1257–1259. [PubMed] [Google Scholar]
- Shaughnessy A., Nickel R. Prescription-writing patterns and errors in a family medicine residency program. 1989;29(3):290–295. [PubMed] [Google Scholar]
- Sonallah H., Ibrahim T., Izham M. Evaluation of medication reconciliation conducted by clinical pharmacists at a newly established hospital in Qatar. Pharmacotherapy. 2014;34(10):e249. [Google Scholar]
- Strand, L.M., Morley, P.C., Cipolle, R.J., Ramsey, R, Lamsam, G.D., Drug-related problems: their structure and function. DICP.1990;24:1093–1097. [DOI] [PubMed]
- Szeinbach, S., Seoane-Vazquez, E., Parekh, A., Herderick, M., 2007. Dispensing errors in community pharmacy: perceived influence of sociotechnical factors. Int. J. Qual. Health Care 19 (4) 203–209. 130. [DOI] [PubMed]
- WHO . World Health Organization; Geneva: 1999. Reporting adverse drug reactions. [Google Scholar]
- WHO. 2014. WHO definitions (cited 2014). <http://www.who.int/medicines/areas/quality_safety_efficacy/trainingcourses/definitions.pdf>.
- WHO Collaborating Centre for International Drug Monitoring., 2017. UMC WHO Programme Members. [ONLINE] Available at: http;//www.who-umc.org/global-pharmacovigilance/members/who-programme-members/. (accessed 9 October 2017).
- World Bank . World Bank; Washington, DC: 2017. The World Bank Annual Report 2017. (c) World Bank. https://openknowledge.worldbank.org/handle/10986/27986 License: CC BY-NC-ND. [Google Scholar]
- World Health Organisation. 2014. http://www.who.int/gho/health_financing/total_expenditure/en/. [ONLINE] Available at: http://www.who.int/gho/health_financing/total_expenditure/en/. (accessed 4 October 2017).
- Youssef, A, Almubarak, A, Aljohnai, M et al., 2015. Contraindicated medications administered to inpatients with renal insufficiency in a Saudi Arabian hospital that has a computerized clinical decision support system. J. Taibah Univ. Med. Sci. 10 (3) 320–26. 132.
Further reading
- NHS (2013). Clinical medication review [online]. http://www.northcumbriaccg.nhs.uk/index.aspx/ProfessionalZone/MedicinesManagement/Guidelines/MedicationReview-PracticeGuide2011.pdf.





