Abstract
This research aimed to study the effects of packaging and storage temperature on the shelf-life of an extra virgin olive oil (EVOO) as it can occur in most points of sale. The evolution of the chemical and sensory characteristics of an EVOO, initially stored in stainless steel silos under nitrogen at 12–18 °C, was evaluated after packaging. Tinplate tin (TT) and greenish glass (GG), the most used packaging containers, and temperatures of 6 and 26 °C were taken into consideration. After 125 days from packaging all the samples maintained clearness, green and yellow reflections and the positive sensory notes of bitterness and pungency of the starting EVOO. Shelf-life of EVOO was significantly affected by different storage conditions: oil samples stored in GG at 6 °C preserved for the most part the positive attributes, whereas those stored in TT at 26 °C showed a significant presence of the rancid flavor due to oxidative processes. Moreover, samples stored in GG at 6 °C maintained the highest bitterness intensity and did not show defects at the end of the storage period. The results suggest that storage in GG at a low temperature could represent a promising storage condition to slow-down the oil degradation during market storage.
Keywords: Food science, Food analysis
1. Introduction
The quality of extra-virgin olive oil (EVOO) depends on a process that begins with the olive ripening and finishes with the packaging. Thus, it is necessary to take care not only of agronomical practices, raw material, harvesting, fruit storage and extraction technology, but also of each factor, which can affect its commercial life. In particular, oxygen, light and temperature are responsible for increasing deteriorative processes in EVOO as a consequence of oxidative and hydrolytic reactions (Lanza et al., 2014). Shelf-life of EVOO has been assessed to be 12–18 months (Cicerale et al., 2013), even if it has been shown that when it is properly stored in well-sealed packages, EVOO can reach the second year of storage maintaining its sensorial properties unaltered (Piscopo and Poiana, 2012).
Oxidation is the main responsible for deterioration of olive oil quality, in turn counteracted by the antioxidant activity determined by the presence of polyphenolic compounds and tocopherols, which increase its shelf-life. The main fraction susceptible to oxidation is the lipid one, which degradation gives rise to the production of carbonyl and aldehyde compounds that lead to the development of off-flavors and, at the end, to “oxidative rancidity”. Auto-oxidation further contributes to the degradative processes of the olive oil. This occurs even in the absence of light, following a free radical mechanism where, initially, the absorption of oxygen results in the formation of hydroperoxides. When the olive oil is exposed to light, photo-oxidation occurs through the action of natural photosensitizers such as chlorophyll, which reacts with triplet oxygen to form excited state singlet oxygen. As a consequence, storage and packing conditions of olive oil become of primary importance (Gargouri et al., 2015; Sanmartin et al., 2018), being it produced in a limited period of time but consumed throughout the year. In fact, to maintain the designation “extra virgin” or even “virgin”, lipid oxidation products (i.e. hydroperoxides, conjugated dienes and trienes) must not exceed maximum threshold limits, and/or rancid off-flavors must not occur (Silva et al., 2015).
As observed in previous studies concerning the storage of wine, characteristics of packaging deeply affect its chemical and sensorial qualities as a function of the storage conditions used (Venturi et al., 2017a); in particular, the packaging that mostly preserved wine from oxidation was the glass bottle (Venturi et al., 2017d).
Packaging can directly influence olive oil quality by protecting the product from two of the main factors inducing oxidative deterioration: oxygen and light (Lanza et al., 2014). Moreover, it is essential to avoid the contact with inadequate materials such as metal containers which can initiate oxidative degradative reactions (Sgherri et al., 2018), thus affecting the shelf-life of the oil. For this reason, metal containers which offer the advantage of total protection against light, oxygen and water vapor, are now made of tin plate or tin-free steel based on chromium instead of aluminum or aluminum alloys. The inside of the tin is also coated with resins which protect the metal surface against corrosion (Silva et al., 2015). Although glass represents a good barrier against moisture and gases (Venturi et al., 2017b), transparent bottles can lead to photo-oxidation, not avoiding exposure to light. Since protection from direct light is required for commercial edible oils (Gargouri et al., 2015), glass with low transmittance of light in the UV range have been realized by means of additives (Limbo et al., 2014).
To determine the effects of packaging on the commercial life of olive oil several studies have been carried out, and different containers such as clear and dark bottles, polyethylene and tin containers have been taken into consideration (Pristouri et al., 2010; Gargouri et al., 2015). In most cases, storage stability of oils in tin or stainless containers and in dark glass was the highest (Dabbou et al., 2011; Gargouri et al., 2015). Besides the type of packaging, also storage temperature plays a fundamental role; Pristouri et al. (2010) observed that the shelf life of oil was improved in dark colored containers stored in the dark at temperatures lower than 22 °C.
The present research aimed to evaluate the effects of packaging and storage conditions on a EVOO as it occurs in most points of sale, that is the usual storage of oil in tanks under nitrogen for a more or less long time (also for several months), after which the packaging and the sale. Since this period (in particular from the bottling phase till to sale) is very critical for the oxidation processes, we think that this moment is one of the most suitable for assessing the ability of the packaging and storage temperature to slow down the oxidation evolution of EVOO, and for this reason we studied the effects of packaging and storage temperature on the shelf life of EVOO, initially stored in stainless steel silos under nitrogen at 12–18 °C, observing the evolution of its chemical and sensory characteristics after packaging. An innovative approach based on the overlapping of information deriving from both chemical and sensorial analysis was used. In order to simulate conditions similar to the market storage, the two most commonly used packaging containers for olive oil, namely tinplate tin (TT) and greenish glass (GG). To evidence the influence of storage temperature on oil evolution, a low-temperature (T = 6 ± 1 °C) was compared with the room temperature condition (T = 26 ± 1 °C) mostly adopted for EVOO storage in sale points.
2. Results and discussion
2.1. Effect of packaging and temperature on EVOO chemical parameters
Before packaging (t0), the olive oil could be labeled as extra virgin since its physicochemical parameters (Table 1) showed values below the legal limits for extra virgin olive oil (LL EVOO) according to the EEC/2568/91 Regulation (EEC, 1991). Moreover, the oil was characterized by a remarkable presence of phenolic compounds leading to a high BI (Bitterness Intensity) (Gutiérrez Rosales et al., 1992) and a high antioxidant activity (Sgherri et al., 2016).
Table 1.
Chemical characterization of EVOO at the time of packaging (t0). LL EVOO, legal limits for extra virgin olive oil according to the Regulation EEC/2568/91 (EEC, 1991).
| Analytical parameters | t 0 | LL EVOO |
|---|---|---|
| Free fatty acids (g oleic acid/kg oil) | 0.28 ± 0.01 | <0.80 |
| Peroxide value (meqO2/kg) | 12.31 ± 0.19 | ≤20.00 |
| K232 | 2.18 ± 0.08 | ≤2.50 |
| K270 | 0.14 ± 0.01 | ≤0.22 |
| ΔK | 0.00 | ≤0.01 |
| Phenolic content (g gallic acid/kg oil) | 0.71 ± 0.01 | - |
| Antioxidant capacity (μmol TEAC/mL) | 0.41 ± 0.01 | - |
| Bitterness Intensity | 10.91 ± 0.01 | - |
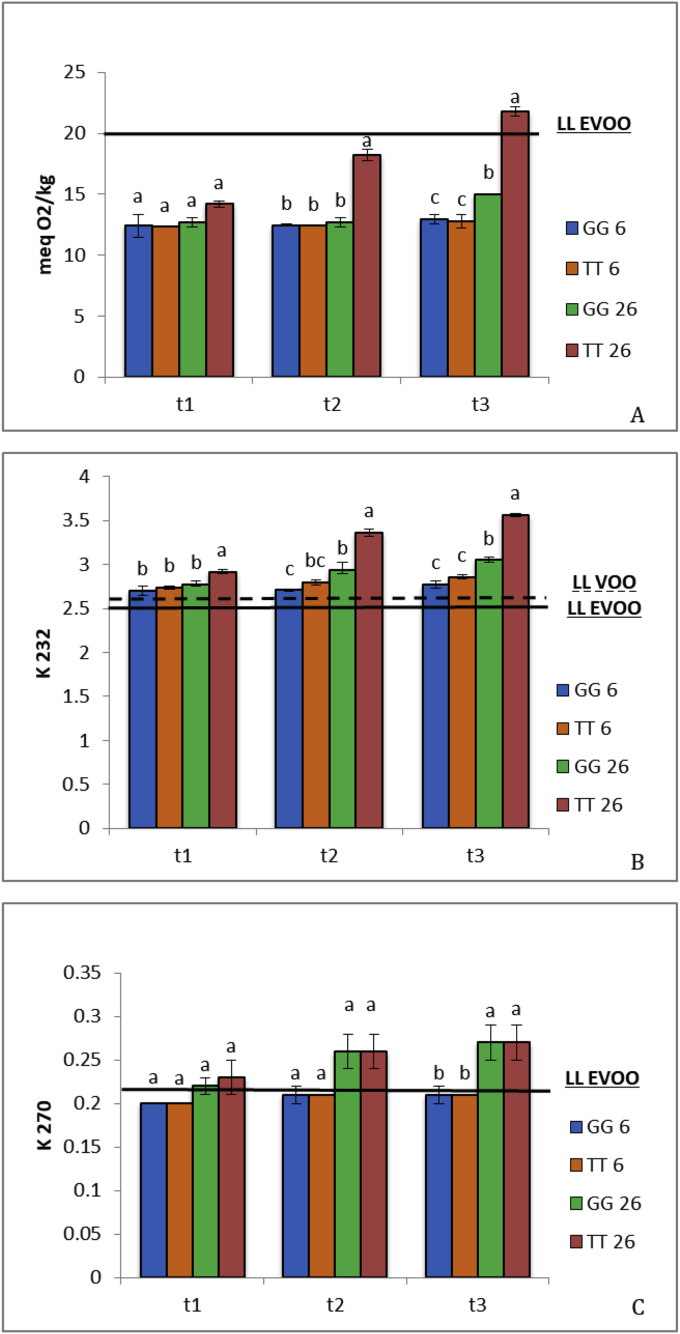
During storage time, different packaging conditions did not affect free acidity, which remained always below the LL EVOO (data not shown). This indicates that in no case hydrolysis of the triacylglycerol matrix occurred significantly, although an impact on PV and quality parameters related to the oxidation level (K232 and K270) could be detected (Fig. 1A–C). In particular, in the sample stored in tinplate tin at 26 °C (TT26) PV increased by 48 and 77% after 78 (t2) and 125 days (t3) of packaging, respectively (Fig. 1A). K232 and K270 also showed the highest increases at t2 and t3 in samples stored at 26 °C both in GG and in TT (Fig. 1B, C), indicating that temperature is critical for the oxidative changes taking place in the EVOO (Mulla et al., 2018). It is noteworthy that after 28 days from packaging (t1) none of the oil samples, regardless of packaging, could be still labeled as EVOO (Fig. 1).
Fig. 1.
Changes in peroxide value (A) and in spectrophotometric indices (K232, B; K270, C) in EVOO samples stored in greenish glass (GG) or tinplate tin (TT) at 6 and 26 °C for 28 (t1), 78 (t2) and 125 days (t3) after packaging. Data are reported as mean ± SD (n = 3). At each sampling time, different letters indicate significant differences among treatments at p < 0.05. The continuous line represents the legal limit for extra virgin olive oil (LL EVOO), whereas the dashed line represents the legal limit for virgin olive oil (LL VOO) according to the EEC/2568/91 Regulation (EEC, 1991).
Occurrence of degradative oxidations could be also evidenced by the decrease in phenolic compounds and thus in the antioxidant activity (Table 2) monitored at the end of the experiment (t3). In fact, the antioxidant activity of phenols such as flavonoids, phenolic acids andanthocyanins, widely distributed in fruits and vegetables, has been extensively reported (Soobrattee et al., 2005). The antioxidant effects are related to the capacity of these compounds to inhibit the initiation of free radical processes or to interrupt the chain reactions in the propagation of oxidation (Rice-Evans et al., 1997), events occurring mainly in the lipid fraction and that, if not counteracted, lead to lipid peroxidation.
Table 2.
Phenolic content (PC, g gallic acid/kg oil), Bitterness Intensity (BI) and Antioxidant capacity (μmol TEAC/mL) of EVOO samples stored in greenish glass (GG) or tinplate tin (TT) at 6 and 26 °C for 125 days (t3) after packaging. Data were reported as mean ± SD (n = 3). In each column, different letters indicate significant differences among treatments at p < 0.05.
| Sample | PC | BI | TEAC |
|---|---|---|---|
| GG 6 | 0.64 ± 0.01 a | 6.20 ± 0.03 a | 0.37 ± 0.01 a |
| GG 26 | 0.61 ± 0.01 b | 3.99 ± 0.04 b | 0.34 ± 0.01 b |
| TT 6 | 0.64 ± 0.02 a | 4.06 ± 0.09 b | 0.37 ± 0.01 a |
| TT26 | 0.60 ± 0.01 b | 4.24 ± 0.13 b | 0.34 ± 0.01 b |
Compared to t0, the decreases in phenolics as well as in antioxidant activity was about 10 and 15% in samples stored at 6 and 26 °C, respectively (Table 2), further confirming the role of low temperature in the shelf life of the product (Fig. 1). BI, linked to the presence in the EVOO of secoridoid derivatives such as the aldehydic form of oleuropein aglycone (Mateos et al., 2004), was particularly sensitive to storage conditions. In fact, except for GG6 (greenish glass at 6 °C), all the other samples showed decreases higher than 60% compared to t0, suggesting that only GG at low temperature (6 °C) could represent a promising storage condition to slow-down the oil degradation during market storage.
2.2. Effect of packaging and temperature on EVOO volatile compounds
All volatile compounds identified by GC-MS analysis are reported in Table 3. Among the twenty-one components, (E)-2-hexenal was the main constituent in all oil samples representing about 40% of the total volatile compounds. Other compounds present in relatively high concentrations were 1-hexanol and (Z)-3-hexenyl acetate accounting for about 13%, whereas 3,7-decadiene, 1-hexyl acetate, (E)-β-ocimene, (E)-2-dodecene and (E,E)-α-farnesene showed values higher than 3%. These results agree substantially with those reported by Ouni et al. (2011), although qualitative and quantitative differences in composition are tightly dependent on the levels and activity of enzymes involved in the synthesis of volatile compounds, in its turn genetically determined. Moreover, the production of these metabolites changes in relation to the ripening degree and storage time of fruits, as well as the operative conditions used during oil extraction (Angerosa et al., 1999).
Table 3.
Relative percentages of volatile compounds extracted by HS-SPME of EVOO at the time of packaging (t0) and of EVOO samples stored in greenish glass (GG) or tinplate tin (TT) at 6 and 26 °C for 125 days (t3) after packaging. LRI: linear retention indices. Data were reported as mean ± SD (n = 3). In each row, different letters indicate significant differences among treatments at p < 0.05.
| Volatile compound | LRI | t 0 | GG 6 | GG 26 | TT 6 | TT 26 |
|---|---|---|---|---|---|---|
| (E)-2-penten-1-ol | 767 | 1.00 ± 014 a | 1.10 ± 0.28 a | 0.90 ± 0.07 a | 1.00 ± 0.14 a | 0.90 ± 0.28 a |
| hexanal | 802 | 2.20 ± 0.35 a | 2.30 ± 0.57 a | 2.80 ± 0.57 a | 2.10 ± 0.28 a | 2.30 ± 0.57 a |
| (E)-2-hexenal | 856 | 40.30 ± 0.92 ab | 41.70 ± 0.85 a | 40.00 ± 1.56 ab | 38.70 ± 1.13 ab | 36.80 ± 1.41 b |
| 1-hexanol | 869 | 13.40 ± 0.71 a | 13.40 ± 0.42 a | 12.20 ± 0.85 a | 11.60 ± 0.85 a | 12.40 ± 0.71 a |
| (Z)-2-pentenyl acetate | 907 | - | - | 0.50 ± 0.01 a | - | 0.70 ± 0.01 a |
| 3-ethyl-1,5-octadiene* | 942 | 1.30 ± 0.28 a | 1.30 ± 0.14 a | 1.30 ± 0.41 a | 1.20 ± 0.28 a | 1.20 ± 0.42 a |
| 3-ethyl-1,5-octadiene* | 950 | 1.60 ± 0.42 a | 1.80 ± 0.57 a | 1.90 ± 0.28 a | 1.60 ± 0.42 a | 2.20 ± 0.57 a |
| 6-methyl-5-hepten-2-one | 987 | 0.80 ± 0.14 b | - | 0.50 ± 0.01 b | 2.70 ± 0.57 a | 3.60 ± 0.42 a |
| 3,7-decadiene* | 998 | 4.20 ± 0.57 a | 4.00 ± 0.57 a | 4.20 ± 0.78 a | 3.80 ± 0.14 a | 3.70 ± 0.57 a |
| 3,7-decadiene* | 999 | 0.40 ± 0.01 a | 0.90 ± 0.14 a | 0.80 ± 0.21 a | 0.80 ± 0.28 a | 0.60 ± 0.01 a |
| (Z)-3-hexenyl acetate | 1008 | 13.70 ± 0.85 a | 13.30 ± 0.85 a | 12.80 ± 0.99 a | 12.70 ± 0.57 a | 12.70±0.85 a |
| 1-hexyl acetate | 1010 | 3.90 ± 0.28 a | 3.50 ± 0.42 a | 3.70 ± 0.57 a | 3.20 ± 0.42 a | 3.30 ± 0.57 a |
| (E)-2-hexenyl acetate | 1017 | 0.50 ± 0.14 a | 0.70 ± 0.01 a | 0.70 ± 0.07 a | 0.70 ± 0.01 a | 0.80 ± 0.14 a |
| limonene | 1032 | - | - | 0.40 ± 0.01 b | 1.20 ± 0.14 a | 0.30 ± 0.01 b |
| (E)-β-ocimene | 1052 | 3.70 ± 0.42 a | 3.60 ± 0.57 a | 3.40 ± 0.71 a | 3.80 ± 0.57 a | 2.60 ± 0.42 a |
| nonanal | 1104 | 1.30 ± 0.42 a | 1.20 ± 0.28 a | 1.70 ± 0.28 a | 1.40 ± 0.42 a | 1.60 ± 0.42 a |
| 4,8-dimethyl-1,3,7-nonatriene* | 1112 | 0.80 ± 0.07 a | 0.90 ± 0.28 a | 0.80 ± 0.41 a | 0.90 ± 0.41 a | 0.80 ± 0.28 a |
| n-dodecane | 1200 | - | - | - | - | 0.70 ± 0.07 a |
| (E)-2-dodecene | 1205 | 5.60 ± 0.71 a | 5.60 ± 0.64 a | 6.00 ± 0.71 a | 6.40 ± 0.57 a | 6.90 ± 0.57 a |
| α-copaene | 1377 | 1.00 ± 0.28 a | 0.90 ± 0.01 a | 1.30 ± 0.42 a | 1.10 ± 0.28 a | 1.40 ± 0.28 a |
| (E,E)-α-farnesene | 1508 | 4.20 ± 0.57 a | 3.70 ± 0.42 a | 4.00 ± 0.42 a | 5.00 ± 0.78 a | 4.30 ± 0.71 a |
| C6-Aldehydes | - | 42.5 ± 1.27 a | 44.0 ± 1.42 a | 42.8 ± 2.13 a | 40.8 ± 1.41 a | 39.1 ± 1.98 a |
| C6-Alcohols | - | 13.40 ± 0.71 a | 13.40 ± 0.42 a | 12.20 ± 0.85 a | 11.60 ± 0.85 a | 12.40 ± 0.71 a |
| C6-Esters | - | 18.10 ± 1.27 a | 17.50 ± 2.27 a | 17.20 ± 1.63 a | 16.60 ± 1.00 a | 16.80 ± 1.56 a |
| Other oxygenated derivatives | - | 3.10 ± 0.70 b | 2.30 ± 0.56 b | 3.60 ± 0.36 b | 5.10 ± 1.13 a | 6.80 ± 1.13 a |
| Other hydrocarbon derivatives | - | 13.90 ± 2.06 a | 14.50 ± 2.34 a | 15.00 ± 2.80 a | 14.70 ± 2.10 a | 16.10 ± 2.49 a |
| Monoterpenes | - | 3.70 ± 0.42 b | 3.60 ± 0.57 b | 3.80 ± 0.72 b | 5.00 ± 0.71 a | 2.90 ± 0.43 b |
| Sesquiterpenes | - | 5.20 ± 0.85 ab | 4.60 ± 0.43 b | 5.30 ± 0.84 ab | 6.10 ± 1.06 a | 5.70 ± 0.99 ab |
| Total identified | - | 99.90 | 99.90 | 99.90 | 99.90 | 99.90 |
Virgin olive oils maintained an almost similar volatile composition in all storage conditions (Table 3), although hierarchical cluster analysis identified two statistical units (Fig. 2). In particular, the first one gathers together t0, GG6 and GG26 (greenish glass at 26 °C), which show great similarities among them, confirming that glass allows to better preserve oil characteristics. Furthermore, in both clusters the oil stored at higher temperature show the greater distance from t0, suggesting that the volatile composition of oil vary faster when storage temperature increases.
Fig. 2.
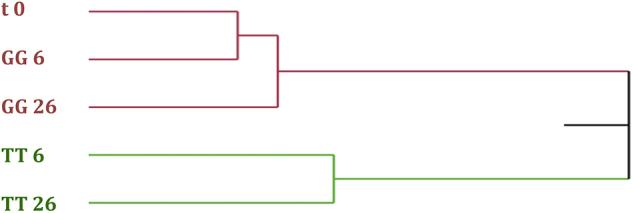
Hierarchical cluster analysis based on volatile compounds of EVOO at the time of packaging (t0) and of samples stored in greenish glass (GG) or tinplate tin (TT) at 6 and 26 °C for 125 days (t3) after packaging.
Among the fatty acid-derived C6-volatiles, synthesized via the LOX (lipoxygenase) pathway from linoleic and linolenic acids (Angerosa et al., 1999) and responsible for the green attributes, (E)-2-hexenal was the only one showing a reduction (-12%) in TT26 compared to GG6 (Table 3). In a previous study (Angerosa et al., 1999) a decrease in the concentration of compounds from the LOX pathway was observed, depending on the storage time of fruits even if it was carried out in ideal conditions (low temperature), being more evident for longer storage times (Oueslati et al., 2018).
The aliphatic ketone 6-methyl-5-hepten-2-one increased during storage in TT, showing increases by 3.3 and 4.5-fold at 6 and 26 °C, respectively (Table 3). It can be suggested that oxidation of 6-methyl-5-hepten-2-ol by sulcatone reductase may be induced by the TT packaging.
Furthermore, among all the volatile compounds detected, the value of the relative percentage of Limonene overcome established exclusion threshold during storage only at the highest temperature and in TT.
2.3. Effect of packaging and temperature on EVOO sensorial parameters
According to the International Olive Council standards for sensory evaluation, the classification of oils in categories such as “extra virgin”, “virgin” or “lampante” is based on the evaluation of both ‘negative’ and ‘positive’ attributes (International Olive Council, 2005, 2011). Among the positive sensory attributes, fruity notes should be identified in the EVOO, whereas negative ones (sensory defects) cannot be present.
As regards the visual descriptors (clearness, green and yellow reflections) and the basic positive sensory notes of bitterness and pungency no significative changes could be observed in the EVOO samples differently stored for 125 days (t3) from packaging compared to the sample at the time of packaging (data not shown). This is in contrast with the decrease in phenolics (Table 2), generally linked to the perception of bitterness, astringency and pungency, and which presence is not only important for the organoleptic and nutritional qualities of the oil but also for its conservation over time (Bertuccioli and Monteleone, 2014).
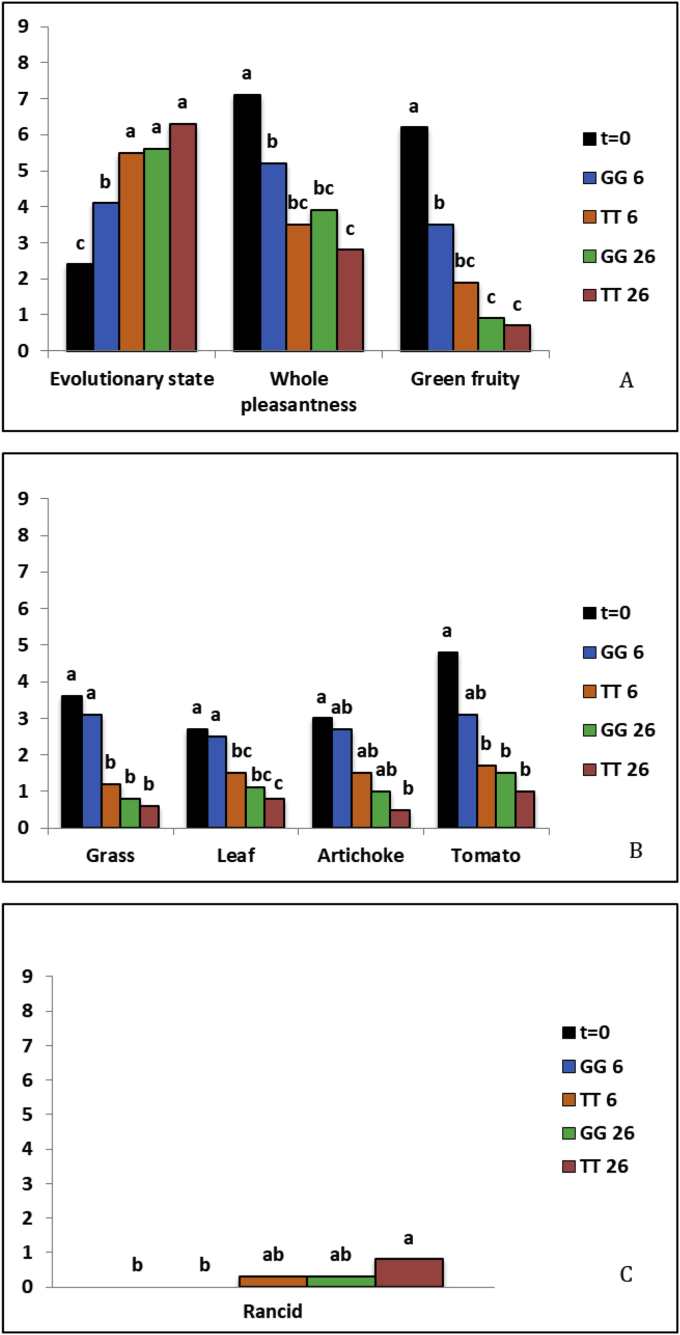
However, in all oil samples the analysis of overall descriptors showed that perception of the oil evolution increased, whereas whole pleasantness and green fruity decreased at the end of storage period in comparison with t0 (Fig. 3A). Notwithstanding samples stored at 26 °C were the most affected, oil stored in GG at 6 °C showed a two-fold increase in the evolutionary state and decreases in whole pleasantness and green fruity perception by 27 and 43%, respectively, compared to t0 (Fig. 3A). This indicates that even storage at low temperature cannot avoid the loss in positive sensory attributes characteristic of EVOO (Bertuccioli and Monteleone, 2014). However, GG6 was the only sample that maintained olfactory sensations of grass, leaf, artichoke and tomato at values close to what observed at t0 (Fig. 3B). Generally, TT26 was the sample more affected by storage conditions (Figs. 1 and 3), and the concomitant decrease in (E)-2-hexenal could, in part, explain the reduction in the perception of green attributes (Table 3, Fig. 3). In fact, volatile compounds responsible for the green attributes of virgin olive oils are produced through the enzymatic oxidation of linoleic and linolenic acids (Angerosa et al., 1999). TT26 also showed the highest presence of rancid flavor (Fig. 3C) indicating that, under these storage conditions, oxidation processes took place in agreement with the increases in PV, K232 and K270 (Fig. 1A, B, C). Thus, temperature represents a critical feature for the oxidative changes taking place in EVOO, oxidation being the main responsible for the qualitative deterioration of olive oil since it brings to the development of off-flavors and thus to “oxidative rancidity”. This latter is, in turn, counteracted by the oil antioxidant activity due to the presence, among the others, of polyphenolic compounds which decreased in comparison with t0 (Table 2), thus decreasing EVOO shelf-life (Bendini et al., 2007). GG6 was the only sample which did not present defects, whereas in TT6 (tinplate tin at 6 °C) and GG26 rancid flavor had an intensity 3-fold lower than in TT 26 (Fig. 3C).
Fig. 3.
Changes in the sensory parameters in EVOO samples stored in greenish glass (GG) or tinplate tin (TT) at 6 and 26 °C for 125 days (t3) after packaging compared to EVOO at the time of packaging (t0). A, overall descriptors; B, smell parameters; C, defects. At each sampling time, means accompanied by different letters indicate significant differences among treatments at p < 0.05.
2.4. Correlations among sensorial and chemical results
Based on Pearson's correlation coefficients, positive sensorial attributes showed strong correlations with some chemical parameters such as BI and phenolic content (PC) involved in the delay of deteriorative processes occurring during EVOO storage (Table 4). Thus, whole pleasantness, green fruity as well as olfactory sensations of grass, leaf, artichoke and tomato were positively correlated with BI and PC. In contrast, the same sensorial attributes were negatively correlated with quality parameters related to the degradation of the lipid matrix. In particular, sensations of leaf, artichoke and tomato showed to be negatively strongly correlated with FFA, PV and the spectrophotometric indices K232 and K270 (Table 4). Because of oxidation processes, the rancid flavour as well as the evolutionary state resulted, instead, positively strongly correlated with the above physiochemical indices, whereas the evolutionary state was negatively correlated with BI and PC (Table 4).
Table 4.
Pearson's correlation coefficients obtained correlating the sensorial with chemical results. Strong correlations are highlighted in bold. BI, bitterness intensity; FFA, free fatty acids; PC, phenolic content; PV, peroxide value.
| Parameter | Evolutionary state | Whole pleasantness | Green fruity | Grass | Leaf | Artichoke | Tomato | Rancid |
|---|---|---|---|---|---|---|---|---|
| K270 | 0.72 | −0.57 | −0.86 | −0.73 | −0.82 | −0.83 | −0.74 | 0.70 |
| K232 | 0.82 | −0.79 | −0.78 | −0.70 | −0.81 | −0.84 | −0.79 | 0.96 |
| PV | 0.74 | −0.71 | −0.68 | −0.59 | −0.72 | −0.76 | −0.70 | 0.92 |
| FFA | 0.77 | −0.65 | −0.85 | −0.73 | −0.83 | −0.85 | −0.77 | 0.80 |
| BI | −0.88 | 0.84 | 0.89 | 0.96 | 0.89 | 0.87 | 0.91 | −0.63 |
| PC | −0.88 | 0.65 | 0.85 | 0.73 | 0.83 | 0.85 | 0.77 | −0.80 |
3. Conclusions
While shelf-life of EVOO was differently affected by packaging and storage temperature, the latter being critical for the oxidative changes taking place in oil, at the end of the observation period none of the oil samples showed significant changes in the visual descriptors of clearness, green and yellow reflections, and the basic positive sensory notes of bitterness and pungency were maintained. In particular, the oil stored in GG at 6 °C mostly preserved positive attributes, whereas the one stored in TT at 26 °C showed an enhancement of oxidative processes leading to a significant presence of the rancid flavour. Moreover, GG6 maintained the highest BI and did not show defects at the end of storage, further suggesting that storage in GG at a low temperature (6 °C), could represent a promising storage condition to slow-down the oil degradation during market storage.
In conclusion, it was observed that despite having used an oil in which the oxidative processes were certainly in place, the subsequent oxidation kinetics appear very different when the experimental parameters (temperature and packaging) were changed. This fact suggests that not only the storage conditions can prevent oxidation processes from occurring, but they can even be usefully used to slow down almost to block them. Although starting from a point where the radical processes were certainly established (see Table 1), once the preservation conditions were modified, the kinetics of degradation were consequently significantly different.
With the aim to individuate the storage conditions which better preserve a product with an already started oxidation state, a new “integrated approach”, deriving from the merging of both chemical and sensorial data, was proposed.
4. Materials and methods
4.1. Preparation of samples
The EVOO, produced during the 2016/17 olive milling, was a blend of three native Apulian cvs: 60% Ogliarola Salentina, 20% Coratina, and 20% Leccino. The EVOO is named “Piana del Lentisco” and is produced and packaged by the oil mill “Frantoio Oleario Melcarne” (Gagliano del Capo, Puglia, Italy).
Olive fruits were processed within 24 h after harvesting with an industrial system consisting of a hammer crusher (Alfa Laval, Tavernelle V.P., Firenze, Italy), a traditional horizontal covered malaxer (Amenduni IBERIA 4V500, Modugno, Bari, Italy), a two-phase decanter extractor (Amenduni ORION 902-S) and a double vertical separator (Alfa Laval UVPX507). The malaxation was carried out for 40 min at 27 °C. The filtration procedure was carried out immediately after fruit processing with a sheet filter (Claudio Vignoli TF SR40/50, Jesi, Ancona, Italy).
The oil was stored at oil mill in stainless steel silos under nitrogen at 12–18 °C before being packaged for the experimental tests. After 63 days of storage, the oil, collected by one single tank, was stored in 40 Greenish glass bottles (GG, 100 mL volume) or in 40 tinplate tins (TT, 175 mL volume) at the same time in a commercial packaging line using a fully automated filling station (Oilmatic, sailed by A.T.E. Elettronica s.r.l., Gubbio (PG), Italy).
One final step before sealing the containers was aimed to completely replace air in the headspace with N2, whose chemical inertia makes it particularly suitable in fields where the high reactivity of oxygen causes unwanted actions.
Three replicates of EVOO were analyzed at the time of packaging (t0) and after 28 (t1), 78 (t2) and 125 (t3) days from packaging at 6 and 26 °C.
4.2. Quality parameters
Free fatty acids (FFA), peroxide value (PV) and spectrophotometric indices (K232, K270 and ΔK) were determined according to the Official EU analytical methods described in the European Commission Regulation (EEC, 1991).
4.3. Analysis of phenolic content
Total phenols were extracted from EVOO following the procedure of Montedoro et al. (1992) slightly modified (Venturi et al., 2017c), and extracts were stored at −20 °C under N2 atmosphere until use. Determination of total phenols was performed according to the Folin-Ciocalteau colorimetric method using gallic acid as standard.
4.4. Bitterness intensity (BI) determination
BI was determined following Gutiérrez Rosales et al. (1992). Bitter components were extracted from 1.0 ± 0.01 g EVOO samples and octadecyl (C18) disposable extraction columns (6 mL) (J.T. Baker Chemical Company, Phillipsburg, NJ, USA) were used. Absorbance was recorded at 225 nm (K225).
The value of Bitterness Intensity was calculated according to Favati et al. (2013).
4.5. Antioxidant capacity assay
Antioxidant capacity of phenolic extracts from EVOO samples was performed following Sgherri et al. (2016) using the radical cation ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)). The radical solution was prepared as described by Pellegrini et al. (1999), and a Trolox dose-response curve in the 0.2–1.5 mM range was used. Antioxidant activity was expressed as Trolox equivalent antioxidant capacity (TEAC) mL−1 extract.
4.6. Volatile compound analyses
The volatile fraction of the EVOO samples was analyzed by head-space Solid Phase Micro-Extraction (SPME) using SPME devices (Supelco, Bellefonte, Pa, USA) coated with polydimethylsiloxane (PDMS, 100 μm) to sample the headspace of olive oil. Prior to analysis, each sample was left to equilibrate for 30 min at room temperature before the fibre insertions. After the equilibration time, the septum of each vial is perforated by the holder (syringe) and the fibre is exposed to the head space of the sample for 30 min at room temperature. Once the sampling is complete, the fibre is retracted into the holder and directly injected in the GC–MS apparatus for separation and analysis. Blanks were performed before each first SPME extraction and randomly repeated during each series. Quantitative comparisons of relative peaks areas were performed between the same chemicals in the different samples.
Gas chromatography–electron impact mass spectrometry (GC–EI-MS) analyses were performed with a Varian CP-3800 gas chromatograph equipped with a DB-5 capillary column (30 m × 0.25 mm; film thickness 0.25 μm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions were as follows: injector and transfer line temperatures 220 and 240 °C, respectively; oven temperature programmed from 60 to 240 °C at 3 °C min−1; carrier gas helium at 1 mL/min; splitless injection. Identification of the constituents was based on a comparison of the retention times with those of the authentic samples, comparing their linear retention indices relative to the series of n-hydrocarbons. Computer matching was also used against commercial (NIST 14 and ADAMS) and laboratory-developed library mass spectra built up from pure substances and components of known oils and MS literature data (Stenhagen et al., 1974; Davies, 1990; Adams, 1995).
4.7. Sensory analysis
Quantitative descriptive analysis of the EVOO samples was performed by a panel of ten trained assessors included in the “expert panel” of the Department of Agriculture, Food and Environment (DAFE) of University of Pisa, according to the internal procedure for assessor selection and training (Venturi et al., 2016). Sensorial characterization followed the method described in the EEC/2568/91 Regulation (EEC, 1991). In order to better describe the organoleptic evolution of the stored oil, during the whole observation period the Panel was provided with a technical sheet specifically developed for this purpose. By utilizing the proposed sheet, it was possible to obtain a sensory profile of the stored oil as a function of the storage conditions on the basis of the first order descriptors of color, flavoring and taste and of the hedonic parameter related to the overall pleasantness.
The panelists ranked the EVOO samples on a scale from 0 (no perception, the lowest intensity) to 10 (the highest intensity) to evaluate the intensity of each parameter. Tasting was carried out in the conditions previously reported (Venturi et al., 2017b).
4.8. Statistical analysis
The homogeneity of variances for all the parameters was evaluated by Barlett's test. Results were expressed as means. The statistical analysis was carried out with Costat version 6.400 (2008 CoHort software). One-way ANOVA was independently applied to the data to evaluate the storage condition effect, and LSD post-hoc test was carried out. For each oil, at each sampling time significant differences among the mean values were assessed on the basis of the least significant difference test at 0.05 level of significance.
Pearson's correlation coefficient test was also carried out selecting parameters that showed differences statistically significant among treatments, in order to measure the strength of the correlation among chemical and sensorial results. Generally, a coefficient of about ±0.7 or more indicates a strong correlation, whereas ±0.9 points out a very strong correlation. In the ±0.5 region the correlation is moderate, and in the range –0.3 to +0.3 it is weak (Leighton et al., 2010).
The Hierarchical Cluster Analysis (HCA) was performed using Ward's method, with squared Euclidian distances as a measure of similarity on unscaled data. The statistical analyses were carried out with the JMP software package (SAS Institue, Cary, NC, USA).
Declarations
Author contribution statement
Chiara Sanmartin: Conceived and designed the experiments.
Francesca Venturi, Angela Zinnai: Conceived and designed the experiments; Wrote the paper.
Anita Nari: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Cristina Sgherri, Guido Flamini, Monica Macaluso, Isabella Taglieri: Performed the experiments.
Mike Frank Quartacci, Gianpaolo Andrich: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adams R.P. Allured Publishing Corporation; Carol Stream, Illinois, USA: 1995. Identification of Essential Oil Components by Gas Chromatography/quadrupole Mass Spectroscopy. [Google Scholar]
- Angerosa F., Basti C., Vito R. Virgin olive oil volatile compounds from lipoxygenase pathwayand characterization of some Italian cultivars. J. Agric. Food Chem. 1999;47:836–839. doi: 10.1021/jf980911g. [DOI] [PubMed] [Google Scholar]
- Bendini A., Cerretani L., Carrasco-Pancorbo A., Gόmez-Caravaca A.M., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuccioli M., Monteleone E. The sensory quality of extra-virgin olive oil. In: Peri C., editor. The Extra-Virgin Olive Oil Handbook. Wiley, Chichester; West Sussex, UK: 2014. pp. 35–56. [Google Scholar]
- Cicerale S., Conlan X.A., Barnett N.W., Keast R.S.J. Storage of extra virgin olive oil and its effect on the biological activity and concentration of oleocanthal. Food Res. Int. 2013;50:597–602. [Google Scholar]
- Dabbou S., Gharbi I., Dabbou S., Brahmi F., Nakbi A., Hammami M. Impact of packaging material and storage time on olive oil quality. Afr. J. Biotechnol. 2011;10:16937–16947. [Google Scholar]
- Davies N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A. 1990;503:1–24. [Google Scholar]
- EEC Characteristics of olive oil and olive-residue oil and the relevant methods of analysis. Regulation EEC/2568/91 and later modifications. Off. J. Eur. Communities. 1991;L24:1–83. [Google Scholar]
- Favati F., Condelli N., Galgano F., Caruso M.C. Extra virgin olive oil bitterness evaluation by sensory and chemical analyses. Food Chem. 2013;139:949–954. doi: 10.1016/j.foodchem.2013.01.098. [DOI] [PubMed] [Google Scholar]
- Gargouri B., Zribi A., Bouaziz M. Effect of containers on the quality of Chemlali olive oil during storage. JFST. 2015;52:1948–1959. doi: 10.1007/s13197-014-1273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez Rosales F., Perdiguero S., Gutierrez R., Olias J.M. Evaluation of the bitter taste in virgin olive oil. J. Am. Oil Chem. Soc. 1992;69:394–395. [Google Scholar]
- International Olive Oil Council . IOOC; Madrid: 2005. Method for the Organoleptic Assessment of Extra-virgin Olive Oil Applying to Use a Designation of Origin, T20/Doc No 22. [Google Scholar]
- International Olive Oil Council . IOOC; Madrid: 2011. Sensory Analysis of Olive Oil. Method for the Organoleptic Assessment of virgin Olive Oil, IOOC/T20/Doc. No 15/Rev. 4. [Google Scholar]
- Lanza B., Di Serio M.G., Giansante L., Di Loreto G., Di Giacinto L. Effect of shelf conditions on the phenolic fraction and oxidation indices of monovarietal extra virgin olive oil from cv. “Taggiasca”. Acta Aliment. 2014;44:585–592. [Google Scholar]
- Leighton C.S., Schönfeldt H.C., Kruger R. Quantitative descriptive sensory analysis of five different cultivars of sweet potato to determine sensory and textural profiles. J. Sensory Stud. 2010;25:2–18. [Google Scholar]
- Limbo S., Peri C., Piergiovanni L. Extra virgin olive oil packaging. In: Peri C., editor. The Extra-virgin Olive Oil Handbook. Wiley, Chichester; West Sussex, UK: 2014. pp. 179–199. [Google Scholar]
- Mateos R., Cert A., Pérez-Camino M.C., Garcia J.M. Evaluation of virgin olive oil bitterness by quantification of secoiridoid derivatives. J. Am. Oil Chem. Soc. 2004;81(1):71–75. [Google Scholar]
- Montedoro G., Servili M., Baldioli M., Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992;40:1571–1578. [Google Scholar]
- Mulla M., Ahmed J., Al-Sharrah T. Effect of hot oven and microwave roasting on garden cress (Lepidium sativum) seed flour quality and fatty acid composition, thermal and dielectric properties of extracted oil. Int. J. Food Sci. Technol. 2018 In press. [Google Scholar]
- Oueslati I., Krichene D., Manaï H., Taamalli W., Zarrouk M., Flamini G. Monitoring the volatile and hydrophilic bioactive compounds status of fresh and oxidized Chemlali virgin olive oils over olive storage times. Food Res. Int. 2018;112:425–433. doi: 10.1016/j.foodres.2018.06.058. [DOI] [PubMed] [Google Scholar]
- Ouni Y., Flamini G., Issaoui M., Nabil B.Y., Cioni P.L., Hammami M. Volatile compounds and compositional quality of virgin olive oil from Oueslati variety: influence of geographical origin. Food Chem. 2011;124:1770–1776. [Google Scholar]
- Pellegrini N., Re R., Yang M., Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,20-azinobis[3-ethylenebenzothiazoline-6-sulfonic acid] radical cation decolorization assay. Methods Enzymol. 1999;299:379–389. [Google Scholar]
- Piscopo A., Poiana M. Packaging and storage of olive oil. In: Mazzalupo I., editor. Olive Germplasm – the Olive Cultivation, Table Olive and Olive Oil Industry in Italy. In Tech; 2012. pp. 217–218. [Google Scholar]
- Pristouri G., Badeka A., Kontominas M.G. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Contr. 2010;21:412–418. [Google Scholar]
- Rice-Evans C., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- Sanmartin C., Venturi F., Macaluso M., Nari A., Quartacci M.F., Sgherri C., Flamini G., Taglieri I., Ascrizzi R., Andrich G., Zinnai A. Preliminary results about the use of argon and carbon dioxide in the extra virgin olive oil (EVOO) storage to extend oil shelf life: chemical and sensorial point of view. Eur. J. Lipid Sci. Technol. 2018;120(9):1800156. [Google Scholar]
- Sgherri C., Micaelli F., Andreoni N., Baldanzi M., Ranieri A. Retention of phenolic compounds and antioxidant properties in potato bread obtained from a dough enriched with a powder from the purple cv. Vitelotte. Agrochimica. 2016;60:312–328. [Google Scholar]
- Sgherri C., Pinzino C., Quartacci M.F. Reactive oxygen species and photosynthetic functioning: past and present. In: Singh V.P., Singh S., Tripathi D.K., Prasad S.M., Chauhan D.K., editors. Reactive Oxygen Species in Plants: Boon or Bane – Revisiting the Role of ROS. J. Wiley & Sons, Ltd; Chichester, UK: 2018. pp. 137–155. [Google Scholar]
- Silva S.F., Anjos C.A.R., Cavalcanti R.N., Celeghini R.M. Evaluation of extra virgin olive oil stability by artificial neural network. Food Chem. 2015;179:35–43. doi: 10.1016/j.foodchem.2015.01.100. [DOI] [PubMed] [Google Scholar]
- Soobrattee M.A., Neergheen V.S., Luximon-Ramma A., Aruoma O.I., Bahorum T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat. Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Stenhagen E., Abrahamsson S., Mc Lafferty F.W. Wiley & Sons; New York, NY: 1974. Registry of Mass Spectral Data. [Google Scholar]
- Venturi F., Sanmartin C., Taglieri I., Nari A., Andrich G., Terzuoli E. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients. 2017;9:916–929. doi: 10.3390/nu9080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi F., Sanmartin C., Taglieri I., Xiaoguo Y., Andrich G., Zinnai A. A kinetic approach to describe the time evolution of red wine as a function of packaging and storage conditions. Acta Aliment. 2017;46:336–345. [Google Scholar]
- Venturi F., Sanmartin C., Taglieri I., Xiaoguo Y., Andrich G., Zinnai A. Glass and wine: a good example of the deep relationship between drinkware and beverage. J. Wine Res. 2016;27(2):153–171. [Google Scholar]
- Venturi F., Sanmartin C., Taglieri I., Xiaoguo Y., Deng S., Andrich G. The influence of packaging on the time evolution of red wine Shelf life of red wine as a function of the storage conditions adopted over a period of 12 months. Agro Food Ind. Hi Tech. 2017;28(4):60–63. [Google Scholar]
- Venturi F., Sanmartin C., Taglieri I., Xiaoguo Y., Quartacci M.F., Sgherri C. A kinetic approach to describe the time evolution of red wine as a function of packaging conditions adopted: influence of closure and storage position. Food Packag. Shelf Life. 2017;13:44–48. [Google Scholar]