Abstract
Genetic and environmental factors contribute to thyroid diseases. Although still debated, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is thought to induce thyroid dysfunction in humans and rodents. The data here reported point out the contribution of the exposure window and genetic background in mediating the low-dose TCDD effects on thyroid. Indeed, early (from E0.5 to PND30) and low-dose (0,001 μg/kg/day) TCDD exposure reduced the circulating fT4 and altered the expression of thyroid specific transcripts. The role of genetic components was estimated monitoring the same markers in Pax8+/− and Nkx2-1+/− mice, susceptible to thyroid dysfunction, exposed to 0, 1 μg/kg/day TCDD from E15.5 to PND60. Haploinsufficiency of either Pax8 or Nkx2-1 genes exacerbated the effects of the exposure impairing the thyroid enriched mRNAs in sex dependent manner. Such effect was mediated by mechanisms involving the Nkx2-1/p53/p65/IĸBα pathway in vitro and in vivo. Foetal exposure to TCDD impaired both thyroid function and genes expression while thyroid development and differentiation did not appear significantly affected. In mouse, stronger effects were related to earlier exposure or specific genetic background such as either Pax8 or Nkx2-1 haploinsufficiency, both associated to hypothyroidism in humans. Furthermore, our data underline that long exposure time are needed to model in vitro and in vivo results.
Introduction
Genetic and environmental factors concur to the development of several pathologies including thyroid diseases1. The incidence of these pathologies is growing worldwide2 and environmental factors, other than iodine deficiency, have been implicated. Genetic factors importantly influence both normal and pathological thyroid functions. Studies on congenital hypothyroidism (CH) have identified different genes (Pax8, Nkx2-1, etc.) as key players in thyroid organogenesis highlighting, moreover, few differences between mouse and human3. Several environmental pollutants, defined THyroid Disrupting Chemicals (THDC), are able to compromise the thyroid functionality acting at different levels on the hypothalamic–pituitary–thyroidal (HPT)-axis in humans and in animal models. Indeed, early-life exposure to THDC might impair thyroid tissue organization and/or the homeostatic regulation of thyroid adaptive processes in rodents4,5. Up to now the data obtained in animal model have been discontinuously confirmed in humans.
The 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is an environmental, ubiquitous and low-level contaminant, the most toxic among the polychlorinated dibenzodioxins (PCDDs), frequently retrieved at low dose in human fluids. TCDD is an endocrine disruptor exerting a pleiotropic activity by mechanism involving the Aryl-Hydrocarbon-Receptor (AhR) networks in human and animal models6. The AhR pathways are activated by chemicals similar to TCDD also in thyrocytes7. Although strongly debated, epidemiological8–10 and experimental11 studies suggest that TCDD and PCBs impair blood thyroxine (T4) and triiodothyronine (T3) levels. Exposure to TCDD has been shown to affect thyroid morphology and functionality in rodents12,13, with rats being more sensitive than mouse14.
The TCDD activity on the HPT axis observed in rodents has been only sporadically confirmed in humans15. This might be due to the variability of the genetic background of individuals, as opposed to the homogeneous genotype of the animal strains used in laboratory, and the relevance of the exposure window clearly evaluable only in animal models. Although pivotal in defining the risk associated to the environmental TCDD exposure, both aspects need further investigation. Indeed, researchers have poorly investigated the role of specific gene mutations, not having effect or inducing sub-clinical thyroid dysfunction, in exacerbating the TCDD activity. Furthermore, the thyroid-specific mechanisms of TCDD toxicity are not clarified yet. Their investigation is complicated in vivo because TCDD exerts its effects on HPT axis by the direct activity on the pituitary, on the thyroid gland or on both by the pituitary/thyroid feedback loop. Despite that, the effects of TCDD exposure on cellular models of thyrocytes have been assessed discontinuously.
We present data obtained both in mice and in vitro (in FRTL5, rat thyroid follicular cells) suggesting that low-dose TCDD carries out a direct effect on thyrocytes by a mechanism involving NF-κB pathway. We show that different TCDD doses, windows of exposure, genetic factors and, finally, sex are involved in mediating the impact of foetal TCDD exposure on the thyroid function.
Results
Exposure to low-dose TCDD since the conception induces phenotypic and molecular signs of hypothyroidism by a mechanism involving the Nkx2-1/p53/p65/IκBα pathway
Effects of early maternal exposure to TCDD on thyroid hormones (TH) status of dams and their offspring have not been detailed, as recently done for other chemicals16,17, because of the stressing administration routes usually adopted for TCDD administration (i.e. gavage). Intending to focus our attention on a “real exposure scenario”, we exposed C57BL/6 mice from E0.5 to the weaning by administration of food medicated with TCDD (TCDD-food) to pregnant dams and, then, feeding directly the offspring till the sacrifice (PND 30). A pilot experiment was done to verify the foetal toxicity of TCDD dosed at 0,1, 0,01 and 0,001 μg/kg/day. We did not obtain pups by pregnant dams exposed to 0, 1 μg/kg/day and 0, 01 μg/kg/day TCDD, in agreement with previous report showing that TCDD, dosed in similar range, exerted a toxic activity mainly on the pre-implantation embryos18.
Hence, TCDD 0,001 μg/kg/day exposure was adopted, a dose far below the ones used to investigate the TCDD-induced thyroid dysfunctions at later life stages (0,1 μg/kg/day)16. Treated dams had none statistical significant differences in the circulating free T4 (fT4, Fig. S1a) and in the expression of thyroid-stimulating hormone (Tsh) gene in pituitary (Fig. S1b), although TCDD activated the AhR pathway as suggested by the upregulation of its target Cyp1A1 in thyroid (Fig. S1f) and pituitary (Fig. S1d).
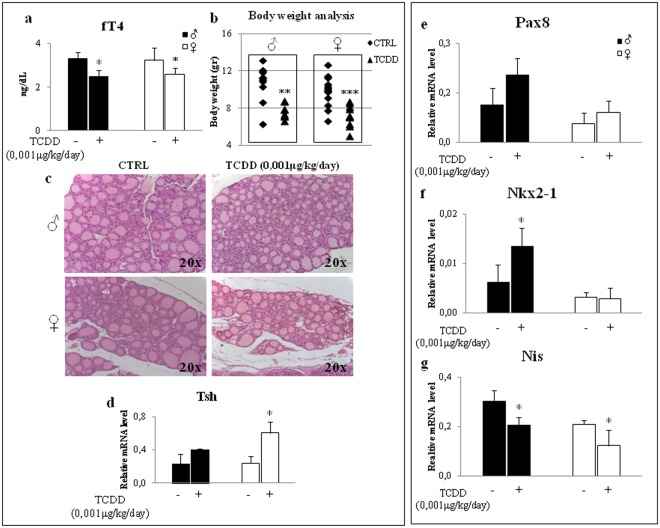
The increase of Cyp1A1 transcript was detected also in the thyroid (Fig. S2b) and pituitary (Fig. S2d) of the exposed offspring of both sexes in which TCDD exposure resulted in the reduction of circulating fT4 and body weight at the sacrifice (Fig. 1a,b, respectively). None major impact was evidenced in thyroid development as well as in thyroid morphology, as analysed by histochemistry (Fig. 1c). Although statistically significant only in females, Tsh transcript showed a trend towards the increase in the pituitary of TCDD treated mice (Fig. 1d). The expression levels of the sodium/iodide symporter (Nis), a TSH regulated gene19 also targeted by TCDD in primary thyrocytes20, and of its main transcriptional regulators Pax8 and Nkx2-1, were analysed in thyroids of control and exposed mice. Despite the increase of Tsh mRNA (Fig. 1d) and of Pax8 and Nkx2-1 transcripts in males (Fig. 1e,f), the Nis mRNA decreased in both sexes (Fig. 1g). Being the level of thyroglobulin (Tg, Fig. S3a) and thyroperoxidase (Tpo, Fig. S3b) transcripts concordant with Pax8 and Nkx2-1 mRNAs, we suggested that TCDD might impair a pathway specifically involved in regulation of Nis expression. We supposed that NF-ĸB might be a mediator of TCDD activity in thyroid. Indeed, this transcriptional factor is involved in the regulation of thyroid specific genes expression21,22, mediates the TSH activity23,24 and interplays with AhR25. In addition, NF-ĸB is activated by pollutants able to increase the cellular reactive oxygen species (ROS), as TCDD26.
Figure 1.
TCDD exposure since the conception impairs thyroid function. (a) fT4 serum levels were determined by ELISA in C57BL/6 males (black bar) and females (white bar) exposed at 0,001 μg/kg/day TCDD (TCDD) or not (CTRL), from E0.5 to PND30. (b) Body weight analysis of CTRL and TCDD-treated mice (male on the left and female on the right). (c) Hematoxylin and Eosin thyroid staining of CTRL- and TCDD-males (top panels) and females (bottom panels). (d–g) RT-qPCR analysis of Tsh transcript in pituitary and Pax8, Nkx2-1 and Nis transcripts in thyroid of CTRL- and TCDD-males (black bar) and TCDD-females (white bar). Data are reported as means ± SD of Gapdh normalized-mRNA levels; n = 5 mice for each group and sex. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001 treated vs control mice.
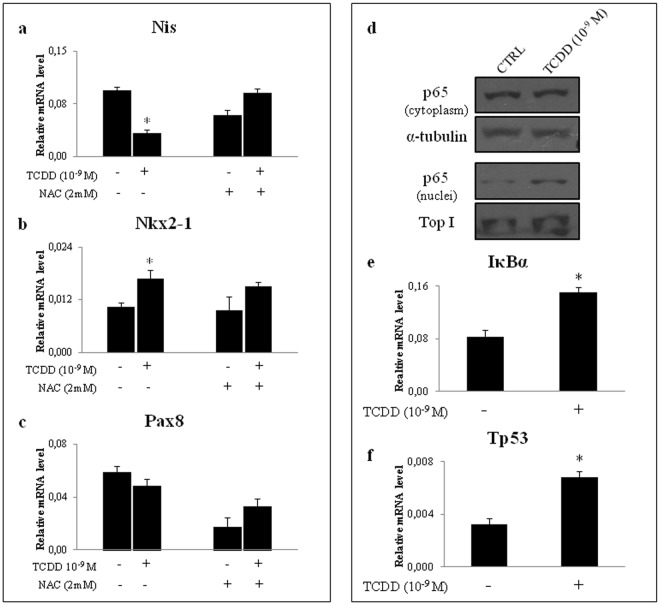
To test our hypothesis, we investigated the direct effects of TCDD on the immortalized rat follicular cell line FRTL-5, an evaluable model to study the response to low-dose environmental pollutants27–29. We exposed the cells to 10−9 M TCDD for 24 hrs, a setting previously reported to be associated with activation of AhR signalling and inhibition of Nis expression in primary thyrocytes20. The cellular content of Nis transcript was inhibited by TCDD as in vivo (Fig. 2a). The Nkx2-1 expression was also increased whereas none significant effect was retrieved for Pax8 messenger (Fig. 2b,c, respectively). TCDD induced the Tg and Tpo mRNAs as in vivo (see Supplementary Fig. S3c,d, respectively). To monitor the contribution of ROS production in the observed effect we co-treated the cells with N-acetyl-L-cysteine (NAC, 2 mM) to block ROS production. NAC treatment reversed the inhibition of Nis mRNA in TCDD-exposed cells, as well as the induction of Tg and Tpo transcripts (Fig. S3c,d), whereas none major effects was observed on the regulation of Nkx2-1 expression (Fig. 2b). Finally, the level of Pax8 transcript was reduced by NAC exposure and TCDD alleviated its inhibition (Fig. 2c).
Figure 2.
Oxidative stress contributes to alteration of thyroid-enriched transcripts in FRTL5 exposed to TCDD. (a–c) RT-qPCR analysis of Nis transcript (a) and thyroid specific regulators Nkx2-1 (b) and Pax8 (c) in FRTL-5 cells treated with TCDD 10−9 M for 24 hrs. When reported, cells were co-treated with NAC 2 mM for 24 hr. (d) Western blotting analysis of nuclear/cytosolic localization of p65 protein in FRTL-5 untreated (CTRL) and exposed to 10−9 M TCDD. β-Tubulin and Topoisomerase 1 were used as loading control of cytoplasmic and nuclear fraction, respectively. A cropped version of the image is shown (full-lenght blots are shown in Supplementary Fig. S7). Cytoplasmic and nuclear fractions were loaded on two different gels. Cellular level of IκBα and Tp53 mRNAs (e and f, respectively) was assayed by RT-qPCR in CTRL- and TCDD-cells. Level of transcripts was determined after exposure to TCDD 10−9 M for 24 hr and reported as means ± SD of Gapdh normalized-mRNA levels of three independent experiments. *p-value < 0.05 compared with controls.
The activation of NF-ĸB pathway was better characterized analysing the localization of its p65 subunit (p65) by western blotting. We found that the exposure to TCDD induced the p65 nuclear translocation (Fig. 2d). Concordantly, the expression of IĸBα mRNA, a NF-ĸB target gene, was increased (Fig. 2e). The reported activation of NF-ĸB together with the up-regulation of Nkx2-1 mRNA suggested that TCDD might activate the Nkx2-1/p53/p65/IĸBα pathway, as previously described in lung carcinogenesis30. Therefore, we monitored the level of Tp53 mRNA in the exposed cells retrieving its increase (Fig. 2f).
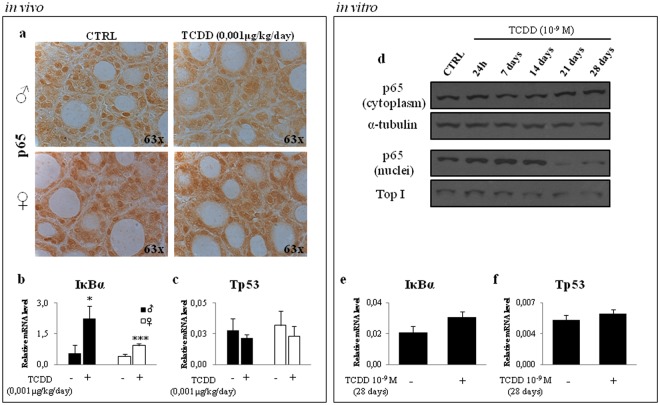
Considering that Nkx2-1 mRNA showed a trend towards the induction in treated males, we verified if the Nkx2-1/p53/p65/IĸBα pathway might be active also in mice exposed to 0, 001 μg/kg/day from E0.5 to PND30 (TCDD-mice). Concordantly, with the induction of Nkx2-1 transcript (Fig. 1f), IκBα mRNA increased in thyroids of TCDD-mice, although strongly in males (Fig. 3b). The p65 localization in the thyroids of control and TCDD-mice was assessed by immunohystochemistry. We observed the reduction of the p65 nuclear staining in thyroid of exposed animals of both sexes (Fig. 3a), which might be explained by the increased transcription of IκBα promoting its cytoplasmic retention. None difference was detected in Tp53 transcripts (Fig. 3c). Since the in vivo data partially confirmed the in vitro results, we verified if this discrepancy might be related to a time-dependent activation of NF-ĸB signalling being longer exposure time applied in vivo. Therefore, we exposed the FRTL-5 cells to 10−9 M TCDD for 1-, 7-, 14-, 21- 28 days and nuclear/cytoplasmic localization of p65 protein was analysed by western blotting (Fig. 3d). The data evidenced that the increase of nuclear level of p65 was on-going till the day 14 and, then, it was reduced at the later time points. We measured the level of Nis mRNA and the transcripts of the Nkx2-1/p53/p65/IκBα pathway at this last time point. The level of Nis and Nkx2-1 mRNAs was slightly affected in FRTL5 exposed to TCDD for 28 days compared to cell treated for 1 day (see Supplementary Fig. S4 and Fig. 2, respectively). The long exposure time (28 days) resulted in milder impairment of IκBα mRNA (Fig. 3e) and none major change was detected for Tp53 transcript (Fig. 3f).
Figure 3.
TCDD exposure induces the Nkx2-1/p53/p65/IĸBα pathway in vivo and in vitro. (a) Nuclear localization of p65 protein was assayed by immune-histochemistry in thyroids of control (CTRL) and 0,001 μg/kg/day TCDD treated-males (top panel) and TCDD-females (bottom panel) from E0.5 to PND30. Negative control was performed using purified IgG and it is shown in Fig. S3g. (b,c) Levels of IκBα (b) and Tp53 (c) transcripts were assayed by RT-qPCR in thyroid of CTRL- and TCDD-mice of both sexes (black bar for male, white bar for female). (d) Nuclear/cytosolic localization of p65 protein in FRTL-5 untreated (CTRL) and exposed to 10−9 M TCDD for 1-, 7-, 14-, 21-, 28- days was assayed by western blotting analysis. β-tubulin and Topoisomerase 1 were used as loading control of cytoplasmic and nuclear fraction, respectively. A cropped version of the image is shown (full-lenght blots are shown in Supplementary Fig. S7). Cytoplasmic and nuclear fractions were loaded on two different gels. (e,f) RT-qPCR analysis of IĸBα (e) and Tp53 (f) transcript levels in FRTL-5 cells treated with TCDD 10−9 M for 28days. In vitro data are reported as means ± SD of Gapdh normalized-mRNA levels of three independent experiments. In vivo data are reported as means ± SD of Gapdh normalized-mRNA levels; 5 mice for each group and sex were analyzed. *p-value < 0.05 and ***p-value < 0.001.
Overall the in vivo and in vitro data suggest that the early foetal exposure to low-dose TCDD affects thyroid function altering the expression of thyroid specific transcripts, mainly Nis, by a mechanism, at least partly, involving the Nkx2-1/p53/p65/IĸBα pathway differently in the early or late phase of the exposure.
Haploinsufficiency of Pax8 and Nkx2-1 predisposes to impairment of thyroid function in TCDD exposed mice
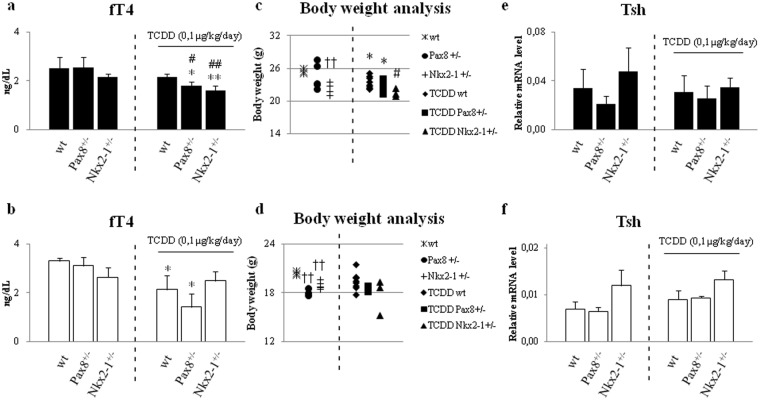
Thyroid diseases have a strong genetic component. In order to evaluate the role of the genotype in determining the effects of TCDD exposure, we used mice heterozygous for deletion of either Pax8 (Pax8+/−) or Nkx2-1 (Nkx2-1+/−). Haploinsufficiency of each of these genes associates with hypothyroidism in human and has slighter effects in mouse31. They might represent a good model to investigate the role of the genotype in the impairment of thyroid function by TCDD exposure. Indeed, the Nkx2-1+/− mice had a slight reduction of the circulating level of fT4 (Fig. 4a,b, at left of the dashed lines), a trend toward the reduction in the body weight (Fig. 4c,d, left side of the graphs) and increased levels of Tsh mRNAs (Fig. 4e,f, at left of the dashed lines). None phenotypic difference was retrieved in the Pax8+/− mice that showed a decreased level of Tsh transcript (Fig. 4e,f, at left of the dashed lines).
Figure 4.
Characterization of thyroid function in mice with different genetic background or foetally exposed to TCDD. (a,b) ELISA assay of fT4 serum level was performed in C57BL/6 wild type (wt), Pax8+/− and Nkx2-1+/− mice (at left of the dashed lines) and in wt, Pax8+/− and Nkx2-1+/− treated with TCDD 0,1 μg/kg/day (at right of the dashed lines), males (a) and females (b). (c,d) Body weight analysis of wt, Pax8+/− and Nkx2-1+/− and TCDD-wt, -Pax8+/− and -Nkx2-1+/− mice, males (c) and females (d). (e,f) RT-qPCR analysis of Tsh in pituitary of wt, Pax8+/− and Nkx2-1+/− and TCDD-wt, -Pax8+/− and -Nkx2-1+/− mice, males (e) and females (f). Data are reported as means ± SD of Gapdh normalized-mRNA levels; n = 4 mice for each group. *p-value < 0.05; **p-value < 0.01 for statistical analysis of TCDD-exposed vs untreated mice having the same genotype; #p-value < 0.05; ##p-value < 0.01 TCDD-exposed vs TCDD-wt mice; ††p-value < 0.01 unexposed mice of different genotype vs wt mice.
To assess the role of either Pax8 or Nkx2-1 haploinsufficiency in exacerbating the TCDD-induced thyroid dysfunction, we delayed the exposure time at E15.5, when the thyroid organogenesis is completed19. TCDD was dosed at 0, 1 μg/kg/day as previously done for this exposure window16,32,33. The impairment of thyroid function by TCDD in the new exposure condition was before tested in wild type C57BL/6 (wt). Pregnant females were fed with food medicated with TCDD (0, 1 μg/kg/day) since E15.5. The offspring was exposed by the mothers till the weaning and, then, by direct feeding till the sacrifice at PND60. TCDD exposure did not impair the levels of circulating fT4 or of the Tsh transcript of the mothers (Fig. S1g,h) although it resulted in the AhR activation, as documented by the increase of the Cyp1A1 mRNA in their pituitary and thyroids and (Fig. S1l,n). Cyp1A1 transcript was increased also in the thyroid (Fig. S5b,d) and pituitary (Fig. S5f,h) of the offspring. Circulating fT4 was weakly reduced in wt mice exposed to TCDD, although the difference was statistically significant only in females (Fig. 4a,b, at right of dashed line). Slight but not statistically significant differences could be detected in body weight and Tsh mRNA expression in the pituitary in wt mice (Fig. 4c–f, at right of dashed line). The reported results suggested that the figured exposure plan might highlight the role of genetic factors, predisposing to thyroid dysfunction, in worsening TCDD effects (i.e. the haploinsufficiency of either Pax8 or Nkx2-1). Two months old wt females were mated with males carrying the heterozygotic loss of function mutation of Pax8 and Nkx2-1 gene. Pregnant dams and offspring were exposed as reported above. Pups were genotyped at PND15 in order to conduct the phenotypic and molecular analysis of the TCDD activity considering genotypes and sex as important factors influencing thyroid dysfunctions.
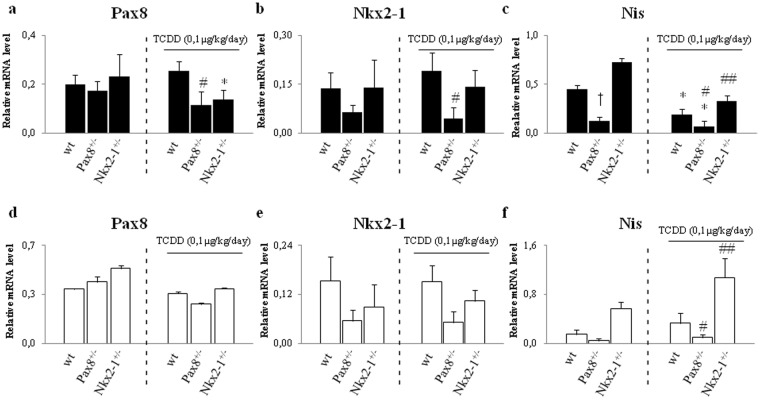
Circulating fT4 was reduced in statistically significant manner in both TCDD-Pax8+/− and TCDD-Nkx2-1+/− males versus (vs) TCDD-wt mice (Fig. 4a). Although TCDD exposure had an effect on the body weight, it was influenced by the genotype only in TCDD-Nkx2-1+/− males vs TCDD-wt mice (Fig. 4c). Surprisingly, we observed less pronounced TCDD effects in females. TCDD exposure resulted in the reduction of circulating fT4 in TCDD-Pax8+/− females vs TCDD-wt (Fig. 4b) but it did not affect the body weight at the sacrifice (Fig. 4d). None clear effect could be detected in pituitary level of Tsh mRNA (Fig. 4e,f), even in males in which the reduction of circulating fT4 was statistically significant. In order to molecularly characterize the effects of TCDD exposure, we analyzed the expression of thyroid specific transcripts and their upstream regulators in exposed animal of each genotype and sex. Focusing on the effect of the exposure, the data revealed that Pax8 transcript was reduced in TCDD-Pax8+/− males vs TCDD-wt mice (Fig. 5a) whereas Nkx2-1 showed a trend towards the down-regulation in the TCDD-males of both genotypes, reaching the statistical significance only in the TCDD-Pax8+/− males vs TCDD-wt mice (Fig. 5b). Concordantly, the inhibition of Tg was statistically significant only in TCDD-Pax8+/− mice of both sexes vs TCDD-wt whereas Tpo was inhibited in TCDD-Pax8+/− males (see Supplementary Fig. S3e,f). Nis mRNA was strongly inhibited in the TCDD-Pax8+/− mice vs TCDD-wt whereas it was induced in TCDD-Nkx2-1+/−, both in males and females (Fig. 5c,f).
Figure 5.
Haploinsufficiency of Pax8 or Nkx2-1 exacerbates the effects of TCDD treatment. Expression of thyroid specific transcripts in unexposed or 0,1 μg/kg/day TCDD exposed mice (from E15.5 to PND60) of each genotype. Thyroid Pax8, Nkx2-1 and Nis transcripts were assayed by RT-qPCR in C57BL/6 wild type (wt), Pax8+/− and Nkx2-1+/− (at left of the dashed lines) and in TCDD-wt, -Pax8+/− and -Nkx2-1+/− (at right of the dashed lines), males (a,b and c) and females (d,e and f). Data are reported as means ± SD of Gapdh normalized-mRNA levels; n = 4 mice for each group. *p-value < 0.05; **p-value < 0.01 for statistical analysis of TCDD-exposed vs untreated mice having the same genotype; #p-value < 0.05; ##p-value < 0.01 TCDD-exposed vs TCDD-wt mice; †p-value < 0.05 unexposed mice of different genotype vs wt mice.
Overall, the data highlighted the interplay between TCDD, genetic factor and sexes and defined them as factors influencing the response to the TCDD exposure.
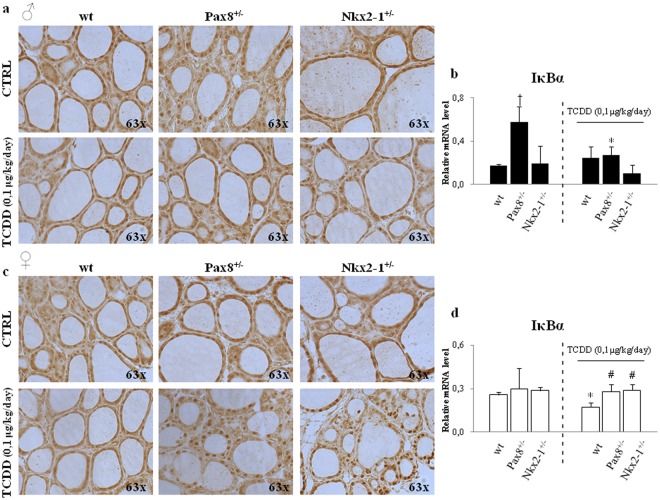
Then, we analyzed the p65 nuclear localization by immune-histochemistry (Fig. 6a,c) and immunofluorescence (see Supplementary Fig. S6a,b) and the expression of its target IκBα (Fig. 6b,d). This transcript was regulated in sex dependent manner by TCDD exposure, being inhibited by TCDD exposure in wt exposed females (Fig. 6d) and showing a trend towards the up-regulation in exposed wt males (Fig. 6b). IκBα was not regulated by TCDD in Pax8+/− and Nkx2-1+/−. The data were corroborated by the analyses of p65 localization by immune-hystochemistry showing that in TCCD-Pax8+/− and TCDD-Nkx2-1+/− females it was localized in the nucleus similarly to unexposed females of the same genotype and more than in TCDD-wt females. These lasts presented a reduced nuclear staining for p65 vs the unexposed wt-females. These last data might explain the milder effect of TCDD exposure in females. As expected, no changes were detected in Tp53 mRNA levels (data not shown).
Figure 6.
Activation of NF-κB pathway in Pax8+/− or Nkx2-1+/− exposed mice. (a,c) Immune-peroxidase analysis of p65 protein in thyroids of CTRL wt, Pax8+/− and Nkx2-1+/− and of TCDD-wt, -Pax8+/− and -Nkx2-1+/−, males (a) and females (c) (n = 4 mice for each group and sex). Negative control was performed using purified IgG and it is shown in Fig. S3h. (b,d) RT-qPCR of IκBα gene in thyroid of C57BL/6 wild type (wt), Pax8+/− and Nkx2-1+/− (at left of the dashed lines) and in TCDD-wt, -Pax8+/− and -Nkx2-1+/− (at right of the dashed lines), males (b) and females (d). Data are reported as means ± SD of Gapdh normalized-mRNA levels; n = 4 mice for each group and sex. *p-value < 0.05 for statistical analysis of TCDD-exposed vs untreated mice having the same genotype; #p-value < 0.05 TCDD-exposed vs TCDD-wt mice; †p-value < 0.05 unexposed mice of different genotype vs wt mice.
Overall, the above reported results underlined that foetal exposure to TCDD resulted in thyroid function impairment whose strength was genotype- and sex-dependent. Indeed, the TCDD exposure was worsened in genetic context characterized by haploinsufficiency of Pax8+/− or Nkx2-1+/−, genetic conditions, associated with hypothyroidism in humans.
Discussion
Although neonatal hypothyroidism has been associated with maternal exposure to TCDD in rodents, this suggestion is strongly debated in humans. The question has been reintroduced by a recent paper describing that the maternal exposure to TCDD resulted in a long-lasting, neonatal thyroid dysfunction in the highly exposed population of Seveso (Italy)15,34. In the 1976, approximately 30 kg of TCDD were released in area surrounding a trichlorophenol manufacturing plant in Seveso (Italy), determining the highest known exposure to TCDD in a residential population. The accident was monitored since the beginning to investigate the effects of TCDD exposure on hormonal axis at different ages. The women were followed through the Seveso Women’s Health Study, revealing that the serum TCDD levels were inversely correlated with total T4 levels in women exposed before the menarche in this population15. These results underscored that the impact on thyroid function of TCDD needs to be investigated with the aim to translate better the results from rodents to humans. This would imply both the consideration of realistic scenario of exposure, characterized by low-dose and foetal exposure of genetically different individuals, and the identification of thyroid molecular targets of TCDD activity that can be verified cross-species35. Here, we tried to address both aspects.
The possible impact of foetal TCDD exposure on thyroid function has been suggested since long time because the cleft palate36, a malformation associated to congenital hypothyroidism37, was recognized as a common feature of high-dose TCDD foetal exposure in C57BL/6 mice. Despite that, the TH status in exposed animals has not been specifically investigated, even in recent studies reporting the molecular mechanisms of TCDD activity38. Although very early foetal exposure (E0.5) to low-dose TCDD did not damage thyroid development, here we report its effects on the thyroid function evidenced by the reduction of circulating fT4 and body weight as well as the increased transcription of Tsh in pituitary in all the exposed mice. The observed effects on the pituitary are in agreement with previous report describing the TCDD impact on pituitary hormone transcripts, even if the Authors analyzed the effects on the hypothalamic–pituitary–gonadal axis (HPG axis) in different exposure settings33. Here we further suggest that the TCDD-induced thyroid dysfunction is mechanistically associated to effects, sometimes slight, on the transcription of several thyroid specific genes. As we have previously proposed for other EDCs, they might cumulatively promote the phenotypic effects in vivo27,34. Some molecular results underscore the peculiarity of TCDD exposure: i.e. the unexpected divergence between the Tsh transcript increase and Nis mRNA inhibition not involving Tg and Tpo mRNAs, whose transcription is also dependent by TSH. This suggests that TCDD alters a mechanism mediating specifically the regulation of Nis expression by TSH.
Considering that the pituitary/thyroid feedback loop complicates mechanistic analysis in vivo, we performed part of our study on FRTL-5, a cellular model different from the ones already adopted20,39. The reported data agreed with previous results that showed the inhibition of Nis transcript in primary thyrocytes exposed to TCDD, associated to activation of the AhR signalling. Using bioinformatics analysis, we failed in finding AhR binding sites in rat or mouse Nis promoter (data not shown). This suggested us that other transcriptional regulators enriched (Pax8, Nkx2-1, etc) or not (NF-κB, etc) in thyroid could have a role in mediating the TCDD thyroid effects. The thyroid gene expression is controlled by their specific combination, established by the respective promoters/enhancers organization21,40. Their exact combination is required to induce/inhibit gene expression. Here, we propose the role of p65 and Pax8 in the regulation of Nis expression by TCDD not excluding the AhR signalling, indeed activated in all the different exposure conditions as stated by up-regulation of Cyp1a1 transcript. Activated AhR might contribute to p65 nuclear activation25 following TCDD exposure. Indeed, both proteins negatively regulate Nis expression in oxidative conditions, such as the iodide-induced ROS production, the last induced by TCDD in FRLT-5. The here retrieved p65 activation and IκBα overexpression has been positively associated to the increase of Nkx2-1 transcript in lung41. These proteins with p53 participate to the Nkx2-1/p53/p65/IκBα pathway involved in lung carcinogenesis30. Here, we show that the same pathway is activated in TCDD-exposed FRTL-5 and in mice, although partially, suggesting a possible mechanism of TCDD-induced thyroid carcinogenesis in human42 and rodents43. Furthermore, the reported results underline that longer exposure times are required to model in vitro the in vivo response to EDCs, as we have previously shown34.
The in vivo data point to the sex as a factor influencing the response to TCDD. This is not a novelty as a sex dependent modification of liver transcriptome by TCDD has been reported44. Here, we show sex related differences in the TCDD regulation of thyroid-enriched mRNAs as well as of the involved molecular mechanisms. Indeed, Nis transcript could not be induced in TCDD-males because of the p65 inhibition whereas in TCDD-females because Pax8 and Nkx2-1 transcripts were not induced. Overall the in vivo and in vitro data suggest a direct, sex and time dependent impairment of Nkx2-1/p53/p65/IκBα axis by TCDD in thyrocytes. As said, its activation was partially confirmed in vivo suggesting that the longer exposure time applied in mouse might promote the compensation processes needed for surviving (i.e. inhibition of Tp53 transcription, to allow the cell proliferation, or inflammation block). Hence, longer exposure times or higher TCDD dose could be needed for a stable activation of Nkx2-1/p53/p65/IκBα pathway in vivo. Both conditions characterize the accidental human exposure already associated to the increased incidence of thyroid cancer45. Its activation during the foetal life might promote the low-dose induced TCDD thyroid carcinogenesis46.
Evidences of long-term and low-dose effects of TCDD on human thyroid are debated. The contrasting results might be largely attributed to the genetic variability in human population and to the impossibility to control precisely the window, the route and the dose of exposure. It was already assumed that genetic factors influenced the response to TCDD because it was differently developed up to the strains adopted, being the C57BL/6 TCDD-sensitive and DBA resistant47. Heterozygous mutations of thyroid enriched master regulators, such as Pax8 and Nkx2-1, have been associated to different grades of human hypothyroidism. Environmental factors could concur to exacerbation of their effects. The presented data corroborate this suggestion showing that TCDD exposure induces a thyroid dysfunction stronger in Pax8+/− or Nkx2-1+/− males vs wild type mice at phenotypic and molecular level. Indeed, haploinsufficiency of Pax8+/− or Nkx2-1+/− resulted in the inability to restore the normal thyroid specific regulation, as shown for Tg or Nis transcripts. Furthermore, the genotype affected in sex dependent manner the impairment of the Nkx2-1/p53/p65/IκBα pathway promoted by TCDD exposure, corroborating the hypothesis that it is a player of TCDD action in mouse thyroid.
Conclusion
The reported in vivo data point to genetic factors in combination with the window of exposure and the sex as key players in the thyroid dysfunction following foetal exposure to low-dose TCDD. In vitro and in vivo evidences suggest the Nkx2-1/p53/p65/IκBα pathway, or its components, as mediator of TCDD effects on thyrocytes, here highlighted by the analysis of several molecular markers of thyroid activity. Thus, this report represents one of the few attempts to identify mechanisms of TCDD thyroid toxicity, showing the role of the in vitro studies in their identification.
Overall, the data here presented underline the complexity to dissect the effects of TCDD giving suggestion of the multiple factors, above all the single genotype, which should be considered in analysing them in humans and in animal models.
Methods
Cell culture and treatment
FRTL-5 cells were grown in Coon’s modified F12 medium (EuroClone) supplemented with 5% newborn bovine serum (HyClone Laboratories) and six hormones mixture48. Cells were exposed to TCDD (Chemical Research), dissolved in DMSO at a concentration of 100 μM then diluted at 1 × 10−9 M in culture medium. Controls were treated with vehicle alone. The ROS inhibitor, N-acetyl-L-cysteine (NAC, Sigma-Aldrich), was dispensed on the FRTL-5 cells at a concentration of 2 mM one hour before starting the co-treatment with TCDD 1 × 10−9 M, for 24 hours.
Isolation of nuclear/cytoplasmic protein fractions and immunoblotting
FRTL-5 cells were scraped with ice-cold PBS, collected by centrifugation and lysed in hypotonic buffer (20 mm HEPES pH 7.8, 10 mM KCl, 1 mM MgCl2, 1 mm PMSF, 0.1% Triton X-100, 20% Glycerol, protease and phosphatase inhibitor cocktail, Roche). Upon incubation on ice for 10 min, cytosolic fractions were prepared by spinning the samples for 1 min at 4 °C at 6000 g and subsequently clarified by centrifugation (13,000 g for 5 min at 4 °C). The nuclear pellets were firstly washed to remove any residual cytosolic contaminations and, then, lysed in nuclear lysis buffer (20 mm HEPES pH 7.8, 0.2 mm EDTA, 400 mm NaCl, 0.1%, 1 mm PMSF, 0.1% Triton X-100, 20% Glycerol, protease and phosphatase inhibitor cocktail). They were incubated for 20 min at 4 °C with gentle shaking and, finally, centrifuged for 5 min at 4 °C at 15,000 g.
Protein concentration was measured by Bradford assay (Biorad Protein Assay) but loaded on gels up to cell equivalent. Nuclear and cytosolic proteins from 1 × 106 cells were separated by SDS–PAGE and transferred onto nitrocellulose membrane. The following primary antibodies were used: rabbit anti-p65 (SantaCruz, sc-372, 1:1000 for p65 determination in nuclear extracts and 1:3000 for cytosolic proteins); mouse anti-β-tubulin (Sigma-Aldrich, 1:5000); rabbit anti-Topoisomerase I (Abcam-Ab109374, 1:1000). Horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) have been used for the detection usually diluted 1.5000. Only for the detection of cytosolic p65 it was used diluted 1:10000.
Animals and treatments
Animal experiments were performed in accordance with the European Council Directive 2010/63/EU following the rules of the D.Lgs 26/14 and procedures were approved by the Ethical committee of the Biogem Institute, Genetics Research “Gaetano Salvatore” (IRGS) and by the Italian Minister of Health (ID number 61-16).
The mice were kept under standard facility conditions and received water and standard diet (#1320, Altromin) or medicated food containing TCDD dosed at 0, 001 μg/kg/day and 0, 1 μg/kg/day (Altromin) “ad libitum”. Food and water consumption was monitored. To expose the animal since the conception, C57BL/6 dams were exposed, 7 days before the mating, to TCDD dosed at 0, 001 μg/kg/day by administration of medicated food. The offspring was exposed by the mothers from gestational day 0 (E0.5) to the weaning (PND21) and, then, by direct feeding till the sacrifice (PND30). In the experiment with genetically modified mice, C57BL/6 females were mated with males of the same strain carrying the double heterozygotic deletion of Pax8 (Pax8+/−)49 and Nkx2-1 genes (Nkx2-1+/−)50. Pregnant dams were feed with 0, 1 μg/kg/day TCDD from E15.5. The offspring was exposed through the mother till the weaning and, then, directly exposed by feeding till the sacrifice, at PND60. The consumption of food and water was recorded weekly for all the mice enrolled in the studies. All the animals were sacrificed by carbon dioxide inhalation. Organ of HPT-axis and blood were sampled and properly stored for further analysis.
fT4 determination and thyroid histology
Venous blood samples were collected in microtubes and centrifuged (5000 rpm for 5 min 4 °C). The serum fraction was kept at −80 °C. Free T4 (fT4) was measured using the fT4 ELISA kit (DiaMetra) following the manufacturer’s instructions. T4-conjugated HRP and TMB substrate system were used for quantification.
For microscopy, thyroids (1 lobe) were embedded in paraffin. 4 µm sections were stained with hematoxylin and eosin (Sigma-Aldrich) according to the manufacturer’s instructions. For immunoperoxidase, target retrieval was performed in TEG buffer pH 9.2, as already described51. Briefly, primary antibody anti-p65 (Abcam, ab7970, 1:500) was incubated overnight at 4 °C followed by incubation with the secondary anti-rabbit HRP-conjugated antibody (Dako, P0488, 1:200). Chromogenic reactions were carried out with DAB Peroxidase Substrate Kit (Vector Laboratories). For immunofluorescence analysis, thyroid sections were incubated overnight at 4 °C with anti-p65 primary antibody (Abcam, ab7970, 1:500). Sections were then incubated with secondary Alexa Fluor 488 antibody (Invitrogen); 1 μg/ml DAPI solution was used as nuclear counterstain. Zeiss Axioplan 2 microscope was used for images acquisition (20x and 63x magnification).
RNA extraction and RT-qPCR
Total RNA, from tissue and cells, was isolated with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription, primers design and qPCR were accomplished using QuantiTect Reverse Transcription Kit (Qiagen), NCBI Primer Blast and Fast SYBR Green Master Mix (Applied Biosystems with Applied Biosystem QuantStudio 7 Flex System) respectively, as already reported22. Primer sequences are listed in Supplemental Material (see Supplementary Table S1).
Data were normalized by the level of internal control Gapdh expression in each sample. The 2−∆∆Ct method was used to calculate relative expression changes.
Statistical analysis
Statistical analysis were performed using two-tailed Student’s t-test. Probability p-values < 0.05 were considered significant. Unless otherwise indicated, at least three independent experiments were considered for in vitro data and not less than four animals for each genotype and sex were used for in vivo analysis. The results are expressed as the mean ± standard deviation.
Electronic supplementary material
Acknowledgements
This work was supported by: the Italian Ministry of Health (grant n. RF 2010-2309484), Legge Regionale 5/02 (CUP: F82I14001270002), the Italian Workers’ Compensation Authority (grant. n. 12010), Sensor Regione Campania (grant n. 23). We thank IRGS (Biogem) for support and the mouse facility for the technical support, in particular Dr. Nicoletta Vito.
Author Contributions
M.D.F. and C.A. developed the rationale of the study; Massimo Mallardo, C.R., I.P., M.D.F. and C.A. wrote the manuscript, which was reviewed by all the authors; C.R. and I.P. performed the majority of the cell culture and mouse experiments with additional experimental contributions of F.R., M.M., L.R., N.A.R. and E.C. C.A. supervised all the experiments.
Data Availability Statement
The datasets generated and materials used during the current study are available from the corresponding author C.A. (email: coambros@unisannio.it) or M.D.F. (email: mario.defelice@ unina.it), on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reale Carla, Porreca Immacolata, De Felice Mario and Ambrosino Concetta contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34427-2.
References
- 1.Samollow PB, et al. Genetic and environmental influences on thyroid hormone variation in Mexican Americans. J Clin Endocrinol Metab. 2004;89:3276–84. doi: 10.1210/jc.2003-031706. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 3.De Felice M, Di Lauro R. Minireview: Intrinsic and extrinsic factors in thyroid gland development: an update. Endocrinology. 2011;152:2948–56. doi: 10.1210/en.2011-0204. [DOI] [PubMed] [Google Scholar]
- 4.Duntas LH. Chemical contamination and the thyroid. Endocrine. 2015;48:53–64. doi: 10.1007/s12020-014-0442-4. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842–52. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 6. Mulero-Navarro, S. & Fernandez-Salguero, P. M. New Trends in Aryl Hydrocarbon Receptor Biology. Front Cell Dev Biol4 (2016). [DOI] [PMC free article] [PubMed]
- 7.Xu B, et al. 2,3′, 4,4′,5 Pentachlorobiphenyl Induces Inflammatory Responses in the Thyroid Through JNK and Aryl Hydrocarbon Receptor-Mediated Pathway. Toxicol Sci. 2016;149:300–11. doi: 10.1093/toxsci/kfv235. [DOI] [PubMed] [Google Scholar]
- 8.Bahn AK, et al. N Hypothyroidism in workers exposed to polybrominated biphenyls. Engl J Med. 1980;302:31–3. doi: 10.1056/NEJM198001033020105. [DOI] [PubMed] [Google Scholar]
- 9.Goodman JE, Kerper LE, Boyce CP, Prueitt RL, Rhomberg LR. Weight-of evidence analysis of human exposures to dioxins and dioxin-like compounds and associations with thyroid hormone levels during early development. Regul Toxicol Pharmacol. 2010;58:79–99. doi: 10.1016/j.yrtph.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Schell LM, Gallo MV. Relationship of putative endocrine disruptors to human sexual maturation and thyorid activity in youth. Physiol Behav. 2010;99:246–53. doi: 10.1016/j.physbeh.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craft ES, DeVito MJ, Crofton KM. Comparative responsiveness of hypothyroxinemia and hepatic enzyme induction in Long-Evans rats versus C57BL/6J mice exposed to TCDD-like and phenobarbital-like polychlorinated biphenyl congeners. Toxicol Sci. 2002;68:372–80. doi: 10.1093/toxsci/68.2.372. [DOI] [PubMed] [Google Scholar]
- 12.Potter CL, Sipes IG, Russell DH. Hypothyroxinemia and hypothermia in rats in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin administration. Toxicol Appl Pharmacol. 1983;69:89–95. doi: 10.1016/0041-008X(83)90123-0. [DOI] [PubMed] [Google Scholar]
- 13.Van Birgelen AP, et al. Toxic potency of 3,3′,4,4′,5-pentachlorobiphenyl relative to and in combination with 2,3,7,8-tetrachlorodibenzo-p-dioxin in a subchronic feeding study in the rat. Toxicol Appl Pharmacol. 1994;127:209–21. doi: 10.1006/taap.1994.1155. [DOI] [PubMed] [Google Scholar]
- 14.McConnell EE. Comparative toxicity of PCBs and related compounds in various species of animals. Environ Health Perspect. 1985;60:29–33. doi: 10.1289/ehp.856029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chevrier J, et al. Serum dioxin concentrations and thyroid hormone levels in the Seveso Women’s Health Study. Am J Epidemiol. 2014;180:490–8. doi: 10.1093/aje/kwu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maranghi F, et al. Dietary exposure of juvenile female mice to polyhalogenated seafood contaminants (HBCD, BDE-47, PCB 153, TCDD): comparative assessment of effects in potential target tissues. Food Chem Toxicol. 2013;56:443–9. doi: 10.1016/j.fct.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 17.Van Esterik JC, et al. 2015. Compound- and sex specific effects on programming of energy and immune homeostasis in adult C57BL/6JxFVB mice after perinatal TCDD and PCB 153. Toxicol Appl Pharmacol. 2015;289:262–75. doi: 10.1016/j.taap.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Hutt Karla J, Shi Zhanquan, Albertini David F, Petroff Brian K. The environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin disrupts morphogenesis of the rat pre-implantation embryo. BMC Developmental Biology. 2008;8(1):1. doi: 10.1186/1471-213X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postiglione MP, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA. 2002;99:15462–7. doi: 10.1073/pnas.242328999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocar P, et al. AhR-agonist-induced transcriptional changes of genes involved in thyroid function in primaryporcine thyrocytes. Toxicol Sci. 2006;89:408–14. doi: 10.1093/toxsci/kfj042. [DOI] [PubMed] [Google Scholar]
- 21.Nicola JP, et al. S-Nitrosylation of NF-κB p65 Inhibits TSH-Induced Na(+)/I(−) Symporter Expression. Endocrinology. 2015;156:4741–54. doi: 10.1210/en.2015-1192. [DOI] [PubMed] [Google Scholar]
- 22.Gentilcore D, et al. Bisphenol A interferes with thyroid specific gene expression. Toxicology. 2013;304:21–31. doi: 10.1016/j.tox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Kikumori T, Kambe F, Nagaya T, Funahashi H, Seo H. Thyrotropin modifies activation of nuclear factor kappaB by tumour necrosis factor alpha in rat thyroid cell line. Biochem J. 2001;354:573–9. doi: 10.1042/bj3540573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voigt C, Prodinger C, Paschke R. The TSH receptor is linked with AP1 and NFkappaB signaling in COS7 cells. Exp Clin Endocrinol Diabetes. 2007;115:590–3. doi: 10.1055/s-2007-981453. [DOI] [PubMed] [Google Scholar]
- 25.Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor dependent nuclear translocationof NFkappaB and expression of Fas ligand in thymic stromalcells and consequent apoptosis in T cells. J Immunol. 2005;175:90–103. doi: 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- 26.Wan C, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS One. 2014;9:e89811. doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porreca I, et al. "Stockpile" of Slight Transcriptomic Changes Determines the Indirect Genotoxicity of Low DoseBPA in Thyroid Cells. PLoS One. 2016;11:e0151618. doi: 10.1371/journal.pone.0151618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, et al. Molecular mechanisms of 2,3′,4,4′,5-pentachlorobiphenyl induced thyroid dysfunction in FRTL-5 cells. PLoS One. 2015;10:e0120133. doi: 10.1371/journal.pone.0120133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coperchini F, et al. Exposure to perfluorinated compounds: in vitro study on thyroid cells. Environ Sci Pollut Res Int. 2015;22:2287–94. doi: 10.1007/s11356-014-3480-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen PM, Wu TC, Cheng YW, Chen CY, Lee H. NKX2-1 mediated p53 expression modulates lung adenocarcinoma progression via modulatingIKKβ/NF-κB activation. Oncotarget. 2015;6:14274–89. doi: 10.18632/oncotarget.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amendola E, et al. A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocrinology. 2005;146:5038–47. doi: 10.1210/en.2005-0882. [DOI] [PubMed] [Google Scholar]
- 32.Formosa R, Vassallo J. The Complex Biology of the Aryl Hydrocarbon Receptor and Its Role in the Pituitary Gland. Horm Cancer. 2017;8:197–210. doi: 10.1007/s12672-017-0300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda T, Taura J, Hattori Y, Ishii Y, Yamada H. Dioxin-induced retardation of development through a reduction in the expression of pituitaryhormones and possible involvement of an aryl hydrocarbon receptor in this defect: a comparative study using two strains of mice with different sensitivities to dioxin. Toxicol Appl Pharmacol. 2014;278:220–9. doi: 10.1016/j.taap.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Baccarelli A, et al. Neonatal thyroid function in Seveso 25 years after maternal exposure to dioxin. PLoS Med. 2008;5:e161. doi: 10.1371/journal.pmed.0050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porreca I, et al. Cross-species toxicogenomic analysis and phenotypic anchoring in response to groundwater low-level pollution. BMC Genomics. 2014;15:1067. doi: 10.1186/1471-2164-15-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CH, He W, Meng T, Shi B. Experimental study on protection of vitamin B6 on TCDD-induced palatal cleft formation in the mice. Birth Defects Res B Dev Reprod Toxicol. 2009;86:357–61. doi: 10.1002/bdrb.20203. [DOI] [PubMed] [Google Scholar]
- 37.De Felice M, et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19:395–8. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- 38.Gao L, Yin J, Wu W. Long non-coding RNA H19-mediated mouse cleft palate induced by 2,3,7,8 tetrachlorodibenzo-p-dioxin. Exp Ther Med. 2016;11:2355–2360. doi: 10.3892/etm.2016.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katarzyńska D, Hrabia A, Kowalik K, Sechman A. Comparison of the in vitro effects of TCDD, PCB 126 and PCB 153 on thyroid restricted gene expression and thyroid hormone secretion by the chicken thyroid gland. Environ Toxicol Pharmacol. 2015;39:496–503. doi: 10.1016/j.etap.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Okladnova O, et al. The genomic organization of the murine Pax8 gene and characterization of its basal promoter. Genomics. 1997;42:452–61. doi: 10.1006/geno.1997.4735. [DOI] [PubMed] [Google Scholar]
- 41.Londhe VA, et al. NF-kB induces lung maturation during mouse lung morphogenesis. Dev Dyn. 2008;237:328–38. doi: 10.1002/dvdy.21413. [DOI] [PubMed] [Google Scholar]
- 42.Le KT, Sawicki MP, Wang MB, Hershman JM, Leung AM. High prevalence of agent orange exposure among thyroid cancer patients in the national va healthcare system. Endocr Pract. 2016;22:699–702. doi: 10.4158/EP151108.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, et al. Male and female mice show significant differences in hepatic transcriptomic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Genomics. 2015;16:625. doi: 10.1186/s12864-015-1840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pesatori AC, et al. Short- and long-term morbidity and mortality in the population exposed to dioxin after the "Seveso accident". Ind Health. 2003;41:127–38. doi: 10.2486/indhealth.41.127. [DOI] [PubMed] [Google Scholar]
- 46.Yoshizawa K, Heatherly A, Malarkey DE, Walker NJ, Nyska A. A critical comparison of murine pathology and epidemiological data of TCDD, PCB126, and PeCDF. Toxicol Pathol. 2007;35:865–79. doi: 10.1080/01926230701618516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umbreit TH, Hesse EJ, Macdonald GJ, Gallo MA. Effects of TCDD-estradiol interactions in three strains of mice. Toxicol Lett. 1988;40:1–9. doi: 10.1016/0378-4274(88)90177-4. [DOI] [PubMed] [Google Scholar]
- 48. Porreca, I. et al. Pesticide toxicogenomics across scales: in vitro transcriptome predicts mechanisms and outcomes of exposure in vivo. Sci Rep6 (2016). [DOI] [PMC free article] [PubMed]
- 49.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- 50.Kimura S, et al. The T/ebp null mouse: thyroid specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–9. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 51.Reale C, et al. NF-κB Essential Modulator (NEMO) Is Critical for Thyroid Function. J Biol Chem. 2016;291:5765–73. doi: 10.1074/jbc.M115.711697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and materials used during the current study are available from the corresponding author C.A. (email: coambros@unisannio.it) or M.D.F. (email: mario.defelice@ unina.it), on reasonable request.