Abstract
Severe tricuspid regurgitation (TR) is reported to represent a hemodynamic pattern similar to that of constrictive pericarditis (CP), which should be clearly differentiated for appropriate management. We report the case of a patient with severe TR due to atrial fibrillation (AF) in whom hemodynamic monitoring played a role in the selection of the management strategy. An 81-year-old Japanese man with chronic AF was admitted due to worsening heart failure. Echocardiography showed the dilation of bilateral atria and a right ventricle with severe TR. The right heart catheterization demonstrated the elevation and equalization of diastolic pressures of four cardiac chambers with impaired diastolic filling pattern, which are hallmarks of pericardial constriction due to CP. Of note, the CP-like hemodynamics were completely normalized by 10 days of medical therapies including diuretics and carperitide. After his discharge and over a 1-year follow-up, he has never experienced worsening heart failure and remained NYHA class II with moderate TR. Medical management targeted at volume reduction and vasodilation can be a therapeutic option for CP-like hemodynamics in isolated severe TR related to AF. Repeated hemodynamic assessment is an appropriate tool to help our understanding of the CP-like physiology caused by severe TR based on chronic AF.
<Learning objective: Atrial fibrillation (AF)-related severe tricuspid regurgitation (TR) is sometimes reported to hemodynamically mimic constrictive pericarditis. However, it has never been described whether such a hemodynamics could be reversed by medical treatment alone. Repeated pressure monitoring may be helpful to obtain important clues for the diagnosis and the therapeutic strategy in pericardial constraint due to AF-related TR.>
Keywords: Tricuspid regurgitation, Atrial fibrillation, Pericardial constraint, Heart failure, Medical management
Introduction
Atrial fibrillation (AF) has been recognized recently as a precipitating factor that triggers tricuspid annular dilation and subsequent tricuspid regurgitation (TR) [1], [2], [3]. However, the standard management of AF-related TR has not been established, partly because the TR sometimes dramatically reduces after medical treatment. In addition, severe TR is reported to represent hemodynamics mimicking those of constrictive pericarditis (CP), which should be clearly differentiated because a misdiagnosis leads to an inappropriate corrective operation [4], [5]. To date, previous case reports regarding CP-like hemodynamics due to severe TR have indicated the need of surgical TR repair or replacement with prosthetic valve [5], [6]. However, to our knowledge, there is no report that examined how such a CP-like hemodynamics will be changed by medical treatment alone. We report the case of a patient with heart failure caused by AF-related TR in whom repeated hemodynamic monitoring showed a disappearance of CP-like physiology after medical treatment.
Case report
An 81-year-old Japanese man was admitted to our hospital because of worsening exertional dyspnea, 3-kg weight gain, and leg edema that began 2 weeks earlier. His medical history included AF, hypertension, and mild renal impairment. His AF was paroxysmal 3 years earlier and had progressed to chronic AF since the last year despite attempts at pharmacological and electrical cardioversion. He had no history of cardiac surgery, radiation therapy, tuberculosis, rheumatic fever, endocarditis, or chest trauma.
The physical examination revealed a distended jugular vein and a pan-systolic heart murmur (Levine III/VI) at the left parasternal border. Coarse crackles in both lung fields and moderate lower-limb edema were observed. His body weight was 67 kg, and his vital signs were blood pressure 116/79 mmHg, heart rate 95 beats per minute, and oxygen saturation 92% on room air. The electrocardiogram showed AF with normal ST segment and T-waves. A chest X-ray revealed an increased cardiothoracic ratio, pulmonary congestion, and pleural effusion (Fig. 1A).
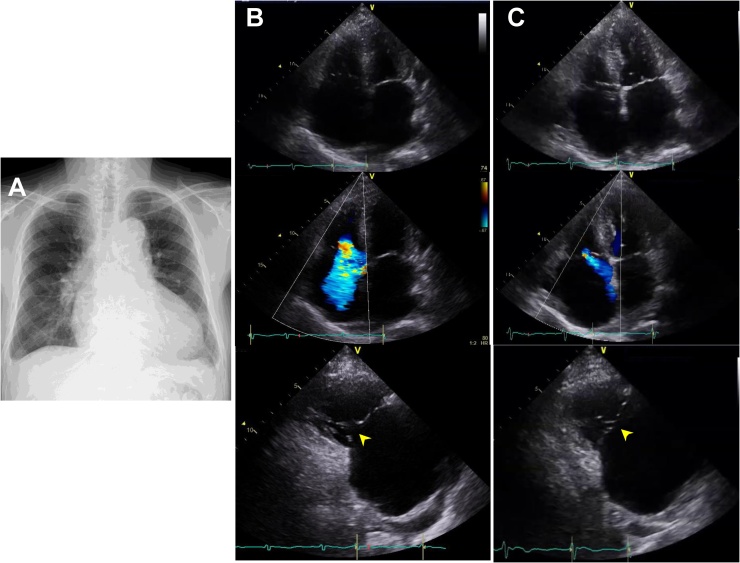
Fig. 1.
Chest X-ray on admission (A). Echocardiographic two-dimensional (B, upper) and color Doppler (B, middle) images of apical four camber view showing the enlargement of both atria and right ventricle that result in severe tricuspid regurgitation (TR). Long-axis view of right ventricular inflow (B, lower) shows an obvious loss of coaptation in the tricuspid valve (yellow arrowhead). Echocardiographic findings after the compensation of heart failure (C). The right ventricle became smaller (C, upper) and the TR was reduced to moderate (C, middle). Long-axis view of right ventricular inflow (C, lower) reveals the improvement of coaptation in the tricuspid valve (yellow arrowhead).
In laboratory tests, the patient’s brain natriuretic peptide was 425 pg/mL; blood urea nitrogen, 38 mg/dL; and serum creatinine, 1.48 mg/dL. Echocardiography revealed a marked enlargement of both atria and the right ventricle, and severe TR caused by coaptation loss due to tethering (Fig. 1B). Although the patient’s pulmonary artery (PA) systolic pressure could not be estimated from the TR velocity due to its laminar behavior, his mean PA pressure was estimated to be 30 mmHg from the peak velocity of pulmonary regurgitation, suggesting the presence of pulmonary hypertension. The inferior vena cava was dilated to 21 mm with reduced respiratory fluctuation. We did not find any abnormal findings in the left ventricle (LV), and mitral annular velocities were preserved, indicating that the pulmonary hypertension was not due to LV myocardial disease. Computed tomography (CT) showed no evidence of thickened or calcified pericardium.
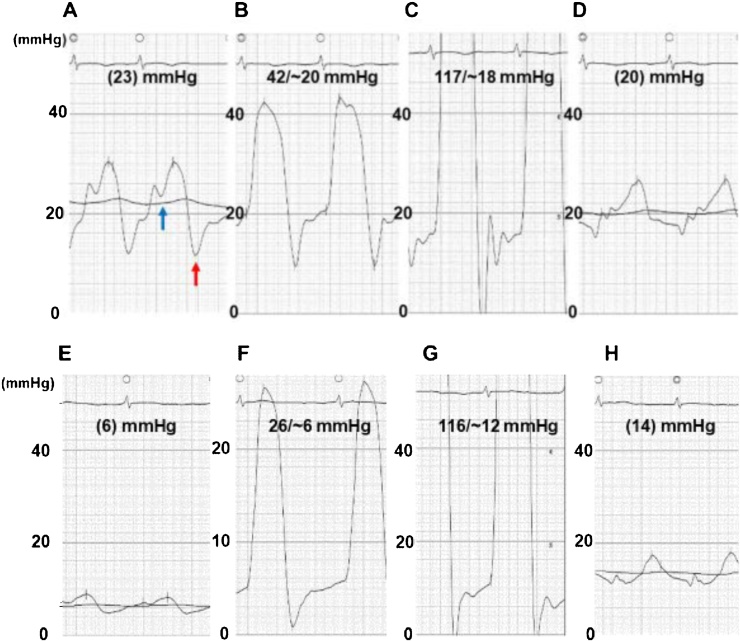
In cardiac catheterization, the elevated right atrial (RA) pressure with a prominent v-wave, as well as a preserved x-descent and a deep y-descent, was apparent (Fig. 2A). The mean RA pressure was 23 mmHg, which was almost identical to the right ventricular (RV) end-diastolic pressure (RVEDP), the LV end-diastolic pressure (LVEDP), and the mean pulmonary capillary wedge pressure (PCWP), indicating the equalization of diastolic pressures in all cardiac chambers (Fig. 2A–D). Both the RV and LV pressure waveforms showed a typical ‘dip and plateau’ pattern (Fig. 2B,C). Together, these data were consistent with CP.
Fig. 2.
Pressure tracings at right and left heart catheterization on admission (A–D) and after compensation (E–H). The right atrial pressure with a preserved x descent (blue arrow) and a deep y descent (red arrow) (A); right ventricular pressure (B) and left ventricular pressure (C) showing ‘dip and plateau’ pattern; and pulmonary capillary wedge pressure (D). The elevation and equalization of diastolic pressures in all cardiac chambers were evident. Decreased right atrial pressure with a return of x- and y-descents (A). The right and left ventricular diastolic pressures (B, C), as well as pulmonary capillary wedge pressure (D), were reduced in conjunction with no apparent ‘dip and plateau’ configuration. Decreased right atrial pressure with a return of x- and y-descents (E). The right and left ventricular diastolic pressures (F, G), as well as pulmonary capillary wedge pressure (H), were reduced in conjunction with no apparent ‘dip and plateau’ configuration.
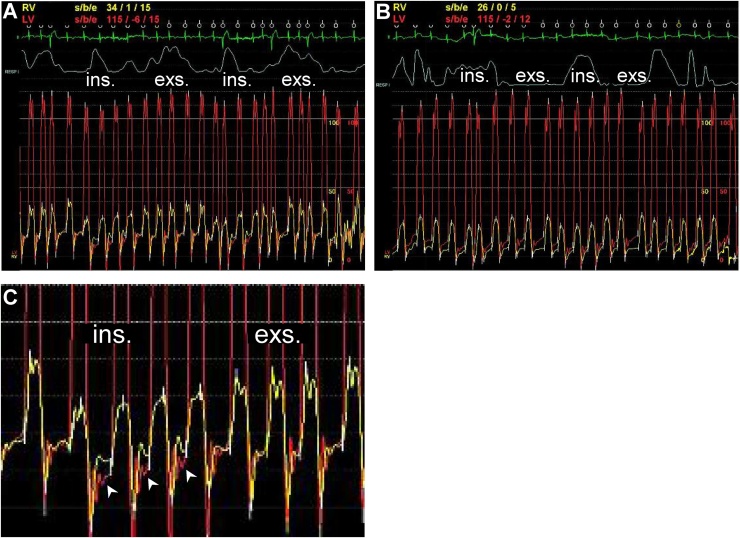
To differentiate TR from CP, we monitored the patient’s biventricular pressures during deep respiration. This monitoring revealed that the systolic area index calculated by the ratio of the RV to LV systolic pressure-time area during inspiration versus expiration was 0.97, suggesting a low probability of CP (Fig. 3A). In addition, the differences in diastolic pressures between the RV and LV became wider with the RV pressure exceeding the LV pressure during inspiration (Fig. 3C), which further confirmed the hemodynamics of severe TR.
Fig. 3.
Simultaneous right (RV) and left ventricular (LV) tracings during a respiration cycle on admission (A) and after compensation (B). (C) An enlarged image of panel (A). During inspiration, the right ventricular diastolic pressure surpasses the LV diastolic pressure (white arrowhead). Ins.: inspiration. Exp.: expiration.
As a therapeutic approach to the patient’s heart failure, furosemide 40 mg/day was administered and titrated up to 60 mg/day. After 3 days, as sufficient diuretic response was not achieved, we added an intravenous infusion of carperitide (0.02 μg/kg/min) for its vasodilatory actions. Immediately, the patient’s urine volume increased and congestive symptoms were relieved.
On the 13th day, the patient’s RV had become smaller and the TR was reduced to moderate (Fig. 1C). In the repeated catheterization, the mean RA pressure declined to 6 mmHg with the absence of accentuated x- and y-descents (Fig. 2E). The ‘dip and plateau’ pressure pattern was not significant, and the RVEDP, LVEDP, and PCWP were reduced to 6, 12, and 14 mmHg, respectively (Fig. 2F–H). The distinct relationship (RVEDP > LVEDP) during inspiration disappeared (Fig. 3B). The patient was discharged on the 25th day and has remained NYHA class II with moderate TR, presenting no congestive findings and edema with plasma BNP 203.8 pg/mL over a 1-year follow-up.
Discussion
This patient’s case fulfilled the conventional diagnostic criteria for CP: elevation and equalization of end-diastolic pressures in four cardiac chambers; a ‘dip and plateau’ pattern in the ventricular pressure; a prominent y-descent with a preserved x-descent in RA pressure; a mild increase in PA systolic pressure (<55 mmHg); and RVEDP more than one-third that of systolic pressure [7]. Although a histological examination was not performed, the patient’s pericardium was intact with echocardiography and CT, and thus CP-like hemodynamics were thought to be due to severe TR.
Several lines of evidence have demonstrated that severe TR mimics physiology seen in CP. Cha et al. first reported two cases presenting the equalization of diastolic pressures among 59 patients with severe TR [4], and four similar cases were reported by others [5], [8]. Such a pseudoconstrictive physiology has been documented in severe TR, but was assessed only at admission and needed surgical tricuspid repair or prosthetic valve replacement. The present case is the first to show a complete disappearance of CP-like hemodynamics after medical treatment by repeated precise pressure monitoring. Severe TR from annulus enlargement due to chronic AF has been increasingly recognized [1], [2]. In the present case, LV function was preserved with no other valvular diseases, and tricuspid annulus was dilated along with the enlargement of both atria, which are typical findings of AF-related TR. In fact, previous reports indicated that severe TR was associated with chronic AF with no fundamental abnormalities of tricuspid valve leaflets and subvalvular tissues [6].
Therapeutic strategies for isolated TR, especially that with surgical indication, have not been established [2]. Rather, our patient’s case underscores the importance of medical management controlling fluid overload in isolated TR. Since tricuspid valve chordae attach directly to RV wall, the volume overload that dilates the RV results in the exacerbation of TR. In addition, distended pericardium attributed to right heart enlargement has a filling-restraining effect, leading to the elevation and equalization of all cardiac chambers. In contrast, discordant changes on inspiration in RV and LV systolic pressures, which are a distinct feature of CP [9], [10], were not evident in the present case.
In addition, our patient’s RVEDP exceeded his LVEDP during inspiration, which could confirm the hemodynamics of severe TR rather than CP [10]. Our experience suggests that carperitide infusion combined with a diuretic may reduce pericardial pressure through venous and arteriolar dilation, contributing to the improvement in pericardial constraint. Notably, once our patient’s CP-like physiology improved, the hemodynamics never relapsed and have been maintained for over a year. Indeed, he remained NYHA class II with moderate TR without any history of worsening heart failure.
Conclusion
Patients with AF-related TR often develop acute heart failure after rapid fluid retention. Exact hemodynamic evaluations would increase our understanding of the CP-like physiology, and medical management might be relevant before surgical intervention in these settings.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Utsunomiya H., Itabashi Y., Mihara H., Berdejo J., Kobayashi S., Siegel R.J. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2017:10. doi: 10.1161/CIRCIMAGING.116.004897. [DOI] [PubMed] [Google Scholar]
- 2.Fender E.A., Zack C.J., Nishimura R.A. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart. 2018;104:798–806. doi: 10.1136/heartjnl-2017-311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi Y., Izumi C., Miyake M., Imanaka M., Kuroda M., Nishimura S. Actual management and prognosis of severe isolated tricuspid regurgitation associated with atrial fibrillation without structural heart disease. Int J Cardiol. 2017;243:251–257. doi: 10.1016/j.ijcard.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Cha S.D., Desai R.S., Gooch A.S., Maranhao V., Goldberg H. Diagnosis of severe tricuspid regurgitation. Chest. 1982;82:726–731. doi: 10.1378/chest.82.6.726. [DOI] [PubMed] [Google Scholar]
- 5.Studley J., Tighe D.A., Joelson J.M., Flack J.E., 3rd The hemodynamic signs of constrictive pericarditis can be mimicked by tricuspid regurgitation. Cardiol Rev. 2003;11:320–326. doi: 10.1097/01.crd.0000089527.66713.7f. [DOI] [PubMed] [Google Scholar]
- 6.Nwiloh J.O. Isolated severe tricuspid regurgitation in a post pneumonectomy patient with chronic atrial fibrillation. Ann Thorac Cardiovasc Surg. 2012;18:132–135. doi: 10.5761/atcs.cr.11.01682. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A., Mehta M., Jain A.C. Constrictive pericarditis. Clin Cardiol. 1999;22:334–344. doi: 10.1002/clc.4960220509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozpelit E., Akdeniz B., Ozpelit M.E., Goldeli O. Severe tricuspid regurgitation mimicking constrictive pericarditis. Am J Case Rep. 2014;15:271–274. doi: 10.12659/AJCR.890092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talreja D.R., Nishimura R.A., Oh J.K., Holmes D.R. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol. 2008;51:315–319. doi: 10.1016/j.jacc.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 10.Jaber W.A., Sorajja P., Borlaug B.A., Nishimura R.A. Differentiation of tricuspid regurgitation from constrictive pericarditis: novel criteria for diagnosis in the cardiac catheterisation laboratory. Heart. 2009;95:1449–1454. doi: 10.1136/hrt.2008.155523. [DOI] [PubMed] [Google Scholar]