Abstract
The use of optical coherence tomography (OCT) has been suggested as a potential biomarker for Alzheimer’s Disease based on previously reported thinning of the retinal nerve fiber layer (RNFL) in Alzheimer’s disease’s (AD) and Mild Cognitive Impairment (MCI). However, other studies have not shown such results. 930 individuals (414 cognitively healthy individuals, 192 probable amnestic MCI and 324 probable AD) attending a memory clinic were consecutively included and underwent spectral domain OCT (Maestro, Topcon) examinations to assess differences in peripapillary RNFL thickness, using a design of high ecological validity. Adjustment by age, education, sex and OCT image quality was performed. We found a non-significant decrease in mean RNFL thickness as follows: control group: 100,20 ± 14,60 µm, MCI group: 98,54 ± 14,43 µm and AD group: 96,61 ± 15,27 µm. The multivariate adjusted analysis revealed no significant differences in mean overall (p = 0.352), temporal (p = 0,119), nasal (p = 0,151), superior (p = 0,435) or inferior (p = 0,825) quadrants between AD, MCI and control groups. These results do not support the usefulness of peripapillary RNFL analysis as a marker of cognitive impairment or in discriminating between cognitive groups. The analysis of other OCT measurements in other retinal areas and layers as biomarkers for AD should be tested further.
Subject terms: Retina, Alzheimer's disease
Introduction
Alzheimer’s disease (AD) is a complex neurodegenerative disease and the most common cause of dementia1. Clinical diagnostic criteria for AD do not discriminate with accuracy between different dementing etiologies2. Before the onset of dementia, cognitive disorders progress slowly with minor cognitive impairment and without significant interference in daily activities. This prodromal phase is known as mild cognitive impairment (MCI), a clinically heterogeneous syndrome whose definition has evolved in last years3–5 and can be due to many different etiologies (AD, vascular damage, depression,…). Although some MCI patients can remain stable for decades or even return to cognitive normality, it is well established that amnestic and multi-domain MCI condition increases the risk of progressing to AD6,7. Given the fact that diagnosis of AD is still complicated especially in the MCI stage, the search of inexpensive and noninvasive biomarkers is a promising area of research8. Even though some biomarkers have been validated and integrated into the new clinical diagnostic criteria9,10, most show suboptimal test accuracy and either are very expensive such as detection of Aβ and Tau deposits in the brain using positron emission tomography (PET) or fairly invasive such as measurement of tau protein and Aβ peptide levels in cerebrospinal fluid (CSF) analysis11,12
The retina is considered an anatomical protrusion of the central nervous system with same embryological origins. Unmyelinated axons of retinal ganglion cells form the retinal nerve fiber layer (RNFL) which prolongs as the optic nerve and connects to the lateral geniculate nucleus (LGN) in the thalamus, which serves as the first relay center of the visual pathway.
Color and contrast sensitivity impairment, worse depth and motion perception, visual field deficits and impaired visual acuity are often seen in AD patients13,14. Visual symptoms in AD are supposedly caused by damage to associative visual cortical areas15,16, however, there is mounting evidence that neuroretinal involvement could also be a contributing factor17 and this has spurred interest in the search for retinal AD biomarkers18,19. Numerous postmortem histopathological studies reported RNFL and ganglion cell layer (GCL) reduction in AD patients18,19 although others demonstrated disparate results20–22.
Optical coherence tomography (OCT) is a relatively inexpensive, innocuous, quick transpupillary technique that permits in vivo objective retinal measurements and quantification. OCT is routinely used in ophthalmology to evaluate retinal integrity through high-resolution cross-sectional scans of retinal layers such as the RNFL and CGL at different locations such as the macula or papilla23,24. OCT is widely used in clinical ophthalmology and is a promising tool for neurological research25 due to its good reliability in a variety of Central Nervous System (CNS) pathologies26–28 and high correlation with several visual electrophysiological techniques29,30.
Peripapillary RNFL thinning is the most common finding in many neurological conditions such as multiple sclerosis, stroke, neuromyelitis optica, Lewy Body Dementia, Parkinson’s disease and AD31–33. It has been postulated to occur due to retrograde degeneration of the retinal ganglion cell axons or retinal deposits of AD pathology. Of note, classical histological studies18,19 have not found beta-amyloid plaques or neurofibrillary tangles in the retina of AD patients but more recent studies claim to detect them34–36.
Studies on peripapillary RNFL are inconclusive as they are based on small size samples and show important methodological heterogeneity37–39 and discrepant results as will be discussed in further sections. Some studies show marked RNFL thinning affecting all retinal quadrants in patients with AD and MCI13,40–43, whereas others do not26,44–46.
Our study aims to assess the clinical usefulness and feasibility of collecting RNFL thickness using OCT in a memory unit (MU) from a large, consecutively recruited cohort.
Results
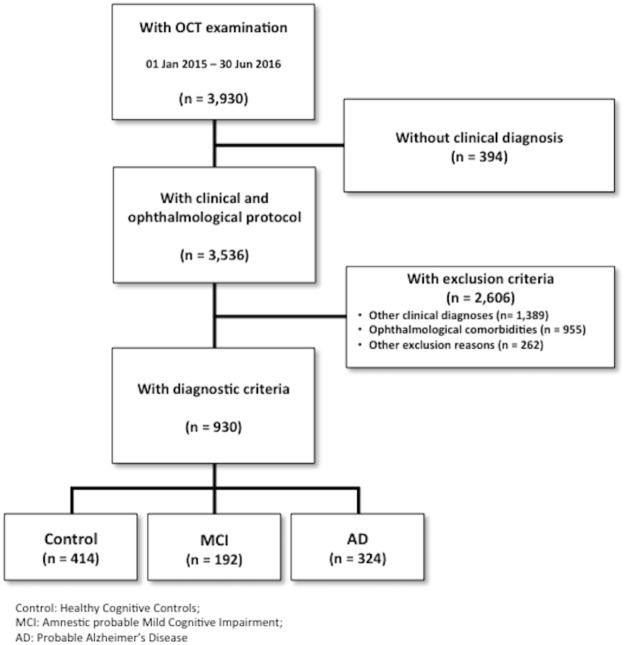
Figure 1 depicts the participant algorithm selection. 3,930 subjects attending a MU from January 2015 to July 2016 were invited to take part in this study and underwent a complete ophthalmological examination. 3,536 individuals (90%) received clinical diagnosis. 955 (24.4%) individuals out of 3,930 patients were discarded owing to several eye diseases (Table 1). Glaucoma and degenerative maculopathy were the main reasons for exclusion and their prevalence was especially high, especially among older and AD patients.
Figure 1.
Patient selection and study cohort flow chart. Eligible population and selection of the study sample for this study through inclusion and exclusion criteria.
Table 1.
Ophthalmological causes of exclusion.
| Causes | Number (% over excluded) | Prevalence in MU |
|---|---|---|
| Glaucoma | 292 (30.6) | 7,4% |
| Macular degeneration | 289 (30.3) | 7,3% |
| Amblyopia | 135 (14.1) | 3.4% |
| Intraocular pressure >24 | 62 (6.5) | 1.6% |
| Retinal surgery | 46 (4.8) | 1.1% |
| OCT artifacts | 39 (4.1) | 1.0% |
| Myopia magna | 36 (3.8) | 0.9% |
| Optic neuropathies | 18 (1.9) | 0.5% |
| Ocular injury | 6 (0.6) | 0.2% |
| Other causes | 61 (6.4) | 1.6% |
| Unknown | 72 (7.6) | 1.8% |
955 out of 3,930 participants were excluded because of ophthalmological causes. Some subjects met more than one ophthalmological exclusion criterion.
After the comprehensive application of inclusion and exclusion criteria depicted in detail below (see Methods), 930 (23.6%) participants in the cohort met all the inclusion criteria and none of the exclusion criteria: 414 subjects were in the control group, 192 in the MCI group and 324 in the AD group. Demographics are described in Table 2.
Table 2.
Baseline demographics.
| Mean | SD | Inter-groups Significance | ||
|---|---|---|---|---|
| Education (years) | Control | 10.96 | 4.13 | |
| MCI | 7.00 | 4.32 | ||
| AD | 6.14 | 4.08 | ||
| Total | 8.46 | 4.72 | <0.001+ | |
| Age (years) | Control | 65.93 | 9.01 | |
| MCI | 76.46 | 7.14 | ||
| AD | 78.99 | 7.87 | ||
| Total | 73.05 | 10.23 | <0.001+ | |
| MMSE (points) | Control | 29.29 | 1.00 | |
| MCI | 25.14 | 2.97 | ||
| AD | 20.28 | 3.98 | ||
| OCT Image Quality (%) | Control | 47.81 | 7.57 | |
| MCI | 44.59 | 8.23 | ||
| AD | 43.23 | 10.33 | ||
| Total | 45.41 | 9.09 | <0.001+ | |
| Gender (% women) | Control | 67.7% | ||
| MCI | 56.2% | |||
| AD | 74.0% | |||
| Total | 67.8% | <0.001* | ||
Demographic features like age, gender and education, MMSE scores, and OCT quality image among groups are summarized. All the analyzed characteristics were significantly different among diagnostic groups.
*Pearson’s Chi2 test.
+1-factor ANOVA.
There were significant differences between all three diagnostic groups in all demographical features (p < 0.001). Women made up most of the total sample and were significantly more prevalent in the AD than in the MCI group. Cognitively healthy individuals were younger and showed higher education levels, higher MMSE scores and a slightly better OCT image quality than both MCI and AD patients. The MCI group was younger and scored higher in the MMSE (Mini-mental State Examination) than the AD group.
Table 3 summarizes each covariate’s contribution to RNFL thickness variance including eta-squared and significance, after using ANCOVA.
Table 3.
Contribution of every covariate to variance of RNFL thickness.
| Covariate | Significance | *D.f. | Partial Eta2 |
|---|---|---|---|
| Education | 0.018 | 1 | 0.006 |
| Gender | 0.516 | 1 | 0.000 |
| Age | 0.0001 | 1 | 0.033 |
| OCT image quality | 0.0001 | 1 | 0.037 |
| Diagnosis | 0.352 | 2 | 0.002 |
Correlation between demographical and opthalmical covariates with the dependent variable is itemized.
*D.f.: Degrees of Freedom.
OCT image quality, followed by age and education turned out to be the most decisive factors for explaining variability of RFNL thickness and showed a very significant correlation and effect size. These three variables were more strongly associated with RNFL measurements than the diagnosis itself, whose correlation was not significant. The effect of gender was not significantly correlated with the peripapillary RNFL thickness.
Due to the differences in demographics between groups, we adjusted overall and sector-specific RNFL thickness in a multivariate model by the following covariates: OCT image quality, age, gender and education. No significant differences in mean overall (p = 0.352), temporal (p = 0.119), nasal (p = 0.151), superior (p = 0.435) and inferior (p = 0.825) RNFL thickness were found after comparing diagnostic groups. Our Mean and SD values are within the range of previously published studies.
Raw and adjusted data is displayed for mean overall (Table 4) and sector-specific (Table 5) RNFL thickness. A boxplot for mean overall RNFL thickness is represented (Fig. 2).
Table 4.
Results of mean RNFL in all diagnostic groups.
| Group (N) | Mean | SD | Meanaa | SEM | p value |
|---|---|---|---|---|---|
| Control (414) | 100.20 | 14.59 | 97.81aa | 0.82 | |
| MCI (192) | 98.54 | 14.43 | 99.86aa | 1.05 | |
| AD (324) | 96.61 | 15.27 | 98.88aa | 0.89 | |
| Total (930) | — | — | — | 0.352 |
Raw and adjusted mean overall RNFL thickness (μm), standard deviation (SD) and standard error of the mean (SEM). After a multivariate adjustment, no significance between any diagnostic groups appeared and dispersion data is shown as SEM.
SD: Standard Deviation; aaafter adjustment; SEM: Standard Error of the Mean; p: Significance; AD: Alzheimer’s Disease; MCI: Mild Cognitive Impairment.
Table 5.
Results of quadrants RNFL in all diagnostic groups.
| p value | Group (N) | Mean | SD | Meanaa | SEM |
|---|---|---|---|---|---|
| Temporal 0.119 | Control (413) | 72.76 | 12.43 | 71.44aa | 0.77 |
| MCI (192) | 72.46 | 13.73 | 73.24aa | 0.99 | |
| AD (324) | 72.82 | 14.63 | 74.04aa | 0.83 | |
| Total (929) | — | — | — | ||
| Superior 0.435 | Control (413) | 117.74 | 24.54 | 114.88aa | 1.34 |
| MCI (192) | 116.31 | 24.17 | 117.74aa | 1.73 | |
| AD (324) | 114.27 | 22.34 | 117.05aa | 1.45 | |
| Total (929) | — | — | — | ||
| Nasal 0.151 | Control (413) | 78.67 | 18.18 | 77.12aa | 1.00 |
| MCI (192) | 78.95 | 17.27 | 79.68aa | 1.29 | |
| AD (324) | 75.18 | 17.65 | 76.72aa | 1.08 | |
| Total (929) | — | — | — | ||
| Inferior0.82 5 | Control (413) | 131.80 | 21.97 | 128.06aa | 1.28 |
| MCI (192) | 126.34 | 21.82 | 128.53aa | 1.65 | |
| AD (324) | 123.82 | 25.25 | 127.28aa | 1.39 | |
| Total (929) | — | — | — |
Raw and adjusted sectorial RNFL thickness (μm), standard deviation (SD) and standard error of the mean (SEM). After a multivariate adjustment, no significance between any diagnostic groups appeared and dispersion is shown as SEM.
SD: Standard Deviation; aaafter adjustment; SEM: Standard Error of the Mean; p: Significance (missing data: 1); AD: Alzheimer’s Disease; MCI: Mild Cognitive Impairment.
Figure 2.
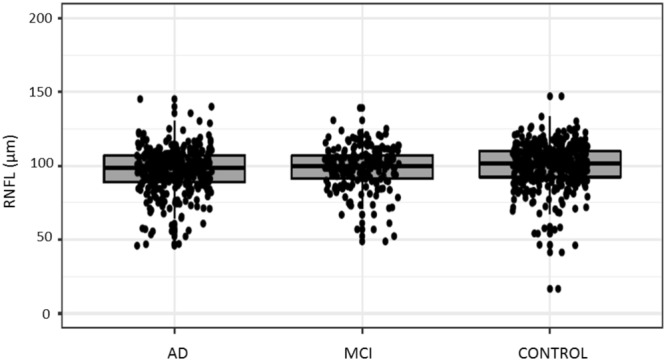
Mean RNFL for each diagnostic group. This boxplot represents the mean RNFL for each diagnostic group (Control, MCI, AD). No differences were found between clinical categories. The bottom and top of the box are the first and third quartiles and the band inside the box represents the median. AD: Alzheimer’s Disease; MCI: Mild Cognitive Impairment.
Since previous studies showing significant differences in RNFL thickness compared data between age and gender matched groups instead of using multivariate regression, we performed a sensitivity analysis in some age and gender groups at different age intervals to verify that the observed lack of significance in RNFL reduction between diagnostic groups was not due to this methodological difference. No significant differences were obtained by comparing age and gender matched groups (Table 6).
Table 6.
Example of mean RNFL thickness in all diagnostic groups using a matching strategy for age.
| Group (N) | Women (%) | p value | Age (years) | SD | p value |
|---|---|---|---|---|---|
| Control(85) | 55.3 | 74.48 | 2.87 | ||
| MCI (84) | 58.3 | 74.55 | 2.64 | ||
| AD (63) | 63.5 | 74.81 | 2.61 | ||
| Total (232) | 58.6 | 0.60 | 74.62 | 2.70 | 0.99 |
| Group (N) | p RNFL | SD | p value | ||
| Control (85) | 97.36 | 13.03 | |||
| MCI (84) | 100.44 | 15.10 | |||
| AD (63) | 99.88 | 12.33 | |||
| Total (232) | 99.16 | 13.65 | 0.185 |
Mean age (years), mean peripapillary RNFL thickness (μm) and standard deviation (SD) are shown. Age (p = 0.99) and gender (p = 0.60) do not differ among all diagnostic groups. Changes in RNFL thickness between any diagnostic groups were not significant (p = 0.185).
SD: Standard Deviation; p: Significance; p RNFL: Peripapillary RNFL; AD: Alzheimer’s Disease; MCI: Mild Cognitive Impairment.
Discussion
In contrast to some previous studies, we have not found significant differences in peripapillary RNFL thickness between cognitively healthy subjects, MCI and AD patients. However, previous literature on this matter is discrepant and inconclusive so far. Most publications showed that peripapillary RNFL thinning might affect all quadrants in the AD as well as in the MCI stage and correlates with functional measurements of visual pathways13,40–43. However, other recent studies do not show a marked reduction of disc RNFL thickness26,44–46. Such discrepancies may be due to several reasons such as lack of a strong involvement of the RNFL in AD, and, therefore, the effect is not detectable.
Due to the difficulty to recruit very old cognitive controls and very young people with dementia, the examined age interval in previous literature was very reduced (mean age between 70 and 80 years). Thus, cognitive impairment happening at very early or very advanced ages was not taken into account and it is known that rates of cognitive decline vary depending on the age47. Other important factors affecting cognition such as education or affecting measurements such as OCT image quality were seldom studied in prior investigations. Our study tests both of these key covariates and in all age intervals showing that OCT image quality appears to be the most influential covariate determining peripapillary RNFL variability, followed by age. OCT signal strength is considered as a proxy of OCT image quality. Its value ranges from 0 (minimal) to 1 (maximal) and is given automatically by the software, after each acquisition. Controls got higher OCT signal than AD patients. This parameter also appeared to be much more influential in OCT protocols assessing disc than macula possibly because the disc protocol requires excentrical fixation and therefore it is more demanding for the cognitively impaired patients than for healthy controls whose attention is better. It is also possible that cognitively impaired patients have more subclinical corneal or lens opacities than healthy individuals, even after having excluded all the subjects with evident ocular etiologies, due to the known comorbidity between AD and degenerative maculopathies48,49. Thus, we consider that image quality values should always be included in future research when studying the optic disc using OCT.
We have conducted a cross-sectional study using a large sample representative of clinical practice in a wide range of ages, cognitive stages and medical etiologies in order to enhance ecological validity. Most patients from our cohort were able to collaborate during OCT performance and this fact supports the feasibility of OCT assessment in a memory clinic setting. However, 24.4% of eligible subjects were excluded due to ophthalmological comorbidities, mainly glaucoma and macular degeneration. Both are diseases associated to age and different authors consider that both diseases share some pathophysiological underlying mechanisms with AD48,49 and it could partly explain the high prevalence observed in our cohort. The fact that these retinal pathologies also affect RNFL thickness might make the application of OCT as biomarker in a memory clinic more complex.
We consider our study counts with various strengths compared to previous investigations that study the retina in AD. Unlike other studies, where diagnostic criteria for cognitive normality and impairment are not detailed or only based on simple screening tests, we applied a comprehensive and standardized diagnostic protocol including extensive neuropsychological testing and neuroimaging biomarkers, as shown in the Methods. Most studies in the past were case-control designs based on small, hyperselected and underpowered sample sizes37–39. Their methods and results were very heterogeneous, given the fact that some studies included only one eye per patient whereas others included both, that different OCT techniques and brands were used, etcetera. Heterogeneity between studies can be measured through the Higgins I2 test50. I2 was calculated in three meta-analysis37–39 showing values over 95%, indicating an extreme lack of homogeneity that makes comparison difficult. Inclusion of participants in previous studies was not consecutive and investigators were not blinded to the clinical diagnosis before OCT execution leading to important risk of bias and overestimating test accuracy51,52. We took special care to avoid these limitations, by including every consecutive patient attending the MU, independently of their clinical presentation, education and age. The cohort of this study is the largest sample size collected in a single site so far. To avoid bias, clinician and optometrist were blinded to all procedures carried out in the same patient by the other. In our results, peripapillary RNFL is not discriminating enough between cognitive groups and its normal variability due to ageing and other factors might be fairly more pronounced than the variability hypothetically due to active neurodegeneration. Our results do not support the usefulness of RNFL thickness as biomarker of cognitive impairment in a MU.
We also acknowledge several limitations of this study. First of all, the results are only cross-sectional, therefore no conclusions can be drawn about the dynamics of RNFL thinning. We envision a longitudinal analysis to elucidate the RNFL changes over time. Second, standard deviation of RNFL measurements showed an important inter-individual variability within each group, similar to the one observed in some population-based or normative studies53,54. However, this higher variability may be related to the fact that the age range in our population is less restricted than in the case-control studies previously mentioned.. Third, given the heterogeneity of clinical and visual symptoms in AD, it may be possible that there are certain subgroups of AD patients with significant lesions of the visual system and this fact could contribute to explain observed discrepancies in the literature45. Fourth, the covariates considered in our model might not be sufficient to control for inter-group variability, as we did not consider important eye parameters that might be confounding factors such as axial length and optic disc area, among others53.
Although controversial, the peripapillary RNFL thinning is the most published retinal discovery in AD. However, many other OCT findings such as reduction in macular volume, ganglionar cell layer thickness, choroid width and some vascular alteration have been described and might be promising biomarkers for dementia staging and AD progression55–59. In fact, it is possible that retinal AD biomarkers can only be achieved after having integrated various of the already cited biomarkers, both neuroretinal (such as RNFL, CGL, macular width) and retinovascular parameters (vessel morphology among others), in a composite biomarker. In any case, rapid advancements both in OCT technology such as automatic segmentation and in biomarkers in PET/CSF to improve diagnostic certainty60,61, can give us a better insight into relationships between brain and eye.
Methods
Participant selection and characterization: the NORFACE cohort
The Neuro-Ophthalmology Research At Fundació ACE (NORFACE) research cohort was founded to search for retinal biomarkers of AD and examine the intriguing relationships between retinal pathophysiology and different types of neurodegenerative disease that cause cognitive impairment including AD, frontotemporal dementia, Lewy body dementia, Parkinson’s Disease and vascular dementia among others. It is characterized by (a) prospective and consecutive recruitment in a memory clinic (b) extensive neurological, neuropsychological and socio-functional evaluations (c) a complete neuro-ophthalmological evaluation including a OCT scan, (d) consensus-based clinical diagnosis made by a multidisciplinary team (e) a longitudinal periodical clinical and neuro-ophthalmological reassessment. Fundació ACE-Institut Català de Neurociències Aplicades, Barcelona, Spain has developed a multidisciplinary approach to diagnose and care for patients with neurodegenerative diseases62 based on standardized neuropsychological and medical examinations63,64. Patients are referred to Fundació ACE-Memory Unit (FACE-MU) by primary care physicians or medical specialists of the Catalan Public Health System and undergo cognitive and psychological screening, including the Mini-Mental State Examination (MMSE)65,66, the 7-minute tests67 and the Hospital Anxiety and Depression Scale (HAD)68. Afterwards, a comprehensive neuropsychological battery called NBACE is administered. NBACE counts with normative data and includes tests sensitive to orientation, memory, language, executive, visuoconstructive, visuospatial and visuoperceptive functions63,64.
Blood tests comprising syphilis screening, liver and renal function, cholesterol profile, thyroid function and serum vitamin B12 and folate levels are analyzed to exclude possible causes of dementia. Brain atrophy, in particular medial temporal lobe (MTL) involvement is assessed by structural MRI and CT to improve diagnostic certainty69. For some selected individuals PET or CSF data is also available60,61. After the neurologist’s and neuropsychologist’s clinical examination made by the neurologist, a consensus-based diagnosis is reached by a multidisciplinary team (neurologist, neuropsychologist, social worker).
Since January 2015 all new and follow-up patients attending the FACE-MU who are able to collaborate and understand instructions, undergo a complete a neuro-ophthalmological history and examination always by the same optometrist, before reaching the definite clinical diagnosis. The assessment includes (i) ophthalmological history, (ii) best-corrected visual acuity assessment by subjective refractometry using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, (iii) slit lamp examination was only used if the optometrist considered that biomicroscopy/ophthalmoscopy would be useful based on the medical history or on any unexpected finding seen in OCT scans, (iv) intraocular pressure measurement by Icare tonometry (v) and spectral domain OCT examination.
The optometrist and neurologist were blinded to all data from each other evaluation.
Optical Coherence Tomography
Patients were imaged with a 3D-OCT Maestro®, Fast Map software version 8.40 (Topcon Co. Tokio, Japan) to assess macular and optic disc regions. Importantly, no pupil dilatation was required because of high resolution Bscan mode which also allows to get also CScan confocal imaging through the EnFace software. The OCT capture is combined with a real color fundus picture obtained through an internal camera.
Retina layer segmentation was performed using the TABS (Topcon Advanced Boundary SegmentationTM) algorithm as part of the Fast Map software. TABS provide accurate and consistent measurements on retina images, improving reproducibility and accuracy together with great stability in performance across blood vessel shadows and it has proved effectiveness in ophthalmological diseases70,71. It also provides multi-layer delineation in one aggregate operation including the internal limiting membrane (ILM), nerve fiber layer (NFL), ganglion cells layer (GCL) boundary, inner plexiform layer (IPL), inner nuclear layer (INL) boundary, retinal pigment epithelium (RPE) and Bruch’s membrane (BM). NFL (from ILM to NFL/GCL), GCL+ (from NFL/GCL to IPL/GCL) and total retinal thickness (from ILM to RPE) was automatically generated by TABS. RNFL parameters evaluated in this study were average or mean overall thickness (360° measurement), temporal quadrant thickness (316–45°), superior quadrant thickness (46–135°), nasal quadrant thickness (136–225°) and inferior quadrant thickness (226–315°). For this study, OCT data was only analyzed from one eye (right). After finishing a OCT imaging session, the same optometrist screened all images searching for abnormalities and sent all images to a consultant ophthalmologist, an expert in retinal pathology, who reviewed them all, one by one and made a diagnostic report.
Eligibility criteria
Patients were included if they were between 50 and 95 years of age and met control, MCI or AD diagnostic criteria described in continuation. The control group was defined by (a) absence of significant symptoms (CDR = 0) and (b) a normal age, gender, and education-adjusted performance on NBACE63,64. The MCI group was defined by (a) meeting Petersen criteria for amnestic MCI72 and (b) absence of significant signs of cerebrovascular or psychiatric disease. The last criterion was applied as described in previous publications6,73 to increase the probability of AD etiology. All subjects in the AD group met National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS- ADRDA) criteria for probable AD74. Note that the chosen inclusion criteria require the absence of other diseases capable of producing similar symptoms, resulting in a study cohort with high probability of “pure” AD etiology. In other words, patients with MCI or dementia and signs of non-AD etiologies (even if AD was also thought to be present, such as in cases of mixed AD and non AD etiology) were excluded to avoid confounding effects on the analysis of the relationship between RNFL thickness and AD-spectrum groups.
Patients were excluded if they could not understand or collaborate in the neuro-ophthalmological evaluation, if there was only data obtained from the left eye or presence of OCT artifacts or diseases that could affect OCT measurement such as glaucoma, maculopathies, neuropathies, prior retinal surgery, an intraocular pressure ≥ 24 mmHg, high myopia (<−6Dp), or hyperopia (>+6Dp), congenital abnormalities of the optic nerve. These excluded patients usually have low OCT signal strength but this parameter was not an exclusion criteria by itself.
Ethical considerations
This study and its informed consent were approved by the ethics committees of both the Hospital Clínic I Provincial and the Hospital Vall D’Hebron, (Barcelona, Spain) in accordance with Spanish biomedical laws (Law 14/2007, July 3rd, about biomedical research; Royal Decree 1716/2011, November 18th) and followed the recommendations of the Declaration of Helsinki. All the participants signed the informed consent.
Statistical analysis
Statistical analyses were performed using IBM SPSS 20 (SPSS Inc., Chicago, IL) and according to APOSTEL guidelines75. All data were examined for normality, skew, and restriction of range. All quantitative variables were normally distributed (Fig. 3). The results are presented as mean ± SD for quantitative variables, range and number and percentage for categorical variables.
Figure 3.
Mean RNFL distribution for all the diagnostic groups. AD: Alzheimer’s Disease; MCI: Mild Cognitive Impairment.
Chi-Squared test and parametric Student t-test were used to compare the demographic characteristics, clinical diagnoses and OCT measurements. The primary analysis of the study, assessing differences in the RNFL between subgroups adjusted by age, education, gender and image quality was tested with ANCOVA, with mean overall RNFL or sector-specific RNFL thickness (the sectorial areas were included in the same multivariate analysis, according to its correlated profile), as dependent factors and clinical groups as independent factors (three categories). Age, gender, years of education and OCT image quality were included in the model as adjustment variables. Eta2 was calculated for every factor of the model, obtaining the explained variance of all the predictors. A significant effect was considered when p < 0.05.
Acknowledgements
This study was supported in part by funds from Fundació ACE, Institut Català de Neurociències Aplicades. Fundació ACE researchers are indebted to Trinitat Port-Carbó and her family who are supporting Fundació ACE scientific programs. The authors thank to Fundació ACE staff, patients and their families, without whose contribution this work would not have been possible. It was also funded by donations of “La Nit de l′Alzheimer”, a charity dinner initiative that took place on the 8th of August 2016 in the frame of the International Festival de Peralada. The OCT MAESTRO device was acquired with a grant EFSD/Lilly Mental Health and Diabetes 2013 Program of the European Foundation for the Study of Diabetes (EFSD), given to the project “Retinal neurodegeneration in type 2 diabetes as biomarker of Alzheimer’s disease”, under leadership of Dr. Cristina Hernández. We also want to thank TOPCON for its reliability as technological partnership. The present work was performed as part of Domingo Sánchez Ruiz’ doctoral program with title “Clinical Utility of OCT in diagnosing and monitoring of cognitive impairment” in the Department of Surgery and Morphological Sciences at Universitat Autonoma Barcelona (Barcelona, Spain).
Author Contributions
D.S.R. and M.C.M. have equally contributed to this work. This study was conceived by D.S.R. and A.R. Data were acquired, prepared, processed, or managed by J.S., J.M., and D.S.R. Ophthalmological screening was performed by M.C.M. Statistics were conceived and data were analysed by S.V. The manuscript was written by D.S.R. Data was interpreted by D.S.R., A.R., S.V., M.C.M. and O.R.G. The manuscript was critically revised by A.P., G.M., S.M.G., B.H.O., I.D.R., I.H., C.A., M.R.R., L.V., A.M., M.A.S.S., M.A., G.O., A.E., A.P.C., A.S., A.C., R.S., C.H., P.V., A.R., L.T. and M.B.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
A.P. is currently an employee of Topcon Spain. All the other authors have no conflict of interests to report.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/8/2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the Clinical Diagnosis of Alzheimer Disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JC, et al. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Arch. Neurol. 2001;58:124–129. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Bondi MW, et al. Neuropsychological Criteria for Mild Cognitive Impairment Improves Diagnostic Precision, Biomarker Associations, and Progression Rates. J. Alzheimers. Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, et al. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014;275:214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa A, et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J. Alzheimers. Dis. 2013;34:769–780. doi: 10.3233/JAD-122002. [DOI] [PubMed] [Google Scholar]
- 7.Gainotti G, Quaranta D, Vita MG, Marra C. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers. Dis. 2014;38:481–495. doi: 10.3233/JAD-130881. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKhann G, et al. The diagnosis of Dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois B, et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 11.Engler H, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 12.Diniz BSO, Pinto JA, Forlenza OV. Do CSF total tau, phosphorylated tau, and β-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J. Biol. Psychiatry. 2008;9:172–182. doi: 10.1080/15622970701535502. [DOI] [PubMed] [Google Scholar]
- 13.Iseri PK, Altinas Z, Tokay T, Yüksel N. Relationship between Cognitive Impairment and Retinal Morphological and Visual Functional Abnormalities in Alzheimer Disease. J. Neuro-Ophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 14.Katz B, Rimmer S. Ophthalmologic manifestations of Alzheimer’s disease. Surv. Ophthalmol. 1989;34:31–43. doi: 10.1016/0039-6257(89)90127-6. [DOI] [PubMed] [Google Scholar]
- 15.Cogan DG. Visual disturbances with focal progressive dementing disease. Am. J. Ophthalmol. 1985;100:68–72. doi: 10.1016/S0002-9394(14)74985-2. [DOI] [PubMed] [Google Scholar]
- 16.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 17.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest. Ophthalmol. Vis. Sci. 2007;48:2285–2289. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 18.MacGillivray TJ, et al. Retinal imaging as a source of biomarkers for diagnosis, characterization and prognosis of chronic illness or long-term conditions. Br. J. Radiol. 2014;87(1040):20130832. doi: 10.1259/bjr.20130832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikram MK, Cheung CY, Wong TY, Chen CPLH. Retinal pathology as biomarker for cognitive impairment and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2012;83:917–922. doi: 10.1136/jnnp-2011-301628. [DOI] [PubMed] [Google Scholar]
- 20.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic- nerve degeneration in Alzheimer’s disease. N. Engl. J. Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 21.Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology. 1990;97:9–17. doi: 10.1016/S0161-6420(90)32621-0. [DOI] [PubMed] [Google Scholar]
- 22.Davies DC, McCoubrie P, McDonald B, Jobst KA. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br. J. Ophthalmol. 1995;79:596–600. doi: 10.1136/bjo.79.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puliafito CA, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. doi: 10.1016/S0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 24.Schuman JS, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography: A pilot study. Arch. Ophthalmol. 1995;113:586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 25.Kardon RH. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J. Neuroophthalmol. 2011;31:353–361. doi: 10.1097/WNO.0b013e318238b9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polo V, et al. Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. Eye. 2014;28:680–690. doi: 10.1038/eye.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Martin E, et al. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology. 2012;119:2161–2167. doi: 10.1016/j.ophtha.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Martin E, et al. Fourier-Domain OCT in multiple sclerosis patients: Reproducibility and ability to detect retinal nerve fiber layer atrophy. Investig. Ophthalmol. Vis. Sci. 2011;52:4124–31. doi: 10.1167/iovs.10-6643. [DOI] [PubMed] [Google Scholar]
- 29.Kromer R, Serbecic N, Hausner L, Froelich L, Beutelspacher SC. Comparison of visual evoked potentials and retinal nerve fiber layer thickness in Alzheimer’s disease. Front. Neurol. 2013;4:203. doi: 10.3389/fneur.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parisi V, et al. Correlation between optical coherence tomography, pattern electroretinogram, and visual evoked potentials in open-angle glaucoma patients. Ophthalmology. 2001;108:905–912. doi: 10.1016/S0161-6420(00)00644-8. [DOI] [PubMed] [Google Scholar]
- 31.Ratchford JN, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302–308. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J. Alzheimers. Dis. 2013;34:659–664. doi: 10.3233/JAD-121975. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Lapiscina EH, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol. 2016;15:574–584. doi: 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed] [Google Scholar]
- 34.Jentsch S, et al. Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer’s disease. Acta Ophthalmol. 2015;93:241–247. doi: 10.1111/aos.12609. [DOI] [PubMed] [Google Scholar]
- 35.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003;9:184–190. [PubMed] [Google Scholar]
- 36.Löffler KU, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest. Ophthalmol. Vis. Sci. 1995;36:24–31. [PubMed] [Google Scholar]
- 37.He XF, et al. Optical coherence tomography assessed retinal nerve fiber layer thickness in patients with Alzheimer’s disease: a meta-analysis. Int. J. Ophthalmol. 2012;5:401–405. doi: 10.3980/j.issn.2222-3959.2012.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppola G, et al. Optical Coherence Tomography in Alzheimer’s Disease: A Meta-Analysis. PLoS One. 2015;10(8):e0134750. doi: 10.1371/journal.pone.0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson KL, Yeo JM, Waddell B, Cameron JR, Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers. Dement. Diagnosis, Assess. Dis. Monit. 2015;1:136–143. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J. Neuro-Ophthalmology. 2013;33:58–61. doi: 10.1097/WNO.0b013e318267fd5f. [DOI] [PubMed] [Google Scholar]
- 41.Ascaso FJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: An optical coherence tomography study. J. Neurol. 2014;261:1522–1530. doi: 10.1007/s00415-014-7374-z. [DOI] [PubMed] [Google Scholar]
- 42.Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin. Neurol. Neurosurg. 2011;113:523–526. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Paquet C, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2007;420:97–99. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 44.Gharbiya M, et al. Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J. Alzheimers. Dis. 2014;40:907–917. doi: 10.3233/JAD-132039. [DOI] [PubMed] [Google Scholar]
- 45.Kromer R, et al. Detection of Retinal Nerve Fiber Layer Defects in Alzheimer’s Disease Using SD-OCT. Front. psychiatry. 2014;5:22. doi: 10.3389/fpsyt.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Martin ES, et al. Macular thickness as a potential biomarker of mild Alzheimer’s disease. Ophthalmology. 2014;121:1149–1151. doi: 10.1016/j.ophtha.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Bernick C, Cummings J, Raman R, Sun X, Aisen P. Age and rate of cognitive decline in Alzheimer disease: implications for clinical trials. Arch. Neurol. 2012;69:901–905. doi: 10.1001/archneurol.2011.3758. [DOI] [PubMed] [Google Scholar]
- 48.Valenti DA. Alzheimer’s disease and glaucoma: imaging the biomarkers of neurodegenerative disease. Int. J. Alzheimers. Dis. 2011;2010:793931. doi: 10.4061/2010/793931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghiso J, Doudevski I, Ritch R, Rostagno A. Alzheimer’s disease and glaucoma: mechanistic similarities and differences. J. Glaucoma. 2013;22:S36–S38. doi: 10.1097/IJG.0b013e3182934af6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. B.M.J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lijmer JG, et al. Empirical evidence of design-related bias in studies of diagnostic tests. J.A.M.A. 1999;282:1061–1066. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 52.Whiting P, et al. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann. Intern. Med. 2004;140:189–202. doi: 10.7326/0003-4819-140-3-200402030-00010. [DOI] [PubMed] [Google Scholar]
- 53.Budenz DL, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rougier MB, et al. Retinal nerve fibre layer thickness measured with SD-OCT in a population-based study of French elderly subjects: the Alienor study. Acta Ophthalmol. 2015;93:539–545. doi: 10.1111/aos.12658. [DOI] [PubMed] [Google Scholar]
- 55.de Jong FJ, et al. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung CYL, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers. Dement. 2014;10:135–142. doi: 10.1016/j.jalz.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Marziani E, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2013;54:5953–5958. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 58.Williams MA, et al. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers. Dement. Diagnosis, Assess. Dis. Monit. 2015;1:229–235. doi: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Bryhim, B. E. et al. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. Published online August 23, 2018. [DOI] [PMC free article] [PubMed]
- 60.Bouwman FH, et al. CSF biomarker levels in early and late onset Alzheimer’s disease. Neurobiol. Aging. 2009;30:1895–1901. doi: 10.1016/j.neurobiolaging.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Ossenkoppele R, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. J.A.M.A. 2015;313:1939–1349. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boada M, et al. Design of a comprehensive Alzheimer’s disease clinic and research center in Spain to meet critical patient and family needs. Alzheimers. Dement. 2014;10:409–415. doi: 10.1016/j.jalz.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alegret M, et al. Normative data of a brief neuropsychological battery for Spanish individuals older than 49. J. Clin. Exp. Neuropsychol. 2012;34:209–202. doi: 10.1080/13803395.2011.630652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alegret M, et al. Cut-off scores of a Brief Neuropsychological Battery (NBACE) for Spanish Individual adults older than 44 years old. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0076436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 66.Blesa R, et al. Clinical validity of the “mini-mental state” for Spanish speaking communities. Neuropsychologia. 2001;39:1150–1157. doi: 10.1016/S0028-3932(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 67.Meulen EFJ, et al. The seven minute screen: A neurocognitive screening test highly sensitive to various types of dementia. J. Neurol. Neurosurg. Psychiatry. 2004;75:700–705. doi: 10.1136/jnnp.2003.021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 69.Reiman E, Jagust W. Brain imaging in the study of Alzheimer’s disease. Neuroimage. 2012;61:505–516. doi: 10.1016/j.neuroimage.2011.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Q, et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt. Express. 2010;18:21293–21307. doi: 10.1364/OE.18.021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hood DC, Raza AS. Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma. Biomed. Opt. Express. 2011;2:1097–1105. doi: 10.1364/BOE.2.001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petersen RC, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/WNL.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 73.Lopez OL, et al. Prevalence and Classification of Mild Cognitive Impairment in the Cardiovascular Health Study Cognition Study. Arch. Neurol. 2003;60:1385. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 74.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 75.Cruz-Herranz A, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86:2303–2309. doi: 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.