Abstract
Male fertility is the ability of sperm to fertilize the egg and sustain embryo development. Several factors determine the fertilizing capacity of mammalian sperm, including those intrinsic to sperm and components of the seminal plasma. The present study analyzed the seminal fluid proteome of Bos taurus and potential associations between proteins and fertility scores. Mass spectrometry coupled with nano HPLC allowed the identification of 1,159 proteins in the dairy bull seminal plasma. There were 50 and 29 seminal proteins more abundant in high (HF) low fertility (LF) bulls, respectively. Based on multivariate analysis, C-type natriuretic peptide, TIMP-2, BSP5 and sulfhydryl oxidase indicated relationship with HF bulls. Clusterin, tissue factor pathway inhibitor 2, galectin-3-binding protein and 5′-nucleotidase were associated with LF bulls. Abundance of NAD(P)(+)-arginine ADP-ribosyltransferase, prosaposin and transmembrane protein 2 proteins had the highest positive correlations with fertility ranking. Quantities of vitamin D-binding protein, nucleotide exchange factor SIL1 and galectin-3-binding protein showed the highest negative correlations with fertility ranking. A fertility ranking score was calculated and the relationship with these proteins was significant (Spearman’s rho = 0.94). The present findings represent a major and novel contribution to the study of bovine seminal proteins. Indicators of fertility can be used to improve reproductive biotechnologies.
Introduction
Reproduction efficiency of the male is one of the most important factors influencing sustainability of livestock as cryopreserved sperm from the elite bulls are widely distributed around the world by means of artificial insemination1. Male fertility is defined as the ability of sperm to fertilize the egg and sustain embryo development2 and accurate prediction of dairy bull fertility is still a significant challenge despite of the advances in genetic selection of dairy herds1. As well established, herd fertility is determined by female performance but aspects of male physiology are also important, as the AI industry still faces challenges related to bull subfertility1,3. Given that semen of dairy bulls are used to inseminate large number of cows, selection of high fertility bulls is crucial because even small increases in conception rates in the herds represent major revenues for the industry and farmers.
Several factors potentially determine the fertilizing capacity of mammalian sperm, including those that are intrinsic to sperm, such as DNA integrity4, RNA5, proteins6 and metabolites7. Sperm physiology and eventually their fertilizing capacity are also modulated by components of the milieu where they are maintained, the seminal plasma3. Seminal fluid is a composite secretion from the accessory sex glands and epididymides, mainly, and it contains organic and inorganic compounds, including proteins, lipids, ions and metabolites8. Proteins of the seminal plasma play vital roles in sperm protection9, capacitation10, acrosome reaction and sperm-egg binding, fertilization and initial embryonic development11–13. Given the importance of seminal plasma proteins, methods based on gel electrophoresis and mass spectrometry have allowed the identification of numerous classes of proteins in the bull seminal plasma14–17. More recently, the shotgun proteomic approach based on LC-MS/MS has been used for the study of protein mixtures18 as this technical strategy potentially allows more efficient identification of molecules in complex biological systems, such as the seminal plasma.
Studies have described statistical associations between components of reproductive fluids and fertility criteria of men19,20, boars21 and bulls22–25, among other species. These researchers used gel electrophoresis, N-terminal sequencing or SDS-PAGE coupled with mass spectrometry to compare and identify proteins of seminal plasma from males with different fertility status. Such studies certainly revealed important pieces of information about the composition of seminal fluid and potential molecular markers of fertility. Although representing remarkable achievements, gel-based strategies have limitations when used as the sole method to describe the whole proteome of a biological entity.
Also, several studies have used semen parameters to distinguish fertility phenotypic status in bulls25 and men as well20. However, such parameters are not reliable predictors of male fertility when compared to in vivo assays26. Working with dairy bulls as a model for fertility studies allows us to have access to an accurate database of sire conception rates calculated from hundreds of artificial inseminations. Thus, the present study was conducted to analyze the seminal plasma proteome of dairy sires (Bos taurus) using a label-free shotgun proteomics approach. We also investigated potential associations between seminal plasma proteins and fertility phenotype of the bulls.
Results
Seminal plasma proteome of Holstein bulls
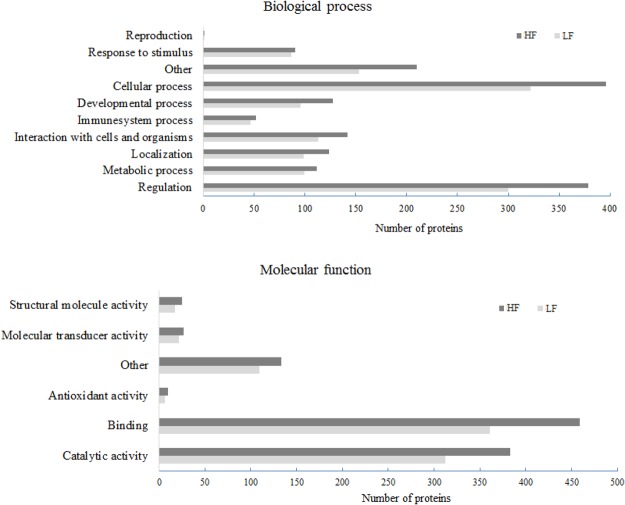
In the present study, 1,159 proteins were identified in the dairy bull seminal plasma, using mass spectrometry coupled with nano HPLC (Supplementary Table S1). Among all listed proteins, 765 were characterized according to UniProt database and 394 were still defined as non-characterized. Gene ontology terms related to biological process and molecular function of dairy bull seminal plasma proteins are presented in Fig. 1. The most important biological processes linked to the identified proteins were cellular process (24.3 and 24.5% in HF and LF bulls, respectively) followed by regulation (23.2 and 22.8% in HF and LF bulls, respectively) and interaction with cells and organisms (8.7 and 8.6% in HF and LF bulls, respectively). Molecular functions of bovine seminal proteins were mainly reported as binding (44.2 and 43.6% in HF and LF bulls, respectively) and catalytic activity (37 and 37.8% in HF and LF bulls, respectively).
Figure 1.
Gene ontology annotations of bull seminal plasma proteins based on biological process and molecular function. Protein data were analyzed using the software for researching annotations of proteins STRAP27. Gene ontology terms were obtained from UniProtKB database.
Comparison between protein profiles of seminal plasma from high and low fertility bulls
Of the 1,159 proteins identified in dairy bull seminal plasma, 949 were found in seminal plasma from HF bulls and 771 in LF sires, with 561 proteins (48.4%) common to both HF and LF phenotypes (Supplementary Table S1; Fig. 2). Thus, 388 proteins were exclusive to HF, while 210 were found in only LF sires. Among the proteins present in the seminal plasma of all bulls (561), there were 79 with different (p < 0.05) quantities in the groups of high and low fertility sires. Then, 50 proteins were more abundant in HF bulls and 29 proteins, in LF bulls (Table 1).
Figure 2.
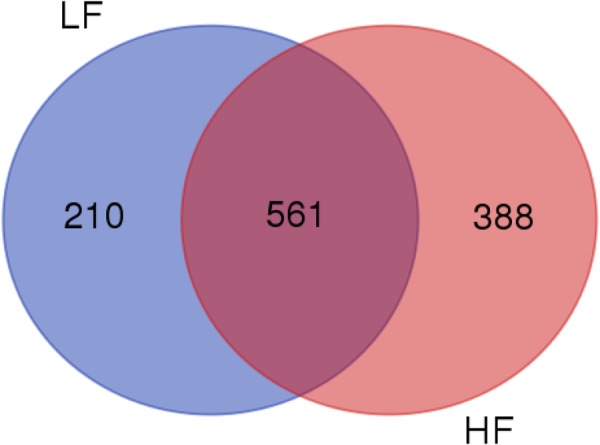
Proteomes of seminal plasma from bulls of high (HF, n = 5) and low (LF, n = 5) fertility scores. The Venn diagram was generated by the Bioinformatics & Evolutionary Genomics platform (http://bioinformatics.psb.ugent.be/webtools/Venn. From a total of 1,159 proteins, 949 were identified in HF group and 771 in LF. The intersection represents proteins (561) conserved to both HF and LF groups.
Table 1.
Proteins of the seminal plasma differentially expressed in bulls with high (HF) and low (LF) fertility scores. Proteins were identified by DDA (data dependent acquisition) label-free mass spectrometry, Progenesis QI software and UniProt database.
| Accession number | Description | Overexpression in | Peptide count | Unique peptides | Confidence score | Anova (p) |
|---|---|---|---|---|---|---|
| Q05927 | 5′-nucleotidase | LF | 63 | 33 | 173.56 | 0.031 |
| Q50HZ8 | Acetyl-CoA carboxylase, type beta (Fragment) | HF | 1 | 1 | 70.17 | 0.023 |
| Q27984 | Alpha1-antichymotrypsin isoform pHHK12 (Fragment) | LF | 1 | 1 | 47.62 | 0.049 |
| F1MVR5 G3N156 | Anion exchange protein | LF | 1 | 1 | 21.11 | 0.026 |
| Q5EA01 | Beta-1,4-glucuronyltransferase 1 | LF | 4 | 4 | 98,80 | 0.043 |
| H7BWW2 | Beta-hexosaminidase | LF | 37 | 26 | 97.55 | 0.049 |
| F1N619 | Cadherin-1 (Fragment) | LF | 174.00 | 0.012 | ||
| P52193 | Calreticulin | HF | 5 | 5 | 61.38 | 0.049 |
| P06833 | Caltrin | HF | 15 | 13 | 138.93 | 0.020 |
| A6BML7 | Carboxypeptidase | HF | 15 | 15 | 39.83 | 0.008 |
| Q17QK3 | Carboxypeptidase Q | LF | 1 | 1 | 47.79 | 0.047 |
| A5PJF7 | C-C motif chemokine | HF | 3 | 3 | 339.39 | 0.009 |
| F1N2J8 | Chromosome 16 open reading frame 89 | HF | 6 | 6 | 75.18 | 0.032 |
| F1MLR4 | Ciliary neurotrophic factor receptor subunit alpha precursor | LF | 6 | 6 | 121.84 | 0.033 |
| P17697 | Clusterin | LF | 85 | 73 | 1233.10 | 0.027 |
| A0A0F6QNP7 | Complement component 3 | LF | 5 | 5 | 467.05 | 0.040 |
| P81187 | Complement factor B | LF | 6 | 6 | 433.91 | 0.047 |
| F1MC45 | Complement factor H (Fragment) | LF | 61 | 5 | 221.93 | 0.004 |
| P55206 | C-type natriuretic peptide | HF | 46 | 44 | 488.24 | 0.032 |
| A7MBJ5 | Cullin-associated NEDD8-dissociated protein 1 | LF | 22 | 20 | 115.93 | 0.033 |
| P81425 | Dipeptidyl peptidase 4 | LF | 25 | 25 | 303.81 | 0.024 |
| O18738 | Dystroglycan | HF | 2 | 2 | 94.94 | 0.011 |
| E1BJV0 | EH domain containing 4 | LF | 14 | 11 | 142.00 | 0.009 |
| A6QR19 | ENO2 protein | HF | 1 | 1 | 33.46 | 0.042 |
| P79345 | Epididymal secretory protein E1 | LF | 29 | 29 | 123.57 | 0.031 |
| A7E3W2 | Galectin-3-binding protein | LF | 23 | 22 | 313.46 | 0.031 |
| E1BA29 | Guanine nucleotide-binding protein G(q) subunit alhpa | LF | 1 | 1 | 97.95 | 0.008 |
| Q0P565 | HD domain-containing protein 2 | HF | 1 | 1 | 70.52 | 0.005 |
| Q76LV2 | Heat shock protein HSP 90-alpha | HF | 29 | 28 | 189.30 | 0.029 |
| F1MNT3 | Hormone-sensitive lipase | LF | 10 | 8 | 52.37 | 0.004 |
| Q7YS45 | Hyaluronidase (Fragment) | LF | 1 | 1 | 107.36 | 0.029 |
| E1B748 | Hypoxia up-regulated protein 1 precursor | HF | 17 | 17 | 289.84 | 0,001 |
| Q70IB2 | Inactive ribonuclease-like protein 10 | HF | 8 | 8 | 57,69 | 0,029 |
| Q95M12 | Legumain | LF | 8 | 8 | 138.49 | 0,022 |
| Q9MYM4 | Lysosomal alpha-glucosidase | LF | 11 | 11 | 76.30 | 0,036 |
| Q3SZI0 | Mannose-6-phosphate isomerase | HF | 6 | 6 | 84.25 | 0,039 |
| A5D7D5 | MATN2 protein | HF | 4 | 4 | 50.06 | 0.022 |
| E1BDF3 | Matrilin 4 | HF | 20 | 20 | 149.27 | 0.017 |
| P16368 | Metalloproteinase inhibitor 2 | HF | 11 | 11 | 1071.83 | 0.034 |
| Q9N282 | MMP-9 (Fragment) | HF | 1 | 1 | 33.60 | 0.014 |
| Q1LZH9 | N-acetylglucosamine-6-sulfatase | HF | 9 | 9 | 62.80 | 0.040 |
| E1BI74 | NAD(P)(+)−arginine ADP-ribosyltransferase (Fragment) | HF | 14 | 13 | 76.94 | 0.002 |
| Q0IIH5 | Nucleobindin 2 | HF | 26 | 25 | 290.90 | 0.021 |
| Q32KV6 | Nucleotide exchange factor SIL1 | LF | 5 | 5 | 57.08 | 0.029 |
| A7MBI8 | NUDT9 protein | LF | 1 | 1 | 39.35 | 0.049 |
| E1B818 | Olfactomedin-like 2A-like | HF | 6 | 6 | 163.47 | 0.001 |
| Q9BGI2 | Peroxiredoxin-4 | HF | 3 | 3 | 66.36 | 0.021 |
| Q32KN6 | Phosphoglycerate kinase | HF | 35 | 27 | 100.42 | 0.045 |
| Q28017 | Platelet-activating factor acetylhydrolase | HF | 61 | 59 | 1162.20 | 0.026 |
| A1L555 | Prosaposin | HF | 35 | 3 | 120.78 | 0.007 |
| P21856 | Rab GDP dissociation inhibitor alpha | HF | 9 | 4 | 64.47 | 0.033 |
| Q0VCQ9 | Reticulocalbin 2, EF-hand calcium binding domain | HF | 9 | 9 | 65.81 | 0.020 |
| Q0III8 | RNASET2 protein (Fragment) | HF | 6 | 6 | 26.70 | 0.042 |
| A7MB70 | Semaphorin-3C | HF | 6 | 5 | 97.87 | 0.011 |
| P81019 | Seminal plasma protein BSP-30 kDa | HF | 85 | 83 | 2706.25 | 0.005 |
| P00669 | Seminal ribonuclease | HF | 92 | 79 | 1070.13 | 0.018 |
| Q29443 | Serotransferrin | LF | 29 | 1 | 30.41 | 0.029 |
| Q2HJF0 | Serotransferrin-like | HF | 33 | 4 | 208.39 | 0.002 |
| Q862P3 | Similar to cyclophilin B (Fragment) | HF | 1 | 1 | 73.31 | 0.021 |
| F1MJI3 | SLIT-ROBO Rho GTPase activating protein 3 | HF | 2 | 1 | 20.89 | 0.036 |
| Q4R0H2 | Spermadhesin 2 | LF | 80 | 29 | 300.31 | 0.015 |
| F1MHF1 | ST6 beta-galactoside alpha-2,6-sialyltransferase 1 | HF | 13 | 13 | 138.21 | 0.024 |
| P82292 | Spermadhesin Z13 | HF | 53 | 4 | 36.67 | 0.038 |
| A6QQA8 | Sulfhydryl oxidase | HF | 38 | 37 | 405.51 | 0.013 |
| F1MJB6; A0JN68 | Targeting protein for Xklp2 | HF | 1 | 1 | 20.33 | 0.024 |
| Q32L40 | T-complex protein 1 subunit alpha | HF | 1 | 1 | 42.71 | 0.034 |
| Q3ZBH0 | T-complex protein 1 subunit beta | HF | 18 | 18 | 58.09 | 0.021 |
| Q7YRQ8 | Tissue factor pathway inhibitor 2 | LF | 34 | 32 | 378.76 | 0,007 |
| F1MNY2 | Transmembrane protein 2 | HF | 1 | 1 | 22.47 | 0.010 |
| Q3T077 | Tubulin polymerization-promoting protein family member 2 | HF | 5 | 5 | 68.68 | 0,050 |
| G3X861 | Uncharacterized protein (Fragment) | HF | 1 | 1 | 55.44 | 0.050 |
| F1MY12 | Uncharacterized protein (Fragment) | HF | 2 | 2 | 91,12 | 0.010 |
| E1BI55 | Uncharacterized protein | HF | 1 | 1 | 21.69 | 0.039 |
| G5E5W7 | Uncharacterized protein | HF | 1 | 1 | 54.09 | 0.013 |
| Q3MHN5 | Vitamin D-binding protein | LF | 1 | 1 | 70.54 | 0.021 |
| Q32LB7 | V-type proton ATPase subunit E 2 | HF | 1 | 1 | 31.85 | 0.044 |
| P40682 | V-type proton ATPase subunit S1 | HF | 1 | 1 | 60.26 | 0.025 |
| Q3T0Z0 | WAP four-disulfide core domain 2 | HF | 3 | 3 | 98.59 | 0.029 |
| Q3ZCH5 | Zinc-alpha-2-glycoprotein | LF | 2 | 2 | 100.34 | 0.006 |
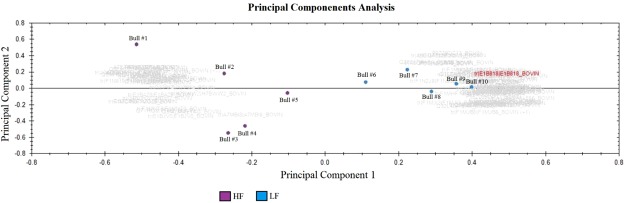
According to the plot based on the principal component analysis, there was a clear distribution of proteins with different quantities in high and low fertility bulls (Fig. 3). Based on multivariate analysis, a score plot of the two components with the highest variability (49.5 and 37.3%, data not shown) was performed (Fig. 4a) and eight proteins had VIP score greater than 1.5 (Fig. 4b), indicating meaningful contributions for definition of the fertility phenotype. Proteins contributing to definition of high fertility are C-type natriuretic peptide (NPPC), metalloproteinase inhibitor 2 (TIMP2), seminal plasma protein −30 kDa (BSP5) and sulfhydryl oxidase (QSOX1). On the other hand, clusterin (CLU), tissue factor pathway inhibitor 2 (TFPI2), galectin-3-binding protein and 5′-nucleotidase (NT5E) are the ones with meaningful contributions to low the fertility phenotype.
Figure 3.
Principal component analysis (PCA) score plot associated with 79 seminal plasma proteins differentially expressed in high and low fertility bulls.
Figure 4.
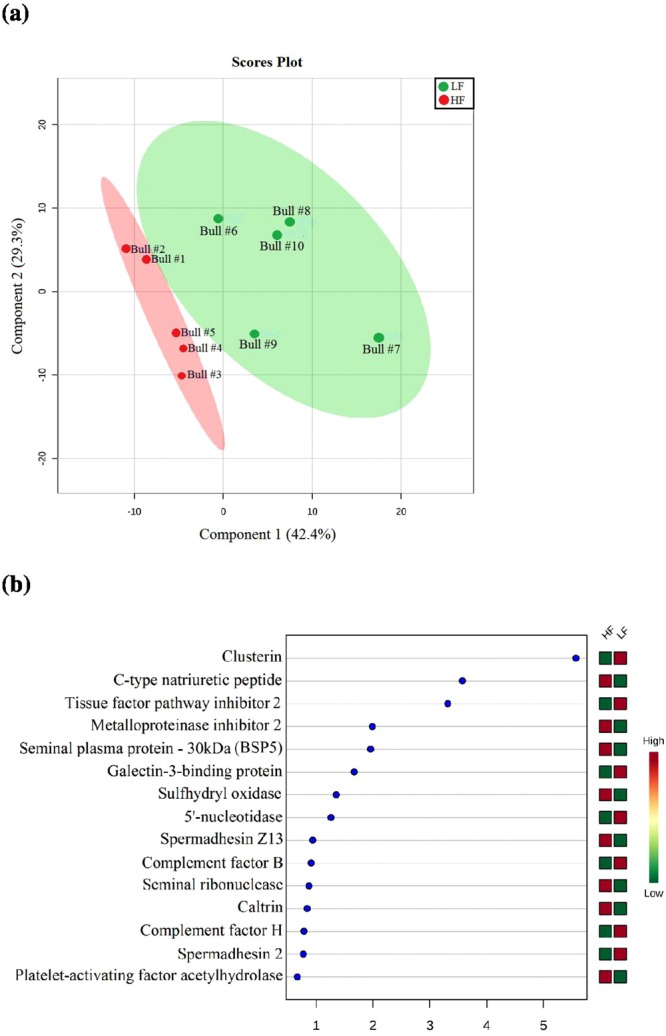
(a) Partial least-squares discriminant analysis (PLS-DA), showing the separation of groups of bulls with high and low fertility scores. The explained variances are shown in brackets. (b) Important features (proteins) based on Variable Importance in Projection (VIP) scores.
Protein-based fertility rank score
Given the 79 seminal plasma proteins with different abundances (p < 0.05) between the groups of high and low fertility bulls, we selected the six proteins showing the highest correlations with fertility ranking. NAD(P)(+)-arginine ADP-ribosyltransferase, prosaposin and transmembrane protein 2 had the highest positive correlations, while vitamin D-binding protein, nucleotide exchange factor SIL1 and galectin-3-binding protein showed the highest negative correlations with fertility of bulls. Using the normalized abundances of all these six proteins, it was possible to calculate a predictive fertility rank score with Spearman’s rho = 0.91 and Pearson’s correlation = 0.83 (Fig. 5).
Figure 5.
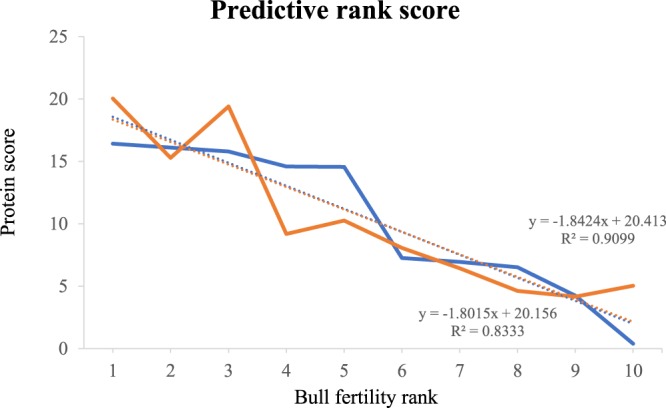
Predictive fertility rank score based on protein score (Y) and bull fertility rank (X). Protein score was obtained using normalized abundances of the six proteins with highest correlation with fertility rank. Bull fertility rank is shown from the highest (1) to the lowest value (10), as defined in Table 2. A predictive fertility rank score was significant with Spearman’s rho = 0.91 and Pearson’s correlation = 0.83. The blue line represents the conception rate difference from average (%) and the orange line represents the protein fertility score. The dotted line represents the linear regression for the respective (blue or orange) curve, showing the correlations between both scores, the conception rate and protein fertility score.
In silico analysis of protein-protein networks
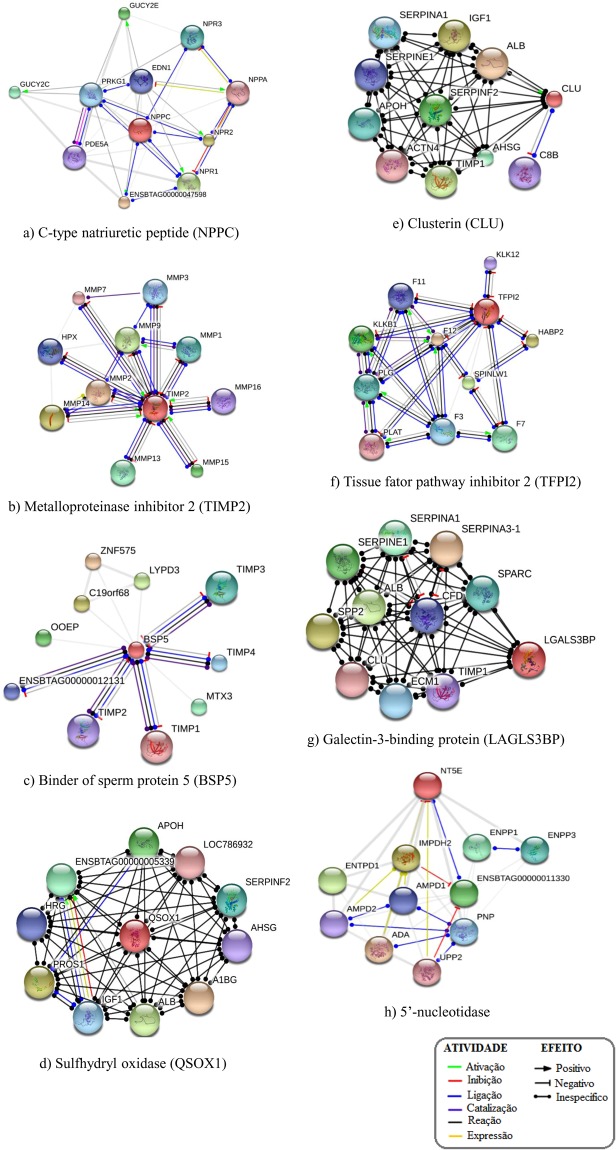
Seminal plasma proteins associated with VIP score >1.5 were NPPC, TIMP2, BSP5, QSOX1, CLU, TFPI2, galectin-3-binding protein and NT5E. Based on in silico analysis of protein-protein network, NPPC interacts with its receptors and with endothelin 1 (Fig. 6a). TIMP2 exhibits associations with several types of metalloproteinases (Fig. 6b) and BSP5 interacts with tissue inhibitor of metalloproteinases (Fig. 6c), as well as with oocyte-expressed protein homolog. QSOX1 interacts with glycoproteins (A1BG, HRG, AHSG), insulin-like growth factor 1 (IGF-1) and albumin (Fig. 6d). Clusterin shows links with albumin, IFG-1, alpha actinin 4, complement component C8 beta and protease inhibitors (Fig. 6e). TFPI2, in turn, interacts with proteins related to coagulation, plasminogen precursor and tissue type plasminogen activator (Fig. 6f). Galectin-3-binding protein interacts with protease inhibitors (serpins and TIMP1) and with clusterin, another protein overexpressed in low fertility animals with VIP score higher than 1.5 (Fig. 6g). Finally, NT5E interacts with enzymes such as deaminases and ectonucleotides (Fig. 6h).
Figure 6.
In silico analysis of protein-protein network as determined by STRING platform (http://string-db.org). Interactions were evaluated for seminal plasma proteins associated with VIP score >1.5: (a) C-type natriuretic peptide (NPPC); (b) Metalloproteinase inhibitor 2 (TIMP-2); (c) Binder of Sperm Protein 5 (BSP5); (d) Sulfhydryl oxidase (QSOX1); (e) Clusterin (CLU); (f) Tissue factor pathway inhibitor 2 (TFPI2); (g) Galectin-3-binding protein (LGAL3SBP); (h) 5′-nucleotidase (NT5E).
Discussion
In the present study, we used a label-free mass spectrometry approach to characterize the seminal plasma proteome of adult Holstein bulls. This strategy allowed the identification of 1,159 proteins and represents a major contribution to the understanding of seminal plasma composition in the Bos taurus species. Moreover, there were specific seminal proteins with different expression profiles in sires with contrasting fertility phenotypes determined in vivo.
The major biological processes (cellular process, regulation and interaction with cells) and molecular functions (binding and catalytic activity) of the seminal proteins related to their participation in events such as cell protection, sperm motility and capacitation, acrosome reaction, fertilization and embryonic development. Also, gene ontology terms related to biological process and molecular function were very similar in bulls of high and low fertility. The reason for this certainly relies on the fact that dairy bulls used in our study have been selected for decades by the AI companies and differences in fertility among them do not relate to general pattern of seminal plasma proteins, such as the pattern defined by gene ontology. Instead, differences in fertility phenotypes of those bulls are associated to very specific molecular aspects of seminal plasma, as we discuss below.
According to multivariate statistical analysis, proteins identified as more abundant in HF bulls were seminal plasma protein −30 kDa (BSP5), metalloproteinase inhibitor 2 (TIMP2), C-type natriuretic peptide (NPPC) and sulfhydryl oxidase (SQOX1). These proteins had VIP >1.5 and, thus, they are the best indicators of the high fertility phenotype of the bulls. BSP5 belongs to the Binder of Sperm Protein (BSP) family and, along with BSP1 and BSP3, represent around 60% of all proteins of bull seminal plasma15,27,28. BSPs are secreted by the accessory sex glands and, after ejaculation, bind to sperm29 and induce cholesterol and phospholipid efflux from the sperm membrane, an essential step for capacitation30. BSPs also mediate sperm interaction with the oviduct epithelium31 and BSP1 affects both fertilization and early development of bovine embryos in vitro13. In silico analysis points out to significant interactions between BSP5 and metalloproteinase inhibitors, such as TIMP-2, both with greater abundance in high fertility bulls. Furthermore, a link seems to exist between BSP5 and the oocyte-expressed protein homolog, which is located in subcortical cytoplasm of early embryos and play roles in cell divisions32, thereby supporting a putative role of BSP5 during fertilization.
Metalloproteinase inhibitors (TIMPs) modulate the activity of matrix metalloproteinases (MMPs), enzymes involved in remodeling of the extracellular matrix (ECM)33. The balance between MMPs and TIMPs is crucial during ECM remodeling33. Certain TIMPs have been suggested to play roles in sperm-egg fusion in the mouse34. TIMP-3 controls the degree of trophoblast implantation in the murine uterus35 and TIMP-2 content in bovine seminal plasma has a negative correlation with post-thaw sperm morphology and membrane stability36. Moreover, treatment of bull sperm with heparin binding proteins, a fertility-associated antigen and TIMP-2, increased pregnancy rates after artificial insemination37. Not only does TIMP-2 interact with several types of metalloproteinases but also with HPX, a protein that transports hemoglobin to the liver for breakdown and iron recovery. According to in silico analysis, TIMP-2 interacts with several types of metalloproteinases and, in fact, some MMPs are involved in reproductive events, such as angiogenesis, implantation and embryogenesis38,39.
NPPC belongs to a family of small peptides that participates in natriuresis and diuresis through vasodilatation40. Authors have described higher amounts of NPPC in reproductive tissues of male pigs when compared to other41. NPR-B (a NPPC receptor) is present in the acrosome and tail of human sperm and, thus, it is plausible that NPPC from seminal plasma binds to its receptor thereby stimulating intracellular cGMP and sperm motility42. In silico analysis showed interactions between NPPC, its receptors and guanylate cyclase, an enzyme involved in cGMP biosynthesis42. Also, NPPC interacts with natriuretic peptide A, which promotes trophoblasts implantation and artery remodeling in uterus43. QSOX1 plays a role in reduction of oxygen molecule to hydrogen peroxide, forming disulfide bonds in proteins and peptides44. In the male reproductive tract, QSOX1 protects spermatozoa structure and function by oxidizing sulfhydryl groups that could cause damage to the cell45. Several authors suggest that QSOX is crucial for sperm physiology and its dysregulation is associated with failures in spermatogenesis in hamsters46 and rats47. Based on in silico analysis, QSOX1 interacts with several types of glycoproteins present in the cellular membrane and with albumin, the most abundant protein of the cauda epididymal fluid in Holstein bulls48. Albumin protects sperm cells against harmful effects of lipid peroxides49 and acts during sperm capacitation50 and acrosome reaction51. QSOX1 also interacts with vascular endothelial growth factor A and with insulin growth factor 1, a protein that improves blastocyst rate formation52 in the bovine species.
Thus, seminal plasma proteins BSP5, TIMP2, NPPC and QSOX1 participate in important events related to reproduction, which explains, at least partially, their empirical associations with fertility. An earlier study described a quadratic relationship between BSP5 content in accessory sex gland fluid and fertility status of bulls15. This indicates that increasing amounts of BSP5 are beneficial but too much BSP5 in semen becomes detrimental to fertility. In fact, in vitro experiments confirm that BSPs are needed for proper sperm function but, when cells are exposed to high amounts of BSPs and for long periods of time, they excessively loose membrane cholesterol and phospholipids and become less viable25,47. Considering these facts, we suggest that the amount of BSP5 present in the bulls of our study was not sufficiently high to exert negative effects on fertility.
Seminal plasma clusterin (CLU), tissue factor pathway inhibitor 2 (TFPI2), galectin-3-binding protein and 5′-nucleotidase had VIP >1.5, indicating their significant contribution for definition of the low fertility phenotype of the dairy bulls. CLU is a chaperone and protects sperm against complement-mediated attack53 and against the effects of protein precipitation54. Clusterin contributes to removal of defective spermatozoa and is an indicator of poor semen quality in bulls55, rams56, men57, stallions58 and peccaries59. Also, seminal plasma CLU is inversely associated with the number of normal sperm in beef cattle60 and a positive association exists between abnormal morphology of sperm head and clusterin expression after scrotal insulation of Holstein bulls55. Based on in silico analysis, CLU interacts with a diverse cohort of molecules, some of which found in the reproductive tract and germ cells of bulls, such as serpins, albumin, TIMP, alpha-2-HS-glycoprotein. CLU also interacts with galectin-3 binding protein, another protein found at high levels in the seminal plasma of low fertility bulls, discussed below. Thus, there is sufficient experimental evidence in support of the inverse association between seminal plasma CLU and fertility of bulls.
TFPI-2 is a serine protease inhibitor also known as matrix-associated serine protease inhibitor (MSPI)61. It has been postulated that TFPI-2 is in fact one of the products of PP5 (placental protein 5) degradation62. PP5 is a placental glycoprotein associated with the coagulation and fibrinolytic system63 and PP5 plays a role in clotting and liquefaction mechanisms in human seminal plasma64. In fact, our in silico analysis indicates that TFPI-2 activity is linked to coagulation proteins, such as coagulation factors (F3, F7, F11 and F12), plasminogen precursor (PLG) and tissue-type plasminogen activator (PLAT). TFPI-2 also interacts with kallikreins, a group of proteins that convert kininogen into kinin, promoting increase in sperm motility65. However, further studies are still needed to confirm if TFPI-2 has a causal relation with low fertility in bulls. NT5E is a glycosylated enzyme already described in seminal plasma of bulls66 and participates in hydrolysis of AMP, stimulating sperm motility and sperm capacitation67. In silico analysis showed interactions of TFPI-2 with deaminases (ADA, AMPD1 and AMP D2), phosphorylases (ENTPD1, ENPP1, ENPP3, PNP and UPP2) and inosine-5′-monophosphate dehydrogenase 2 (IMPDH2). These enzymes that interact with TFPI-2 act through different intracellular signaling events that may lead to activation of sperm motility and capacitation. Galectin-3-binding protein is a member of beta-galactoside binding lectins expressed in various cells and tissues68 and this molecule has been previously found in epidydimal fluid of dairy bulls48. In humans, seminal galectin-3-binding protein plays multiple roles associated with semen liquefaction, sperm motility, angiogenesis in the female reproductive tract and as a pro-inflammatory agent69. In silico analysis detected an interaction between galectin-3 binding protein and clusterin, which is also over expressed in low fertility dairy bulls. Like clusterin, galectin-3-binding protein also interacts with albumin, a molecule involved in sperm capacitation and acrosome reaction, as we mentioned above. In addition, galectin-3 binding protein interacts with complement factor D (CFD), an activator of the immune system in the female reproductive tract70. As well known, seminal plasma components interact with the female reproductive tract, stimulating gene expression and the immune system, influencing fertility and embryo development71. Proteins from seminal plasma interact with endometrium epithelial cells, inducing or suppressing several mRNAs. This event causes synthesis of cytokines and chemokines that recruit immune cells from the blood to the endometrial lumen72. Besides cleaning the environment, such immune cells play roles in selection of the most competent sperm for fertilization73. Dendritic cells, a type of immune cells, carry seminal fluid antigens to the local lymph node activating Treg cell (regulatory T cells) population. Treg cells migrate, via blood, to the endometrium and promote endometrial receptivity for embryo implantation once the embryo expresses the same paternally derived antigens present in seminal plasma.
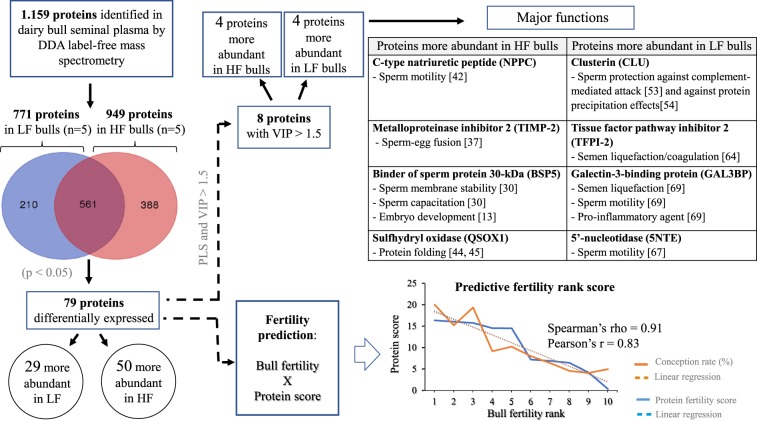
In conclusion, the present study is a comprehensive overview of the proteome of bull seminal plasma. An approach based on DDA label-free mass spectrometry allowed the description of 1,159 proteins and this is, so far, the broadest inventory of the bovine seminal plasma proteome. At this point, we cannot precisely make inferences about the full protein composition of the seminal plasma of dairy bulls but it is certain that seminal fluid is a very complex milieu, containing components yet to be identified. Statistical analyses indicated eight proteins with significant contributions for definition of the fertility phenotype of dairy bulls. Moreover, we describe two distinct fertility indicators: a discriminator of high and low fertility bulls and a rank predictor. The entire approach used in our study and functional aspects of potential indicators of bull fertility are depicted in Fig. 7. Studies about the composition of seminal fluid set the foundations for the understanding of mechanisms regulating male fertility, which in turn has major economic impact for the industry and farmers. Also, definition of molecular indicators of bull fertility can be used to enhance reproductive biotechnologies for cattle.
Figure 7.
A graphical abstract showing the main findings of this study. Using DDA label-free mass spectrometry, 1,159 proteins were identified in dairy bull seminal plasma. Of the 1,159 proteins, 949 were found in seminal plasma from high fertility (HF, n = 5) bulls and 771 proteins in low fertility (LF, n = 5) sires. while 561 proteins were common to both HF and LF phenotypes. There were 50 and 29 seminal proteins more abundant in HF and LF bulls, respectively. Based on multivariate analyses, there were eight proteins with VIP score greater than 1.5 (Fig. 4b), indicating meaningful contributions of such proteins for definition of the fertility phenotype. Among them, four proteins were more abundant in either HF or LF bulls. DDA: dependent data acquired; PLS: partial least square; VIP: variable influence in projection.
Materials and Methods
Experimental design
Analysis of the seminal plasma proteome from Holstein bulls (Bos taurus) with contrasting in vivo fertility rates was conducted using high performance liquid chromatography combined with mass spectrometry. Computational biology as well as univariate and multivariate analyses were performed to compare the seminal proteome of high (HF) and low (LF) fertility dairy bulls and to detect molecular indicators for bull fertility. Then, we evaluated the correlation between normalized abundance of seminal proteins to create an equation of predictive fertility score.
In vivo bull fertility and semen samples
Semen samples from ten Holstein bulls with reliable fertility phenotypes (Table 2) were provided by Alta Genetics (Watertown, WI, USA). Individual fertility scores of bulls used in the present study were calculated using Probit.F90 software, based on the average conception of at least 674 breeding outcomes per bull6. The population standard deviations were used as criteria to define bull fertility74 and, for the present study, high and low fertility sires differed from the mean by at least 1.3 standard deviations. Factors that influence fertility performance of sires (breeding event, environmental factors and herd management) were adjusted to determine reliable fertility scores using threshold models69.
Table 2.
Fertility phenotypes of Holstein bulls. Bulls 1–5 are defined as high fertility (HF) and bulls 6–10, as low fertility (LF).
| Bull # | Fertility status | Number of breedings | Conception rates (% difference from average) | Std of difference |
|---|---|---|---|---|
| 1 | HF | 5293 | 5.42 | 2.0238 |
| 2 | HF | 825 | 5.1 | 1.9034 |
| 3 | HF | 2032 | 4.8 | 1.7931 |
| 4 | HF | 2487 | 3.59 | 1.3415 |
| 5 | HF | 5751 | 3.56 | 1.3304 |
| 6 | LF | 1604 | −3.75 | −1.4014 |
| 7 | LF | 2276 | −4.06 | −1.5159 |
| 8 | LF | 967 | −4.49 | −1.6762 |
| 9 | LF | 5603 | −6.76 | −2.5239 |
| 10 | LF | 674 | −10.61 | −3.9624 |
Semen from the five high and five low fertility bulls were collected using an artificial vagina and treated with a protease inhibitor, as reported before24,74. Right after semen collection, seminal plasma was subjected to a 10-min. centrifugation at 700 × g (4 °C). Afterwards, the resulting supernatant (seminal plasma) was transferred to a new tube and centrifuged again at 10,000 × g for 60 min., at 4 °C27. Following the second centrifugation, the supernatant was pipetted out into a cryotube, covered with Parafilm®M (Sigma-Aldrich, Darmstadt, Germany), then subjected to lyophilization using a Freeze Drier System (Labconco, Kansas City, MO, USA) (vacuum of 133 × 10−3 mBar, −40 °C). The samples were then stored at −80 °C for further analysis.
Protein quantification, trypsinization and desalting
Lyophilized seminal plasma samples were suspended in 0.02 M TEAB and soluble protein content was quantified using QubitTM assay (Thermo Fisher Scientific, Waltham, MA, USA). Twenty-five micrograms of seminal plasma proteins were aliquoted in a microtube and dried in vacuum. To each sample, 15 µl of lysis buffer containing 8 M urea, 0.02 M TEAB and 0.5 M DTT (dithiothreitol) were added, followed by incubation at 55 °C and 400 rpm agitation (Eppendorf® Thermomixer® R, Sigma-Aldrich, Darmstadt, Germany) for 25 min. Further, as an alkylation process, a volume of IAA (iodoacetamide) was added to reach a final concentration of 0.014 M. The mixture was maintained at 21 °C and 400 rpm in the dark for 40 min. Prior to protein digestion, a volume of digestion buffer was added to reach a final concentration of 0.005 M DTT, 0.001 M CaCl2 and 0.02 M TEAB in a final volume of 75 µl. All samples were digested with trypsin (Promega, Fitchburg, WI, USA) with a 1/50 (w/w) enzyme/substrate ratio and incubated at 37 °C for 18 h. A solution of TFA (trifluoroacetic acid) was added to a final concentration of 1% to stop tryptic activity75.
Ten stage tip C18 columns were manually made to perform peptide desalting using Empore TM SPE disks (Sigma-Aldrich, Darmstadt, Germany), as previously described76. Briefly, to prepare a stage tip C18 membranes 100% methanol was added to the column at centrifuged at 1,000 × g for 3 min. The same procedure was repeated twice: first with a solution containing 80% acetonitrile and 0.5% acetic acid and second with 5% acetic acid. Finally, tryptic-digested samples were added to columns and centrifuged at 900 × g during 5 min, followed by washing twice with 0.5% acetonitrile at 1,000 × g for 3 min. To elute peptides, the columns were centrifuged at 600 × g for 3 min with increasing concentrations of acetonitrile (25% to 80%) with 0.5% acetic acid. Then, samples were again subjected to peptide quantification prior to mass spectrometry analysis (QubitTM; Thermo Fisher, Waltham, MA, USA).
Label-free mass spectrometry
Three micrograms of tryptic digested peptides from each sample were individually applied to a Dionex Ultimate 3,000 liquid chromatographer (Thermo Scientific, Waltham, MA, USA) for reversed phase nano-chromatography. The peptides were injected into a 2 cm × 100 µm trap-column containing C18, 5 µm particles (Dr. Maisch GmbH, Germany). The peptides were eluted from this column to another analytical one (32 cm × 75 µm) containing C18, 3 µm particles (Dr. Maisch GmbH, Germany) and finally eluted to the spectrometer’s ionization source. The elution gradient was composed of 0.1% formic acid in water (solvent A), and 0.1% formic acid in acetonitrile (solvent B), in a gradient of 2 to 35% solvent B for 170 min.
Samples were analyzed in positive DDA (data dependent acquisition) mode in a label-free mass spectrometric approach using an Orbitrap Elite instrument (Thermo Fisher, Waltham, MA, USA), as previously reported77. The eluted fractions generated MS1 spectra between 300–1,650 m/z with a resolution of 120,000 FWHM at 400 m/z. The twenty most abundant ions from MS1 with charges larger than two were automatically selected to fragmentation (MS2) by higher-energy collisional dissociation (HCD) with an automatic gain control (AGC) of 1 × 106 and dynamic exclusion of 10 ppm for 90 s. HCD isolation window was set for 2.0 m/z, with 5 × 104 AGC, normalized collision energy of 35% and threshold for detection of 3,000.
Data analyses
MS1 spectra found in the chromatograms were aligned and, according to integrated intensity area from the XIC peaks generated by the respective ion, quantified using Progenesis QI software Nonlinear Dynamics (Waters, Milford, MA, USA). The protein identification was performed using Peaks software, which deduces sequences from the fragmentation information and searches in UniProt database. Protein identification information was inserted again in Progenesis QI program and combined with quantitative data generated previously.
Multivariate statistical analysis was performed using Progenesis QI software to evaluate differences in protein abundance in bulls of high and low fertility. Normalized abundances of proteins were plotted against fertility scores of each bull. A first statistical analysis was performed before protein identification to filter the MS1 features presenting ANOVA p-values < 0.05. Peaks 7.0 software was used with the fragmentation spectra and searched the Bos taurus Uniprot database, downloaded on 01/nov/2016. Parameters were set as following: precursor ion mass error tolerance of 10 ppm, MS/MS mass tolerance of 0.5 Da, carbamidomethylation of cysteine residues (fixed modification), deamidation and methionine oxidation (variable modifications). Trypsin was selected as the digestion enzyme, and up to two missed cleavage sites per peptide were allowed. The identified proteins were filtered at a rate of 1% for false discovery rate (FDR), and a minimum of 1 unique peptide per protein was required for identification. The protein input from Peaks were imported into the Progenesis QI software to generate quantitative data at the protein level. Multivariate Principal Component Analysis (PCA) was performed in Progenesis QI to evaluate protein abundances as related to phenotypes. Proteins were considered differentially abundant when presented p ≤ 0.05 after the ANOVA test at the protein level.
An additional multivariate analysis was carried out using MetaboAnalyst 3.0 (http://www.metaboanalyst.ca)78 considering the proteins significantly related to bull phenotypes. The protein dataset was normalized by sum, and Pareto-scaling was used to reduce relative importance of MS large values. Partial-Least Squares Discriminant Analysis (PLS-DA) was applied to differentiate classes in highly complex protein datasets, despite variability within each class. Variable Importance in Projection (VIP) based on the PLS-DA was used for the identification of biologically relevant features to categorize indicators of fertility. Then, variables with VIP >1.5 were considered important for group separation (high vs low fertility).
Normalized relative abundances of the regulated proteins were tested for correlation with the individual fertility scores using Spearman and Pearson correlation coefficient. The abundances of the three better positively correlated proteins and the three better negatively correlated proteins were used to calculate a rank score. Then, a protein-based fertility rank score was calculated based on a curve-fitting model of the protein abundances.
Functional clustering and networking of bull seminal plasma proteins
Gene ontology (GO) analysis was carried out using STRAP software, gathering information about biological process and molecular function from UniProtKB and EBI databases. Moreover, in silico analysis of protein-protein network was performed using STRING (http://string-db.org) version 9.0 database27. Interactions were evaluated for seminal plasma proteins associated with VIP score >1.5.
Electronic supplementary material
Acknowledgements
The present study was funded in part by Alta Genetics, Inc. and Mississippi Agricultural and Forestry Experiment Station, Selcuk University, Federal Research Councils of Brazil (CAPES, CNPq) and Research Foundation of Ceará State (FUNCAP). Grants 0439/11 and 0694/13 from “Financiadora de Estudos e Projetos” (FINEP, Brazil) were conceded to Sousa, M.V. through PROINFRA program. Scholarships for graduate students (Viana, A.G.A. and Pontes, A.F.) were provided by CAPES.
Author Contributions
The present study is the result of a multidisciplinary effort, with researchers from Brazil, USA and Holland. Samples were collected from bulls housed in the US (Alta Genetics Inc.) and analyzed in Brazil. A. Moura, E. Memili, A. Kaya and E. Topper defined the original concept of the study. Strategy for the proteomics analyses was defined by A. Moura, A. Martins, W. Fontes, M. Castro, C. Ricart and M. Sousa. Also, A. Viana, A. Pontes and A. Martins prepared the samples, including trypsinization, and carried out bioinformatics analysis. W. Fontes did the mass spectrometry analysis. The article was initially written by A. Viana, A. Martins, A. Moura, W. Fontes, C. Ricart and E. Memili, with subsequent significant revision by M. Sousa. All of the co-authors have reviewed the final revision of the submitted manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A.G.A. Viana and A.M.A. Martins contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34152-w.
References
- 1.Kaya A, Memili E. Sperm macromolecules associated with bull fertility. Anim Reprod Sci. 2016;169:88–94. doi: 10.1016/j.anireprosci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Parisi AM, Thompson SK, Kaya A, Memili E. Molecular, cellular, and physiological determinants of bull fertility. Turk J Vet Anim Sci. 2014;38:637–642. doi: 10.3906/vet-1404-76. [DOI] [Google Scholar]
- 3.Moura AA, Memili E. Functional aspects of seminal plasma and sperm proteins and their potential as molecular markers of fertility. Anim Reprod. 2016;13(3):191–199. doi: 10.21451/1984-3143-AR884. [DOI] [Google Scholar]
- 4.Salehi M, et al. Correlation between human clusterin in seminal plasma with sperm protamine deficiency and DNA fragmentation. Mol Reprod Dev. 2013;80:718–724. doi: 10.1002/mrd.22202. [DOI] [PubMed] [Google Scholar]
- 5.Feugang JM, et al. Transcriptome analysis of bull spermatozoa: implications for male fertility. Reprod Biomed Online. 2010;21:312–324. doi: 10.1016/j.rbmo.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Peddinti D, et al. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with bull fertility. BMC Syst Biol. 2008;2:19. doi: 10.1186/1752-0509-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velho, A. L. C. et al. Applications of metabolomics in reproductive biology. In: H Schatten & G. M. Constantinscu (Eds) Animal Models and Human Reproduction. Fisrt Edition. 509–517 (2017).
- 8.Juyena NS, Stelletta C. Seminal plasma: an essential attribute to spermatozoa. J Androl. 2012;33:536–551. doi: 10.2164/jandrol.110.012583. [DOI] [PubMed] [Google Scholar]
- 9.Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays. 1994;16:259–267. doi: 10.1002/bies.950160409. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath P, Thérien I. Role of seminal plasma phospholipid-binding proteins in sperm membrane lipid modification that occurs during capacitation. J Reprod Immunol. 2002;53:109–119. doi: 10.1016/S0165-0378(01)00098-5. [DOI] [PubMed] [Google Scholar]
- 11.Henault MA, Killian GJ, Kavanaugh JF, Griel LC. Effect of accessory sex gland fluid from bulls of differing fertilities on the ability of cauda epididymal sperm to penetrate zona-free bovine oocytes. Biol Reprod. 1995;52:390–397. doi: 10.1095/biolreprod52.2.390. [DOI] [PubMed] [Google Scholar]
- 12.Hao Y, et al. Osteopontin reduces polyspermy during in vitro fertilization of porcine oocytes. Biol Reprod. 2006;75:726–733. doi: 10.1095/biolreprod.106.052589. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Villamil P, et al. Purification of binder of sperm protein 1 (BSP1) and its effects on bovine in vitro embryo development after fertilization with ejaculated and epididymal sperm. Theriogenology. 2016;85:540–554. doi: 10.1016/j.theriogenology.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Assumpção TI, Fontes W, Sousa MV, Ricart CAO. Proteome analysis of Nelore bull (Bos taurus indicus) seminal plasma. Protein Pept Lett. 2005;12:813–817. doi: 10.2174/0929866054864292. [DOI] [PubMed] [Google Scholar]
- 15.Moura AA, Chapman DA, Koc H, Killian GJ. Proteins of the cauda epididymal fluid associated with fertility of mature dairy bulls. J Androl. 2006;27:534–541. doi: 10.2164/jandrol.05201. [DOI] [PubMed] [Google Scholar]
- 16.Kelly VC, et al. Characterization of bovine seminal plasma by proteomics. Proteomics. 2006;6:5826–5833. doi: 10.1002/pmic.200500830. [DOI] [PubMed] [Google Scholar]
- 17.Westfalewicz B, et al. Analysis of bull (Bos taurus) seminal vesicle fluid proteome in relation to seminal plasma proteome. J. Dairy Sci. 2016;100:1–17. doi: 10.3168/jds.2016-11866. [DOI] [PubMed] [Google Scholar]
- 18.Carrell DT, Aston KI, Oliva R, Emery BR, De Jonge CJ. The “omics” of human male infertility: integrating big data in a systems biology approach. Cell Tissue Res. 2016;363:295–312. doi: 10.1007/s00441-015-2320-7. [DOI] [PubMed] [Google Scholar]
- 19.Cadavid JAP, Alvarez A, Markert UR, Maya WC. Differential protein expression in seminal plasma from fertile and infertile males. J Hum Reprod Sci. 2014;7:206–211. doi: 10.4103/0974-1208.142485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacomini E, et al. Comparative analysis of the seminal plasma proteomes of oligoasthenozoospermic and normozoospermic men. Reprod Biomed Online. 2015;30:522–531. doi: 10.1016/j.rbmo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 21.González-Cadavid V, et al. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology. 2014;82:697–707. doi: 10.1016/j.theriogenology.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Killian GJ, Chapman DA, Rogowski LA. Fertility-associated proteins in Holstein bull seminal plasma. Biol Reprod. 1993;49:1202–1207. doi: 10.1095/biolreprod49.6.1202. [DOI] [PubMed] [Google Scholar]
- 23.Moura AA, Chapman DA, Koc H, Killian GJ. A comprehensive proteomic analysis of the accessory sex gland fluid from mature Holstein bulls. Anim Reprod Sci. 2007;98:169–188. doi: 10.1016/j.anireprosci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Rego JP, et al. Proteomic analysis of seminal plasma and sperm cells and their associations with semen freezbility in Guzerat bulls. J Anim Sci. 2016;94:5308–5320. doi: 10.2527/jas.2016-0811. [DOI] [PubMed] [Google Scholar]
- 25.Menezes EB, et al. Proteomic analysis of seminal plasma from locally-adapted “Curraleiro Pé-Duro bulls” (Bos taurus): identifying biomarkers involved in sperm physiology in endangered animals for conservation of biodiversity. Anim Reprod Sci. 2017;183:86–101. doi: 10.1016/j.anireprosci.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Tanghe S, Van Soom A, Sterckx V, Maes D, de Kruif A. Assessment of different sperm quality parameters to predict in vitro fertility of bulls. Reprod Domest Anim. 2002;37:127–132. doi: 10.1046/j.1439-0531.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 27.Rego JPA, et al. Seminal plasma proteome of electro ejaculated Bos inducus bulls. Anim Rep Sci. 2014;148:1–17. doi: 10.1016/j.anireprosci.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Manjunath P, Lefebvre J, Jois PS, Fan J, Wright MW. New nomenclature for mammalian BSP genes. Biol Reprod. 2009;80:394–397. doi: 10.1095/biolreprod.108.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souza CAE, Moura AA, Monaco E, Killian GJ. Binding patterns of bovine seminal plasma proteins A1/A2, 30 kDa and osteopon tin on ejaculated sperm before and after incubation with isthmic and ampullary oviductal fluid. Anim Reprod Sci. 2008;105:72–89. doi: 10.1016/j.anireprosci.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Thérien. I, Moreau R, Manjunath P. Bovine seminal plasma phopholipid binding proteins stimulate phospholipid efflux from epididymal sperm. Biol Reprod. 1999;61:590–598. doi: 10.1095/biolreprod61.3.590. [DOI] [PubMed] [Google Scholar]
- 31.Suarez SS. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016;363:185–194. doi: 10.1007/s00441-015-2244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bebbere D, et al. Expression of maternally derived KHDC3, NLRP5, OOEP and TLE6is associated with oocyte developmental competence in the ovine species. BMC Dev Biol. 2014;14:40. doi: 10.1186/s12861-014-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Correa LM, Cho C, Myles DG, Primakoff P. A role for a TIMP-3-sensitive, Zn(2+)−dependent metalloprotease in mammalian gamete membrane fusion. Dev Biol. 2000;225:124–134. doi: 10.1006/dbio.2000.9825. [DOI] [PubMed] [Google Scholar]
- 35.Leco KJ, Edwards DR, Schultz GA. Tissue inhibitor of metalloproteinases-3 is the major metalloproteinase inhibitor in the decidualizing murine uterus. Mol Reprod Dev. 1996;45:458–465. doi: 10.1002/(SICI)1098-2795(199612)45:4<458::AID-MRD8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.McCauley TC, Zhang HM, Bellin ME, Ax RL. Identification of a heparin-binding protein in bovine seminal fluid as tissue inhibitor of metalloproteinases-2. Mol Reprod Dev. 2001;58:336–341. doi: 10.1002/1098-2795(200103)58:3<336::AID-MRD12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Gallardo H, et al. Gamete therapeutics: recombinant protein adsorption by sperm for increasing fertility via artificial insemination. Plos One. 2013;8:1–7. doi: 10.1371/journal.pone.0065083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amălinei C, Căruntu I, Bălan RA. Biology of metalloproteinases. Rom J Morphol Embryol. 2007;48(4):323–334. [PubMed] [Google Scholar]
- 39.Hashizume K. Analysis of utero-placental-specific molecules and their functions during implantation and placentation in the bovin. J Reprod Dev. 2007;53(1):1–11. doi: 10.1262/jrd.18123. [DOI] [PubMed] [Google Scholar]
- 40.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen SJ, et al. Measurement of pro-C-type natriuretic peptide in plasma. Clin Chem. 2005;51:2173–2176. doi: 10.1373/clinchem.2005.053488. [DOI] [PubMed] [Google Scholar]
- 42.Xia H, et al. Role of C type natriuretic peptide in the function of normal human sperm. Asian J Androl. 2016;18:80–84. doi: 10.4103/1008-682X.154993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui Y, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostrowski MC, Kistler WS, Williams-Ashman HG. A flavoprotein responsible for the intense sulfhydryl oxidase activity of rat seminal vesicle secretion. Biochem Biophys Res Commun. 1979;87:171–176. doi: 10.1016/0006-291X(79)91662-0. [DOI] [PubMed] [Google Scholar]
- 45.Chang TS, Morton B. Epididymal sulfhydryl oxidase: a sperm-protective enzyme from the male reproductive tract. Biochem Biophys Res Commun. 1975;66:309–315. doi: 10.1016/S0006-291X(75)80329-9. [DOI] [PubMed] [Google Scholar]
- 46.Cornwall GA, Vindivich D, Tillman S, Chang TS. The effect of sulfhydryl oxidation on the morphology of immature hamster epididymal spermatozoa induced to acquire motility in vitro. Biol Reprod. 1988;39:141–155. doi: 10.1095/biolreprod39.1.141. [DOI] [PubMed] [Google Scholar]
- 47.Tury A, et al. Cell-specific localization of the sulphydryl oxidase QSOX in rat peripheral tissues. Cell Tissue Res. 2006;323:91–103. doi: 10.1007/s00441-005-0043-x. [DOI] [PubMed] [Google Scholar]
- 48.Moura AA, Souza CE, Stanley BA, Chapman DA, Killian GJ. Proteomics of cauda epididymal fluid from mature Holstein bulls. J Proteomics. 2010;73:2006–2020. doi: 10.1016/j.jprot.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez JG, Storey BT. Differential incorporation of fatty-acids into and peroxidative loss of fatty-acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–346. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 50.Go KJ, Wolf DP. Albumin-mediated changes in sperm sterol con-tent during capacitation. Biol Reprod. 1985;32:145–153. doi: 10.1095/biolreprod32.1.145. [DOI] [PubMed] [Google Scholar]
- 51.Singleton CL, Killian GJ. A study of phospholipase in albumin andits role in inducing the acrosome reaction of guinea pig spermatozoain vitro. J. Androl. 1983;4:150–156. doi: 10.1002/j.1939-4640.1983.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 52.Dhali A, Anchamparuthy VM, Butler SP, Pearson RE, Gwazdauskas FC. In vitro development of bovine embryos cultured with stem cell factor or insulin-like growth factor-I following IVF with semen of two bulls having different field fertility. Animl Reprod Sci. 2009;116:188–195. doi: 10.1016/j.anireprosci.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- 54.Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem. 1999;274:6875–6881. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- 55.Shojaei Saadi HA, et al. Proteins associated with critical sperm functions and sperm head are differentally expressed in morphlogocally abnormal bovine sperm induced by scrotal insulation. J Proteomics. 2013;82:64–80. doi: 10.1016/j.jprot.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim NM, Roman OJE, Troedsson MH, Crabo BG. Effect of scrotal insulation on clusterin-positive cells in ram semen and their relationship to semen quality. J Androl. 2001;22:863–77. [PubMed] [Google Scholar]
- 57.Zalata A, et al. Seminal clusterin gene expression associated with seminal variables in fertile and infertile men. J Urol. 2012;188:1260–1264. doi: 10.1016/j.juro.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Novak S, et al. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology. 2010;74:956–967. doi: 10.1016/j.theriogenology.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 59.Santos EA, et al. Protein profile of the seminal plasma of collared peccaries (Pecari tajacu Linnaeus, 1758) Reproduction. 2014;147:753–764. doi: 10.1530/REP-13-0220. [DOI] [PubMed] [Google Scholar]
- 60.Boe-Hansen GB, et al. Seminal plasma proteins and their relationship with percentage of morphologically normal sperm in 2-year-old Brahman (Bos indicus) bulls. Anim Reprod. 2015;162:20–30. doi: 10.1016/j.anireprosci.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Sprecher CA, Kisiel W, Mathewes S, Foster DC. Molecular cloning, expression, and partial characterization of a second human tissue-factor-pathway inhibitor. Proc Natl Acad Sci USA. 1994;91:3353–3357. doi: 10.1073/pnas.91.8.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyagi Y, et al. cDNA cloning and mRNA expression of a serine proteinase inhibitorsecreted by cancer cells: identification as placental protein 5 and tissue factor pathway inhibitor-2. J Biochem. 1994;116:939–942. doi: 10.1093/oxfordjournals.jbchem.a124648. [DOI] [PubMed] [Google Scholar]
- 63.Salem HT, Menabawey M, Seppala M, Shaaban MM, Chard T. Human seminal plasma contains a wide range of trophoblast-‘specific’ proteins. Placenta. 1981;5:413–418. doi: 10.1016/S0143-4004(84)80021-1. [DOI] [PubMed] [Google Scholar]
- 64.Salem HT, Menabawey M, Seppälä M, Shaaban MM, Chard T. Human seminal plasma contains a wide range of trophoblast-‘specific’ proteins. Placenta. 1984;5:413–417. doi: 10.1016/S0143-4004(84)80021-1. [DOI] [PubMed] [Google Scholar]
- 65.Schill WB, Miska W. Possible effects of the kallikrein-kinin system on male reproductive functions. Andrologia. 1992;24(2):69–75. doi: 10.1111/j.1439-0272.1992.tb02613.x. [DOI] [PubMed] [Google Scholar]
- 66.Schiemann PJ, Aliante M, Wennemuth G, Fini C, Aumiller G. Distribution of endogenous and exogenous 5′-nucleotidase on bovine spermatozoa. Histochemistry. 1994;101:253–262. doi: 10.1007/BF00315912. [DOI] [PubMed] [Google Scholar]
- 67.Adeoya-Osiguwa SA, Fraser LR. Fertilization promoting peptide and adenosine, acting as first messengers, regulate cAMP production and consequent protein tyrosine phosphorylation in a capacitation-dependent manner. Mol Reprod Dev. 2000;57:384–392. doi: 10.1002/1098-2795(200012)57:4<384::AID-MRD11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 68.Krześlak A, Lipińska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004;9:305–328. [PubMed] [Google Scholar]
- 69.Kovak MR, Saraswati S, Schoen DJ, Diekman AB. Investigation of galectin-3 function in the reproductive tract by identification of binding ligands in human seminal plasma. Am J Reprod Immunol. 2014;72:403–412. doi: 10.1111/aji.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanderpuye OA, Labarrere CA, McIntyre JA. The complement system in human reproduction. Am J Reprod Immunol. 1992;27(3-4):145–55. doi: 10.1111/j.1600-0897.1992.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 71.Robertson SA, Sharkey DJ. Seminal fluid and fertility in women. Fertil Steril. 2016;106:511–519. doi: 10.1016/j.fertnstert.2016.07.1101. [DOI] [PubMed] [Google Scholar]
- 72.Schjenken JE, Glynn DJ, Sharkey DJ, Robertson SA. TLR4 signaling is a major mediator of the female tract response to seminal fluid in mice. Biol Reprod. 2015;93:68. doi: 10.1095/biolreprod.114.125740. [DOI] [PubMed] [Google Scholar]
- 73.Moldenhauer LM, et al. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182:8080–8093. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- 74.De Oliveira RV, et al. Molecular morphology and function of bull spermatozoa linked to histones and associated with fertility. Reprod. 2013;146:263–272. doi: 10.1530/REP-12-0399. [DOI] [PubMed] [Google Scholar]
- 75.Arshid S, et al. High performance mass spectrometry based proteomics reveals enzyme and signaling pathway regulation in neutrophils during the early stage of surgical trauma. Proteomics Clin Appl. 2017;11:1–2. doi: 10.1002/prca.201600001. [DOI] [PubMed] [Google Scholar]
- 76.Arshid S, et al. Neutrophil proteomic analysis reveals the participation of antioxidant enzymes, motility and ribosomal proteins in the prevention of ischemic effects by preconditioning. J Proteomics. 2017;151:162–173. doi: 10.1016/j.jprot.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Gomes HAR, et al. Identification of multienzymatic complexes in the Clonostachys byssicola secretomes produced in response to different lignocellulosic carbon sources. J Biotechnol. 2017;254:51–58. doi: 10.1016/j.jbiotec.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Antoniassi MP, et al. Analysis of the functional aspects and seminal plasma proteomic profile of sperm from smokers. BJU Int. 2016;118:814–822. doi: 10.1111/bju.13539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.