Abstract
The ethanol yields from lignocellulo-starch biomass (peels of sweet potato, elephant foot yam, tannia, greater yam and beet root) by fed-batch separate hydrolysis and fermentation (F-SHF) and simultaneous saccharification and fermentation (F-SSF) using Saccharomyces cerevisiae were compared. Fed-batch saccharification of steam or dilute sulphuric acid pretreated biomass enhanced the reducing sugar yield which resulted in high RS consumption, volumetric ethanol productivity and ethanol yield during the first 24 h fermentation under F-SHF mode, while continuous production and utilization of reducing sugars occurred up to 72 h in F-SSF. Dilute sulphuric acid pretreated residues under F-SHF gave higher ethanol yield (34–43 g/L) and productivity (274–346 ml/kg dry biomass) than steam pretreatment (27–36 g/L and 223–295 ml/kg respectively), while F-SSF was superior for steam pretreated peels of sweet potato, elephant foot yam and tannia giving ethanol yields from 281 to 302 ml/kg. Glucose and xylose were present in all the hydrolysates with a preponderance of glucose and fermentation resulted in significant reduction in glucose levels in both F-SHF and F-SSF. Higher levels of total soluble phenolics and hydroxymethyl furfural were observed in the hydrolysates from dilute sulphuric acid pretreatment and yeast assimilated/detoxified part of the inhibitors, while only trivial amounts of furfural were present due to the low xylose content in the hydrolysates. Continuous formation led to higher accumulation of inhibitors in F-SSF despite supplementation with the detoxification mix comprising Tween 20, polyethylene glycol and sodium borohydride. F-SHF of dilute sulphuric acid pretreated biomass could be considered as a comparatively advantageous process where only one time feeding of enzyme cocktail and yeast was adopted compared to multiple feeds of enzymes and yeast along with other additives such as detoxification mix or nutrient solution in F-SSF.
Keywords: Chemical engineering, Biotechnology
1. Introduction
The rapid depletion of fuel resources such as coal, oil or natural gas coupled with the climate change related threats have triggered global research efforts on alternative fuel sources that are environmentally and ecologically safe (Sarkar et al., 2012; Zhang and Shahbazi, 2011). Global warming which is reported to affect the quality of human life results mainly from the burning of fossil fuels and transportation sector accounts for a major part of environmental pollution due to the emission of greenhouse gases (GHGs). Bioethanol produced from lignocellulosic biomass (LCB) such as agricultural and forest residues, dedicated grass or waste paper is reported as the potential transportation fuel of future due to its ability to reduce GHG emission by 86% (Sun and Cheng, 2002; Taherzadeh and Karimi, 2008). Despite the several advantages of bioethanol such as high octane number avoiding the need for methyl tertiary butyl ether (MTBE) in petrol, high oxygen content (35%) reducing emission of carbon monoxide and non-combusted hydrocarbons, ease of blending with petrol etc. (Farrel et al., 2006; Öhgren et al., 2006), the high operational cost of second generation (2G) ethanol from LCBs restricts its commercialization in a big way (Janssen et al., 2013). The economic and competitive bioethanol production from LCBs is challenged by various technological barriers such as high biomass recalcitrance, rigorous pretreatment processes needed to deconstruct lignocellulose, enzyme costs and efficiency of biological conversion of carbohydrates to sugars etc. (Himmel et al., 2007; Wyman, 1999). The enzymatic conversion of structural polysaccharides such as cellulose and hemicellulose is performed by a major multienzyme cocktail containing β-1-4 endoglucanase, β-1-4 exoglucanase, cellobiohydrolase (CBH) and xylanase. The efficiency of enzymatic saccharification which is the key step in the bioconversion of LCBs to ethanol is affected by several factors such as the cellulose crystallinity, severity of pretreatment process, choice of enzymes in the cocktail, extent of formation of saccharification/fermentation inhibitors etc. (Alvira et al., 2010; Yang and Wyman, 2008). Inhibitory products such as furan aldehydes and phenolic compounds are reported to exert differential effects on fermentation that include longer lag-phase, slower growth and lower cell density and decreased ethanol productivity (Heipieper et al., 1991; Modig et al., 2008; Palmqvist et al., 1999a). Many detoxification methods ranging from chemical, physical, and biological methods have been studied to minimize these effects and enhance the ethanol yield (Jönsson et al., 1998; Larsson et al., 1999a,b; Mussatto and Roberto, 2004). Achieving an ethanol concentration of >40 g/L in the fermentation broth is of prime importance to make bioethanol cost competitive as it reduces the recovery cost (Zacchi and Axelsson, 1989). This necessitates a high reducing sugar (RS) concentration of above 80 g/L in the saccharified hydrolysate which is possible only through high initial substrate loading density (Ballesteros et al., 2009). Although the high substrate loading could enhance the RS concentration and consequently result in high ethanol yield the level of toxic inhibitors is also simultaneously increased which coupled with the enzyme inhibition from high level of end products such as cellobiose and glucose are major problems (Hodge et al., 2008; Rudolf et al., 2005). Besides there are also other issues such as poor mixing and heat transfer problems due to high viscosity of substrate solutions, restricted enzyme movement, decreased cellulase adsorption, osmotic stress etc. associated with high solids loading (Koppram et al., 2014; Rudolf et al., 2005) and such problems could be minimised by adopting the fed-batch mode of saccharification (Chen et al., 2007; Rudolf et al., 2005).
Biological conversion of carbohydrates in LCBs to ethanol could be done by either Separate hydrolysis and fermentation (SHF) or Simultaneous saccharification and fermentation (SSF) and both processes have merits and demerits (Galbe and Zacchi, 2002). Whilst the pretreated biomass is enzymatically hydrolysed to glucose (and pentose) and subsequently fermented to ethanol in two separate steps in SHF, both are combined in SSF necessitating only one reactor (Galbe and Zacchi, 2002). Inhibition of cellulase activity by cellobiose and glucose is a major drawback of SHF which decelerates the hydrolysis rate (Hodge et al., 2008). Although simultaneous utilization of sugars by fermenting organisms could reduce the extent of feedback inhibition of enzymes and chances of contamination are also minimal due to presence of ethanol in SSF, it has a major disadvantage that both saccharification and fermentation are carried out under suboptimal conditions (Galbe and Zacchi, 2002; Stenberg et al., 2000). Fed batch saccharification is reported to overcome these problems and helps mitigate the inhibitory effect on enzymes as well as increase the cumulative substrate loading and economize enzyme loading (Gao et al., 2014; Wanderley et al., 2013; Zhang and Zhu, 2017). It has several advantages such as mitigation of enzyme inhibitory effect as the fermentable sugars formed are simultaneously converted to ethanol, reduction in viscosity problems due to pulsed addition of substrate and detoxification of part of the inhibitors such as furfural or hydroxymethyl furfural (HMF) by yeast so that better fermentation performance could be achieved (Hodge et al., 2008; Taherzadeh et al., 2000).
Although high solids loading coupled with minimum enzyme usage could enhance the fermentable sugar yield in a cost effective manner (Hodge et al., 2008), ensuring appropriate water availability during enzyme action has also been reported as essential for successful catalytic action (Horn et al., 2012). Supplementing the saccharification system with additional xylanases, ferulic acid esterase, acetyl xylan esterase etc. was reported to enhance glucose yields from hydrothermally-pretreated brewers spent grain (Wilkinson et al., 2016).
Extensive studies have been carried out on LCBs for ethanol production by different saccharification and fermentation approaches; nevertheless literature on lignocellulo-starch biomass (LCSB) such as peels of root and vegetable crops containing starch as a major polysaccharide besides cellulose and hemicellulose is scanty. Many of these wastes discharged from domestic activities cause environmental problems resulting from their non-judicious disposal and harbouring of pests, insects and microorganisms (Singh et al., 2012). Although starch present in raw materials such as corn, potato or cassava could be easily converted to glucose by enzymes, LCBs containing holocellulose (cellulose + hemicellulose) as well as LCSBs containing starch also as a major polysaccharide require different pretreatment and saccharification strategies. Furthermore in the latter case the liquid fraction after pretreatment containing many inhibitor compounds has to be saccharified along with the solid fraction as the liquid fraction is enriched with fermentable sugars (Mithra et al., 2017). The potential of peels of root crops such as sweet potato, elephant foot yam, tannia, greater yam and beetroot for bioethanol production was investigated by Mithra and Padmaja (2016a, 2017a) and the various strategies for enhancing fermentable sugar yield from them were optimized (Mithra and Padmaja, 2017b; Mithra et al., 2017). Previous studies on steam (ST) and dilute sulphuric acid (DSA) pretreated LCSBs showed that Tween 20 in combination with polyethylene glycol (PEG 4000) could remove 70–82% of phenolic compounds and thus help in reducing the enzyme dosage during saccharification (Mithra and Padmaja, 2016b). The present study aims at a comparison of ethanol production from steam and DSA pretreated biomass residues by performing fed-batch reaction under SHF or SSF modes in presence of detoxification chemical mix.
2. Materials and methods
2.1. Materials
Peels from root crops such as sweet potato (SP; Ipomoea batatas), elephant foot yam (EFY; Amorphophallus paeoniifolius), tannia (Xanthosoma sagittifolium), greater yam (GY; Dioscorea alata) and beet root (BR; Beta vulgaris) were collected by manually peeling them. Peels were washed in running tap water to remove adhering dirt and then drained and dried in sun light for 36–48 h. The dry peels were powdered in a hammer mill to particles of ca. 2–3 mm size and unscreened powder was used for the study. Previous studies on the composition of dry peels showed that they contained cellulose (13–19%), hemicellulose (13–20%), starch (27–32%) and lignin (4–8%) besides reducing and non-reducing sugars and ash (Table 1) (Mithra and Padmaja, 2016a, 2017a).
Table 1.
Compositional profile∗ of the selected root processing residues (expressed as g/100 g dry basis).
| Parameters | SP peel | EFY peel | Tannia peel | GY peel | BR Peel |
|---|---|---|---|---|---|
| Cellulose | 13.31 ± 0.03 | 15.63 ± 0.20 | 17.32 ± 0.34 | 18.02 ± 0.58 | 18.94 ± 0.20 |
| Hemicellulose | 13.32 ± 0.14 | 14.00 ± 0.00 | 14.48 ± 0.35 | 20.02 ± 0.57 | 19.17 ± 0.55 |
| Starch | 32.05 ± 0.09 | 28.96 ± 0.42 | 30.46 ± 0.37 | 28.84 ± 0.44 | 27.13 ± 0.00 |
| Lignin | 8.15 ± 0.43 | 7.01 ± 0.13 | 8. 26 ± 0.11 | 6.72 ± 0.17 | 3.87 ± 0.34 |
| Ash | 3.77 ± 0.15 | 9.67 ± 0.12 | 5.27 ± 0.31 | 3.29 ± 0.24 | 5.66 ± 0.10 |
| Total sugars | 11.21 ± 0.01 | 5.53 ± 0.05 | 2.42 ± 0.05 | 4.33 ± 0.00 | 17.07 ± 0.12 |
| Reducing sugars | 6.22 ± 0.03 | 2.58 ± 0.00 | 1.34 ± 0.00 | 2.17 ± 0.00 | 6.91 ± 0.04 |
Mean ± SD from three replicates; Source: (Mithra and Padmaja, 2016a, 2017a).
2.2. Enzymes and chemicals
The hydrolytic enzymes used for the study included Ecozyme RT80 (cellulolytic enzyme complex), Ecozyme XY50 (Xylanase) and Stargen™002 (granular starch-hydrolysing enzyme) and the former two enzymes were supplied by M/s Ecostar Ltd., Chennai, India while Stargen was gifted by M/s Genencor International Inc., USA (presently Genencor-Danisco, USA). Ecozyme RT80 was earlier reported to contain 22 FPU cellulase activity per millilitre besides 328 units of β-glucosidase activity per millilitre and 126 units of α-amylase activity per millilitre (Mithra et al., 2017). Stargen™002 had an activity of 570 glucoamylase units (GAU) per gram, and one GAU is the amount of enzyme that will liberate one gram of reducing sugars (as glucose) per hour from soluble starch substrate under the conditions of the assay (Anon, 2009) and Ecozyme RT80, Stargen and Ecozyme XY50 respectively contained 78.8 mg, 216.0 mg and 5.25 mg crude protein per millilitre (Mithra et al., 2017).
2.3. Pretreatment
Based on earlier studies two best pretreatments were selected which included steam pretreatment of moist biomass (moisture content: 40%) at 100 °C for 45 min (ST) in a vegetable steamer (M/S Prestige India Ltd; India) and dilute sulphuric acid (0.1 mol/L H2SO4) pretreatment at 121 °C (0.102 MPa pressure) for 60 min (time after pressure build up) in a pressure cooker (M/s TTK Prestige India Ltd., India). In the case of steam (ST) pretreatment the moist steam pretreated biomass was reconstituted in distilled water (90 ml) and the whole slurry was used, while for the DSA pretreatment, 10 g dry biomass in 90 ml 0.1 mol/L DSA was subjected to pretreatment. These levels were standardized earlier for all the biomass residues under study (Mithra and Padmaja, 2016a) and used as such to start the fed-batch studies.
2.4. Fed-batch separate hydrolysis and fermentation (F-SHF)
The fed batch saccharification was initiated with 10 g/90 ml solids loading of both the pretreated slurries in 250 ml Erlenmeyer flasks. The slurries were treated with a detoxification chemical mix containing Tween 20 (0.25% v/v), Polyethylene glycol 4000 (PEG; 0.25% w/v) and sodium borohydride (NaBH4; 0.15% w/v) and incubated at room temperature (30 ± 1 °C) for 30 min. The levels of the detoxifying chemicals were optimized through an earlier study and selected based on their ability to channel out maximum quantity of phenolic compounds (Mithra and Padmaja, 2016b). The treated slurries after pH adjustment to 5.0 and volume increase to 100 ml were equilibrated in a shaking water bath (M/s Julabo Industries, Germany) for 10 min at 50 °C and at a shaking speed of 100 rpm.
An enzyme cocktail containing Ecozyme RT80 (16 FPU/g cellulose in the system), Ecozyme XY50 (3 mg protein/g hemicellulose in the system) and Stargen (0.25 ml equivalent to 54 mg protein/10 g biomass) was added to steam pretreated slurries. In the case of DSA pretreated slurries the dose level of Stargen was reduced to half (0.125 ml/10 g biomass) while the dose levels of Ecozyme RT 80 and Ecozyme XY50 were maintained as before. Both the slurries were incubated at 50 °C for 24 h. The levels of enzymes were optimized based on earlier studies on steam and DSA pretreatments and the latter pretreatment hydrolysed as high as 86–94% starch from the residues permitting dose level reduction for Stargen (Mithra and Padmaja, 2017b).
Three graded levels of pretreated slurries such as 5 g/20 ml, 5 g/20 ml and 2.5 g/10 ml were added consequently at 24 h, 48 h, and 72 h of incubation without any additional enzyme loading but with exposure to the detoxification chemicals at proportionate levels for 30 min as mentioned earlier. The cumulative substrate loading was 22.5 g/150 ml (equivalent to 15% w/v). Incubation was continued up to 96 h after which the slurries were centrifuged at 3000 rpm for 15 min and the clear supernatant was used for the fermentation experiment.
The reducing sugar content in the hydrolysates after 96 h was quantified using arsenomolybdate reagent (Nelson, 1944) and expressed as g/L (equivalent to that released from 150 g biomass as 15% w/v solid loading was adopted). All the experiments were run in triplicates and enzyme blanks as well as substrate blanks were kept to eliminate the interference from the sugars already present in the enzyme samples and original biomass respectively. The combined Hydrolysis yield (%) was computed from the RS values in the 96 h hydrolysates (which included the sugars formed after pretreatment as well) using the formula:
| (1) |
where C: cellulose, HC: hemicellulose, S: starch and TS: total sugars; 1.11 is the conversion factor for cellulose and starch to sugars and 1.14 is the conversion factor for hemicellulose to sugars; 1.5 is the factor for 150 g biomass per litre.
2.5. Fermentation set up for F-SHF
The fermentation experiments were conducted in 250 ml Erlenmeyer flasks using 150 ml clear hydrolysate. The hydrolysates were adjusted to pH 4.5 and equilibrated in a thermostatic water bath at 37 °C for 10 min at a shaking speed of 100 rpm.
2.5.1. Activation of yeast
Twenty grams of dry Baker's yeast (Saccharomyces cerevisiae) were suspended in 100 ml solution containing 10 g sucrose. Yeast was allowed to proliferate at 37 °C for 1 h (Shanavas et al., 2011) and from this 7.5 ml yeast suspension was used for each 150 ml hydrolysate.
2.5.2. Nutrient solution
A nutrient solution containing ammonium sulphate (1.0 g), copper sulphate (0.004 g), magnesium sulphate (0.35 g) and calcium chloride (0.055 g) was prepared in one litre distilled water (Russel, 2003). All the chemicals used were of analytical grade.
2.5.3. Fermentation to ethanol
Activated yeast suspension (7.5 ml) along with 0.25 ml nutrient solution was added to 150 ml each of the 96 h fed-batch hydrolysate. The flasks after covering with aluminium foil were allowed to ferment for 72 h. Destructive sampling was adopted at 24 h, 48 h and 72 h so that the chances of entry of air into the fermentation system could be avoided. The fermented broth was centrifuged at 3000 rpm for 15 min and the cell free supernatant was used for the determination of RS and ethanol content. Ethanol content was assayed by the spectrophotometric method of Caputi et al. (1968) using potassium dichromate reagent. The broth after 72 h was also distilled using rotary evaporator (Ms BUCHI India Pvt. Ltd., India) at 70 °C to quantify the recovery of ethanol. The distilled ethanol was mixed with anhydrous sodium sulphate (5 g/100 ml distillate) to eliminate the last traces of water and then volume was measured.
2.6. Fed-batch simultaneous saccharification and fermentation (F-SSF)
In contrast to F-SHF where there was only substrate feeding along with detoxification chemicals at three time points (24 h, 48 h and 72 h) without further addition of enzyme, a proportionate feeding strategy for enzymes and detoxification chemicals as well as yeast and nutrient solution along with each substrate addition was adopted for F-SSF.
The fed batch SSF experiment for ST was started with 40% moisture-conditioned biomass (equivalent to 10 g dry weight) which was subjected to steam pretreatment as earlier for 45 min. After pH adjustment to 5.0 and reconstitution to 90 ml slurry volume with distilled water, the samples were equilibrated in a thermostatic water bath at 37 °C. Sodium azide (0.25% w/v) and detoxification mix [Tween 20 (0.25% v/v) + PEG (0.25% w/v) + NaBH4 (0.15% w/v)] were added (Mithra and Padmaja, 2016b) and after exposure to chemicals at room temperature for 30 min, the triple enzyme cocktail (16 FPU of Ecozyme RT80/g cellulose, 3 mg protein of Ecozyme XY50/g hemicelluloses and 0.25 ml equivalent to 54 mg protein of Stargen/10 g biomass)was added to each system (Mithra et al., 2017). After thorough mixing 10 ml yeast suspension prepared as described under SHF was added making the total volume to 100 ml. Nutrient solution (0.20 ml; volume reduced compared to 0.25 ml in F-SHF due to the subsequent additions in the next two steps of substrate loading) with the same composition as given above was also added. The flasks were closed with aluminium foil and incubated for 24 h at a shaking speed of 100 rpm. Residual sugars and alcohol contents were determined at 24 h as per the methods described earlier.
Two more flasks of whole slurry of the steam pretreated biomass (each 50 ml containing 10 g dry weight) were prepared and after pH adjustment to 5.0, half the quantity of detoxification chemicals was also added and exposed to room temperature for 30 min.
Immediately after 24 h incubation and sampling, the first batch of 50 ml pH adjusted slurry along with yeast suspension (2.0 ml), nutrient solution (0.05 ml) and one-fourth the dose of enzymes (4 FPU Ecozyme RT80/g cellulose, 0.75 mg Ecozyme XY50/g hemicellulose and 0.06 ml Stargen/10 g biomass) was added and incubation continued up to 48 h and the residual sugar and alcohol contents were determined at 48 h. Immediately the second batch of pH adjusted 50 ml whole slurry (containing the next 10 g dry weight + detoxification chemicals), along with yeast, enzymes and nutrient solution at levels mentioned above as in the first batch was added. The slurries were incubated up to 120 h with sampling for ethanol and RS assays at 72 h, 96 h and 120 h. The cumulative biomass addition was 30 g in 200 ml (15% w/v) although at any point of sampling the weight may not be equivalent to 15% (w/v) as it was continuously hydrolysed by the enzymes.
The same study was repeated for the DSA pretreated biomass also, except that the enzyme cocktail for first enzyme feeding had full dose Ecozyme RT80 and Ecozyme XY50 along with half dose of Stargen (16 FPU Ecozyme RT80/g cellulose, 3.0 mg Ecozyme XY50/g hemicellulose and 0.125 ml Stargen/10 g biomass) as most of the starch was hydrolysed during the pretreatment stage itself by dilute sulphuric acid (Mithra and Padmaja, 2017b). Accordingly the enzyme dosages for the next two levels (10 g biomass in 50 ml at each time) of application were also adjusted (4 FPU of Ecozyme RT80/g cellulose, 0.75 mg of Ecozyme XY50/g hemicellulose and 0.03 ml of Stargen/10 g biomass). Yeats and nutrient addition were maintained at the same level as in the case of steam pretreatment for the three stages. Residual reducing sugars and ethanol was quantified at 24 h intervals upto120 h fermentation as described before.
The broth after 120 h F-SSF (steam and DSA set) was also distilled using rotary evaporator (Ms BUCHI India Pvt. Ltd., India) at 70 °C to quantify the recovery of ethanol as described earlier.
2.7. Calculation for ethanol yield related parameters
The reducing sugar consumption in F-SHF was worked out from the initial sugar concentration in the hydrolysate and the residual sugar concentration in the fermented broth. The other parameters related to ethanol fermentation were computed based on the previous reports (Barcelos et al., 2011; Pereira et al., 2015; Yadav et al., 2011; Pooja et al., 2018) as given under:
| (2) |
| (3) |
| (4) |
| (5) |
where Ef is the ethanol concentration (g/L) in fermented broth and W1 is the weight of dry biomass in one litre slurry
| (6) |
where 0.82 is the specific gravity of ethanol.
In order to compute sugar consumption in F-SSF, the initial available total sugars were computed from the potential sugar yielding carbohydrate content as:
Initial sugars (g/L) available for fermentation (A) = Total potential sugars (g/150 g) in biomass, which was computed as:
| [(cellulose + starch) × 1.11 + hemicellulose × 1.14 + Total sugars)% × 1.5 | (7) |
where 1.5 is the factor for converting to 150 g biomass.
The unutilized sugars in the residue left after saccharification and fermentation were quantified by determining the total sugars by extracting them with 80% ethanol, converting the non-reducing sugars to reducing sugars using acid hydrolysis and then estimating the RS using arsenomolybdate reagent (Nelson, 1944) and the total carbohydrate by using anthrone reagent (Hedge and Hofreiter, 1962). The biomass residue remaining after SSF was quantified and based on this, the sugars in the residue from 150 g biomass was worked out.
| Unutilized sugars (g/L) (B) = (Total sugars in fermented residue from 150 g biomass + total sugars (g/L) remaining in the fermented broth after 120 h) | (8) |
| Sugar consumption (g/L) during SSF (C) = A − B | (9) |
2.8. HPLC characterization of monosaccharides and furan aldehydes
Sugar profile was analysed by HPLC in the 96 h hydrolysates from steam and DSA pretreated residues as well as after 72 h fermentation in F-SHF. In F-SSF, the fermented broth after 120 h was used for characterization of monosaccharides. The samples (single pooled sample from three replicates) were centrifuged at 3000 rpm for 10 min. and the clear supernatant was stored at −4 °C until use. At the time of assay, the hydrolysates were again filtered through 0.2 μm sterile filters (Millipore) and used for the HPLC characterization of sugars. Analysis of monomeric sugars was performed on an isocratic mode using HPLC (M/s Shimadzu, Kyoto, Japan) having a computer software based integration system. The conditions were: Column: SUPELCOSIL LC-NH2 (250 × 4.6 mm), mobile phase: acetonitrile:water (75:25), flow rate: 1.0 ml/min; column temperature: ambient (30 ± 1 °C); Detector: RID-10 A; injection volume: 20 μl and run time: 30 min.
Furfural and HMF were quantified in the same samples as above (single pooled sample from three replicates) using HPLC with Zorbax-SB-C18 reverse phase column and Photodiode array (PDA-960) UV detector for HMF and Aminex-HPX-87 H column (250 × 4.6 mm) along with a Guard column (Aminex-HPX-87 H) and SPD-M20 A PDA UV detector for furfural. Other conditions were: mobile phase: Ultrapure water and 0.25 mM H2SO4 (4:1), injection volume: 20 μl; flow rate: 0.6 ml/min and total run time 40 min (furfural) and 55 min (HMF).
Peaks were identified and quantified by comparing with the retention times of authentic standards (glucose, xylose, arabinose, galactose, mannose, furfural and HMF), procured from M/s SIGMA, St. Louis, USA.
2.9. Total soluble phenolics content
Total soluble phenolics (TSPs) in the hydrolysates after saccharification (96 h for F-SHF experiment) as well as after fermentation [both F-SHF (after 72 h) and F-SSF (after 120 h) were determined using Folin-Ciocalteu reagent (Singleton and Rossi, 1965) and expressed as gallic acid equivalents (g/L) computed using pure gallic acid standard (M/s SIGMA). Any interference from the detoxification agents in the assay was nullified by keeping a blank containing the same concentration of detoxification chemicals as in the test samples.
2.10. Statistical analysis
The data from three replicates were subjected to Analysis of Variance (ANOVA) for statistical testing of the mean values and was followed by least significant difference (LSD) for pair-wise comparison of mean values by using the statistical package, SAS 9.3 (SAS, 2010).
3. Results and discussion
3.1. Reducing sugar changes and fermentative performance during F-SHF
The initial RS levels available for fermentation by S. cerevisiae in the various root peel hydrolysates, combined (from pretreatment and saccharification) Hydrolysis yield (HY %) in the 96 h hydrolysate expressed as the percentage of RS formed from theoretically possible RS in the original biomass as well as the RS consumption during fermentation during 24–72 h are depicted in Table 2. It was observed that most of the LCSBs gave higher RS content after 96 h saccharification of steam pretreated biomass compared to the respective levels in DSA pretreatment, while in the case of SP peel, the two values were insignificant (Table 2). It may be noted that SP peel had the highest starch content (32%) among the residues (Table 1) and DSA pretreatment was earlier reported to result in very high hydrolysis of starch (Mithra and Padmaja, 2016a) and hence the pretreated liquor itself had high RS content. This might have resulted in higher HY (%) in DSA pretreated SP peel than the other residues. Previous studies showed that even after 120 h batch saccharification at high solids loading (15% w/v), only 72–100 g/LRS were released from steam pretreated biomass while 60–88 g/L RS were released from DSA pretreated biomass (Mithra et al., 2018) and higher RS levels obtained in the fed-batch saccharification compared to batch saccharification indicated that the former was effective in enhancing the RS release from pretreated biomass (Table 2). It was reported from previous studies that RS release was proportionate with time up to 96 h after which the production rate slowed down up to 120 h possibly due to feedback inhibition on cellulases (and also other enzymes in the cocktail) by the RS formed (Mithra et al., 2018). During enzymatic hydrolysis, oligosaccharides, disaccharides and monomers are formed which may cause feedback inhibition on the respective enzymes ultimately affecting the hydrolysis yield. The major inhibition of cellulose is caused by end products such as cellobiose and glucose (Lammirato et al., 2010; Shi et al., 2009), while xylose causes feedback inhibition of hemicellulase.
Table 2.
Pattern of sugar consumption and ethanol yield during the course of fermentation (72 h) in Steam/DSA pretreated and saccharified hydrolysates under F-SHF.
| Biomass | Initial sugars (g/L)∗ | HY (%)∗∗ | Reducing sugar consumption during fermentation (g/L) |
Volumetric ethanol productivity (g/L/h) |
Ethanol yield (YE)∗∗∗ |
Ethanol content (g/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |||
| (a) Steam pretreated and saccharified biomass | ||||||||||||||
| SP peel | 99.56c | 86.48b | 75.15fg | 75.59ef | 76.16e | 1.15e | 0.59d | 0.397d | 0.368c | 0.374b | 0.375b | 27.67f | 28.27f | 28.58f |
| EFY peel | 90.12d | 84.64c | 75.89f | 76.29e | 76.80e | 1.15e | 0.59d | 0.395d | 0.364c | 0.369b | 0.370b | 27.62f | 28.17f | 28.45f |
| Tannia peel | 86.56e | 80.19e | 74.11g | 74.49f | 74.99f | 1.11e | 0.57d | 0.381d | 0.359c | 0.365b | 0.366b | 26.63g | 27.15f | 27.43f |
| GY peel | 105.91b | 89.18a | 85.70c | 86.17c | 86.77c | 1.33d | 0.68cd | 0.458c | 0.374b | 0.379b | 0.380b | 32.01e | 32.65e | 32.98e |
| BR peel | 117.54a | 87.01ab | 93.53a | 94.05a | 94.72a | 1.47c | 0.75b | 0.505a | 0.377b | 0.382b | 0.383b | 35.24c | 35.95cd | 36.32c |
| (b) DSA pretreated and saccharified biomass | ||||||||||||||
| SP peel | 101.44bc | 88.12a | 81.39d | 81.82d | 82.40d | 1.413c | 0.719bc | 0.484b | 0.417ab | 0.422a | 0.423a | 33.90d | 34.50d | 34.82d |
| EFY peel | 78.96f | 74.16f | 69.08i | 69.48h | 70.00h | 1.411cd | 0.717bc | 0.482b | 0.490a | 0.495a | 0.496a | 33.86d | 34.40d | 34.69d |
| Tannia peel | 79.90f | 74.02f | 71.81h | 72.19g | 72.68g | 1.370d | 0.696c | 0.468c | 0.458a | 0.463a | 0.463a | 32.87e | 33.39de | 33.66de |
| GY peel | 96.22c | 81.03d | 80.36e | 80.83d | 81.44d | 1.594b | 0.810ab | 0.545a | 0.476a | 0.481a | 0.482a | 38.25b | 38.89b | 39.22b |
| BR peel | 107.35b | 79.46e | 87.70b | 88.21b | 88.89b | 1.728a | 0.879a | 0.591a | 0.473a | 0.478a | 0.479a | 41.48a | 42.19a | 42.56a |
Initial reducing sugars available for fermentation (g/L) in saccharified liquor from fed-batch system (after 96 h saccharification).
indicates the final saccharification yield as per Eq. (1) (includes the pretreatment yield also).
YE: g ethanol produced/g sugar consumed; statistical comparison was made column-wise and values with different superscripts are significant at p < 0.05.
The Hydrolysis yield (HY %) was also very high for the steam pretreated biomass, except for SP peel where ca. 88% HY was obtained for DSA pretreated biomass as against 86.5% from steam pretreated sample. As high as 80–89% of the potential RS in the biomass could be recovered in the hydrolysates from steam pretreated biomass by adopting the fed-batch approach, where the substrate feeding was distributed from 24 h to 72 h as three pulsed additions giving adequate time for the enzymes to act on the polysaccharides, while the HY (%) ranged from 74–88% in the DSA pretreated and saccharified biomass. As different from the typical lignocellulosic biomass (LCBs), the lignocellulo-starch biomass (LCSBs) under study had high content of starch as well and triple enzyme cocktail also had Stargen (amylolytic enzyme) in the F-SHF system with full dose (54 mg protein/10 g biomass) while for DSA pretreated system only half dose was used (27 mg protein/10 g biomass) due to the extensive hydrolysis of starch at the pretreatment stage reported earlier (Mithra et al., 2017).
Previous studies on the LCSBs also showed that the triple enzyme cocktail containing cellulase, xylanase and Stargen (starch hydrolysing enzyme) could facilitate maximum release of RS (Mithra et al., 2017). It was reported earlier that steam pretreatment of 40% moist residues for 45 min removed 10–12% cellulose, 17–23% hemicellulose and 35–37% starch from the selected biomass, while DSA pretreatment for 60 min removed 3.5–15% cellulose, 41–47% hemicellulose and 86–94% starch (Mithra and Padmaja, 2016a, 2017a). Accordingly the enzyme levels were optimized in a subsequent study and based on this, the Stargen levels could be halved (Mithra and Padmaja, 2017b). Furthermore the systems were alos supplemented with detoxification chemical mix comprising surfactants and sodium borohydride which along with the balanced enzyme cocktail might have helped to enhance the RS yield. Zhou et al. (2008) observed that a well balanced enzyme cocktail is of prime importance to realise optimum fermentable sugars from LCBs. Tween 20 along with PEG 4000 could remove 73–82% of total soluble phenolics from steam or DSA pretreated LCSBs, while sodium borohydride removed up to 50% phenolics (Mithra and Padmaja, 2016b) from the selected residues and the optimized level of the two surfactants and sodium borohydride was used in the present study as well. There are several reports on the beneficial effects of surfactants such as Tween or Polyethylene glycol (PEG) (Börjesson et al., 2007; Eriksson et al., 2002; Tejirian and Xu, 2011) or sodium borohydride (Cavka and Jönsson, 2013) in enhancing the saccharification yield from LCBs.
The reducing sugar consumption by S. cerevisiae during the fermentation period of 24 h–72 h as presented in Table 2 indicated that the maximum RS consumption occurred in the initial 24 h and further changes in the next 48 h were negligible. A uniform utilization pattern was obtained for the two pretreatments and all the biomass residues, indicating that fermentation could be curtailed at 24 h thereby leading to saving of energy also. This is further supported by the high volumetric ethanol productivity (VEP) in the first 24 h, which sharply decreased afterwards (Table 2). The VEP values were significantly higher for the DSA pretreated biomass compared to the steam pretreated one and this resulted from the higher ethanol production (g/L) from the former pretreatment (Table 2). The sharp decrease in VEP during 24–48 h indicated that most of the ethanol production occurred within the first 24 h of fermentation. The high starch content in the LCSB residues yielded hydrolysates rich in glucose unlike many LCBs having only cellulose and hemicellulose as polysaccharides and this might have resulted in the high VEP values. Yadav et al. (2011) reported VEP values of 0.33 g/L/h from acid hydrolysed rice straw fermented using co-culture of S. cerevisiae and Scheffersomyces stipitis (Pichia stipitis). Significantly higher VEP values were obtained in the present study from F-SHF compared to this report using S. cerevisiae alone. Approximately 11–23 g and 7–19 g RS remained unutilized respectively in steam and DSA pretreated and saccharified hydrolysates of LCSBs, possibly because of the non-utilization of pentose sugars by S. cerevisiae.
The ethanol yield (YE) expressed as gram ethanol produced per gram RS consumed was higher for the DSA pretreated and saccharified biomass hydrolysates and there was only insignificant change in YE after 24 h for all the residues (Table 2). Accordingly the ethanol content (g/L) in the fermented broth was also higher for the DSA system (F-SHF).
As proportionate increase in ethanol content with fermentation time was not observed, fermentation could be curtailed at 24 h from the economic point of view. The highest ethanol yields were obtained from GY and BR peels in the case of both steam and DSA pretreatments, which was evidently due to the higher initial RS contents in these hydrolysates (Table 2). However despite the high RS content in SP peel hydrolysates, the ethanol content after fermentation was similar to that from EFY or tannia peel hydrolysates (Table 2). The possible reason for this may be the high level of pentose sugars (arabinose + xylose) in the SP peel hydrolysates (Table 4 discussed below), which also had very high levels of furfural (Table 6) compared to the other samples and this also might have adversely affected ethanol production. Furthermore BR peel hydrolysates contained mannose and galactose and both of these along with ca. 17% total sugars (Tables 1 and 4) could be converted to ethanol by S. cerevisiae which led to the highest ethanol content in the hydrolysates (ca. 36.3 g/L from steam and 42.6 g/L from DSA). Zacchi and Axelsson (1989) observed that the RS content in the hydrolysate has to be more than 80 g/L in order to achieve an ethanol content of >40 g/L, which is essential to reduce the distillation costs and it was found that out of the five residues only BR peel hydrolysates from DSA pretreatment could yield >40 g/L ethanol in SHF process.
Table 4.
Monosaccharide sugar profile (g/L) in hydrolysates (96 h) and fermented broth (72 h) from LCSBs under F-SHF and 120 h fermented broth under F-SSF.a
| Biomass |
Mannose |
Galactose |
Glucose |
Arabinose |
Xylose |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| H |
FB |
H |
FB |
H |
FB |
H |
FB |
H |
FB |
|
| F-SHF | ||||||||||
| (a) Steam (ST) pretreatment | ||||||||||
| SP peel | - | - | - | - | 56.64 | 8.60 | 9.40 | 8.11 | 3.94 | 3.11 |
| EFY peel | 8.30 | 0.64 | - | - | 40.33 | 2.30 | - | - | 9.56 | 8.22 |
| Tannia peel | 10.47 | 0.69 | - | - | 48.66 | 1.35 | - | - | 9.54 | 8.64 |
| GY peel | 17.25 | 2.35 | - | - | 50.44 | 6.50 | - | - | 9.88 | 8.46 |
| BR peel | 13.07 | 1.50 | 7.10 | 2.33 | 61.20 | 11.40 | - | - | 3.11 | 3.01 |
| (b) DSA pretreatment | ||||||||||
| SP peel | - | - | - | - | 50.31 | 3.60 | 10.60 | 10.00 | 3.80 | 2.90 |
| EFY peel | 5.89 | 0.44 | - | - | 34.42 | 1.40 | - | - | 8.30 | 7.00 |
| Tannia peel | 8.08 | 0.65 | - | - | 42.01 | 1.10 | - | - | 5.60 | 5.00 |
| GY peel | 13.13 | 1.79 | - | - | 44.12 | 4.14 | - | - | 9.50 | 8.65 |
| BR peel |
7.70 |
1.69 |
4.31 |
1.41 |
52.11 |
8.66 |
- |
- |
2.12 |
1.94 |
| F-SSF | ||||||||||
|
Mannose |
Galactose |
Glucose |
Arabinose |
Xylose |
||||||
| (a) Steam (ST) pretreatment | ||||||||||
| SP peel | - | - | 2.54 | 2.51 | 0.94 | |||||
| EFY peel | 0.31 | - | 0.78 | - | 2.56 | |||||
| Tannia peel | 0.32 | - | 0.71 | - | 4.36 | |||||
| GY peel | 0.25 | - | 0.64 | - | 0.76 | |||||
| BR peel | 0.46 | 0.55 | 3.21 | - | 0.81 | |||||
| (b) DSA pretreatment | ||||||||||
| SP peel | - | - | 2.03 | 6.30 | 1.92 | |||||
| EFY peel | 0.57 | - | 1.85 | - | 9.77 | |||||
| Tannia peel | 1.04 | - | 1.45 | - | 8.18 | |||||
| GY peel | 1.34 | - | 3.08 | - | 6.76 | |||||
| BR peel | 1.17 | 0.98 | 6.00 | - | 1.46 | |||||
H: hydrolysate (96 h) and FB: Fermented broth (72 h); Each value represents the data from single pooled analysis of three replicates.
Table 6.
HMF and furfural in the hydrolysate (96 h; F-SHF) and fermented broth [after fermentation (72 h; F-SHF) and 120 h (F-SSF)] from steam/DSA pretreated biomass.
| Biomass | 96 h hydrolysate (F-SHF) |
Fermented broth (72 h; F-SHF) |
Fermented broth (120 h; F-SSF) |
|||
|---|---|---|---|---|---|---|
| ST | DSA | ST | DSA | ST | DSA | |
| (a) HMF (mg/L) | ||||||
| SP peel | 61.95 | 85.65 | 50.88 (17.87)a | 74.73 (12.75) | 67.59 | 89.97 |
| EFY peel | 56.53 | 85.45 | 45.18 (20.08) | 74.93 (12.31) | 62.16 | 89.59 |
| Tannia peel | 58.55 | 76.95 | 47.30 (19.21) | 65.90 (14.36) | 64.19 | 81.19 |
| GY peel | 34.75 | 87.35 | 22.29 (35.86) | 76.09 (12.89) | 40.39 | 91.67 |
| BR peel | 62.76 | 87.56 | 51.73 (17.57) | 76.73 (12.37) | 68.40 | 88.88 |
| (b) Furfural (mg/L) | ||||||
| SP peel | 59.54 | 64.16 | 50.29 (15.54) | 57.62 (10.19) | 65.46 | 69.11 |
| EFY peel | 43.05 | 37.56 | 37.21 (13.57) | 31.89 (15.10) | 35.44 | 37.66 |
| Tannia peel | 42.96 | 25.78 | 39.04 (9.12) | 23.17 (10.12) | 26.11 | 27.13 |
| GY peel | 44.45 | 42.79 | 38.26 (13.93) | 39.08 (8.67) | 43.11 | 44.66 |
| BR peel | 14.93 | 10.61 | 14.49 (2.95) | 9.82 (7.45) | 10.54 | 11.54 |
Figures in parentheses indicate the percentage decrease in HMF and furfural during fermentation (72 h) by yeast; Each value represents the data from single pooled analysis of three replicates.
3.2. Reducing sugar changes and fermentative performance during F-SSF
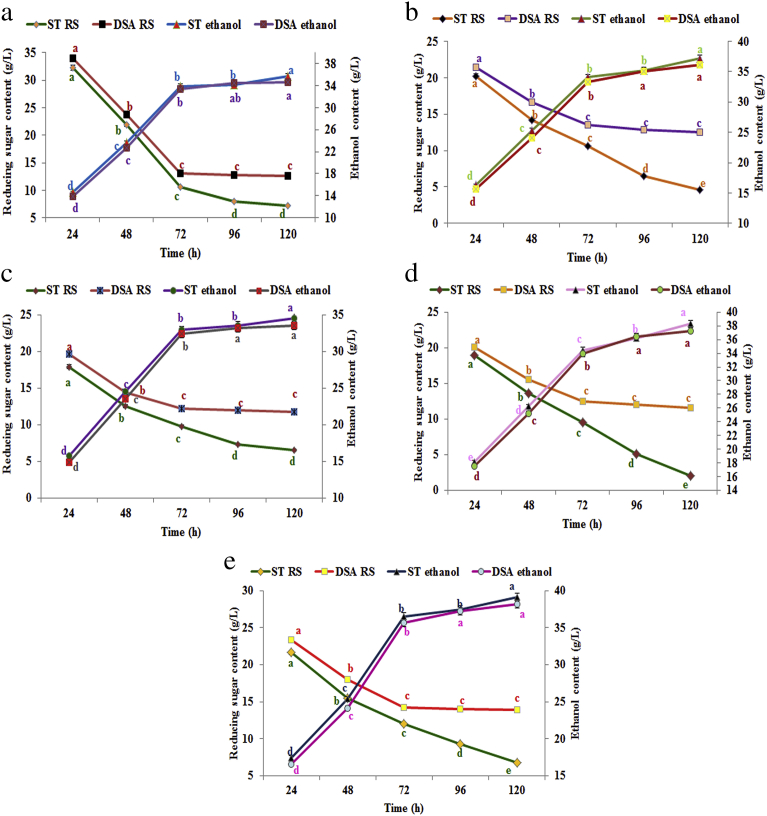
The time course utilization of RS by yeast along with the production pattern of ethanol during F-SSF of steam or DSA pretreated biomass is presented in Fig. 1(a–e) and progressive utilization of RS from 24 to 120 h could be seen in all the biomass samples with a greater extent of conversion to ethanol in the initial phase (24–72 h). In the case of all the residues, initial RS utilization was close in both the pretreatments while higher utilization was observed towards the last phase (72–120 h) in steam pretreated biomass compared to the DSA pretreatment (Fig. 1a–e). Nevertheless the increased RS utilization obtained during 72–120 h F-SSF in steam pretreated residues did not result in increased ethanol production as evidenced from the close values for ethanol in both the pretreatments for all the residues (Fig. 1a–e). Ethanol production steadily increased during the first 24–72 h of F-SSF indicating that the RS produced by enzymatic hydrolysis was continuously converted to ethanol by yeast. Unlike in batch SSF the pulsed addition of substrate along with enzymes, detoxification chemicals etc. in F-SSF prevented the feedback inhibition of enzymes. Furthermore ethanol production was more from steam pretreated residues subjected to F-SSF than F-SHF, while significantly higher ethanol content was observed for DSA pretreated GY and BR peels in F-SHF. It was further observed that although the RS consumption was higher after F-SSF (120 h) of steam pretreated residues than the DSA counterparts, there were only insignificant differences between the two pretreatments in the VEP, ethanol yield and ethanol content (Table 3 and Fig. 1a–e). McIntosh et al. (2017) also reported that the available glucose was rapidly converted to ethanol by the yeast in the first 6–12 h after inoculation in SSF and that 90% of ethanol production occurred during this period. However in the present study, as fed-batch substrate feeding strategy was adopted, continuous ethanol production was observed up to 72 h which then tapered off.
Fig. 1.
a. Time course utilization of reducing sugars and production of ethanol during fed-batch SSF of pretreated SP peel, b. Time course utilization of reducing sugars and production of ethanol during fed-batch SSF of pretreated EFY peel, c. Time course utilization of reducing sugars and production of ethanol during fed-batch SSF of pretreated tannia peel, d. Time course utilization of reducing sugars and production of ethanol during fed-batch SSF of pretreated GY peel, e. Time course utilization of reducing sugars and production of ethanol during fed-batch SSF of pretreated BR peel.
Table 3.
Sugar consumption (g/L) and fermentation parameters after 120 h F-SSF of steam/DSA pretreated biomass.*
| Biomass | Reducing sugar consumption (g/L) |
Volumetric ethanol productivity (g/L/h) |
Ethanol yield (YE) |
|||
|---|---|---|---|---|---|---|
| ST | DSA | ST | DSA | ST | DSA | |
| SP peel | 100.29c | 95.35c | 0.297b | 0.288b | 0.355c | 0.363c |
| EFY peel | 96.26d | 89.57d | 0.310a | 0.302a | 0.386a | 0.404a |
| Tannia peel | 93.93e | 89.28d | 0.288b | 0.280b | 0.368b | 0.376b |
| GY peel | 107.73b | 99.61b | 0.319a | 0.311a | 0.356c | 0.375b |
| BR peel | 118.59a | 112.08a | 0.326a | 0.318a | 0.330d | 0.340d |
Statistical comparison was made column-wise and values with different superscripts are significant at p < 0.05.
3.3. HPLC sugar profile
HPLC sugar profile of the hydrolysates (96 h) from F-SHF of steam pretreated biomass as well as the fermented broth [72 h (F-SHF) and 120 h (F-SSF)] as given in Table 4 indicated that glucose and xylose were uniformly present in all the hydrolysates. Galactose was present in only BR peel hydrolysates, while arabinose was present in only SP peel hydrolysates and mannose was present in all the hydrolysates except that from SP peel. Tannia and EFY peel hydrolysates did not contain arabinose, although these and GY peel hydrolysates had high levels of xylose. This indicated the structural differences in the hemicellulose in the residues under study, which was hitherto not reported. The HPLC sugar profile after 72 h fermentation of the hydrolysates from steam pretreated biomass (F-SHF mode) showed that there was drastic reduction in glucose levels in all the hydrolysates (Table 4). Mannose was also significantly reduced in the fermented broth from EFY, tannia and GY peels, while both galactose and mannose were reduced in BR peel hydrolysates after fermentation. Arabinose (SP peel hydrolysate) and xylose in all the hydrolysates showed only insignificant changes after fermentation under the F-SHF mode because of the inability of S. cerevisiae to utilize the pentose sugars as reported by others (Chen, 2011; Lin and Tanaka, 2006). The trivial reduction that was observed in the content of xylose or arabinose in fermented broth might have occurred from the adhesion on the surface of the proliferated yeast which was filtered off prior to HPLC characterization.
A similar monosaccharide profile was observed in the hydrolysates from DSA pretreated biomass also under the F-SHF mode, with slightly lower levels of hexoses and xylose in all the samples, but higher level of arabinose in SP peel hydrolysate (Table 4). Accordingly the residual hexoses in the 72 h fermented broth from DSA pretreatment was also lower than the steam treated counterparts. Despite the lower levels of hexose sugars in the hydrolysates from DSA pretreatment (F-SHF mode), the ethanol yield (YE) and ethanol production (g/L) were higher (Table 2) than from steam pretreatment indicating that some of the partially hydrolysed di- or oligosaccharides were also directly converted to ethanol by the yeast.
As compared to the fermented broth from F-SHF, the broth after 120 h F-SSF had significantly lower levels of both hexoses and pentoses. The low content of arabinose and xylose (which could not be utilized by yeast) indicated that the formation of pentose sugars itself was less in F-SSF in most residues than under F-SHF. The slow saccharification was further supported by the low VEP values under F-SSF (Table 3) compared to F-SHF (Table 2). Nevertheless the ethanol content in the broth from F-SSF was higher (steam pretreatment) than F-SHF or similar to F-SHF in DSA pretreatment (except GY and BR peels) and this indicated that hexose conversion to ethanol was better in steam pretreated biomass under F-SSF than F-SHF. Despite the high RS consumption, ethanol production (ml/kg) was poor from DSA pretreated biomass under F-SSF compared to the respective F-SHF and this resulted possibly from the inhibition of yeast enzymes by the higher levels of phenolics and HMF (discussed below).
Most of the studies on the HPLC sugar profile have focused on the monosaccharides in the liquid fraction from pretreated material. Stenberg et al. (2000) reported high content of mannose (ca. 22 g/L) in the liquor from steam pretreated SO2 impregnated spruce compared to glucose (ca. 16 g/L) even though native spruce had 40% glucan and 13% mannan. The very high glucose levels in F-SHF resulted from the high content of starch as well in the LCSBs under study (Table 1). Nguyen et al. (2018) reported ethanol yields of 0.31 g/g and production of 20.8 g/L from dilute acid pretreated soybean residue using a high solids loading of 20% (w/v) and the traditional yeast, S. cerevisiae. However when galactose adapted yeast was used, they could obtain much higher yields of ethanol (33.9 g/L), although similar or higher yields were obtained in the present study using traditional yeast adopting the fed-batch approach. It may also be noted that galactose was present in only DSA pretreated BR peel hydrolysates in the present study and that too in small amounts (7.1 and 4.3 g/L respectively in steam and DSA pretreatments under F-SHF).
3.4. Inhibitor profile in F-SHF and F-SSF
The level of three types on inhibitors such as total soluble phenolics (TSPs), furfural and 5-hydroxymethyl furfural (5-HMF) was monitored in the 96 h hydrolysates from fed-batch SHF as well as in the fermented broth (72 h for F-SHF and 120 h for F-SSF) from both pretreatments. Total soluble phenolics (TSPs) were very high in the hydrolysates and fermented broth from DSA pretreated biomass compared to steam pretreatment (Table 5). Nevertheless these values were significantly lower than those present in the pretreated liquor from the residues reported earlier where the pretreated liquor from steam pretreatment was found to contain as high as 1.07–2.88 g/L TSPs when 10% loading was adopted, while in DSA system, 1.17–3.18 g/L were observed (Mithra and Padmaja, 2016b). This meant that the pretreated slurries at 15% cumulative loading in the present study should have ca. 1.6–4.32 g/L and 1.76–4.76 g/L in steam and DSA pretreatments respectively (based on mathematical computation). However significantly lower values were only observed in the 96 h hydrolysates in F-SHF (0.32–0.60 g/L in steam and 0.74–0.86 g/L in DSA respectively), which was evidently due to the action of detoxification chemical mix containing surfactants such as Tween 20 and PEG 4000 and sodium borohydride. Previous studies showed that these chemicals were highly effective in channelling out the soluble phenolics from the pretreated liquor (Mithra and Padmaja, 2016b). Although the most popular method of pretreatment of lignocellulosic biomass is the use of dilute sulphuric acid, it leads to the accumulation of inhibitors such as furfural, HMF and phenolics (Nguyen et al., 2018). Much of the inhibitors are removed in conventional LCB technology in the washing step after pretreatment. However in the case of LCSBs, this was impossible as the pretreated liquor was enriched with RS from the hydrolysis of starch and hemicellulose.
Table 5.
Total soluble phenolics (TSPs; g/L) in the hydrolysate (96 h; F-SHF) and fermented broth [after fermentation (72 h; F-SHF) and 120 h (F-SSF)] from steam/DSA pretreated biomass.
| Biomass | 96 h hydrolysate (F-SHF) |
Fermented broth (72 h; F-SHF) |
Fermented broth (120 h; F-SSF) |
|||
|---|---|---|---|---|---|---|
| ST | DSA | ST | DSA | ST | DSA | |
| SP peel | 0.593d | 0.740b | 0.481e (18.83)∗ | 0.648c (12.45) | 0.718b | 0.856a |
| EFY peel | 0.539d | 0.738b | 0.438e (18.74) | 0.658c (10.90) | 0.651c | 0.864a |
| Tannia peel | 0.559d | 0.783b | 0.468e (16.24) | 0.673c (14.01) | 0.681c | 0.919a |
| GY peel | 0.321e | 0.757b | 0.301e (6.36) | 0.625c (17.44) | 0.494d | 0.853a |
| BR peel | 0.601d | 0.858b | 0.502e (16.48) | 0.731c (14.79) | 0.744c | 1.024a |
Figures in parentheses indicate the percentage decrease in TSPs during fermentation (72 h) by yeast; statistical comparison was made row-wise and values with different superscripts are significant at p < 0.05.
Phenolics were present in appreciable levels in the 96 h hydrolysates from steam pretreated residues as well under F-SHF mode while very high levels were observed in both the pretreatments in F-SSF (Table 5). It was also observed that during fermentation yeast assimilated/detoxified some of the TSPs (Table 5). In steam pretreated residues, ca. 16–19% TSPs were eliminated during fermentation of residues other than GY peel, in which only ca. 6.4% phenolic removal was observed, which was due to the low levels of TSPs in the hydrolysates from this residue. This indicated that a threshold level of TSPs was possible in the hydrolysates beyond which only yeast assimilated or detoxified them for its growth. In the DSA system, ca. 11–15% removal of TSPs was observed in four residues while 17% was removed from GY peel. The high levels of phenolics in F-SSF (both the pretreatments) resulted from the cumulative release from the pretreated biomass, because of the competitive binding of lignin to enzymes than the surfactants.
The levels of HMF and furfural produced as degradation products of hexoses and pentoses respectively were monitored in the 96 h hydrolysates from F-SHF of steam or DSA pretreated biomass and compared with the respective levels after 72 h fermentation and also with the 120 h F-SSF broth. HMF levels were significantly lower in the hydrolysates from steam treatment than DSA treatment (Table 6). Furfural levels were high in the 96 h hydrolysate from DSA pretreated SP peel only, while in the other cases lower levels than steam pretreatment were observed. During fermentation (72 h) ca. 11 mg/L HMF were assimilated/detoxified by S. cerevisiae in both the pretreated samples irrespective of the differences in HMF content in the hydrolysates (Table 6). In contrast to HMF, furfural levels were very low in the fermented broth from BR peel hydrolysates (both steam and DSA). Despite the high sugar (17%) and hemicellulose (19%) content in BR peels (Table 1), the low conversion to furfural during pretreatment indicated that the pentose sugars were possibly protected from dehydration by the high antioxidant activity in BR peels. There were also differences in the detoxification/assimilation of furfural from the various hydrolysates during fermentation by yeast and higher quantities (6–9 mg/L) were removed from SP and EFY peel hydrolysates while only negligible amounts were removed from BR peel hydrolysates.
This indicated that the selective assimilation pathways of yeast were activated only if the levels of HMF or furfural exceeded a certain threshold limit. The significance of threshold levels of phenolics and furfural in influencing ethanol formation by yeast has been highlighted in many studies (Larsson et al., 1999a,b). The fermented broth from F-SSF (120 h) contained significantly higher levels of HMF compared to the respective F-SHF samples, while furfural levels were high in F-SSF broth from SP and GY peels only (Table 6). The very high RS consumption in the first 24 h in F-SHF, which then tapered off during 48–72 h (Table 2) also indicated that HMF or furfural at the levels present did not significantly affect the fermentative performance of yeast. This was again supported by the high VEP in the first 24 h in F-SHF (Table 2).
The levels of HMF and furfural in the present study were lower than those reported in several studies. Öhgren et al. (2007) obtained HMF and furfural levels of 0.6 g/L and 0.7 g/L respectively in the liquid prehydrolysates from steam pretreated corn stover. Stenberg et al. (2000) reported values of 1.6 g/L and 0.9 g/L for HMF and furfural respectively in the liquid fraction from steam pretreated spruce, while very low values of 35–88 mg/L and 15–64 mg/L were obtained in the steam or DSA pretreated LCSB hydrolysates in the present study. Cavka and Jönsson (2013) reported that sodium borohydride was very effective in reducing the high levels of furfural and HMF in sugarcane bagasse hydrolysates. Sodium borohydride along with the two surfactants at levels reported earlier to effectively channel out the soluble phenolics (Mithra and Padmaja, 2016b) might have helped to reduce the levels of the inhibitors. While phenolic compounds were reported to increase the biological membrane fluidity and cause loss of cellular integrity in yeast (Heipieper et al., 1991) furfural and HMF were shown to decrease the fermentability by reducing the activity of various yeast enzymes (Modig et al., 2008; Palmqvist et al., 1999a).
The relative toxicity of inhibitors to ethanol fermentation was phenolics > furfural > HMF and synergistic effect when present together has also been reported (Mussatto and Roberto, 2004). Nevertheless S. cerevisiae has the ability to biotransform or metabolise these inhibitors although this could lead to delayed ethanol production (Taherzadeh and Karimi, 2008). Though several reports indicated that higher inhibitor (furfural, HMF and phenolic lignin degradation products) levels might possibly affect the fermentation performance of yeast (Modig et al., 2008; Palmqvist et al., 1999b). Hodge et al. (2008) observed that acetic acid, phenolic compounds and furanoids at levels of 15 g/L, 9 g/L and 8 g/L respectively had only slight inhibitor effect on the enzyme kinetics. Zha et al. (2012) studied the inhibitory effect of a number of compounds present in LCB hydrolysates potentially toxic to yeast growth and found that only furfural and benzoic acid significantly affected yeast growth.
3.5. Fermentation efficiency and comparative ethanol recovery under F-SHF and F-SSF from steam or DSA-pretreated biomass
The fermentation efficiency (%) was compared for the pretreated LCSBs subjected to F-SHF or F-SSF. Very high FE (%) was observed for DSA pretreated residues subjected to F-SHF indicating its efficiency in obtaining ca. 83–97% of theoretical ethanol yield from this process (Table 7). Among the four systems, the lowest FE values were obtained for F-SSF of steam pretreatment. Despite this, the comparative ethanol recovery (ml/kg dry biomass) from steam and DSA pretreated biomass under F-SHF and F- SSF as given in Table 7 showed that F-SSF was definitely advantageous in the case of steam pretreatment. Although the RS consumption in DSA pretreated EFY and tannia peels was low (70% and 72.68% respectively) compared to 81–89% in the other residues, the FE for the former residues from F-SHF was very high (Tables 2 and 7). Furthermore these residues also had lower content of glucose and mannose (Table 4) that are utilized by yeast and high content of xylose which cannot be utilized and this indicated that there was a high conversion of sugars to ethanol in these residue hydrolysates. Among the LCSBs, the highest ethanol yields were obtained from DSA pretreated GY and BR peels under F-SHF and these residues also had the highest cellulose and hemicellulose content (18–20%) with BR peel having very high (17%) total sugars as well (Table 1). It was found that FE was not directly correlated to ethanol productivity. In the case of GY peel having almost similar FE values (73–74%) under F-SHF (steam pretreatment) and F-SSF (DSA pretreatment), the former gave only 268 ml/kg while the latter gave 303 ml/kg. Similarly for BR peel having FE of 75% (F-SHF; steam) and 67% (F-SSF; DSA) the former gave 295 ml/kg ethanol while the latter gave 310 ml/kg ethanol. This meant that the entire RS consumed in F-SHF (steam) was not converted to ethanol, while in F-SHF (DSA) a higher conversion was achieved possibly because oligosaccharides such as cellobiose or cellodextrins also were directly converted to ethanol by yeast. F-SSF was evidently better for steam pretreated SP, EFY and tannia peels with higher ethanol production and RS consumption than DSA pretreatment. Distillation yielded slightly lower recovery of ethanol for all the samples (Table 7) and this resulted from the smearing loss on the walls of the receiver flasks.
Table 7.
Comparative Fermentation Efficiency (%) and ethanol productivity (ml/kg dry biomass) from steam and DSA pretreated biomass under F-SHF and F-SSF.
| Biomass | Steam pretreated |
DSA pretreated |
||
|---|---|---|---|---|
| F-SHF | F-SSF | F-SHF | F-SSF | |
| (a) Fermentation efficiency (%) | ||||
| SP peel | 73.44b | 69.53d | 82.69a | 71.03c |
| EFY peel | 72.49d | 75.55c | 96.99a | 79.05b |
| Tannia peel | 71.58c | 72.01c | 90.64a | 73.52b |
| GY peel | 74.39b | 69.62d | 94.26a | 73.30c |
| BR peel | 75.04b | 64.59d | 93.69a | 66.63c |
| (b) Ethanol productivity (ml/kg dry biomass) | ||||
| SP peel | 232.36d (221.01)∗ | 289.67a (276.98) | 283.09b (272.50) | 281.38c (271.02) |
| EFY peel | 231.30d (217.98) | 302.11a (290.23) | 282.03c (273.48) | 294.14b (280.94) |
| Tannia peel | 223.01c (212.66) | 280.97a (267.32) | 273.66b (263.38) | 272.68b (261.81) |
| GY peel | 268.13d (256.20) | 311.62b (301.97) | 318.86a (306.67) | 303.33c (292.56) |
| BR peel | 295.28d (281.66) | 318.21b (307.21) | 346.01a (335.48) | 310.24c (298.22) |
Figures in parentheses indicate the recovery through distillation (mean from two replicates); Other values are mean from three replicates; means with different superscripts in each row are significant at p < 0.05.
Rudolf et al. (2005) reported an increase in initial ethanol production in fed-batch SSF with only one enzyme feeding strategy at the start compared to batch SSF and found that yeast was inhibited to a greater degree in batch SSF than F-SSF. While some reports indicated higher ethanol yields from F-SSF (Söderström et al., 2004; Tomás-Pejó et al., 2009), others reported that F-SSF was not superior to batch SSF, unless otherwise the enzyme feeding strategy was altered to counteract the inhibitors formed (Hoyer et al., 2010). In the present study, enzymes at one-fourth the initial level, yeast along with the nutrient solution at one-fifth the initial level and detoxification chemicals at half the initial level were supplemented along with each substrate feeding in both the pretreatments in F-SSF, while in the parallel study on F-SHF, only detoxification chemicals were fed at 24, 48 and 72 h substrate feeding stage. The higher level of enzyme and yeast population in the F-SSF coupled with the lower inhibitor levels (phenolics and HMF) in steam pretreated samples might have contributed to the higher ethanol content after 120 h. In DSA pretreated samples F-SHF gave higher ethanol productivity from greater yam and beet root peels than others while the yield from the other samples was almost similar for both the modes. Olofsson et al. (2008) reported that F-SSF was advantageous due to the low inhibitor accumulation at each point of time compared to batch SSF. The fermented broth from F-SSF of DSA pretreated biomass in the present study had higher levels of inhibitors especially TSPs than the broth from F-SHF which might have decelerated ethanol production by yeast. The major disadvantage of SSF is reported as the sub-optimal performance of enzymes as well as yeast under the compromising temperature (35–37 °C) and pH (4.5) often provided (Sassner et al., 2006). Nevertheless these factors did not adversely affect the F-SSF of steam pretreated residues as higher ethanol recovery was obtained for this system compared to the respective F-SHF. However as enzyme and yeast as well all other additives such as detoxification mix, nutrient solution etc. were also added in proportionate doses along with the pulsed addition of substrate in F-SSF which could negatively influence the process costs, F-SHF of DSA pretreated biomass could be considered as a comparatively advantageous process.
4. Conclusions
The comparative ethanol production from steam or dilute sulphuric acid (DSA) pretreated lignocellulo-starch biomass (peels of root crops such as sweet potato, elephant foot yam, tannia, greater yam and beet root) by fed-batch SHF or SSF was investigated. Maximum reducing sugars (RS) were consumed by Saccharomyces cerevisiae during the first 24 h of fermentation in F-SHF, while continuous production and utilization of RS occurred up to 72 h in F-SSF. The volumetric ethanol productivity (VEP) and ethanol yield (YE) were more for DSA pretreated biomass under F-SHF thereby resulting in higher ethanol content (g/L) in this system than the corresponding steam pretreated system. HPLC sugar profile indicated that glucose and xylose were uniformly present in all the hydrolysates with a preponderance of glucose and fermentation resulted in significant reduction in glucose levels in both F-SHF and F-SSF. Higher levels of total soluble phenolics (TSPs) and HMF were observed in hydrolysates from DSA pretreatment and yeast assimilated or detoxified part of the inhibitors. Despite supplementation with detoxification chemicals (Tween 20 + PEG4000 + sodium borohydride), the fermented broth from F-SSF had high levels of TSPs. Furfural levels were the highest in SP peel hydrolysates and lowest in BR peel hydrolysates. Approximately 8.6–15% removal of furfural was observed during fermentation by yeast in F-SHF from residues other than BR peel while very low reduction (3–7%) was observed in BR peel hydrolysates indicating that only when threshold levels were exceeded yeast detoxification pathways were activated. The study showed that although F-SSF of steam pretreated residues gave higher ethanol recovery than F-SHF (281–318 ml/kg dry biomass vs 223–295 ml/kg), the use of repeated doses of enzyme, yeast, nutrient solution and detoxification chemicals made the process less effective than F-SHF of DSA pretreated biomass, where only one time feeding of enzyme cocktail and yeast was adopted.
Declarations
Author contribution statement
M. G. Mithra: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. L. Jeeva: Conceived and designed the experiments; Wrote the paper.
M. S. Sajeev: Contributed reagents, materials, analysis tools or data; Wrote the paper.
G. Padmaja: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Kerala State Council for Science, Technology & Environment (KSCSTE) (Grant No. 853/2015/KSCSTE).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the Director, ICAR-CTCRI for the facilities provided. The help extended by Dr. J. Sreekumar, Principal Scientist (Agricultural Statistics) for the statistical analysis is gratefully acknowledged. Thanks are also due to Dr. A.N. Jyothi, Principal Scientist (Organic Chemistry) and Mr. V. R. Vishnu, Senior Research Fellow, ICAR-CTCRI for the held extended in the HPLC analysis.
References
- Alvira P., Tomás-Pejó E., Ballesteros M., Negro M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 2010;101(13):4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- Anon . Product Information Published by Genencor International, a Division of Danisco, Danisco US Inc; 2009. STARGEN™ 002: Granular Starch Hydrolyzing Enzyme for Ethanol Production.http://www.genencor.com Available from: [Google Scholar]
- Ballesteros M., Oliva J.M., Manzanares P., Negro M.J., Ballesteros I. Ethanol production from paper materials using a simultaneous saccharification and fermentation system in a fed batch basis. World J. Microbiol. Biotechnol. 2009;18:559–561. [Google Scholar]
- Barcelos C.A., Maeda R.N., Betancur G.J.V., Pereira N., Jr. Ethanol production from sorghum grains [Sorghum bicolor (L.) Moench]: evaluation of the enzymatic hydrolysis and the hydrolysate fermentability. Braz. J. Chem. Eng. 2011;28:597–604. [Google Scholar]
- Börjesson J., Peterson R., Tjerneld F. Enhanced enzymatic conversion of softwood lignocelluloses by poly (ethylene glycol) addition. Enzyme Microb. Technol. 2007;40(4):754–762. [Google Scholar]
- Caputi A., Jr., Ueda M., Brown T. Spectrophotometric determination of ethanol in wine. Am. J. Enol. Vitic. 1968;19:160–165. [Google Scholar]
- Cavka A., Jönsson L.J. Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour. Technol. 2013;136:368–376. doi: 10.1016/j.biortech.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Chen M., Xia L., Xue P. Enzymatic hydrolysis of corncob and ethanol production from cellulosic hydrolysate. Int. Biodeterior. Biodegrad. 2007;59(2):85–89. [Google Scholar]
- Chen W.H. Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: a systematic review. J. Ind. Microbiol. Biotechnol. 2011;38:581–597. doi: 10.1007/s10295-010-0894-3. [DOI] [PubMed] [Google Scholar]
- Eriksson T., Börjesson J., Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol. 2002;31(3):353–364. [Google Scholar]
- Farrel A.E., Pelvin R.J., Turner B.T., Jones A.D., O'Hare M., Kammen D.M. Ethanol can contribute to energy and environmental goals. Science. 2006;311:506–508. doi: 10.1126/science.1121416. [DOI] [PubMed] [Google Scholar]
- Galbe M., Zacchi G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002;59(6):618–628. doi: 10.1007/s00253-002-1058-9. [DOI] [PubMed] [Google Scholar]
- Gao Y., Xu J., Yuan Z., Zhang Y., Liu Y., Liang C. Optimization of fed-batch enzymatic hydrolysis from alkali-pretreated sugarcane bagasse for high-concentration sugar production. Bioresour. Technol. 2014;167:41–45. doi: 10.1016/j.biortech.2014.05.034. [DOI] [PubMed] [Google Scholar]
- Hedge J.E., Hofreiter B.T. In: Whistler R.L., Be Miller J.N., editors. vol. 17. Academic Press; New York: 1962. (Carbohydrate Chemistry). [Google Scholar]
- Heipieper H., Keweloh H., Rehm H. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl. Environ. Microbiol. 1991;57:1213–1217. doi: 10.1128/aem.57.4.1213-1217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel M.E., Ding S.Y., Johnson D.K., Adney W.S., Nimlo M.R., Brady J.W., Foust T.D. Biomass recalcitrance, engineering plants and enzymes for biofuel production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Hodge D., Karim M.N., Schell D.J., McMillan J.D. Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2008;99:8940–8948. doi: 10.1016/j.biortech.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Horn S.J., Vaaje-Kolstad G., Westereng B., Eijsink V.G. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels. 2012;5:45. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer K., Galbe M., Zacchi G. Effects of enzyme feeding strategy on ethanol yield in fed-batch simultaneous saccharification and fermentation of spruce at high dry matter. Biotechnol. Biofuels. 2010;3:14. doi: 10.1186/1754-6834-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R., Turhollow A.F., Rutz D., Mergner R. Production facilities for second-generation biofuels in the USA and the EU–current status and future perspectives. Biofuels Bioprod. Biorefin. 2013;7:647–665. [Google Scholar]
- Jönsson L.J., Palmqvist E., Nilvebrant N.O., Hahn-Hägerdal B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1998;49:691–697. [Google Scholar]
- Koppram R., Tomás-Pejó E., Xiros C., Olsson L. Lignocellulosic ethanol production at high-gravity, challenges and perspectives. Trends Biotechnol. 2014;32(1):46–53. doi: 10.1016/j.tibtech.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Larsson S., Palmqvist E., Hahn-Hägerdal B., Tengborg C., Stenberg K., Zacchi G., Nilvebrant N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999;24(3–4):151–159. [Google Scholar]
- Larsson S., Reimann A., Nilvebrant N.O., Jönsson L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 1999;77(1):91–103. [Google Scholar]
- Lammirato C., Miltner A., Wick L.Y., Kästner M. Hydrolysis of cellobiose by β- glucosidase in the presence of soil minerals-interactions at solid- liquid interfaces and effects on enzyme activity levels. Soil Biol. Biochem. 2010;42:2203–2210. [Google Scholar]
- Lin Y., Tanaka S. Ethanol fermentation from biomass resources, current state and prospects. Appl. Microbiol. Biotechnol. 2006;69:627–642. doi: 10.1007/s00253-005-0229-x. [DOI] [PubMed] [Google Scholar]
- McIntosh S., Palmer J., Zhang Z., Doherty W.O.S., Yazdani S.S., Sukumaran R.K., Vacov T. Simultaneous saccharification and fermentation of pretreated Eucalyptus grandis under high solids loading. Indus. Biotechnol. 2017;13(3):131–140. [Google Scholar]
- Mithra M.G., Padmaja G. Phenolic inhibitors of saccharification and fermentation in lignocellulo-starch prehydrolysates and comparative efficacy of detoxification treatments. J. Biomass Biofuel. 2016;3 [Google Scholar]
- Mithra M.G., Padmaja G. Compositional profile and ultrastructure of steam and dilute sulfuric acid pretreated root and vegetable processing residues. Curr. Biotechnol. 2016 [Google Scholar]
- Mithra M.G., Padmaja G. Comparative alterations in the compositional profile of selected root and vegetable peels subjected to three pretreatments for enhanced saccharification. Int. J. Environ. Agric. Biotechnol. 2017;2(4):1732–1744. [Google Scholar]
- Mithra M.G., Padmaja G. Strategies for enzyme saving during saccharification of pretreated lignocellulo-starch biomass, effect of enzyme dosage and detoxification chemicals. Heliyon. 2017;3 doi: 10.1016/j.heliyon.2017.e00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithra M.G., Sreekumar J., Padmaja G. Binary- and triple-enzyme cocktails and their application mode affect fermentable sugar release from pretreated lignocellulo-starch biomass. Biomass Convers. Biorefin. 2017;8:97–111. [Google Scholar]
- Mithra M.G., Sajeev M.S., Padmaja G. Fed-batch saccharification as a strategy towards reducing enzyme dosage and enhancing fermentable sugar yield from pretreated lignocellulo-starch biomass. Waste Biomass. Valor. 2018 [Google Scholar]
- Modig T., Almeida J.R., Gorwa-Grauslund M.F., Lidén G. Variability of the response of Saccharomyces cerevisiae strains to lignocellulose hydrolysate. Biotechnol. Bioeng. 2008;100:423–429. doi: 10.1002/bit.21789. [DOI] [PubMed] [Google Scholar]
- Mussatto S.I., Roberto I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour. Technol. 2004;93:1–10. doi: 10.1016/j.biortech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for determination of glucose. J. Biol. Chem. 1944;153:375–380. [Google Scholar]
- Nguyen T.H., Ra C.H., Sunwoo I.Y., Sukwong P., Jeong G.T., Kim S.K. Bioethanol production from soybean residue via separate hydrolysis and fermentation. Appl. Biochem. Biotechnol. 2018;184:513–523. doi: 10.1007/s12010-017-2565-6. [DOI] [PubMed] [Google Scholar]
- Öhgren K., Bura R., Lesnicki G., Saddler J.N., Zacchi G. A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam pretreated corn stover. Process Biochem. 2007;42:834–839. [Google Scholar]
- Öhgren K., Bengtsson O., Gorwa-Grauslund M.F., Galbe M., Hahn-Hägerdal B., Zacchi G. Simultaneous saccharification and co-fermentation of glucose and xylose in steam pretreated corn stover at high fiber content with S. cerevisiae TMB3400. J. Biotechnol. 2006;126:488–498. doi: 10.1016/j.jbiotec.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Olofsson K., Rudolf A., Lidén G. Designing simultaneous saccharification and fermentation for improved xylose conversion by a recombinant strain of Saccharomyces cerevisiae. J. Biotechnol. 2008;134:112–120. doi: 10.1016/j.jbiotec.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Palmqvist E., Almeida J.S., Hahn-Hägerdal B. Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiae in batch culture. Biotechnol. Bioeng. 1999;62(4):447–454. doi: 10.1002/(sici)1097-0290(19990220)62:4<447::aid-bit7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Palmqvist E., Grage H., Meinander N.Q., Hahn-Hägerdal B. Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol. Bioeng. 1999;63(1):46–55. doi: 10.1002/(sici)1097-0290(19990405)63:1<46::aid-bit5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Pereira S.C., Maehara L., Machado C.M.M., Farinas C.S. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol. Biofuels. 2015;8:44. doi: 10.1186/s13068-015-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooja N.S., Sajeev M.S., Jeeva M.L., Padmaja G. Bioethanol production from microwave-assisted acid or alkali-pretreated agricultural residues of cassava using separate hydrolysis and fermentation (SHF) 3 Biotech. 2018;8:69. doi: 10.1007/s13205-018-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf A., Alkasrawi M., Zacchi G., Lidén G.A. A comparison between batch and fed-batch simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb. Technol. 2005;37:195–204. [Google Scholar]
- Russel I. Understanding yeast fundamentals. In: Jacques K.A., Lyons T.P., Kelsall D.R., editors. The Alcohol Textbook. fourth ed. Nottingham University Press; U.K: 2003. [Google Scholar]
- Sarkar N., Ghosh S.K., Bannerjee S., Aikat K. Bioethanol production from agricultural wastes: an overview. Renew. Energy. 2012;37:19–27. [Google Scholar]
- SAS, 2010. Cary NC. USA, SAS Institute Inc.
- Sassner P., Galbe M., Zacchi G. Bioethanol production based on simultaneous saccharification and fermentation of steam-pretreated Salix at high dry-matter content. Enzyme Microb. Technol. 2006;39(4):756–762. [Google Scholar]
- Shanavas S., Padmaja G., Moorthy S.N., Sajeev M.S., Sheriff J.T. Process optimization for bioethanol production from cassava starch using novel eco-friendly enzymes. Biomass Bioenergy. 2011;35:901–909. [Google Scholar]
- Shi J., Sharma-Shivappa R.R., Chinn M., Howell N. Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenergy. 2009;33:88–96. [Google Scholar]
- Singh A., Kuila A., Adak S., Bishai M., Banerjee R. Utilization of vegetable wastes for bioenergy generation. Agric. Res. 2012;1:213–222. [Google Scholar]
- Singleton V.L., Rossi A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Söderström J., Galbe M., Zacchi G. Effect of washing on yield in one- and two-step steam pretreatment of softwood for production of ethanol. Biotechnol. Progr. 2004;20:744–749. doi: 10.1021/bp034353o. [DOI] [PubMed] [Google Scholar]
- Stenberg K., Bollók M., Réczey K., Galbe M., Zacchi G. Effect of substrate and cellulase concentration on simultaneous saccharification and fermentation of steam-pretreated softwood for ethanol production. Biotechnol. Bioeng. 2000;68(2):204–210. doi: 10.1002/(sici)1097-0290(20000420)68:2<204::aid-bit9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sun Y., Cheng J. Hydrolysis of lignocellulosic materials for ethanol production, a review. Bioresour. Technol. 2002;83:1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Taherzadeh M.J., Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production, a review. Int. J. Mol. Sci. 2008;9:1621–1651. doi: 10.3390/ijms9091621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherzadeh M.,J., Niklasson C., Lidén G. On-line control of fed-batch fermentation of dilute-acid hydrolyzates. Biotechnol. Bioeng. 2000;69(3):330–338. doi: 10.1002/1097-0290(20000805)69:3<330::aid-bit11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tejirian A., Xu F. Inhibition of enzymatic cellulolysis by phenolic compounds. Enzyme Microb. Technol. 2011;48(3):239–247. doi: 10.1016/j.enzmictec.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Tomás-Pejó E., Garcia-Aparicio M., Negro M.J., Oliva J.,M., Ballesteros M. Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresour. Technol. 2009;100:890–895. doi: 10.1016/j.biortech.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Wanderley M.C.A., Martin C., Rocha G.J.M., Gouveia E.R. Increase in ethanol production from sugarcane bagasse based on combined pretreatments and fed-batch enzymatic hydrolysis. Bioresour. Technol. 2013;128:448–453. doi: 10.1016/j.biortech.2012.10.131. [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Smart K.A., James S., Cook D.J. Maximising high solid loading enzymatic saccharification yield from acid-catalysed hydrothermally-pretreated brewers spent grain. Biofuel Res. J. 2016;10:417–429. [Google Scholar]
- Wyman C.,E. Biomass ethanol, technical progress, opportunities and commercial challenges. Annu. Rev. Energy Environ. 1999;24:189–226. [Google Scholar]
- Yadav S.,K., Naseeruddin S., Prashanthi G.,S., Sateesh S., Rao L.,V. Bioethanol fermentation of concentrated rice straw hydrolyzate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour. Technol. 2011;102(11):6473–6478. doi: 10.1016/j.biortech.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Yang B., Wyman C.,E. Pretreatment, the key to unlocking low cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008;2(1):26–40. [Google Scholar]
- Zacchi G., Axelsson A. Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol. Bioeng. 1989;34(2):223–233. doi: 10.1002/bit.260340211. [DOI] [PubMed] [Google Scholar]
- Zha Y., Muilwijk B., Coulier L., Punt P.J. Inhibitory compounds in lignocellulosic biomass hydrolysates during hydrolysate fermentation processes. J. Bioprocess. Biotech. 2012;2:1–11. [Google Scholar]
- Zhang B., Shahbazi A. Recent developments in pretreatment technologies for production of lignocellulosic biofuels. J. Petrol Environ. Biotechnol. 2011;2:108–115. [Google Scholar]
- Zhang T., Zhu M.J. Enhanced bioethanol production by fed-batch simultaneous saccharification and co-fermentation at high solid loading of Fenton reaction and sodium hydroxide sequentially pretreated sugarcane bagasse. Bioresour. Technol. 2017;229:204–210. doi: 10.1016/j.biortech.2017.01.028. [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang Y.H., Chu J., Luo L.Z., Zhuang Y.P., Zhang S.L. Optimization of cellulase mixture for efficient hydrolysis of steam-exploded corn stover by statistically designed experiments. Bioresour. Technol. 2008;100:819–825. doi: 10.1016/j.biortech.2008.06.068. [DOI] [PubMed] [Google Scholar]