Abstract
The genus Hibiscus contains about 275 species of flowering plants widely grown in the tropics and sub-tropics. The available literature revealed that several Hibiscus species exhibited excellent anticancer activity against several cancer cells like lung, breast, and liver. This motivated the authors to explore the anticancer property of other Hibiscus species (Hibiscus calyphyllus, H. deflersii and H. micranthus) along with development of a validated HPTLC method for the concurrent analysis of three anticancer biomarkers (ursolic acid, β-sitosterol and lupeol) in different Hibiscus species. The anticancer activity of various fractions (petroleum ether, toluene, dichloromethane, ethyl acetate and n-butanol) of all the Hibiscus species (aerial parts) were evaluated in vitro against HepG2 and MCF-7 cell lines using MTT assay. The HPTLC analysis was carried out using chloroform and methanol as mobile phase (97:3; v/v) on 20 × 10 cm glass-backed silica gel 60F254 plates and analyzed different phytoconstituents present in all fractions at λ = 575 nm wavelength. Of the tested fractions of H. calyphyllus, H. deflersii and H. micranthus, HdP (H. deflersii petroleum ether fraction) exhibited the most potent cytotoxic effect on HepG2 and MCF-7 (IC50: 14.4 and 11.1 μg/mL, respectively) cell lines. Using the developed HPTLC method a compact and intense peak of ursolic acid, β-sitosterol and lupeol were obtained at Rf = 0.22, 0.39 and 0.51, respectively. The LOD/LOQ (ng) for ursolic acid, β-sitosterol and lupeol were found as 42.30/128.20, 13.20/40.01 and 31.57/95.68, respectively in the linearity range 100–1200 ng/spot. The obtained result showed maximum presence of ursolic acid, β-sitosterol and lupeol (5.50, 11.85 and 7.47 μg/mg, respectively) in HdP which also supported its strong anticancer effect. Our data suggest that H. deflersii petroleum ether fraction (HdP) can be further subjected to the isolation of active cytotoxic phytoconstituents and establishment of their mechanism of action. The maiden developed HPTLC method for concurrent analysis of anticancer biomarkers may be further employed in the in process quality control of herbal formulation containing the said biomarkers.
Keywords: Ursolic acid, β-sitosterol, Lupeol, Hibiscus spp., HPTLC, MTT assay
1. Introduction
The genus Hibiscus contains about 275 species of flowering plants in the tropics and sub-tropics. Its vitamin C rich flowers are edible with distinct tangy flavor that can be dried, candied, baked as cakes and blended into tea. The calyces are generally decocted and consumed as cold or hot beverage (Sayago-Ayerdi et al., 2014). Cancer, considered as a major health problem worldwide which is responsible for approximately 7.6 million deaths (13% of all deaths) per annum. In spite of the advancement in the area of cancer probe there is still an urgency to find new anti-cancer agents. Taking into account of the progressing requirement for the potent anticancer agents, and relationship of nutritional therapy with diminished cancer risk, eatable plants are progressively considered as good source of anticancer agents (Lin et al., 2005). Several Hibiscus species such as H. syriacus reported to possess excellent cytotoxic effect on lung, breast and liver cancer cells (Cheng et al., 2008, Liang et al., 2017), and its phytoconstituent betulin-3-caffeate (triterpene) showed strong cytotoxic potential against human lung cancer cells, A549 (IC50, 4.3 μM) (Shi et al., 2014); H. sabdariffa L., exhibited excellent cytotoxic property against human gastric carcinoma cells (Lin et al., 2005) and its constituent delphinidin 3-sambubioside (anthocyanin) induced apoptosis in human leukemia cells (Hou et al., 2005). H. deflersii was found to possess antidiarrhetic and antiphologistic activities while the leaves were very effective in heart disorders and diabetes (Lakshman et al., 2014). H. micranthus widely available in southern and western province of Saudi Arabia (Kirtikar and Basu, 1984) reported to contain stronger anti-fungal, antiviral and anti-tumor activity (Rekha, 2017) as well as antibacterial and wound healing properties (Begashaw et al., 2017).
The HPTLC (High Performance Thin Layer Chromatography) has been widely employed these days in the quality control of herbs and its formulations due to its small mobile phase requirement and multi sample analysis which reduces the cost and time of study. It provides a complete profile of a plant extract by using different wavelengths of light that is typically observed with more specific types of analyses. It is more precise and calibrated, and has several advantages over other analytical technique like HPLC (high performance liquid chromatography) in quantification of different markers (both UV active or inactive). The broad dimensions of stationary phases has increased the utilization of HPTLC for a wide range of samples (Siddiqui et al., 2018, Alam et al., 2017, Alam et al., 2015a, Alam et al., 2015b, Alam et al., 2015c, Siddiqui et al., 2015, Alajmi et al., 2015, Alam et al., 2014).
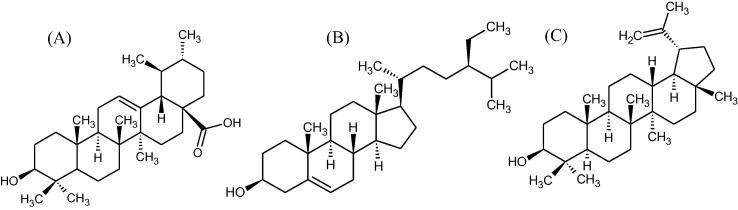
The excellent pharmacological properties shown by Hibiscus species motivated the authors to explore the anticancer property of H. calyphyllus, H. deflersii and H. micranthus grown in Saudi Arabia, including concurrent analysis of cytotoxic biomarkers ursolic acid (A), β-sitosterol (B) and lupeol (C) (Fig. 1) by validated HPTLC method.
Fig. 1.
Anticancer biomarkers of plant origin.
2. Experimental
2.1. Apparatus and reagents
The three anticancer biomarkers, ursolic acid, β-sitosterol and lupeol were procured from Sigma Aldrich (USA). The solvents used (petroleum ether, ethyl acetate, toluene, chloroform, ethyl acetate, methanol and n-butanol; AR grade) were purchased from BDH (UK). The HPTLC plates (glass-backed silica gel 60F254) were purchased from Merck (Germany). The application of biomarkers and extracts (band wise) to the plate was carried out by Automatic TLC Sampler-4 (CAMAG, Switzerland) while the development took place in automatic development chamber (ADC2, Switzerland). The developed plate was derivatized with p-anisaldehyde reagent [the reagent was prepared according to the Wagner and Bladt (2004) by using anisaldehyde, glacial acetic acid, methanol and concentrated sulphuric acid. Initially anisaldehyde (0.5 mL) was mixed with glacial acetic acid (10 mL) followed by addition of methanol (85 mL) and then sulphuric acid (5 mL)]. The scanning and documentation of developed HPTLC plate was carried out by CATS 4 (CAMAG, Switzerland) and TLC Reprostar 3 (CAMAG, Switzerland), respectively.
2.2. Plant material
Aerial parts of H. calyphyllus Cav. (Voucher No. HA-234), H. deflersii Schweinf. ex Cufod. (Voucher No. HA-567) and H. micranthus L. (Voucher No. HA-16240) were collected from As-Sahla mountain of Asir region (Kingdom of Saudi Arabia), authenticated and the specimen were deposited in the herbarium of Pharmacognosy Department, Pharmacy College (King Saud University, Riyadh). The aerial parts of all samples were washed thoroughly, chopped into small pieces and spread uniformly on aluminum trays. Further the samples were shade dried, coarsely powdered and stocked in sealed container for other use.
2.3. Extraction of plant material
The extraction of powder (400 g) of all three Hibiscus species was carried out in 95% ethanol according to the Siddiqui et al. (2018). The obtained extracts were fractionated using various solvents (petroleum ether, toluene, chloroform, ethyl acetate, and n-butanol), yield was calculated and stored at 4 °C in refrigerator as H. calyphyllus [HcP (petroleum ether fraction, 2.25 g), HcT (toluene fraction, 2.21 g), HcC (chloroform fraction, 3.32 g), HcE (ethyl acetate fraction, 2.3 g) and HcB (n-butanol fraction, 2.01 g)]; H. deflersii [HdP (petroleum ether fraction, 1.19 g), HdT (toluene fraction, 2.10 g), HdC (chloroform fraction, 2.46 g), HdE (ethyl acetate fraction, 2.37 g) and HdB (n-butanol fraction, 1.98 g)] and H. micranthus [HmP (petroleum ether fraction, 1.96 g), HmT (toluene fraction, 2.36 g), HmC (chloroform fraction, 2.55 g), HmE (ethyl acetate fraction, 2.86 g) and HmB (n-butanol fraction, 2.01 g)] until the time of use.
2.4. Anticancer activity of different fractions of all Hibiscus species
A human liver cancer cell line (HepG2) and breast cancer cell line (MCF-7) were grown and preserved in culture media DMEM-Glutmax, supplemented with 10% bovine calf serum and 1x penicillin–streptomycin (all from Invitrogen, USA) at 37 °C with 5% CO2 supply. The cells were seeded (0.5x105 cells/well in triplicate) in a 96-well flat-bottom plate (Becton-Dickinson Labware) a day before treatment and grown. The preparation of stock solution (1.0 mg/mL) of all the compounds were accomplished in DMSO (Sigma) and additional working dilutions were prepared in culture media. Cells were treated with three different doses of HcP, HcT, HcE, HcC, HcB, HdP, HdT, HdE, HdC, HdB, HmP, HmT, HmE, HmC and HmB (300, 150 and 75 mg/mL; in triplicate) including vinblastin (standard) and an untreated control, and further incubated for 48 h. The TACS MTT Cell Proliferation and Viability Assay Kit (TACS) were used to perform the Cell proliferation and viability test as per the instructions of manufacturer. The cancer cell lines survival curve was obtained by plotting the relationship between surviving fraction and extract concentration. The estimation of response parameter IC50 value (the concentration required for 50% inhibition of cell viability) was carried out by using the best fit regression curve method in Excel.
2.5. Concurrent analysis of biomarkers ursolic acid, β-sitosterol and lupeol in different Hibiscus species by High Performance Thin Layer Chromatography (HPTLC)
2.5.1. HPTLC instrumentation and conditions
The HPTLC analyses of ursolic acid, β-sitosterol and lupeol in sample (15 fractions) were accomplished on normal phase (NP) HPTLC plates (20 × 10 cm). All the markers as well as the extract were applied on HPTLC plate at a rate of 160 nL/s using microlitre syringe fitted with the automatic TLC Sampler-4. The plate development took place in a pre-saturated twin-trough glass chamber (20 × 10 cm) under the chamber saturation condition (at 25 ± 2 °C and 60 ± 5% humidity). Further, the plates were dried and derivatized with p-anisaldehyde reagent to furnish compact spots of the biomarkers and the phytoconstituents available in the different fractions. The plates were then analyzed quantitatively at λ = 575 nm (wavelength) in absorbance mode.
2.5.2. Preparation of stock solutions (standards)
A stock solution (1 mg/mL) of standard (ursolic acid, β-sitosterol and lupeol) was prepared in chloroform followed by further dilution to make seven different concentrations (10–120 μg/mL). 10 μL of each concentration of ursolic acid, β-sitosterol and lupeol were applied on the HPTLC plate to provide a linearity range of 100–1200 ng/band.
2.5.3. Method validation
The validation of the HPTLC method was carried out as per the ICH guideline (ICH, 2005) for LOD (limit of detection), LOQ (limit of quantification), linearity range, precision, recovery as accuracy and robustness determination.
2.6. Statistical analysis
The one-way analysis of variance (ANOVA) and Dunnet’s test was used in statistical analysis to estimate the total variation in a set of data. The obtained results were denoted as mean ± SD where, P < 0.05 was considered as significant.
3. Results and discussion
3.1. Anticancer activity of different fractions of all Hibiscus species
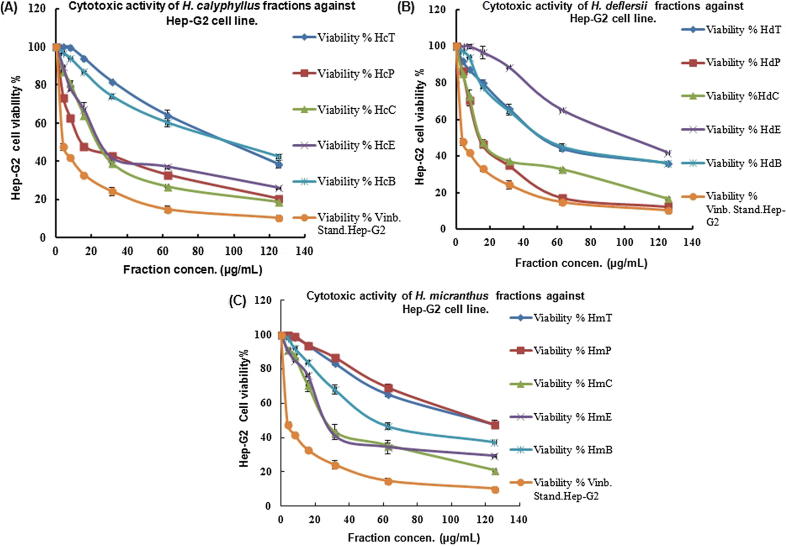
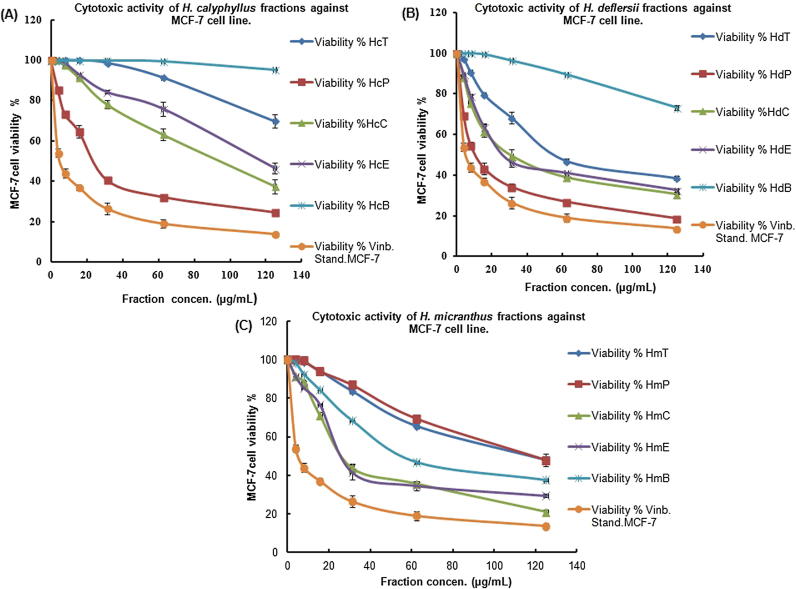
The different fractions of H. calyphyllus (HcP, HcT, HcE, HcC, HcB), H. deflersii (HdP, HdT, HdE, HdC, HdB) and H. micranthus (HmP, HmT, HmE, HmC, HmB) were evaluated for their in vitro anticancer activities against HepG2 and MCF-7 cells showed marked toxicity (Table 1; Fig. 2, Fig. 3). Various fractions of Hibiscus species exhibited strong anticancer property against HepG2 [(IC50): HdP(14.4) > HcP(14.5) > HdC(14.8) > HcC(24.4) > HcE(26) > HmE(27.4) > HmC(27.6)] as well as MCF-7 cells [(IC50): HdP(11.1) > HmC(24.1) > HcP(25.1) > HdE(27.8) > HdC(30.6)]. This result indicated that the petroleum ether fraction of H. deflersii (HdP; IC50: 14.4 and 11.1 μg/mL) possessed the strong anticancer property against both cells in comparison to the standard vinblastin (IC50: 3.48 and 5.44 μg/mL). This was supported by the results of HPTLC analysis in which the maximum presence of cytotoxic biomarkers ursolic acid, β-sitosterol and lupeol was found in HdP fraction.
Table 1.
The estimated IC50 (μg/mL) values of different fractions of H. calyphyllus, H. deflersii, and H. micranthus.
| IC50 (μg/mL) values of different fractions of H. calyphyllus |
IC50 (μg/mL) values of differentfractions of H. deflersii |
IC50 (μg/mL) values of different fractions of H. micranthus |
||||||
|---|---|---|---|---|---|---|---|---|
| Fractions | IC50 (µg/mL) ± SD (HepG2 cells) | IC50 (µg/mL) ± SD (MCF-7 cells) | Fractions | IC50 (µg/mL) ± SD (HepG2 cells) | IC50 (µg/mL) ± SD (MCF-7 cells) | Fractions | IC50 (µg/mL) ± SD (HepG2 cells) | IC50 (µg/mL) ± SD (MCF-7 cells) |
| HcT | 96.9 ± 1.3 | 224 ± 5.8 | HdT | 54.1 ± 2.5 | 58 ± 3.4 | HmT | 117 ± 8.2 | 216 ± 8.6 |
| HcP | 14.5 ± 0.8 | 25.1 ± 1.1 | HdP | 14.4 ± 0.8 | 11.1 ± 0.5 | HmP | 118 ± 4.5 | 104 ± 5.2 |
| HcC | 24.4 ± 1.2 | 94.6 ± 1.4 | HdC | 14.8 ± 0.6 | 30.6 ± 0.8 | HmC | 27.6 ± 1.2 | 24.1 ± 0.6 |
| HcE | 26 ± 1.8 | 118 ± 4.6 | HdE | 103 ± 9.2 | 27.8 ± 1.1 | HmE | 27.4 ± 1.8 | 54.1 ± 3.8 |
| HcB | 98.8 ± 3.6 | 520 ± 3.0 | HdB | 54.8 ± 0.9 | 265 ± 5.9 | HmB | 118 ± 4.5 | 411 ± 12.3 |
| Vinblastin (Stand.) | 3.48 ± 0.22 | 5.44 ± 0.57 | Vinblastin (Stand.) | 3.48 ± 0.22 | 5.44 ± 0.57 | Vinblastin (Stand.) | 3.48 ± 0.22 | 5.44 ± 0.57 |
Fig. 2.
Cytotoxic activity of different fractions of H. calyphyllus, H. deflersii and H. micranthus against Hep-G2 cell line at different concentrations (3.9–125 µg/mL). (A) The% viability of HepG2 cell treated with H. calyphyllus fractions. (B) The% viability of HepG2 cell treated with H. deflersii fractions. (C) The% viability of HepG2 cell treated with H. micranthus fractions.
Fig. 3.
Cytotoxic activity of different fractions of H. calyphyllus, H. deflersii and H. micranthus against MCF-7 cell line at different concentrations (3.9–125 µg/mL). (A) The% viability of MCF-7 cell treated with H. calyphyllus fractions. (B) The% viability of MCF-7 cell treated with H. deflersii fractions. (C) The% viability of MCF-7 cell treated with H. micranthus fractions.
Available literature revealed that ursolic acid exhibited excellent anticancer properties against HepG2 and MCF-7 cells, by down regulating the expression of COX-2 (Liu et al., 2014) and by inducing cell cycle G1/G2 arrest and apoptosis (Chakravarti et al., 2012), respectively. β-sitosterol was found to exhibit anticancer properties against breast, prostate, colon, lung, stomach and ovarian cancer by interfering with multiple cell signaling pathways, including cell cycle and apoptosis (Bin Sayeed and Ameen, 2015). The possible mechanism of anticancer property of β-sitosterol against human colon cancer cells (HT116) was induction of caspase-3/9 activation, decreasing the anti-apoptotic Bcl-2 protein and mRNA expression, and increasing the release of cytochrome c (Choi et al., 2003). Lupeol is widely distributed in several plants and fruits possess many pharmacological properties such as anti-diabetic, antioxidant, cardio protective and anticancer (Tsai et al., 2016). It was found to induce apoptosis of MCF-7 cells by down regulating the Bcl-2 and Bcl-xL proteins expressions (Pitchai et al., 2014). Lupeol exhibited strong anticancer property against human osteosarcoma cells (MNNG/HOS and MG-63) by inducing apoptosis and cell cycle arrest in G0/G1 phase along with down regulation of the PI3-kinase, phospho-protein kinase B, cyclin D1 expression and upregulation of the p21 and p27 expressions (Liu et al., 2016). Taken together the presence of all the three biomarkers in the petroleum ether extracts of H. deflersii strongly endorses its strong anticancer property.
3.2. Concurrent analysis of biomarkers ursolic acid, β-sitosterol and lupeol in different Hibiscus species by High Performance Thin Layer Chromatography (HPTLC)
3.2.1. HPTLC method development and validation
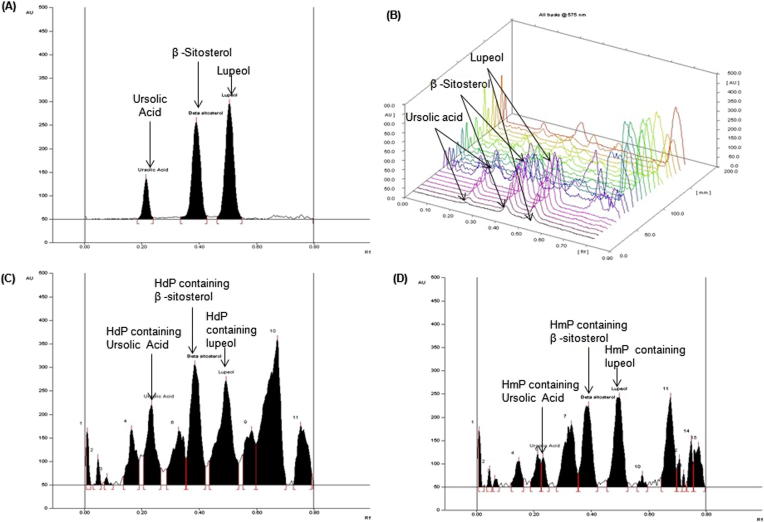
The mobile phase selection for the HPTLC analysis was carried out by testing various solvents combinations. Out of these, the combination of chloroform and methanol (97:3, v/v) was found as best mobile phase for the estimation of ursolic acid, β-sitosterol and lupeol. Sharp and compact peaks of ursolic acid, β-sitosterol and lupeol were obtained at Rf = 0.22, 0.39 and 0.51, respectively (Fig. 4A) with clear separation of the biomarkers from matrix and other phytoconstituents (Fig. 4B) using optimized mobile phase volume (20 mL) and saturation time (20 min). The developed method was found to be quite selective with high baseline resolution. The regression equation/correlation co-efficient (r2) for ursolic acid, β-sitosterol and lupeol were found as Y = 1.076X + 58.384/0.9947, Y = 3.741X + 695.05/0.9967 and Y = 5.352X + 209.346/0.9957, respectively in the linearity range 100–1200 ng/spot while the LOD/LOQ (ng) were found as 42.30/128.20, 13.20/40.01 and 31.57/95.68, respectively (Table 2). The recovery/RSD (%) for ursolic acid, β-sitosterol and lupeol were found as 99.09–100.13/1.089–1.24, 98.40–99.63/1.105–1.538 and 99.01–99.75/1.351–1.562, respectively (Table 3). The% RSD for intra-day/inter-day precisions (n = 6) of ursolic acid, β-sitosterol and lupeol were recorded as 1.039–1.106/1.043–1.102, 1.121–1.277/1.116–1.274, and 1.168–1.316/1.160–1.312, respectively, exhibited good precision of the proposed method (Table 4). The robustness of proposed method was checked by making a small deliberate change in the mobile phase composition, saturation time and mobile phase volume and the obtained data were reported in the Table 5. The low values of SD and% RSD indicated that the proposed method was robust.
Fig. 4.
Quantification of ursolic acid, β-sitosterol and lupeol in different fractions of H. calyphyllus, H. deflersii and H. micranthus by HPTLC at λ = 575 nm [mobile phase: chloroform: methanol (97:3)]. (A) Chromatogram of standard ursolic acid (Rf = 0.22), β-Sitosterol (Rf = 0.39) and lupeol (Rf = 0.51) (B) 3-D display of all tracks (C) Chromatogram of H. deflersii petroleum ether fraction [HdP (ursolic acid, spot 5, Rf = 0.22; β-Sitosterol, spot 7, Rf = 0.39; lupeol, spot 8, Rf = 0.51)]. (D) Chromatogram of H. micranthus petroleum ether fraction [HmP (ursolic acid, spot 6, Rf = 0.22; β-Sitosterol, spot 8, Rf = 0.39; lupeol, spot 9, Rf = 0.51)].
Table 2.
Rf, Linear regression data for the calibration curve of ursolic acid, β-sitosterol and lupeol (n = 6).
| Parameters | Ursolic acid | β-sitosterol | Lupeol |
|---|---|---|---|
| Linearity range (ng/spot) | 100–1200 | 100–1200 | 100–1200 |
| Regression equation | Y = 1.076X + 58.384 | Y = 3.741X + 695.05 | Y = 5.352X + 209.346 |
| Correlation (r2) coefficient | 0.9947 ± 0.005 | 0.9967 ± 0.0001 | 0.9957 ± 0.002 |
| Slope ± SD | 1.076 ± 0.013 | 3.741 ± 0.014 | 5.352 ± 0.051 |
| Intercept ± SD | 58.384 ± 8.131 | 695.05 ± 11.32 | 209.346 ± 8.727 |
| Standard error of slope | 0.005 | 0.006 | 0.021 |
| Standard error of intercept | 3.318 | 4.62 | 3.562 |
| Rf | 0.22 ± 0.002 | 0.39 ± 0.001 | 0.51 ± 0.002 |
| LOD (ng) | 42.30 | 13.20 | 31.57 |
| LOQ (ng) | 128.20 | 40.01 | 95.68 |
Table 3.
Recovery as accuracy studies of the proposed HPTLC Method (n = 6).
| Percent (%) of ursolic acid, β-sitosterol and lupeol added to analyte | Theoretical concentration of ursolic acid, β-sitosterol and lupeol (ng/μL) | Ursolic acid |
β-sitosterol |
Lupeol |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration found (ng/μL) ± SD | % RSD | % Recovery | Concentration found (ng/μL) ± SD | % RSD | % Recovery | Concentration found (ng/μL) ± SD | % RSD | % Recovery | ||
| 0 | 200 | 198.18 ± 2.16 | 1.089 | 99.09 | 197.14 ± 2.18 | 1.105 | 98.57 | 199.03 ± 2.69 | 1.351 | 99.51 |
| 50 | 300 | 298.02 ± 3.57 | 1.197 | 99.34 | 297.8 ± 4.19 | 1.407 | 99.26 | 299.27 ± 4.18 | 1.396 | 99.75 |
| 100 | 400 | 400.52 ± 4.89 | 1.220 | 100.13 | 398.53 ± 5.81 | 1.457 | 99.63 | 396.07 ± 6.09 | 1.537 | 99.01 |
| 150 | 500 | 498.79 ± 6.19 | 1.240 | 99.75 | 492.03 ± 7.57 | 1.538 | 98.40 | 498.58 ± 7.79 | 1.562 | 99.71 |
Table 4.
Precision of the proposed HPTLC Method (n = 6).
| Conc. of standard added (ng/spot) | Ursolic acid |
β-sitosterol |
Lupeol |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-day Precision |
Inter-day Precision |
Intra-day Precision |
Inter-day Precision |
Intra-day Precision |
Inter-day Precision |
|||||||
| Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | |
| 200 | 197.25 ± 2.05 | 1.039 | 192.61 ± 2.01 | 1.043 | 198.88 ± 2.23 | 1.121 | 196.20 ± 2.19 | 1.116 | 196.02 ± 2.29 | 1.168 | 190.42 ± 2.21 | 1.160 |
| 400 | 399.59 ± 4.19 | 1.048 | 396.80 ± 4.13 | 1.040 | 397.21 ± 4.79 | 1.205 | 394.54 ± 4.73 | 1.198 | 398.95 ± 5.21 | 1.305 | 397.08 ± 5.17 | 1.301 |
| 600 | 595.33 ± 6.59 | 1.106 | 591.61 ± 6.52 | 1.102 | 595.74 ± 7.61 | 1.277 | 593.07 ± 7.56 | 1.274 | 597.08 ± 7.86 | 1.316 | 595.21 ± 7.81 | 1.312 |
Table 5.
Robustness of the proposed HPTLC Method (n = 6).
| Optimization condition | Ursolic acid |
β-sitosterol |
Lupeol |
|||
|---|---|---|---|---|---|---|
| SD | %RSD | SD | %RSD | SD | %RSD | |
| Mobile phase composition; (Chloroform: methanol; 97:3) | ||||||
| (96.5: 3.5) | 2.89 | 0.994 | 3.87 | 1.296 | 5.11 | 1.717 |
| (97: 3) | 2.99 | 1.022 | 3.92 | 1.315 | 5.19 | 1.739 |
| (97.5: 2.5) | 3.02 | 1.029 | 3.99 | 1.336 | 5.27 | 1.771 |
| Mobile phase volume (for saturation) | ||||||
| (18 mL) | 2.89 | 0.997 | 3.76 | 1.265 | 5.26 | 1.746 |
| (20 mL) | 2.87 | 0.978 | 3.79 | 1.274 | 5.29 | 1.756 |
| (22 mL) | 2.91 | 0.988 | 3.81 | 1.280 | 5.31 | 1.763 |
| Duration of saturation | ||||||
| (10 min) | 2.82 | 0.955 | 3.82 | 1.281 | 5.07 | 1.683 |
| (20 min) | 2.86 | 0.965 | 3.84 | 1.290 | 5.03 | 1.670 |
| (30 min) | 2.78 | 0.945 | 3.88 | 1.304 | 5.09 | 1.690 |
3.2.2. HPTLC analysis of anticancer biomarkers ursolic acid, β-sitosterol and lupeol in different fractions of Hibiscus species
The developed HPTLC method was employed in the estimation of ursolic acid, β-sitosterol and lupeol in the different fractions of Hibiscus species (Table 6). The quantities (μg/mg of the dried weight of extracts) of biomarkers ursolic acid/β-sitosterol/lupeol in different fractions were found in the following order: HdP (5.50/11.85/7.47) > HmP (4.03/8.57/5.37) > HcP (1.51/5.43/0.42) > HmT (3.20/0.23/0.81) > HdC (1.27/0.59/0.74) > HdE (1.19/0.049/0.11) > HcC (0.06/0.098/0.06). This finding clearly indicated that out of fifteen fractions of all the three Hibiscus species, – H. deflersii petroleum ether fraction (HdP) and H. micranthus petroleum ether fraction (HmP) contains the highest quantity of all the biomarkers (Fig. 4C and D) followed by other fractions. These biomarkers were found completely absent in H. deflersii toluene fraction (HdT), H. calyphyllus toluene fraction (HcT), H. micranthus ethyl acetate fraction (HmE) and the n-butanol fractions of all three species. Several analytical methods like HPLC, HPTLC has been reported for the quantitative analysis of ursolic acid, β-sitosterol and lupeol in different plant extracts like, estimation of β-sitosterol by HPTLC in the aerial parts of Tinospora cordifolia and Calotropis gigantia (Alajmi et al., 2017), lupeol estimation by HPTLC in the leaves of different species of genus Ficus (Alam et al., 2015a, Alam et al., 2015b, Alam et al., 2015c), estimation of ursolic acid in Rabdosia rubescens by RP-HPLC (Yang et al., 2012) and in human plasma by UPLC-MS (Xia et al., 2011), but we did not find any literature on the concurrent estimation of these biomarkers in Hibiscus species. Hence we are privileged to report this maiden research on the estimation of cytotoxic biomarkers ursolic acid, β-sitosterol and lupeol in H. calyphyllus, H. deflersii and H. micranthus fractions along with their comparative cytotoxic potential against MCF-7 and HepG2 cells.
Table 6.
HPTLC analysis of ursolic acid, β-Sitosterol and lupeol in different fractions of H. calyphyllus, H. deflersii and H. micranthus.
| S. No. | Samples | Ursolic acid content (µg/mg of dried weight of extract) | β-sitosterol content (µg/mg of dried weight of extract) | Lupeol content (µg/mg of dried weight of extract) |
|---|---|---|---|---|
| 1 | H. deflersii petroleum ether fraction (HdP) | 5.50 ± 0.17 | 11.85 ± 0.26 | 7.47 ± 0.23 |
| 2 | H. micranthus petroleum ether fraction (HmP) | 4.03 ± 0.11 | 8.57 ± 0.25 | 5.37 ± 0.16 |
| 3 | H. calyphyllus petroleum ether fraction (HcP) | 1.51 ± 0.03 | 5.43 ± 0.19 | 0.42 ± 0.004 |
| 4 | H. micranthus toluene fraction (HmT) | 3.20 ± 0.08 | 0.23 ± 0.003 | 0.81 ± 0.007 |
| 5 | H. deflersii toluene fraction (HdT) | Not detected | Not detected | Not detected |
| 6 | H. calyphyllus toluene fraction (HcT) | Not detected | Not detected | Not detected |
| 7 | H. micranthus chloroform fraction (HmC) | Not detected | 0.062 ± 0.0009 | 0.41 ± 0.006 |
| 8 | H. deflersii chloroform fraction (HdC) | 1.27 ± 0.03 | 0.59 ± 0.01 | 0.74 ± 0.01 |
| 9 | H. calyphyllus chloroform fraction (HcC) | 0.06 ± 0.001 | 0.098 ± 0.001 | 0.06 ± 0.001 |
| 10 | H. micranthus ethyl acetate fraction (HmE) | Not detected | Not detected | Not detected |
| 11 | H. deflersii ethyl acetate fraction (HdE) | 1.19 ± 0.02 | 0.049 ± 0.0005 | 0.11 ± 0.001 |
| 12 | H. calyphyllus ethyl acetate fraction (HcE) | 0.015 ± 0.0001 | Not detected | Not detected |
| 13 | H. micranthus n-butanol fraction (HmB) | Not detected | Not detected | Not detected |
| 14 | H. deflersii n-butanol fraction (HdB) | Not detected | Not detected | Not detected |
| 15 | H. calyphyllus n-butanol fraction (HcB) | Not detected | Not detected | Not detected |
4. Conclusion
With the knowledge about the mechanism of anticancer activity of all the three biomarkers we postulates that the H. deflersii exhibited its anticancer effect by inducing cell cycle (G1/G2) arrest and apoptosis, inducing the activation of caspase-3/9, increasing the release of cytochrome c and down regulating the Bcl-2 and Bcl-xL proteins expressions. We suggest that H. deflersii (Petroleum ether fraction) can be further subjected to the isolation of active cytotoxic phytoconstituents and establishment of their mechanism of action. The maiden HPTLC method developed for the concurrent analysis of ursolic acid, β-sitosterol and lupeol may be further employed for the in-process quality control of herbal formulation containing the said biomarkers.
Acknowledgments
Acknowledgement
“The authors would like to extend their sincere appreciation to the Research Center, College of Pharmacy and Deanship of Scientific Research at King Saud University for providing the financial support.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Perwez Alam, Email: aperwez@ksu.edu.sa.
Hanan M. Al-Yousef, Email: halyousef@ksu.edu.sa.
Tawfeq A. Alhowiriny, Email: talhowiriny@ksu.edu.sa.
Saleh I. Alqasoumi, Email: sqasoumi@ksu.edu.sa.
Musarat Amina, Email: mamina@ksu.edu.sa.
References
- Alajmi M.F., Alam P., Siddiqui N.A., Basudan O.A., Hussain A. Quantitative analysis of biomarker rutin in different species of genus Ficus by validated NP and RP-HPTLC methods. Pak. J. Pharm. Sci. 2015;28S:2213–2220. [PubMed] [Google Scholar]
- Alajmi M.F., Hussain A., Alam P. Concurrent analysis of biologically active markers β-amyrin and β-sitosterol by applying validated HPTLC method in the aerial parts of Tinospora cordifolia and Calotropis gigantia. J. Planar Chromatogr. 2017;30(3):175–180. [Google Scholar]
- Alam P., Siddiqui N.A., Al-Rehaily A.J., Alajmi M.F., Basudan O.A., Khan T.H. Stability indicating densitometric HPTLC method for quantitative analysis of biomarker naringin in the leaves and stems of Rumex vesicarius L. J. Planar Chromatogr. 2014;27:204–209. [Google Scholar]
- Alam P., Siddiqui N.A., Basudan O.A., Al-Rehaily A.J., Alqasoumi S.I., Alam P., Abdel-Kader M.S., Donia Abd El, R. M., Shakeel, F., Comparative profiling of biomarker psoralen in antioxidant active extracts of different species of genus Ficus by validated HPTLC method. Afr. J. Tradit. Complement Altern. Med. 2015;12:57–67. [Google Scholar]
- Alam P., Basudan O.A., Siddiqui N.A., Alqasoumi S.I., Abdel-Kader M.S., Raheim Donia Abd El, M., Alam, P. Development of densitometric HPTLC method for quantitative analysis of biomarker Lupeol in the leaves of different species of genus Ficus. J. Planar Chromatogr. 2015;28(1):30–35. [Google Scholar]
- Alam P., Al-Rehaily A.J., Siddiqui N.A., Al-Sheddi E.S., Shakeel F. A stability-indicating assay of biomarker bergenin in the aerial parts of Flueggea virosa by a validated high-performance thin-layer chromatographic-densitometric method. J. Planar Chromatogr. 2015;28:54–60. [Google Scholar]
- Alam P., Alajmi M.F., Arbab A.H., Parvez M.K., Siddiqui N.A., Alqasoumi S.I., Al-Rehaily A.J., Al-Dosari M.S., Basudan O.A. Comparative study of antioxidant activity and validated RP-HPTLC analysis of rutin in the leaves of different Acacia species grown in Saudi Arabia. Saudi Pharm. J. 2017;25(5):715–723. doi: 10.1016/j.jsps.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begashaw B., Mishra B., Tsegaw A., Shewamene Z. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Complement Altern Med. 2017;17(1):337. doi: 10.1186/s12906-017-1841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Sayeed M.S., Ameen S.S. Beta-sitosterol: a promising but orphan nutraceutical to fight against cancer. Nutr Cancer. 2015;67(8):1214–1220. doi: 10.1080/01635581.2015.1087042. [DOI] [PubMed] [Google Scholar]
- Chakravarti B., Maurya R., Siddiqui J.A., Bid H.K., Rajendran S.M., Yadav P.P., Konwar R. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: role of pro-apoptotic effects of oleanolic acid and urosolic acid. J. Ethnopharmacol. 2012;142(1):72–79. doi: 10.1016/j.jep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Cheng Y.L., Lee S.C., Harn H.J., Huang H.C., Chang W.L. The extract of Hibiscus syriacus inducing apoptosis by activating p53 and AIF in human lung cancer cells. Am. J. Chin. Med. 2008;36(1):171–184. doi: 10.1142/S0192415X08005680. [DOI] [PubMed] [Google Scholar]
- Choi Y.H., Kong K.R., Kim Y.A., Jung K.O., Kil J.H., Rhee S.H., Park K.Y. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int. J. Oncol. 2003;23(6):1657–1662. [PubMed] [Google Scholar]
- Hou D.X., Tong X., Terahara N., Luo D., Fujii M. Delphinidin 3-sambubioside, a Hibiscus anthocyanin, induces apoptosis in human leukemia cells through reactive oxygen species-mediated mitochondrial pathway. Arch. Biochem. Biophys. 2005;440(1):101–109. doi: 10.1016/j.abb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human use, Harmonised Triplicate Guideline on Validation of Analytical Procedures: Text and Methodology Q2 (R1), Complementary Guideline on Methodology incorporated in November 2005 by the ICH Steering Committee, IFPMA, Geneva.
- Kirtikar, K.R., Basu, B.D., 1984. Indian medicinal plants, vol. 1, second ed., Periodical expert book agency, Delhi, p. 293.
- Lakshman S., Jyothi A.B., Mounica V., Ravi Kumar A., Subbu Rathinam K.M., Rajesh K. Antidiabetic activity of methonolic extract of in streptozotocin induced diabetic rats Hibiscus deflersii. Int. Res. J. Pharm. App. Sci. 2014;4(1):1–3. [Google Scholar]
- Liang C., Pan H., Li H., Zhao Y., Feng Y. In vitro anticancer activity and cytotoxicity screening of phytochemical extracts from selected traditional Chinese medicinal plants. J. BUON. 2017;22(2):543–551. [PubMed] [Google Scholar]
- Lin H.H., Huang H.P., Huang C.C., Chen J.H., Wang C.J. Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Mol. Carcinog. 2005;43(2):86–99. doi: 10.1002/mc.20103. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Bi, T., Dai, W., Wang, G., Qian, L., Shen, G., Gao, Q., 2016. Lupeol Induces Apoptosis and Cell Cycle Arrest of Human Osteosarcoma Cells through PI3K/AKT/mTOR Pathway. Technol Cancer Res Treat. 15(6), NP16-NP24. [DOI] [PubMed]
- Liu L., Zhang J., Li M., Zhang X., Zhang J., Li Z., Wang L., Wu J., Luo C. Inhibition of HepG2 cell proliferation by ursolic acid and polysaccharides via the down regulation of cyclooxygenase-2. Mol. Med. Rep. 2014;9(6):2505–2511. doi: 10.3892/mmr.2014.2059. [DOI] [PubMed] [Google Scholar]
- Pitchai D., Roy A., Ignatius C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J. Adv. Pharm. Technol. Res. 2014;5(4):179–184. doi: 10.4103/2231-4040.143037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha T. Comparative pharmacological study of Aerial parts and roots of ethanolic extract of Hibiscus micranthus Linn. J. Med. Pharm. Allied Sci. 2017;1:581–587. [Google Scholar]
- Sayago-Ayerdi S.G., Velazquez-Lopez C., Montalvo-Gonzalez E., Goni I. By-product from decoction process of Hibiscus sabdariffa L. calyces as a source of polyphenols and dietary fiber. J. Sci. Food Agric. 2014;94(5):898–904. doi: 10.1002/jsfa.6333. [DOI] [PubMed] [Google Scholar]
- Shi L.S., Wu C.H., Yang T.C., Yao C.W., Lin H.C., Chang W.L. Cytotoxic effect of triterpenoids from the root bark of Hibiscus syriacus. Fitoterapia. 2014;97:184–191. doi: 10.1016/j.fitote.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.A., Alam P., Al-Rehaily A.J., Al-Oqail M.M. Simultaneous quantification of biomarkers bergenin and menisdaurin in the methanol extract of aerial parts of Flueggea virosa by validated HPTLC densitometric method. J. Chromatogr. Sci. 2015;53(2015):824–829. doi: 10.1093/chromsci/bmu231. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.A., Al-Yousef H.M., Alhowiriny T.A., Alam P., Hassan W.H., Amina M., Hussain A., Abdelaziz S., Abdallah R.H. Concurrent analysis of bioactive triterpenes oleanolic acid and β-amyrin in antioxidant active fractions of Hibiscus calyphyllus, Hibiscus deflersii and Hibiscus micranthus grown in Saudi Arabia by applying validated HPTLC method. Saudi Pharm. J. 2018;26:266–273. doi: 10.1016/j.jsps.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F.S., Lin L.W., Wu C.R. Lupeol and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;929:145–175. doi: 10.1007/978-3-319-41342-6_7. [DOI] [PubMed] [Google Scholar]
- Wagner, H., Bladt, S., 2004. Plant drug analysis (A Thin Layer Chromatography Atlas). Springer (India) Private Limited, Akash Deep Building, Barakhamba Road, New Delhi, India, second ed., Appendix A: Spray Reagents (No. 3).
- Xia Y., Wei G., Si D., Liu C. Quantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879(2):219–224. doi: 10.1016/j.jchromb.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Wei M.C., Huang T.C. Optimisation of an ultrasound-assisted extraction followed by RP-HPLC separation for the simultaneous determination of oleanolic acid, ursolic acid and oridonin content in Rabdosia rubescens. Phytochem. Anal. 2012;23(6):627–636. doi: 10.1002/pca.2365. [DOI] [PubMed] [Google Scholar]