Abstract
Rationale
Addiction theories posit that drug-related cues maintain and contribute to drug use and relapse. Indeed, our recent study in cocaine-dependent patients demonstrated that subliminally presented cocaine-related stimuli activate reward neurocircuitry without being consciously perceived. Activation of reward neurocircuitry may provoke craving and perhaps prime an individual for subsequent drug-seeking behaviors.
Objectives
Using an equivalent paradigm, we tested whether cannabis cues activate reward neurocircuitry in treatment-seeking, cannabis-dependent individuals and whether activation was associated with relevant behavioral anchors: baseline cannabis craving (drug-seeking behavior) and duration of use (degree of conditioning).
Methods
Twenty treatment-seeking, cannabis-dependent individuals (12 males) underwent event-related blood oxygen level-dependent functional magnetic resonance imaging during exposure to 33-ms cannabis, sexual, and aversive cues presented in a backward-masking paradigm. Drug use history and cannabis craving were assessed prior to imaging.
Results
Participants showed increased activity to backward-masked cannabis cues in regions supporting reward detection and interoception, including the left anterior insula, left ventral striatum/amygdala, and right ventral striatum. Cannabis cue-related activity in the bilateral insula and perigenual anterior cingulate cortex was positively associated with baseline cannabis craving, and cannabis cue-related activity in the medial orbitofrontal cortex was positively correlated with years of cannabis use. Neural responses to backward-masked sexual cues were similar to those observed during cannabis cue exposure, while activation to aversive cues was observed only in the left anterior insula and perigenual anterior cingulate cortex.
Conclusions
These data highlight the sensitivity of the brain to subliminal reward signals and support hypotheses promoting a common pathway of appetitive motivation.
Keywords: Addiction, Cannabis cues, Neuroimaging, Cannabis craving, Subliminal, Marijuana cues, Marijuana craving, Reward
Introduction
Cannabis is the most widely used illicit substance in the USA (Substance Abuse and Mental Health Services Administration 2012). Although commonly considered a “soft option” drug (Raphael et al. 2005), chronic cannabis use is associated with several negative consequences, including an increased likelihood of experiencing depression and anxiety (Pacek et al. 2012), high-risk sexual behavior (Schuster et al. 2012), and a range of physiological, cognitive, and neural abnormalities (Crane et al. 2012; Jacobus et al. 2012). Despite these negative consequences, cannabis users often report multiple quit attempts without achieving prolonged abstinence (Budney et al. 2008).
According to theoretical models of addiction, one factor contributing to continued drug use and relapse is exposure to drug-related stimuli, or cues (Milton and Everitt 2012; O'Brien et al. 1998). Exposure to cannabis-related cues, such as seeing cannabis or cannabis-related paraphernalia, elicits increased physiological arousal (Wolfling et al. 2008), subjective craving (Lundahl and Johanson 2011; Nickerson et al.2011, and increased neural activity in reward-related brain regions (Cousijn et al. 2012; Filbey et al. 2009; Goldman et al. 2012. As such, cannabis cues are often defined as conscious triggers for cannabis craving and use, and strategies to avoid and manage exposure to cannabis cues are part of many treatment approaches.
Although coping with conscious triggers may be effective for some individuals, it is possible that the brain's response to reward cues may begin outside conscious awareness (Berridge and Winkielman 2003; Winkielman et al. 2003) and, consequently, may not be amenable to existing cognitive treatment approaches (Asensio etal. 2010). Indeed, functional magnetic resonance imaging (fMRI) studies using subliminal paradigms (i.e., brief presentation of stimuli that are attended to but not consciously perceived) support the notion that exposure to drug-related cues, even when presented outside awareness, activates reward neurocircuitry (Childress et al. 2008; Zhang et al. 2009). Using fast event-related fMRI and a backward-masking paradigm, we previously demonstrated that cocaine cues activated the insula, ventral striatum (VS), and amygdala—without being consciously perceived (Childress et al. 2008). Though this study demonstrated that subliminal cocaine cues can trigger reward circuitry, it is not known whether these findings generalize to other drugs of abuse and populations, such as cannabis-dependent individuals.
Thus, the aim of the present study was to examine whether cannabis-dependent individuals respond to cannabis cues presented in the backward-masking paradigm with activation of reward neurocircuitry and to investigate the relationships between applicable behavioral end points (i.e., cannabis craving and years of cannabis use). Based on our previous findings, we hypothesized that cannabis-dependent individuals would show increased activity to backward-masked cannabis cues in the insula, VS, and amygdala (Childress et al. 2008) and potentially in additional regions involved in salience and reward, such as the anterior cingulate cortex, the medial prefrontal cortex, and the hippocampus. Given that an extensive literature suggests that heightened motivational/emotional states (e.g., hunger, drug craving) lead to heightened sensitivity to associated cues (Martens et al. 2013; Siep et al. 2009), we hypothesized that cannabis craving reported prior to the imaging session would positively correlate with these cannabis cue-related activations. Further, based on the principles of simple Pavlovian conditioning, we hypothesize that drug cues acquire the ability to trigger brain reward circuitry such that greater drug experience should lead to stronger responses. Support for these hypotheses would improve our understanding of the automatic processes underlying cannabis use and help guide treatment strategies.
A secondary aim was to examine whether cannabis-dependent individuals respond similarly to emotionally evocative cues. One hypothesis debated in the field is that chronic drug use leads to the devaluation of natural rewards; however, research in this area has provided mixed results (Cabeza de Vaca and Carr 1998; Goldstein et al. 2010; Grigson and Twining 2002; Klucken et al. 2013). In our previous work in cocaine-dependent patients (Childress et al. 2008), increased brain responses in reward neurocircuitry were observed to both backward-masked cocaine and sexually evocative cues. Thus, we examined whether chronic cannabis use altered reactivity to natural reward and emotionally evocative stimuli by including sexual and nonrewarding, aversive cues in our backward-masking paradigm.
Methods
Participants
Participants were cannabis-dependent individuals who were taking part in the neuroimaging cannabis outpatient treatment study of a larger, ongoing three-arm (i.e., cocaine, cannabis, prescription opiates) study examining neural and behavioral features predictive of treatment outcomes. For the current study, only baseline neuroimaging data from 23 chronic, heavy cannabis smokers (i.e., cannabis use on 10 or more days in the last month or >240 days in the past 2 years (Pope et al. 2001)) who responded to radio advertisements and local list serves describing a neuroimaging outpatient treatment study for cannabis dependence and met the DSM-IV (American Psychiatric Association 1994) criteria for cannabis dependence are presented. Data for the current study were collected at the baseline session, and as such, participants had not begun treatment nor had they set a quit date. Exclusion criteria included current DSM-IV Axis I diagnoses (other than cannabis or nicotine dependence), lifetime history of head injury with loss of consciousness for more than 3 min, contraindications for magnetic resonance imaging, current treatment for cannabis dependence, clinically significant medical conditions, and use of medication interacting with the central nervous system. Individuals were excluded due to structural artifact (n =1) or experiencing claustrophobia upon entering the scanner (n =2). The remaining data from 20 participants (12 males) were used in statistical analyses.
Procedures
This study adhered to the Declaration of Helsinki and was approved by the University of Pennsylvania Institutional Review Board. After completing a detailed telephone screen, participants gave informed consent, completed psychological and medical screening, and participated in an MRI session. To minimize potential substance-induced cerebral blood flow changes, participants were asked to abstain from alcohol and illicit substances for 24 h prior to the MRI session (average self-reported abstinence of cannabis use was 1.5±1.5 days). Of note, urinalysis cannot detect 24-h abstinence of Δ9-tetrahydrocannabinol (THC) metabolites; however, urinalyses increase accuracy of self-reported substance use (Roese and Jamieson 1993). Thus, participants were tested for substance use with a urine drug test and alcohol breathalyzer. All participants were positive for cannabis use and negative for other substance use. Participants met with clinical staff prior to scanning to review MRI scanning tasks and to assess for acute cannabis effects. MRI scans were rescheduled for participants under the influence of drugs or alcohol (n =1). Participants received $135 for completing the screening and MRI sessions.
Clinical assessments
All participants were screened for the presence of Axis I psychiatric disorders using the Mini International Neuropsychiatric Interview (Sheehan et al. 1997). Cannabis and other drug use during the preceding 30 days was quantified using the Timeline Followback method (Sobell and Sobell 1991). Cannabis use and cannabis-related problems, as well as other illicit drug use and problems, were assessed with the Addiction Severity Index (ASI) (McLellan et al. 1992). The Marijuana Craving Questionnaire-Short Form (MCQ-SF) (Heishman et al. 2009) measured self-reported baseline cannabis craving before the neuroimaging session. The MCQ-SF is a four-factor structured scale covering behavioral experiences associated with aversive and appetitive aspects of drug motivation (i.e., compulsivity, emotionality, expectancy, purposefulness). The magnitude of cannabis craving was determined by summing all items for a total score, which results in a more reliable, internally consistent measure of the phenomenon. The Fagerstrom Test for Nicotine Dependence (FTND) measured nicotine dependence severity (Heatherton et al. 1991).
Stimuli and fMRI backward-masking task
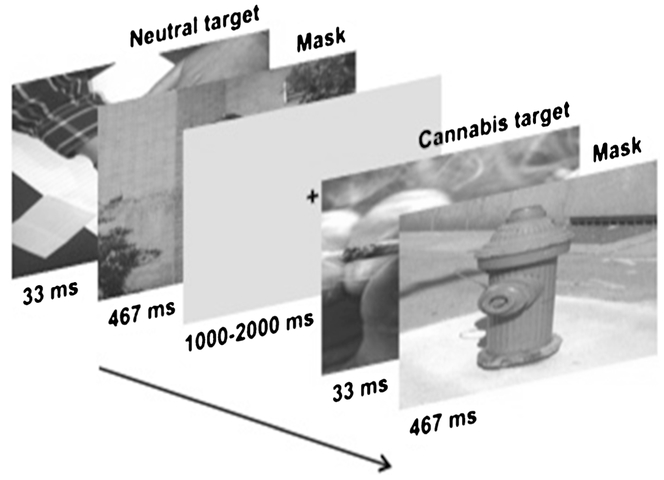
To measure neural responses to subliminally presented stimuli, we used fMRI during a backward-masking paradigm (Childress et al. 2008) designed to prevent conscious processing of targets. In backward-masking paradigms, conscious visual processing of a very brief target is eclipsed if a longer “masking” stimulus is presented directly after the target, causing a disruption in the relay of the original stimulus from the retina to the visual cortex (Brooks et al. 2012). The effectiveness of our backward-masking procedures for 33-ms targets and 467-ms masking stimuli was demonstrated with a forced-choice categorization test in our previous research (Childress et al. 2008). Consequently, in an event-related fMRI design (Fig. 1), each target image was displayed for 33 ms followed by a 467-ms masking stimulus, for a total of 500 ms of visual stimuli. Importantly, the stimulus-onset asynchrony (SOA) between target and mask was 33 ms (Brooks et al. 2012; Carlsson et al. 2004), with the mask immediately following the target. The interstimulus interval (ISI) was of random duration between 1,000 and 2,000 ms. As in Childress et al. (2008), target stimuli consisted of 24 drug-related (in this case, cannabis), 24 sexual, 24 aversive, and 24 neutral images. Cannabis images were pictures of cannabis, cannabis-related paraphernalia, and individuals engaged in cannabis smoking using various modes of administration. Sexual (mean arousal rating = 6.36) and aversive (mean arousal rating = 6.23) images were taken from the International Affective Pictures System (IAPS) (Lang et al. 2008). Neutral targets and masking stimuli were taken from our laboratory archive of nondrug images (e.g., building facades, people engaged in everyday activities, etc.). Images were matched on size, average luminosity, and context/complexity. Targets and masks were paired randomly, and each target–mask pair was presented quasi-randomly (no more than two targets of the same category in succession) without replacement until all target–mask pairs were shown (1 cycle: 24 target–mask pairs × 4 categories = 96 trials); a second cycle followed. Prior to the task, participants were told the following: “Pay attention to each picture. There is a small test later to see which pictures you remember.”
Fig. 1.
Backward-masking cue paradigm. Twenty-four targets from four categories (i.e., cannabis, aversive, sexual, neutral) and neutral masks were paired randomly, and each target–mask pair was presented quasi-randomly (no more than two targets of the same category in succession) without replacement. Targets were presented for 33 ms followed by a 467-ms neutral mask. As requested by the International Affective Picture System (IAPS), sexual and aversive images are not depicted. All task images are presented in color, but are shown here in grayscale
Guided by OptSeq, 33 null stimuli (gray screens with a small black fixation cross) were inserted into each cycle of the stimulus sequence (quasi-random, no more than two in succession) to improve the efficiency of the event-related design, but were not part of the formal statistical analysis. Total task time was approximately 8.5 min.
MR acquisition
Imaging data were acquired on a 3.0-T Trio whole-body scanner (Siemens AG, Erlangen, Germany) equipped with a standard eight-channel receive-only array head coil. Scan sessions began with shimming and sagittal localization. Three-dimensional T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE) structural images were collected transaxially (repetition time (TR) 1, 510 ms, echo time (TE) 3.7 ms, 160 slices, slice thickness 1 mm, field of view (FOV) 192×256 mm, flip angle 90°). Whole-brain gradient-echo, echo-planar images (EPI) sensitive to blood oxygen level-dependent (BOLD) signal were acquired with the following parameters: TR 2,000 ms, TE 30 ms, 32 slices, slice thickness 4.5 mm, FOV 64×64 mm, and flip angle 90°.
Data processing and analysis
Imaging data were analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK). After slice timing correction, functional images of each participant were realigned and unwarped, co-registered to the structural MRI image, normalized to the Montreal Neurological Institute (MNI) standard space, and smoothed using an 8-mm full-width at half maximum Gaussian kernel. First-level (within-subject) analyses were conducted individually for each participant with a general linear model (GLM) to quantify the relationship between the observed event-related (BOLD) signals and regressors encoding experimental condition conditions (i.e., cannabis cues, sexual cues, aversive cues). Regressors were created by convolving experimental condition functions with the canonical hemodynamic response function (Friston et al. 1998). The six motion estimates created during motion correction were entered as covariates of no interest. First-level (subject specific) contrast images were entered into the second-level (random effects) group-level analyses. In addition to the contrast of interest, correlations between subliminal cannabis-cue-induced brain activity (relative to neutral cues) and (1) baseline cannabis craving and (2) years of cannabis use were computed for all subjects by performing regression analysis within an a priori region of interest (ROI) mask to explore brain-behavioral associations.
Imaging analyses were focused on ROIs associated with processing of reward-related stimuli, specifically the medial orbitofrontal cortex (mOFC), insula, perigenual anterior cingulate cortex (pgACC), VS, hippocampus, and extended amygdala (i.e., amygdala, bed nucleus of stria terminalis) (Brooks et al. 2012; Chase et al. 2011; Kuhn and Gallinat 2011a). These ROIs were joined into one mask of 9,602 (2× 2×2 mm3) voxels (see Online Resource 1 or our interactive website: http://franklinbrainimaging.com). The ROI mask was created using the Harvard-Oxford probabilistic anatomical atlas provided with the FMRIB Software Library (FSL) (Smith et al. 2004). To control for type 1 error, neural activity within the ROI mask of each voxel was considered significant at a nominal alpha level of p <0.01 and a cluster extent of 121 contiguous resampled voxels as determined via Monte Carlo simulations using 3dClustSim Analysis of Functional NeuroImages (AFNI) software (Cox 1996) (http://afni.nimh.nih.gov/).
Secondary analyses examined neural responses to backward-masked cues using the following contrasts: (1) sexual versus neutral cues, (2) cannabis versus sexual cues, (3) aversive versus neutral cues, and (4) cannabis versus aversive cues, following the same first- and second-level analyses described above.
Results
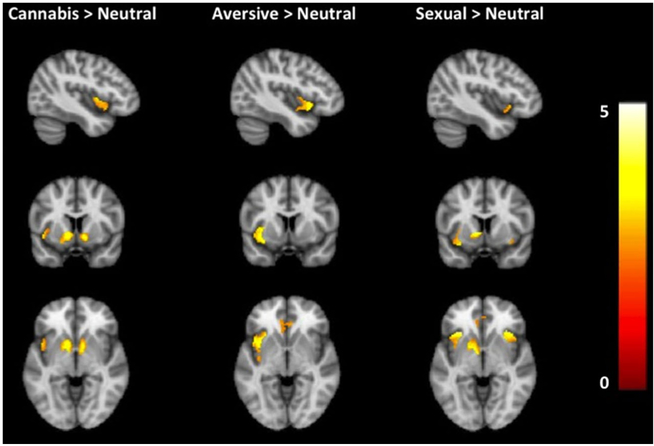
Demographic, self-report, and substance use characteristics are presented in Table 1. Subjects reported no illicit substance use other than cannabis in the past 30 days. Cannabis-dependent individuals displayed enhanced responses to backward-masked cannabis versus neutral cues in the left anterior insula, left VS/amygdala, and right VS (Table 2, Fig. 2). There were no areas wherein activation was greater to neutral cues relative to cannabis cues.
Table 1.
Participant characteristics
| N=20 | Means (SD) | |
|---|---|---|
| Sex | 12 males (60 %) | |
| Race | 18 African American (90 %) | |
| Report drinking alcohol | 7 (35 %, 7 males) | |
| Cigarette smokers | 8 (40 %, 5 males) | |
| Age | 29.1 (9.7) | |
| Years of education | 12.4 (3.0) | |
| Alcohol use (days/past 30 days) | 1.5 (2.3) | |
| Alcohol use (drinks/drinking day) | 1.3 (0.5) | |
| Cigarette dependence (FTND) | 1.5 (2.2) | |
| Duration of heavy cannabis use (years) | 11.4 (7.9) | |
| Cannabis use (days/30 days) | 27.8 (3.7) | |
| Cannabis use (g/day) | 2.3 (2.7) | |
| Cannabis craving (MCQ-SF)a | 40.5 (14.0) | |
| ASI Composite Scoresb | ||
| Alcohol | 0.05 (0.05) | |
| Drug | 0.20 (0.10) | |
| Family/social | 0.16 (0.20) |
SD standard deviation, FTND Fagerstrom Test for Nicotine Dependence, MCQ-SF Marijuana Craving Questionnaire-Short Form
MCQ-SF craving scores can range from 12 to 84 (minimal to maximal intensity). Current sample ranged from 12 to 63
Addiction Severity Index Composite Scores in each domain range from 0 (no problems) to 1 (severe problems)
Table 2.
Neural activation to backward-masked cannabis, sexual, and aversive cues in a priori regions
| Region | Cluster size (voxels) | MNI coordinates |
T values | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Cannabis cues > neutral cues | |||||
| L ventral striatum/amy | 579 | −10 | 10 | −4 | 4.25 |
| R ventral striatum | 209 | 10 | 8 | −6 | 4.14 |
| L insula | 150 | −42 | 2 | 4 | 3.52 |
| Sexual cues > neutral cues | |||||
| L ventral striatum | 893 | −8 | 6 | 2 | 4.40 |
| L insula | 378 | −30 | 26 | −6 | 4.71 |
| R insula | 287 | 36 | 22 | −8 | 4.42 |
| R hippo/amy | 187 | 18 | −14 | −24 | 3.86 |
| L perigenual ACC | 745 | −10 | 36 | 12 | 3.84 |
| Aversive cues > neutral cues | |||||
| L insula | 591 | −32 | 12 | −8 | 4.35 |
| L perigenual ACC | 344 | −10 | 28 | 20 | 3.50 |
MNI coordinates and T values of local maxima are shown for each cluster. Significant regions of interest level, p <0.01; cluster was corrected at p <0.05 (k >121 voxels)
MNI Montreal Neurological Institute, L left, R right, amy amygdala, hippo/amy hippocampus/amygdala, ACC anterior cingulate cortex
Fig. 2.
Neural activation during exposure to backward-masked cannabis, sexual, and aversive cues in cannabis-dependent treatment seekers. Cannabis cues (versus neutral) elicited greater activation in the left anterior and bilateral ventral striatum. Sexual cues (versus neutral) elicited greater activation in the left ventral striatum, bilateral anterior insula, right hippocampus/amygdala, and left perigenual anterior cingulate cortex. Aversive cues (versus neutral) elicited greater activation in the left insula and perigenual anterior cingulate cortex. Data are displayed neurologically (left is left). The color bar represents T values. MNI coordinate of all images displayed at −42, 8, and −4. An interactive visual display of unmasked brain data in all three planes at p =0.01 can be found at http://franklinbrainimaging.com
Next, we examined the extent to which MCQ-SF scores assessed just prior to the imaging session and number of years of cannabis use was correlated with neural activation to cannabis cues (compared to neutral cues). β coefficients were extracted from significant clusters and averaged to calculate the association between behavioral scores and brain responses. Baseline cannabis craving scores were positively correlated with brain activation to backward-masked cannabis cues in the bilateral insula (right: r =0.64, p =0.002; left: r =0.57, p =0.008) and pgACC (r =0.56, p =0.01) (Fig. 3a, b). Number of years of cannabis use positively correlated with brain activation to cannabis cues in a cluster spanning the mOFC (r =0.69, p =0.001) (Fig. 3c). We observed no inverse correlations with the MSQ-SF craving scores or years of cannabis use. Cannabis craving and cannabis use history did not correlate with brain activation to sexually evocative or aversive pictures.
Fig. 3.
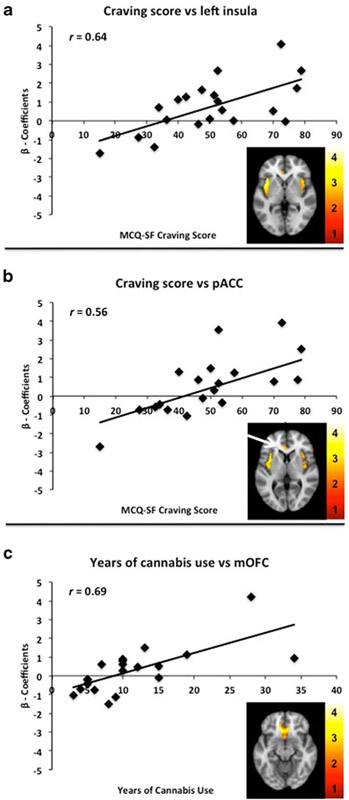
Correlations between brain responses during backward-masked cannabis cues and cannabis craving or years of cannabis use. Scatterplots showing significant correlations between MCQ-SF craving scores and β coefficients extracted from the cluster and averaged. Illustrated are statistical maps in the axial view: a Cannabis craving versus left insula, x = −40; b cannabis craving versus perigenual anterior cingulate cortex (pgACC), x = −4; and c years of cannabis use versus medial orbitofrontal cortex (mOFC), x = 8. Data are displayed neurologically (left is left). The color scale represents the T values. An interactive visual display of unmasked brain data in all three planes at p =0.01 can be found at http://franklinbrainimaging.com
Secondary analyses examined neural responses to backward-masked sexual and nonrewarding, aversive stimuli compared to neutral cues and cannabis cues. Cannabis-dependent individuals displayed enhanced responses to backward-masked sexual cues relative to neutral cues in the left VS, bilateral anterior insula, right hippocampus/amygdala, and pgACC (Table 2, Fig. 2); however, there were no areas wherein activation was significantly different during subliminal exposure to cannabis cues compared to sexual cues. In the aversive cue versus neutral cue contrast, cannabis-dependent individuals displayed enhanced responses to backward-masked aversive cues in the left anterior insula and pgACC (Table 2, Fig. 2), but did not show VS activity that was observed during the subliminal cannabis and sexual cue contrasts. In the cannabis cue versus aversive cue comparisons, there were no significant differences in activation. Online Resource 2 provides a table of these whole-brain analyses.
Discussion
Using visual backward masking of cannabis-related cues, we found additional evidence to support the hypothesis that drug-related cues presented outside awareness activate reward-related circuitry. Specifically, our data indicate that cannabis-related cues evoke neural activity in the left anterior insula, left VS/amygdala, and right VS. Thus, our results replicate and extend previous research in cocaine-dependent individuals (Childress et al. 2008) by using the same backward-masking task with cannabis-related cues and finding similar reward-related activations in a different patient population, specifically those who are cannabis dependent. Further, our findings provide further support for one role of the anterior insula, which is bottom-up detection of salient stimuli and drug craving (Menon and Uddin 2010; Naqvi and Bechara 2010). Our data also provide support for the involvement of the mOFC in reward learning and incentive salience, as evidenced by the positive correlation between brain responses to cannabis cues presented outside awareness and years of cannabis use. Lastly, the demonstration that cannabis-dependent individuals display enhanced reward-related responses to sexually evocative cues supports hypotheses promoting a common pathway of appetitive motivation.
Neural responses to backward-masked cues
Among treatment-seeking, cannabis-dependent participants, backward-masked cannabis cues (relative to neutral) elicited greater neural responses in the left anterior insula, left VS/amygdala, and right VS. The involvement of the VS in subliminal processing of drug-related cues is consistent with an extensive literature indicating that this region, as part of the mesolimbic dopamine (DA) pathway (i.e., DA cells in the ventral tegmental area project into the VS), plays a crucial role in reward processes (Koob and Volkow 2010; Wise 2009), especially cue reactivity (Caggiula et al. 2001; Franklin et al. 2007; Schacht et al. 2013). Drugs of abuse stimulate surges of DA in the VS, thus strengthening stimulus–drug associations (Berridge and Robinson 1998; Robinson and Berridge 2003). Through repeated drug use and stimulus–drug pairings, drug-related stimuli acquire powerful incentive properties and motivate drug-seeking behavior (Berridge and Robinson 1998; Robinson and Berridge 2003). As such, our findings suggest that even subliminal exposure to stimuli associated with one's drug of choice activates this conditioned salience and reward neurocircuitry, perhaps eliciting craving and “priming” an individual for subsequent drug-seeking behaviors.
One hypothesis debated in the field is that chronic drug use leads to the devaluation of natural rewards; however, research in this area has provided mixed results (Cabeza de Vaca and Carr 1998; Goldstein et al. 2010; Klucken et al. 2013). Secondary analyses of neural responses to backward-masked sexual cues versus neutral cues revealed robust limbic activations, including the left VS, bilateral anterior insula, right hippocampus/amygdala, and pgACC. In addition, direct comparisons between neural activation to backward-masked cannabis cues relative to sexual cues revealed no significant differences, suggesting that in this sample of cannabis-dependent individuals, responses to natural rewards are not diminished. These findings are consistent with our previous research examining neural responses to backward-masked sexual cues among cocaine-dependent patients (Childress et al. 2008). Further, we observed a substantial anatomical overlap in the brain response to subliminal cannabis and sexual cues, which may be expected given that both cannabis and sexual cues are known to evoke reward-related responses and evidence suggesting common substrates for both drug and natural rewards (Pessiglione etal. 2007; Schroeder et al. 2001; Tang et al. 2012), although see Zombeck et al. (2008). These results are also in line with the findings of Versace et al. (2011) that showed cigarette-dependent individuals who showed brain responses to smoking cues also showed similar brain responses to sexually evocative stimuli. Although these data suggest a common substrate for drug and natural rewards, firmer conclusions could be drawn if an association between craving for sex and presentation of backward-masked sexual stimuli was observed; however, craving to sexual cues was not assessed.
Additional analyses explored neural responses to backward-masked aversive versus neutral cues and revealed greater responses in the left insula and pgACC; however, aversive cues did not activate the VS. Thus, cannabis-dependent individuals showed specific ventral striatal responses to both drug and natural subliminal reward cues, whereas aversive cues elicited responses in the brain regions involved in interoception and behavioral regulation (Menon and Uddin 2010). Studies have shown activation of the amygdala and/or VS during exposure to aversive stimuli (Klucken et al. 2009), yet others have not (Klucken et al. 2012). Indeed, comparisons of neural activation to backward-masked cannabis cues versus aversive cues revealed no areas wherein cannabis cues elicited greater activity relative to aversive cues. While there are many potential explanations for this null finding (e.g., conditioning and personal histories), our findings should be examined further in a in a larger, more gener-alizable sample prior to drawing definitive conclusions.
The insula, particularly the anterior portion, is a highly interconnected brain region that detects and integrates emotionally salient sensory and visceral stimuli and, as such, is associated with feeling states and interoceptive awareness (Craig 2009, 2011). For example, anterior insula activation has been observed during the presentation of fearful stimuli (Klucken et al. 2009), sexual stimuli (Kuhn and Gallinat 2011b), and drug-related stimuli (Franklin et al. 2009b; Potenza et al. 2012). Consistent with these findings, the anterior insula showed enhanced responses to cannabis, sexual, and aversive subliminal stimuli compared to neutral stimuli and, consequently, may reflect bottom–up detection and internal somatosensory responses to backward-masked emotionally relevant stimuli.
Correlations with cannabis craving and years of cannabis use
Brain activation does not provide information about hedonic tone or reward value. For example, regions that typically activate in response to rewarding stimuli also activate to fearful stimuli (Klucken et al. 2009). Thus, to test whether the brain activation is related to these important properties, we utilized relevant behavioral anchors, such as craving and years of use. Cannabis craving prior to the imaging session was positively correlated with activation in the bilateral insula and pgACC. These findings are consistent with research indicating that drug-related cues activate the insula and that activity in the insula is positively correlated with drug craving (Bonson et al. 2002; Franklin et al. 2009b). The anterior cingulate cortex is involved in several monitoring, decision-making, and cognitive control processes (Isomura and Takada 2004; Rushworth et al. 2011), and the correlation between subliminal cannabis cue reactivity in the pgACC and cannabis craving may reflect interindividual variability in attempts to regulate responses to subliminal cannabis cues detected by the anterior insula. Indeed, a recent theoretical model (Menon and Uddin 2010) posits that the insula and anterior cingulate cortex form a “salience network” that detects externally salient stimuli, modulates subsequent autonomic reactivity, and prepares for appropriate behavioral responses.
In addition, the total number of years of cannabis use was positively correlated with activation in a cluster spanning the mOFC. The mOFC is implicated in reward processing, which includes reward valuations (Diekhof et al. 2012; Elliott et al. 2000). Thus, our findings may suggest that the longer an individual has used cannabis, which suggests a greater number of “pairings” between cannabis cues and drug reward, the greater opportunity there is for conditioned modifications to occur in reward-related brain regions. Together, our data provide support for theories purporting that drug dependence is a disorder of aberrant learning and modification of neural pathways due to repeated pairings of drug-related stimuli and drug use outcomes (Milton and Everitt 2012).
Potential limitations
A limitation to consider when interpreting the findings is the small sample size, with most participants identifying themselves as African-American. To this end, future studies should include a larger sample with greater diversity to validate these findings and ensure generalizability. In addition, 40 % of the cannabis-dependent smokers in the current study also smoked cigarettes, and although it has not been shown that cannabis cues can mimic cigarette cues, it is conceivable. As such, the inclusion of cigarette smokers may confound the findings; however, the low dependence on cigarettes in subjects who participated in this study suggests that any potential confounding influence of cigarette smoking is minimal. It is also conceivable that greater response to the cannabis cues could be contributed to greater complexity of cannabis cues compared to the other emotionally evocative cues; however, all images were carefully matched on context, complexity, size, and luminosity. This study did not assess cannabis withdrawal symptoms on all subjects, and as such, it remains unclear as to whether withdrawal symptoms could have influenced findings. Additional studies are needed in order to examine the effects of withdrawal on cannabis cue reactivity. In addition, this study did not include a non-cannabis-using control group to explicitly show specificity of response to cannabis cues; however, it has been shown repeatedly that nonusers do not exhibit neural responses to drug cues (Childress et al. 1999; George et al. 2001; Grant et al. 1996). Consequently, we did not acquire data on nonusing controls. It is important to note that attention may influence our findings. As such, future research should examine attentional processes during the backward-masking task or following the scan session through an image recollection test. Finally, participants in this study were not immediately tested with a forced choice recognition task, but remained in the scanner for additional data acquisition required as part of a larger, ongoing three-arm study. We have previously shown that our backward-masking paradigm is effective in masking brief drug cues (Childress et al. 2008), and further support is provided by a recent meta-analysis across 12 studies showing that backward-masked stimuli were not consciously perceived even when presentations last for up to 50 ms (Brooks et al. 2012).
Clinical implications
One of the most commonly used evidence-based behavioral treatments for drug dependence is cognitive behavioral therapy (CBT), in which an individual learns how to identify triggers (e.g., cues) for drug use and how to reduce craving and drug use through specific strategies and skills (Carroll 1998). Although there is empirical evidence that CBT and other behavioral treatments are effective, they yield modest effect sizes and outcomes vary widely across individuals (Dutra et al. 2008). Based on our findings, behavioral treatment approaches that focus on the conscious identification of cues for drug use and/or feeling states may face an inherent limitation: cue-triggered reward neurocircuitry may be activated outside conscious awareness. Thus, in some cases, reward neurocircuitry is already stimulated before triggers can be consciously identified or avoided, and in other cases, cognitive control circuitry is recruited too slowly for effective management of emergent craving. Alternative or supplemental interventions to standard treatments might aim to address the neuropsychological mechanisms underlying the rapid, automatic neural response to reward-related stimuli through cognitive training and pharmacological interventions. For example, a recent study of cognitive bias modification (CBM), an approach that aims to modify automatic action tendencies, showed that four brief sessions of retraining automatic action tendencies (from approach bias to avoidance bias) improved treatment outcomes at 1-year follow-up in alcohol-dependent patients (Wiers et al. 2011). Similarly, a pharmacological intervention with a medication such as the purported anti-craving/anti-relapse agent baclofen, which blocks drug-motivated behaviors in animals (Brebner et al. 2002; Fattore et al. 2009) and humans (Addolorato et al. 2011; Franklin et al. 2009a) and reduces “resting” brain activity in reward-related circuitry in humans (Franklin et al. 2011, 2012), may prove effective in drug-dependent individuals who are responsive to subliminal cues. Thus, cognitive and pharmacological interventions aimed at altering or reversing the automatic, aberrant learning processes associated with repeated drug use may provide tailored, comprehensive relapse protection and improve drug use outcomes.
Summary and conclusions
Our findings represent the first evidence that cannabis-dependent individuals exhibit increased brain responses to backward-masked cannabis cues in the left anterior insula, left VS/amygdala, and right VS. Reactivity to cannabis cues was associated with relevant behavioral anchors including cannabis craving and years of cannabis use. These findings are consistent with, and extend, previous research demonstrating that drug cues presented outside awareness can activate the brain's reward network. The data highlight the sensitivity of the brain to reward-related cues, and the brain response to such cues may be both a meaningful target for cannabis-dependence treatments and a screening tool for the discovery of therapeutics that address unconscious drug motivation. Ongoing research will help establish the link between the brain response to subliminal drug cues and subsequent drug use/relapse.
Acknowledgments
This research was made possible by the Pennsylvania Department of Health Commonwealth Universal Research Enhancement (CURE) funding.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00213-013-3342-z) contains supplementary material, which is available to authorized users.
Contributor Information
Reagan R. Wetherill, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA
Anna Rose Childress, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA; Philadelphia VA Medical Center, Philadelphia, PA 19104, USA.
Kanchana Jagannathan, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA.
Julian Bender, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA.
Kimberly A. Young, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA
Jesse J. Suh, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA; Philadelphia VA Medical Center, Philadelphia, PA 19104, USA
Charles P. O’Brien, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA; Philadelphia VA Medical Center, Philadelphia, PA 19104, USA
Teresa R. Franklin, Department of Psychiatry, University of Pennsylvania, 3900 Chestnut Street, Philadelphia, PA 19104, USA
References
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F et al. (2011) Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol 46:312–317 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). American Psychiatric Association, Washington, DC [Google Scholar]
- Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D et al. (2010) Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse 64:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Winkielman P (2003) What is an unconscious emotion? (the case for unconscious “liking”). Cogn Emot 17:181–211 [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL et al. (2002) Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26:376–386 [DOI] [PubMed] [Google Scholar]
- Brebner K, Childress AR, Roberts DC (2002) A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol 37:478–484 [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Savov V, Allzen E, Benedict C, Fredriksson R, Schioth HB (2012) Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. Neuroimage 59:2962–2973 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z (2008) Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat 35:362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD (1998) Food restriction enhances the central rewarding effect of abused drugs. J Neurosci 18:7502–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA et al. (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530 [DOI] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Ohman A (2004) Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion 4:340–353 [DOI] [PubMed] [Google Scholar]
- Carroll KM (1998) A cognitive-behavioral approach: treating cocaine addiction (NIH publication 98–4308). National Institute on Drug Abuse, Rockville, MD [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L (2011) The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry 70:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J et al. (2008) Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One 3:e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW (2012) Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol 18:570–580 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comp Biomed Res 29: 162–173 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009) How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- Craig AD (2011) Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225:72–82 [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R (2012) Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev 23:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O (2012) The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50:1252–1266 [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW (2008) A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 165:179–187 [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD (2000) Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex 10:308–317 [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P (2009) Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol 19:487–498 [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE (2009) Marijuana craving in the brain. Proc Natl Acad Sci U S A 106: 13016–13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y et al. (2007) Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology 32:2301–2309 [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG et al. (2009a) The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend 103:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y et al. (2009b) DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology 34:717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Sciortino N, Harper D, Li Y, Hakun J et al. (2011) Modulation of resting brain cerebral blood flow by the GABA B agonist, baclofen: a longitudinal perfusion fMRI study. Drug Alcohol Depend 117:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Shin J, Jagannathan K, Suh JJ, Detre JA, O'Brien CP et al. (2012) Acute baclofen diminishes resting baseline blood flow to limbic structures: a perfusion fMRI study. Drug Alcohol Depend 125:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998) Event-related fMRI: characterizing differential responses. Neuroimage 7:30–40 [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP et al. (2001) Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 58:345–352 [DOI] [PubMed] [Google Scholar]
- Goldman M, Szucs-Reed RP, Jagannathan K, Ehrman RN, Wang Z, Li Y et al. (2012) Reward-related brain response and craving correlates of marijuana cue exposure: a preliminary study in treatment-seeking marijuana-dependent subjects. J Addict Med 7:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Moeller SJ, Telang F, Jayne M, Wong C et al. (2010) Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J Psychopharmacol 24: 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al. (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A 93:12040–12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC (2002) Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci 116:321–333 [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127 [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA (2009) Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend 102:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Takada M (2004) Neural mechanisms of versatile functions in primate anterior cingulate cortex. Rev Neurosci 15:279–291 [DOI] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF (2012) Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl) 222:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S et al. (2009) Neural activations of the acquisition of conditioned sexual arousal: effects of contingency awareness and sex. J Sex Med 6:3071–3085 [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Koppe G, Merz CJ, Kagerer S, Walter B et al. (2012) Neural correlates of disgust- and fear-conditioned responses. Neuroscience 201:209–218 [DOI] [PubMed] [Google Scholar]
- Klucken T, Alexander N, Schweckendiek J, Merz CJ, Kagerer S, Osinsky R et al. (2013) Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Soc Cogn Affect Neurosci 8:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2011a) Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur JNeurosci 33:1318–1326 [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2011b) A quantitative meta-analysis on cue-induced male sexual arousal. J Sex Med 8:2269–2275 [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B (2008) International affective picture system (IAPS): affective ratings of pictures and instruction manual Technical report A-8. University of Florida, Gainesville, FL [Google Scholar]
- Lundahl LH, Johanson CE (2011) Cue-induced craving for marijuana in cannabis-dependent adults. Exp Clin Psychopharmacol 19: 224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens MJ, Born JM, Lemmens SG, Karhunen L, Heinecke A, Goebel R et al. (2013) Increased sensitivity to food cues in the fasted state and decreased inhibitory control in the satiated state in the over-weight. Am J Clin Nutr 97:471–479 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G et al. (1992) The fifth edition of the Addiction Severity Index. J Subst Abuse Treat 9:199–213 [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ (2012) The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev 36:1119–1139 [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2010) The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct 214:435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson LD, Ravichandran C, Lundahl LH, Rodolico J, Dunlap S, Trksak GH et al. (2011) Cue reactivity in cannabis-dependent adolescents. Psychol Addict Behav 25:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22 [DOI] [PubMed] [Google Scholar]
- Pacek LR, Martins SS, Crum RM (2012) The bidirectional relationships between alcohol, cannabis, co-occurring alcohol and cannabis use disorders with major depressive disorder: Results from a national sample. J Affect Disord 148:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ et al. (2007) How the brain translates money into force: a neuroimaging study of subliminal motivation. Science 316:904–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001) Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58:909–915 [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012) Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael B, Wooding S, Stevens G, Connor J (2005) Comorbidity: cannabis and complexity. J Psychiatr Pract 11:161–176 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54:25–53 [DOI] [PubMed] [Google Scholar]
- Roese N, Jamieson D (1993) Twenty years of bogus pipeline research: a critical review and meta-analysis. Psych Bull 114:363–375 [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE (2011) Frontal cortex and reward-guided learning and decision-making. Neuron 70:1054–1069 [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE (2001) A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience 105:535–545 [DOI] [PubMed] [Google Scholar]
- Schuster RM, Crane NA, Mermelstein R, Gonzalez R (2012) The influence of inhibitory control and episodic memory on the risky sexual behavior of young adult cannabis users. J Int Neuropsychol Soc 18: 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A et al. (1997) The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry 12:232–241 [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A (2009) Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 198:149–158 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–219 [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1991) Timeline followback: a technique for assessing self-reported alcohol consumption. Humana, Totowa [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2012) Results from the 2011 National Survey on Drug Use and Health: summary of national findings NSDUH series H-44, HHS publication no. (SMA) 12–4713. Substance Abuse and Mental Health Services Administration, Rockville, MD [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A (2012) Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 106:317–324 [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Jackson EF, Costa VD, Robinson JD, Lam CY et al. (2011) Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur J Neurosci 34:2054–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J (2011) Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci 22:490–497 [DOI] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL (2003) Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull 31: 121–135 [DOI] [PubMed] [Google Scholar]
- Wise RA (2009) Roles for nigrostriatal—not just mesocorticolimbic— dopamine in reward and addiction. Trends Neurosci 32:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM (2008) Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci 27:976–983 [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen X, Yu Y, Sun D, Ma N, He S et al. (2009) Masked smoking-related images modulate brain activity in smokers. Hum Brain Mapp 30:896–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS (2008) Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav 93:637–650 [DOI] [PubMed] [Google Scholar]