Abstract
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute myocardial ischemia. Identification of intimal flap, true and false lumens in coronary angiogram (CAG) is the standard method to diagnose SCAD. In cases of obscure intimal flap, intravascular ultrasound (IVUS) is a useful method to diagnose, although crossing the wire and IVUS in the dissected lesion is invasive. Multidetector computed tomography (MDCT) provides valuable information in any clinical setting less invasively. We report here a rare case of spontaneous dissecting coronary artery pseudoaneurysm diagnosed by CAG and MDCT, healed by medical treatment, and followed up by MDCT over a 2-year period.
<Learning objective: Spontaneous coronary artery dissection (SCAD) is usually diagnosed by the findings of intimal flap, true and false lumens in coronary angiogram (CAG). In case intimal flap is not obvious in CAG, intravascular ultrasound (IVUS) is a useful method to diagnose, although crossing the wire and IVUS in the dissected lesion is invasive. Multidetector computed tomography is an alternative useful method to obtain valuable information to diagnose SCAD less invasively.>
Keywords: Coronary artery aneurysm, Spontaneous coronary artery dissection, Pseudoaneurysm, Multidetector computed tomography
Introduction
Coronary artery aneurysms (CAAs) are localized dilatation in a coronary artery more than 1.5-fold compared with adjacent normal segments, and classified as true aneurysms or pseudoaneurysms depending on the integrity of the vessel wall. Spontaneous coronary artery dissection (SCAD) is a rare cause of coronary artery aneurysms in adults. We report here a rare case of SCAD complicated with pseudoaneurysm which was diagnosed by coronary angiogram (CAG) and multidetector computed tomography (MDCT), healed by medical treatment, and followed up by MDCT over 2 years.
Case report
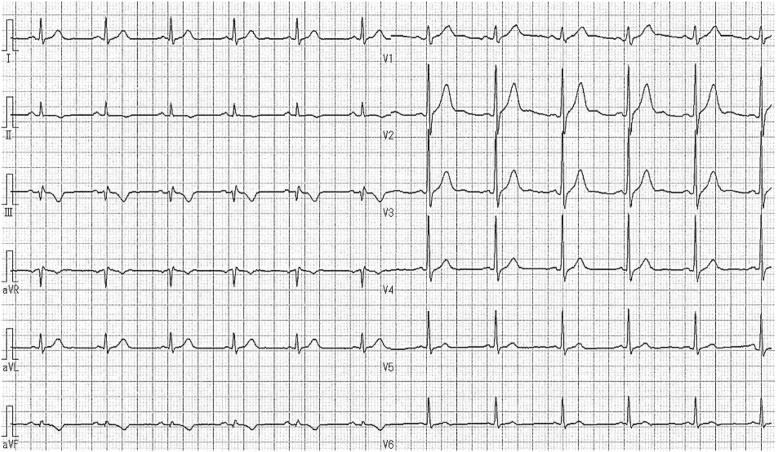
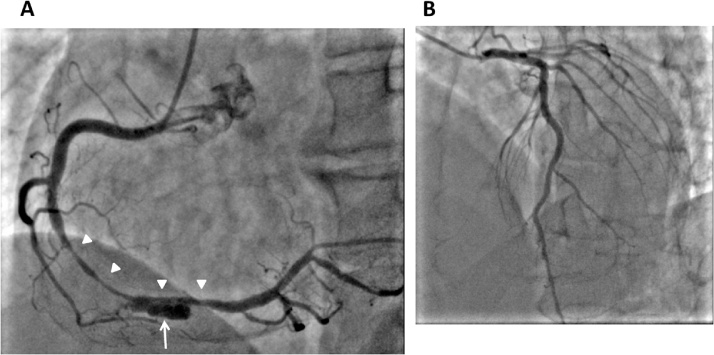
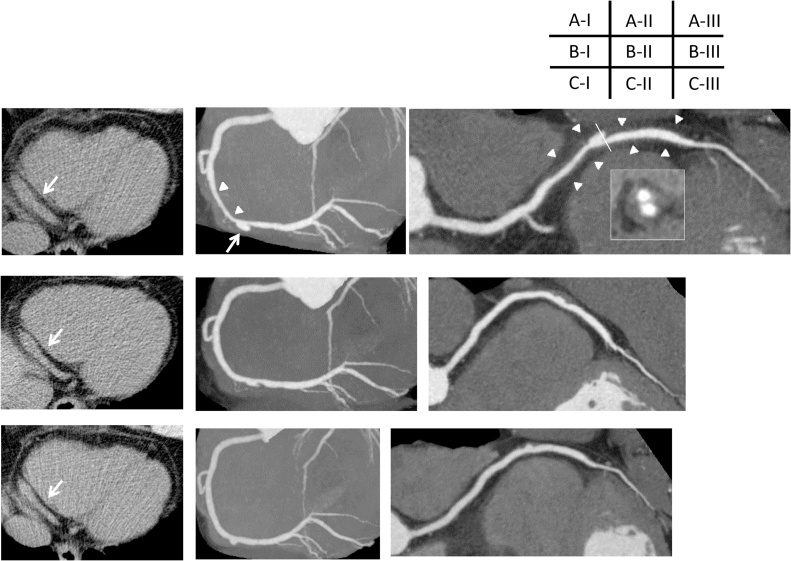
A 55-year-old man was referred to our hospital because of faintness with cold sweat while playing golf two days before. He had no risk factors for coronary artery disease. In physical examination, blood pressure was 139/98 mmHg and heart rate was 75/min. There was no significant abnormality at pulmonary and cardiac auscultation. Electrocardiography showed T wave inversion in II, III, aVF leads (Fig. 1) and transthoracic echocardiography revealed hypokinesis of inferior left ventricular wall. Laboratory testing showed positive troponin test, and elevated level of creatine kinase with 316 IU/L (14–170 IU/L), lactate dehydrogenase with 326 IU/L (106–220 IU/L), and aspartate aminotransferase with 40 IU/L (7–38 IU/L). Although severe myocardial ischemia was suspected, CAG was performed 9 days later because of the patient’s personal reason. CAG revealed diffuse stenosis unresponsive to nitroglycerin infusion with an aneurysm in segment 3 of right coronary artery (RCA), although no significant lesions were shown in left coronary artery (Fig. 2). At that time, we thought that there could be two possible etiologies of the aneurysm, one was a true aneurysm caused by atherosclerosis, the other was a pseudoaneurysm complicated by spontaneous coronary artery dissection. Because of no symptoms and the Thrombolysis in Myocardial Infarction flow grade was three, the decision was made to prescribe medical treatment with aspirin and nitrate. To obtain more detailed information, MDCT was performed 5 days later. A perivascular attenuation was confirmed coinciding with the diffuse stenosis of the segment 3 of RCA, and the outer diameter dilated to approximately 9 mm. In a part of the perivascular attenuation area, contrast media pooling which demonstrated a double-lumen feature on cross-sectional imaging was observed, and it corresponded to an aneurysm in CAG (Fig. 3A-I–A-III). 50 days later, follow-up MDCT was performed. The stenosis was improved with diminished space of perivascular attenuation, contrast media pooling, and outer diameter at the segment 3 of RCA (Fig. 3B-I–B-III). Then, we were confident the etiology of our case was a spontaneous dissecting coronary artery pseudoaneurysm. Subsequently, follow-up MDCT was performed every 6 months with ever more improved findings (Fig. 3C-I–C-III). The patient did not experience any symptoms over a 2-year period.
Fig. 1.
Electrocardiography at initial examination. Electrocardiography showed T wave inversion in II, III, aVF leads.
Fig. 2.
Coronary angiogram (CAG). (A) Right coronary artery (RCA). Diffuse stenosis (white arrowheads) with an aneurysm (white arrow) in segment 3 of RCA. (B) Left coronary artery. No significant lesions in left coronary artery.
Fig. 3.
Multidetector computed tomography (MDCT). (A) Initial MDCT performed 5 days after coronary angiogram. (B) Follow-up MDCT performed 2 months later. (C) Follow-up MDCT performed 1 year later. (I) Transaxial non-enhanced imaging. (II) Angiographic view. (III) Curved planar reconstruction (CPR).
A-I–A-III
In initial MDCT, angiographic view revealed diffuse stenosis (white arrowheads) with an aneurysmal formation (white arrow) in segment 3 of the right coronary artery, in which segment, perivascular attenuation was observed. Cross-sectional imaging demonstrated double-lumen features at the aneurysmal formation. Transaxial imaging showed the dilatation of segment 3 approximately 9 mm (white arrow).
B-I–B-III
Follow-up MDCT was performed 2 months later, the diffuse stenosis and the dilatation of segment 3 were diminished, and the aneurysmal formation was not evident.
C-I–C-III
1 year later follow up MDCT, the stenosis and aneurysmal formation were obscure, and the dilatation of segment 3 diminished to 6.9 mm.
Discussion
CAAs are localized dilatation in a coronary artery more than 1.5-fold compared with adjacent normal segments. The overall incidence of CAAs ranges from 0.37 to 2.53% in patients undergoing CAG [1]. Aneurysms are classified as true aneurysms or pseudoaneurysms depending on the integrity of the vessel wall. True aneurysms are dilatation of the vessel wall with all normal three-layered structure, in contrast, pseudoaneurysms are with single or double layers of the vessel wall. The most common cause of CAAs in adults is atherosclerosis, which accounts for at least 50% of CAAs. Other causes of CAAs include Kawasaki disease, previous angioplasty, congenital malformation, vasculitic disorders (Takayasu arteritis, systemic lupus erythematosus, polyarteritis nodosa), hereditary connective tissue disorders (Marfan syndrome, Ehlers–Danlos disease), infection (mycotic aneurysm), and rarely SCAD as in the present case [2], [3], [4].
SCAD is a rare cause of acute myocardial infarction, unstable angina, and sudden cardiac death. The majority of SCAD patients are young to middle aged people without many risk factors of coronary artery disease. Although the etiology of SCAD is not well elucidated, atherosclerosis and the peripartum period are considered to be common causal factors for SCAD. Connective tissue diseases such as Marfan syndrome and Ehler–Danlos syndrome, hypersensitivity vasculitis, oral contraceptive use, exercise, prolonged sneezing, and spasm are also described as etiologies of SCAD [5].
SCAD is defined as dissection of the adventitia from the media of coronary artery resulting in accumulation of blood in a dissected area (false lumen). The false lumen can lead to compress the true coronary artery lumen and cause severe stenosis or occlusion. CAG is a useful method to detect coronary artery dissection. Finding of double contrast enhanced lumens (true and false lumens) which are distinguished by linear structure (intimal flap) is pathognomonic of coronary artery dissection [6], [7], [8]. In case of obscure intimal flap or severe narrowing of true lumen, the dissection may not be evident, and intravascular ultrasound (IVUS) can be helpful to detect the true and false lumens, although crossing the wire and IVUS in the dissected lesion is invasive [5].
MDCT provides valuable information in any clinical setting less invasively. Finding of hypodense line parallel to vessel longitudinal axis in the contrast enhanced coronary artery indicates the communicating coronary artery dissection with obvious intimal flap [6], [7]. In the case of thrombosed false lumen with intimal defect, MDCT finding is a narrowing contrast enhanced lumen with a perivascular low attenuation corresponding to hematoma [5]. If high blood flow fills into the false lumen, the coronary artery can be seen larger. In the case of thrombosed false lumen communicating with true lumen, MDCT finding is a perivascular low attenuation with ulcer-like contrast enhanced projection. If the ulcer-like projection and dissected portion of the coronary artery are large, it can mimic an aneurysm as in the present case [3], [4].
Conservative therapy is the first-line therapy for SCAD patients with hemodynamically stable and not-ongoing myocardial ischemia, although no therapeutic guideline has yet been established [5]. Percutaneous coronary intervention is considered if ongoing myocardial ischemia is persistent, although involving risk of propagating the dissection by the wire passing through the false lumen and displacing the intramural hematoma with stent placement [4]. In the present case, as the patient’s clinical condition was stable and he was free from any symptom, medical therapy was selected. There are no uniform recommendations for the management of SCAD, therefore, medication for SCAD may be similar to that of acute coronary syndrome including nitrates, calcium channel blockers, beta-blockers, and antiplatelet agents. Thrombolytic therapy should be avoided because of worsened clinical condition due to dissection extension. In the present case, aspirin and nitrate were initiated to avoid thrombus formation at the affected lesion and to prevent spasm which is thought to be one of the possible causes of SCAD, respectively. Beta-blocker was not initiated due to the possibility of inducing spasm, although it is known to reduce vascular shear force. The patient has been uneventful over a 2-year follow up period.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Syed M., Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997;40:77–84. doi: 10.1016/s0033-0620(97)80024-2. [DOI] [PubMed] [Google Scholar]
- 2.Abou Sherif S., Ozden Tok O., Taşköylü Ö., Goktekin O., Kilic I.D. Coronary artery aneurysms: a review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med. 2017;4:24. doi: 10.3389/fcvm.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman S., Abdul-Waheed M., Helmy T., Huffman L.C., Koshal V., Guitron J. Spontaneous left main coronary artery dissection complicated by pseudoaneurysm formation in pregnancy: role of CT coronary angiography. J Cardiothorac Surg. 2009;4:15. doi: 10.1186/1749-8090-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawano H., Matsumoto Y., Satoh O., Arakawa S., Hayano M., Suyama H. Successful treatment of a ruptured spontaneous dissecting coronary artery pseudoaneurysm with a covered stent in a patient with cardiac tamponade. Intern Med. 2014;53:1067–1070. doi: 10.2169/internalmedicine.53.1322. [DOI] [PubMed] [Google Scholar]
- 5.Jinnouchi H., Sakakura K., Matsuda J., Wakabayashi Y., Wada H., Momomura S. Recurrent spontaneous coronary artery dissection observed with multiple imaging modalities. Int Heart J. 2013;54:182–183. doi: 10.1536/ihj.54.181. [DOI] [PubMed] [Google Scholar]
- 6.Gunes H., Kucukdurmaz Z., Seker E., Kurt R., Salk I., Karapinar H. Spontaneous coronary artery dissection diagnosed by multislice computed tomography. Postepy Kardiol Inter. 2013;9:111–113. doi: 10.5114/pwki.2013.34038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Ayala S.C., Maldonado J., Bolton J.S., Bhalla S. Coronary computed tomography angiography of spontaneous coronary artery dissection: a case report and review of the literature. Am J Case Rep. 2015;16:130–135. doi: 10.12659/AJCR.892805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohlmann P., Weigold G., Kim S.W., Hassani S.E., Escolar E., Pichard A.D. Spontaneous coronary dissection: computed tomography appearance and insights from intravascular ultrasound examination. Circulation. 2006;113:e403–e405. doi: 10.1161/CIRCULATIONAHA.105.572313. [DOI] [PubMed] [Google Scholar]