Abstract
The roots of Valeriana officinalis L. (Valerianaceae) are used for treating sleep disorders and/or mild nerve tension. The effect of valerenic acid on brain-derived neurotrophic factor (BDNF) has not yet been studied, although it is known that gamma-amino butyric acid A (GABAA) receptor is regulated by BDNF, which modulates the depressive-like behavior and neurogenesis. The purpose of this study is to determine the effect of V. officinalis root extract (VO), its main constituents valerenic acid (VA) and acetoxy valerenic acid (AVA) as well as valerenic acid-free (VAF), acetoxy valerenic acid-free (AVAF) extracts and increasing amounts of valerenic acid containing extracts on the BDNF expression in SH-SY5Y cell lines. The effect of methanolic extracts of VO, VA, AVA, VAF, AVAF, and the extracts whose amount of VA were increased gradually, were tested using a Human BDNF ELISA kit with 17β-estradiol as a positive control. The VO and VA extracts caused a significant (p < 0.001) increase in the BDNF expression in SH-SY5Y cells compared to control. This effect completely disappeared when cells were treated with VAF extract. AVA alone did not show any significant change in the BDNF levels. The extracts with increasing amount of VA led to a concentration- dependent effect on the cells. In conclusion, our findings suggest that the antidepressant-like effect of the VO extract is also related to BDNF expression, and that this is mainly due to the presence of VA in the extract. Removing VA from VO extract leads to a loss of activity. Moreover, the concentration of VA plays a role for BDNF expressions in SH-SY5Y cells, which demonstrates the importance of quality control on the commercially available products.
Keywords: Valerenic acid, Valerian, Valeriana officinalis L., BDNF, SH-SY5Y
1. Introduction
Insomnia is a common condition in the elderly population, which disrupts the quality of life (Lustberg and Reynolds, 2000). Valeriana officinalis is a well-known traditional medicinal plant used for treating sleep disorders and/or mild nerve tension (Houghton, 1997). As a result of clinical investigations, VO has been found to be effective and approved by Commission E for use in the treatment of sleep disorders (Blumenthal, 1998). VO has some advantages compare to benzodiazepines because it is well tolerated and is effective against insomnia (Gerhardt et al., 1996, Mahady et al., 2003).
VO products are used as a mild sedative throughout the world (ESCOP Monographs, 2003). Valerenic acid (VA), which has a sesquiterpene carboxylic acid skeleton isolated from Valeriana officinalis, is regarded as the major active compound. The anxiolytic potential of VA has been demonstrated with in vitro and in vivo experiments (Yuan et al., 2004, Khom et al., 2007, Sichardt et al., 2007, Trauner et al., 2008, Benke et al., 2009, Khom et al., 2010, Murphy et al., 2010). Studies have shown that VO modulates GABAA receptors due to VA. AVA and hydroxy valerenic acid (HVA) do not modulate GABAA receptors but are linked to the identical binding sites (Felgentreff et al., 2012). The main inhibitory neurotransmitter in the brain is GABA, which is important for balance between neuronal excitation and inhibition; therefore, GABA is crucial for normal brain function. If the balance is disturbed, disorders such as depression, sedation, anxiety, restlessness, and insomnia may occur (Sieghart, 1995). To date, the effect of V. officinalis and valerenic acid on GABAA receptors has been investigated (Felgentreff et al., 2012, Dietz et al., 2005). On the other hand, Brain-Derived Neurotrophic Factor (BDNF) is the most active growth factor in the neurotrophin family that is found in small amounts in the brain. By keeping BDNF at a satisfactory level, neurotransmission occurs at an optimal level and potential physical and mental illnesses are prevented. Therefore, BDNF has an important role in the central nervous system. Alzheimer's, obesity, depression and even schizophrenia can occur with low levels of BDNF (Björkholm and Monteggia, 2016). In particular, the treatment of Alzheimer's disease (AD), which has a multi-factorial and multistage nature, has not yet been fully exploited. For this reason, intensive research is being conducted to find new drug candidates of natural or synthetic origin in AD treatment whose pathogenesis is still not determined.
The aim of this study is to evaluate the effects of VO, its major components VA and AVA as well as VAF and AVAF on the BDNF expression in SH-SY5Y cell lines. The sedative role of valerenic acid will be determined by adding the increasing amounts of valerenic acid to the VAF extract in the Human BDNF ELISA kit using positive control 17 β-estradiol.
2. Methods
2.1. Plant material
Fresh roots of Valeriana officinalis L. were purchased during the flowering period from the cultured areas of Selcuk University Faculty of Agriculture Department of Medicinal Plants. The plant specimens were deposited in the medicinal and aromatical plants herbarium (TBÇ-V-001) of Selcuk University.
2.2. Thin Layer Chromatography (TLC) – According to PhEur (Pharmacopoeia Europea 9th edition, 2017).
Test solution:1 g of the powdered herbal drug was suspended in 10 mL of methanol R and sonicated for 10 min and filtered.
Plate: Kieselgel 60 F254, 20 cm; Mobile phase: glacial acetic acid R/ethyl acetate R/ cyclohexane R (2:38:60 V/V/V).
Reference solution: 5 mg of VA and 5 mg of AVA were dissolved in 20 mL methanol R; then 20 μL of the solution was applied to the plate as 10 mm bands. Anisaldehyde solution R was used for detection. The solution was heated at 100–105 °C for 5–10 min and examined in daylight. VA and AVA were detected as violet zones.
2.3. HPLC studies
Analyses were performed using a Dionex P680 series HPLC (Dionex, Germany), including a binary pump, vacuum degasser, ASI-100 Injektor autosampler, and diode array detector. The analysis method of Pharmacopoeia Europea-9 [18] was applied herein. Chromatographic separations were performed on a Supelco C18 column (4.6 mm × 250 mm × 5 μL). A mobile phase consisting of aqueous solution of phosphoric acid (pH: 2.5) and acetonitrile (20:80 v/v) (A) and aqueous solution of phosphoric acid (pH: 2.5) and acetonitrile (80:20 v/v) (B) was used for separation with a gradient elution at a flow rate of 1.5 mL/min. Gradient elution was performed as follows: from 0 min to 5 min, isocratic 45% solvent B; linear gradient from 5 min to 18 min, from 45% to 80% solvent B; from 18 min to 22 min isocratic 80% solvent B; linear gradient from 22 min to 23 min, from 80% to 45% solvent B; from 23 min to 30 min, isocratic 45% solvent B, and re-equilibration period of 2.5 min with solvent B used between individual runs. Detecion was at 220 nm. The injection volume was 20 μL. Column temperature was set to 30 °C. Identification of the valerenic acid was made by comparing the retention times and UV spectra of the peaks in the valerian samples and the standard. Standard solution was then added to the sample; the increase in the intensity of the peaks verified the identification. All of the calculations concerning the quantitative analysis were performed with external standardization by measurement of peak areas. Standard solutions of valerenic acid (0.5 mg/mL) were prepared in methanol. Each injection was achieved in triplicate to establish reproducibility of detector response at each concentration level.
2.4. Sample preparation
2.4.1. Preparation of VAF and AVAF extracts
One gram of the powdered Valeriana officinalis root was suspended in 10 mL of methanol R and sonicated for 10 min and filtered (VO). The filtered extract was applied as a band form to the plate and the sample band was allowed to migrate with mobile phase (glacial acetic acid R/ethyl acetate R/ cyclohexane R; 2:38:60 V/V/V) in a TLC chamber, up to the upper edge of the band. It was then removed from the plate, the solvent evaporated and visualized under UV. After the VA and AVA bands had separated from each other, the VA and/or AVA zones were scraped from the plates. The other parts of the plate were also removed to obtain VAF and/or AVAF extracts. The scraped parts were dissolved in methanol and filtered. All extracts, as well as VA and AVA gained from the VO, were confirmed by HPLC to make sure that VAF and AVAF extracts were obtained.
2.4.2. Preparation of gradually increased valerenic acid containing VAF extracts
To obtain extracts containing 0.025%, 0.05% and 0.1% VA, the VA reference compound was added separately at concentrations of 0.025%, 0.05% and 0.1% to the VAF extract.
2.5. BDNF expression in SH-SY5Y cells
SH-SY5Y cells were cultured in a T-25 flask in DMEM 10% FBS with the supplements and incubated in 3 mL media at 37 °C. Cells were passaged into a T-75 flask when the confluence reached 80%. Then cells were counted by hemocytometer and 3 × 105 cells were seeded in each well in 6-well plates. In this experiment, three different batches of cells were used (ņ = 3). After the cells incubated in 6-well plates for 24 h, the cells were treated with extracts/compounds and 17-β-estradiol for 72 h in a free serum medium containing 2 mL/well. The final concentrations of the extracts were 100 μg/mL; the final concentrations of the pure compounds were 25 µg/mL; the final concentration of the positive control 17β-Estradiol was 1.10 nM. Three wells were treated only with the carrier solvent as a negative control (blank). After 48 h, the supernatant was removed and the cells were washed with PBS; then PBS was removed and chemical cell lysis was performed by adding 100 µL of RIPA Lysis Buffer System to each well and removed with a cell scraper. Then the mixtures were transferred into labeled tubes and vortex mixed for a few seconds and incubated for 30 min at 4 ˚C. After incubation, the tubes were vortexed again and centrifuged at 14,000g for 30 min at 0 ˚C. After the tubes were centrifuged, the supernatants were transferred into new labeled tubes. Cell lysates were stored at −20 ˚C until the time of use. To assess BDNF expression, a Boster BDNF Human Elisa Kit, which is a standard sandwich enzyme-linked immune-sorbent technique, was used. Instructions in the manual were followed to measure BDNF levels in the cell lysate.
The effectiveness of the Elisa Kit was validated by using a positive control provided by the company. 578 pg of BDNF protein was reconstituted with 1 mL of sample diluent and mixed thoroughly. 100 µL of the mixture was added into the precoated 96-well plate. Instructions on the manual were followed to measure BDNF levels in the cell lysate. The stop solution changes the color from blue to yellow, and the intensity of the color is measured at 450 nm.
2.6. Statistical analysis
The results from each group were presented as a mean ± standard error of the mean [SEM (n = 3)]. Differences among groups were compared using one-way analysis of variance (One-way ANOVA and non-parametric), followed by Tukey testing for multiple comparisons (Prism 5.0 software (GraphPad San Diego, CA, USA). Values of p < 0.05 or less were regarded statistically significant.
3. Results
3.1. Effects of VO, VA, AVA, VAF and AVAF extracts on BDNF expression
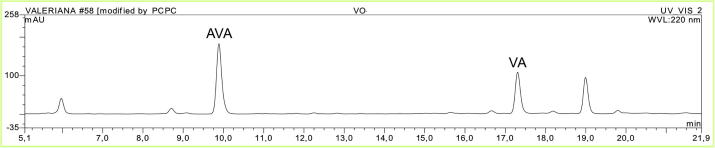
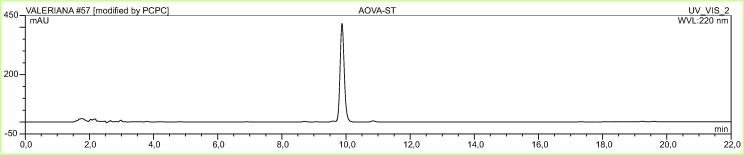
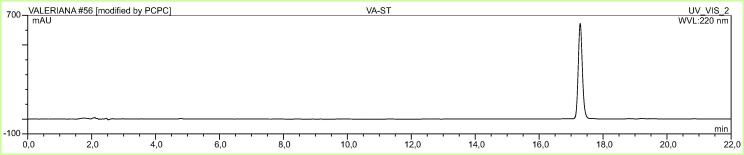
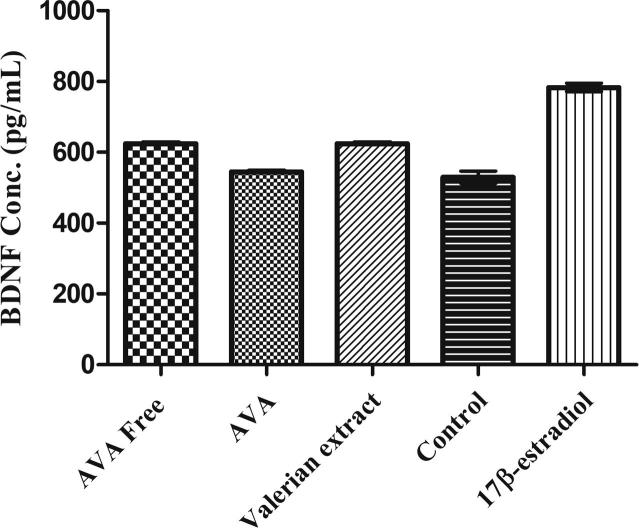
The effect of methanolic extract of Valeriana officinalis (VO) (Fig. 1), valerenic acid free extract (VAF) and acetoxyvalerenic acid-free extract (AVAF), acetoxyvalerenic acid (AVA) (Fig. 2) and valerenic acid (VA) (Fig. 3) on BDNF expression are shown in Table 1. The original valerian methanolic extract (VO) at 25 µg/mL, increased intracellular BDNF levels in SHSY-5Y cells by 17.92% (p < 0.001) compared to the blank. At 25 µg/mL the VAF extract decreased the level of BDNF by 1.34%, while the VA itself at 25 µg/mL increased the level of BDNF by 19.98% (p < 0.001) compared to the blank. On the other hand, at the same concentration AVA increased the BDNF level only by 2.77% while AVAF showed a BDNF increase of 17.80% (p < 0.001) (Fig. 4, Fig. 5; Table 1).
Fig. 1.
HPLC Chromatogram of sesquiterpenic acids in VO.
Fig. 2.
Chromatogram of AVA.
Fig. 3.
Chromatogram of VA.
Table 1.
Effects of VA and AVA on BDNF levels in SH-SY5Y cells.
| Extract conc. μg/mL | BDNF conc.pg/mL | BDNF increase % | |
|---|---|---|---|
| VO | 25 | 635.3 | 19.98 |
| VA | 25 | 522.4 | −1.34 |
| VAF | 25 | 624.4 | 17.92 |
| AVA | 25 | 544.2 | 2.77 |
| AVAF | 25 | 624.0 | 17.80 |
| Blank | – | 529.5 | 0 |
| 17 β-estradiol | 1 μM | 783.0 | 47.8 |
Fig. 4.
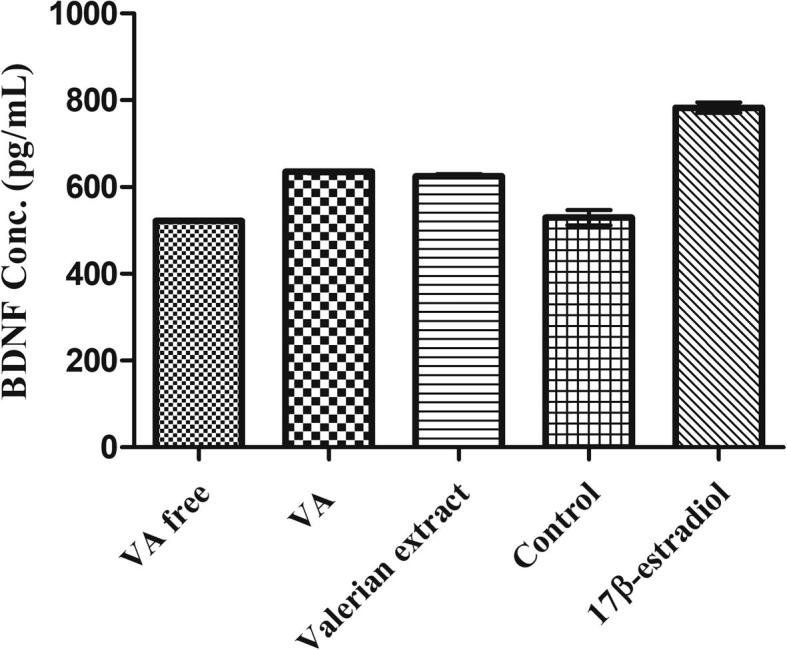
Effects of VA on BDNF levels in SH-SY5Y cells; VA: valerenic acid. Data are expressed as mean ± SEM (n = 3 in each group) **p < 0.001, ***p < 0.0001.
Fig. 5.
Effects of AVA on BDNF levels in SH-SY5Y cells; AVA: acetoxyvalerenic acid. Data are expressed as mean ± SEM (n = 3 in each group) **p < 0.001, ***p < 0.0001.
3.2. Effect of gradually increasing valerenic acid in VAF extract to BDNF expression
Four groups of extracts were prepared with increasing amounts of VA and assesed for their effects on SH-SY5Y cells. Group I: VA-free extract; Group II: % 0.025 VA added extract; Group III: % 0.05 VA added extract; Group IV: % 0.1 VA added extract. BDNF expressions of these extracts were calculated as 2.4%, 19.1%, and 29.1%, respectively whereas 46.5% for positive control and 0 for the blank (Fig. 6, Table 2).
Fig. 6.
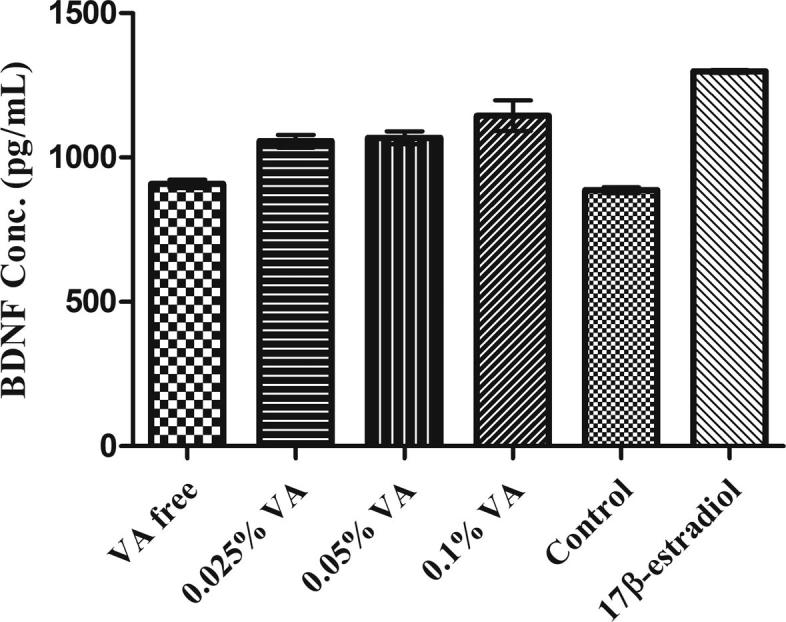
Concentration-response effect of valerenic acid on BDNF levels; VA: valerenic acid. Data are expressed as mean ± SEM (n = 3 in each group) **p < 0.001, ***p < 0.0001.
Table 2.
Concentration-response effect of valerenic acid on BDNF levels in SH-SY5Y cells.
| Extract conc. μg/mL | BDNF conc.pg/mL | BDNF increase % | |
|---|---|---|---|
| VA free extract | 15 | 908.57 | 2.4 |
| 0.025% VA | 15 | 1056.44 | 19.1 |
| 0.050% VA | 15 | 1067.89 | 20.4 |
| 0.100% VA | 15 | 1144.99 | 29.1 |
| Blank | – | 886.86 | 0 |
| 17 β-estradiol | 1 μM | 1298.33 | 46.5 |
4. Discussion
Having the utmost level of phytochemical constituents, Valerian contains more than 150 chemical compounds with different physiological activity (Neamati et al., 2014). Some of these chemicals upregulate the BDNF pathway which was observed in this study. Recent studies have shown that BDNF expression decrease in the brain during depression. Numerous studies have been published on the role of BDNF in the pathophysiology of mood, including major depressive disorders. It has been reported that injection of BDNF in the mesencephalon has behavioral antidepressant effect in learned helplessness paradigms and forced swim test. This suggests that increased BDNF expression has an antidepressant effect (Nibuya et al., 1995). All kind of antidepressants treatment, including lithium, and electroconvulsive shock treatment increase BDNF expression in the cortex and hippocampus (Castrén et al., 2007). Moreover, it has been shown that antidepressant treatment does not decrease immobility in forced swimming test in BDNF knockout mice and there is no any reduction in immobility during forced swimming test with the administration of antidepressant, which indicates that the positive behavioral effect of antidepressant is completed by BDNF signaling (Martinowich et al., 2007). As a result, BDNF has become one of the major targets of antidepressant medications, recently (Chen et al., 2001, Hashimoto et al., 2004). Until now, valerenic and acetoxy valerenic acid associations with the GABA receptor have been investigated to explain the effects of valerian on anxiety and insomnia. The antidepressant effects of the valerian have been attributed to valerenic acid however, the effect of V. officinalis extracts and its components on BDNF expression has not been investigated. This study was thought to be useful for gaining a new perspective on valerian’s activity mechanism in this respect. Therefore, the effects of VO, VA, AVA, VAF, AVAF on BDNF expression were evaluated by using SH-SY5Y cells. Separately, the effect of gradually increasing concentration of VA on BDNF levels was studied. The methanolic extract of V. officinalis, carrying VA, increased the BDNF level while the BDNF level decreased when VA was removed from the VO extract; this suggests that valerenic acid is essential for the activity. Moreover, when the concentration of VA in the extract was increased gradually, a gradual increase in BDNF levels was observed as well (Table 2, Fig. 6). The presence or absence of acetoxy valerenic acid was found to be unrelated to the activity.
In conclusion, our result showed V. officinalis extracts increase BDNF level in SH-SY5Y cells and the effect of V. officinalis extracts on BDNF was demolished completely when valerenic acid was removed from the extract, which indicates that valerenic acid is crucial for the neuronal activity.
Conflict of interest
No conflict to disclose.
Acknowledgement
This study was supported by grants from Hacettepe University Scientific Research Projects (Project No: THD-2016-9171)
We would like to thank Prof. Dr. Gerald Ramelow for language editing.
Footnotes
Peer review under responsibility of King Saud University.
References
- Benke D., Barberis A., Kopp S., Altmann K.H., Schubiger M., Vogt K.E., Rudolph U., Möhler H. GABAA receptors as in vivo substrate fort he anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology. 2009;56(1):174–181. doi: 10.1016/j.neuropharm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Björkholm C., Monteggia L.M. BDNF-a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal M., editor. The Complete German Commission E Monographs Therapeutic Guide to Herbal Medicines. American Botanical Council; Austin, TX: 1998. [Google Scholar]
- Castrén E., Võikar V., Rantamäki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chen B., Dowlatshahi D., MacQueen G.M., Wang J.F., Young L.T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Dietz B.M., Mahady G.B., Pauli G.F., Farnsworth N.R. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Mol. Brain Res. 2005;138:191–197. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCOP Monographs, 2nd ed., 2003. Thieme, New York, NY.
- Felgentreff F., Becker A., Meier B., Brattström A. Valerian extract characterized by high valerenic acid and low acetoxy valerenic acid contents demonstrates anxiolytic activity. Phytomedicine. 2012;19:1216–1222. doi: 10.1016/j.phymed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Gerhardt U., Linnenbrink N., Geroghiadou Ch., Hobi V. Vigilanz- mindernde Effekte zweier pflanzlicher Schlafmittel (Effects of two plant-based remedies on vigilance) Schweiz. Rundsch. Med. 1996;85:473–481. [PubMed] [Google Scholar]
- Hashimoto K., Shimizu E., Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Houghton P.J. Harwood Academic Publishers; Singapore: 1997. Valerian the Genus Valeriana. [Google Scholar]
- Khom S., Baburin I., Timin E., Hohaus A., Trauner G., Kopp B., Hering S. Valerenic acid potentiates and inhibits GABAA receptors: molecular mechanism and subunit specificity. Neuropharmacology. 2007;53:178–187. doi: 10.1016/j.neuropharm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Khom S., Strommeier B., Ramharter J., Schwarz T., Shwarzer C., Erker T., Ecker G.F., Mulzer J., Hering S. Valerenic acid derivatives as novel subunit-selective ligands – in vitro and in vivo characterization. Br. J. Pharmacol. 2010;161:65–78. doi: 10.1111/j.1476-5381.2010.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustberg L., Reynolds C.F. Depression and insomnia: questions of cause and effect. Sleep Med. Rev. 2000;4:253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- Mahady G.B., Parrot J., Lee C., Yun G.S., Dan A. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65–72. doi: 10.1097/00042192-200310010-00011. [DOI] [PubMed] [Google Scholar]
- Martinowich K., Manji H., Lu B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Murphy K., Kubin Z.J., Shepherd J.N., Ettinger R.H. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine. 2010;17:674–678. doi: 10.1016/j.phymed.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Neamati A., Chaman F., Hosseini M., Boskabady M.H. The effects of Valeriana officinalis L. hydro-alcoholic extract on depression like behavior in ovalbumin sensitized rats. J. Pharm. Bioallied Sci. 2014;6(2):97. doi: 10.4103/0975-7406.129174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M., Morinobu S., Duman R. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmacopoeia Europea 9th ed., 2017. Council of Europe, Strasbourg.
- Sichardt K., Vissiennon Z., Koetter U., Brattström A., Nieber K. Modulation of postsynaptic potentials in rat cortical neurons by valerian extracts macerated with different alcohols: involvement of adenosines A1- and GABAA receptors. Phytotherapy Res. 2007;21:932–937. doi: 10.1002/ptr.2197. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acid A receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Trauner G., Khom S., Baburin I., Benedek B., Hering S., Kopp B. Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med. 2008;74:19–24. doi: 10.1055/s-2007-993761. [DOI] [PubMed] [Google Scholar]
- Yuan C.S., Mehendale S., Xiao Y., Aung H.H., Xie J.T., Ang-Lee M.K. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth. Analg. 2004;98:353–358. doi: 10.1213/01.ANE.0000096189.70405.A5. [DOI] [PubMed] [Google Scholar]