Abstract
Objectives
We determined and structurally analyzed the reported effect of hydroxyapatite (HA) bone substitute on alveolar bone regeneration. To the best of our knowledge, no systematic reviews have previously reported the bone regenerative effect of the HA bone substitute.
Materials and methods
A literature search was performed for articles published up to August 2015 using MEDLINE with the search terms “hydroxyapatite,” “bone regeneration,” and “alveolar bone” as well as their known synonyms. The inclusion criteria were set up for human trials with at least five patients. The literature search, eligible article selection, and data extraction were independently performed by two readers, and their agreement was reported by κ value.
Results
Of the 504 studies found using the MEDLINE literature search, 241 were included for further steps (inter-reader agreement, κ = 0.968). Abstract screening yielded 74 studies (κ = 0.910), with 42 completely fulfilling the inclusion criteria (κ = 0.864). In a final step, 42 studies were further analyzed, with 17 and 25 studies with and without statistical analysis, respectively. The 17 studies reporting similar outcome measures were compared using the calculated 95% confidence intervals. The effect of HA on ridge preservation could not be evaluated.
Conclusions
The use of the HA bone substitute interfered with the normal healing process, with significant differences found for sinus augmentation but not for periodontal bone defects. Thus, a bone substitute with optimal bone regenerative properties for alveolar ridge or socket preservation, sinus augmentation, and periodontal bony defect should be developed.
Keywords: Dentistry, Materials science
1. Introduction
In daily clinical practice, dentists usually face alveolar bone loss problems caused by tooth extraction, severe periodontitis, or tumor surgery [1, 2]. The resulting bone defect not only hampers prosthetic reconstruction but also has an aesthetic effect in cases of periodontal defects. Therefore, bone surgery is performed to regenerate the lost bone and restore the alveolar ridge contour. Several grafting materials are used during surgery [2, 3, 4, 5, 6, 7, 8, 9, 10].
The four basic categories of bone grafting materials are autograft, allograft, xenograft, and alloplastic graft. Autograft is still considered the gold standard because it provides a good scaffolding for osteoconduction and contains growth factors for osteoinduction as well as progenitor cells for osteogenesis. However, autograft procedures risk donor site morbidity and can be limited by graft availability. Allografts and xenografts risk disease transmission and can evoke an immunologic reaction [11, 12, 13]. Due to these problems, there is an increasing interest in the use of alloplastic (synthetic) grafting materials.
The first documented use of a synthetic bone graft was reported in 1892 by Van Meekeren [14], who treated a large bone defect with calcium sulfate. Since then, materials classified as bioceramics have been used extensively as bone graft substitutes in humans. The most widely used bioceramics material for bone grafting in humans is hydroxyapatite (HA) [15], which has a chemical composition and crystalline structure similar to those of bone [16].
HA and some other calcium-based ceramic materials can be regarded as bioactive materials since they have been reported to support bone ingrowth [17, 18, 19]. Their bioactivity is related to their osteoconductive properties, which allow apposition and migration of osteoblasts at the material surface [20, 21]. HA is known to be able to bond directly to bone [17, 22]. HA, alone or combined with an auto/allo/xenograft, has been used with adequate clinical success rates in dentistry and maxillofacial surgery to support alveolar bone regeneration [3, 22, 23, 24, 25, 26]. HA is available in a wide variety of forms (powders, porous blocks, or beads).
Several reviews on the use of HA as bone replacement graft to treat bone defects have already been reported [27, 28, 29]. However, none has analyzed the bone regenerative effect of the HA bone substitute. Thus, the fundamental question of whether HA has significant clinical effect on alveolar bone regeneration remains unclear. Therefore, we determined and analyzed structurally the effect of HA bone substitute on alveolar bone regeneration in the case of ridge preservation, sinus augmentation, and periodontal bone defect treatment. For this purpose, all clinical HA applications for these three dental applications were considered.
2. Materials and methods
The method of this systematic review is based on the guidelines of the Preferred Reporting of Systematic Review and Meta-Analysis (PRISMA) statement and modified from previous reviews [30, 31]. The format was followed according to the focused question approach called Population, Intervention, Comparison, and Outcomes (PICO). The focused question was “Is HA bone substitute effective in alveolar bone regeneration?”
2.1. Population, intervention, comparison, and outcomes
All studies involving healthy individuals, without any age limit, who underwent any type of alveolar ridge or socket preservation, sinus augmentation, and periodontal bone defect treatment were included in the systematic review. Only studies involving a comparison between test (treatment using alloplastic material based on HA) and control (unassisted socket healing; autograft; allograft; xenograft; socket sealing, such as soft tissue graft; membrane barrier; biological active agent, such as platelet-rich plasma [PRP]; growth factor; or a combination of the above techniques/materials) groups were included. When the treatment outcomes in the studies were related to alveolar bone regeneration (clinically, radiographically, histologically, and histomorphometrically), the studies were included in the systematic review.
For further consideration in this systematic review, a variety of outcome measures of interest could be considered to assess the effectiveness of HA-based material for alveolar bone regeneration. For this systematic review, studies were included when the primary outcome measure was: (1) radiographic assessment: changes in bone density or changes in bone volume; and (2) histologic and histomorphometric assessment: formation of new alveolar bone in the defect area. In addition, secondary outcome measures were also considered in some studies, which included clinical assessment, such as periodontal pocket depth (PPD), clinical attachment level (CAL), bleeding on probing, plaque index (PI), and gingival index (GI).
2.2. Search strategy
Electronic databases were used to search literature for articles published from 1981 up to and including August 2015. The search was performed using MEDLINE via PUBMED, EMBASE, and Cochrane databases. A combination of search terms (key words and MeSH terms) was used to identify the proper studies, including hydroxyapatite OR hydroxy apatite OR apatite OR calcium hydroxyapatite OR hydroxyapatite-bioglass OR nano-hydroxyapatite OR hydroxyapatite cement OR coralline hydroxyapatite AND bone regeneration OR bone healing OR bone response OR osseointegration AND ridge preservation OR periodontal defect OR alveolar bone defect OR sinus augmentation OR maxillary sinus augmentation OR sinus floor augmentation OR extraction site OR implant site.
2.3. Eligibility criteria for study inclusion
The selection was limited to English language-only human studies related to alveolar bone treatment. Longitudinal prospective studies (randomized [RCTs] and clinical [CCTs] controlled trials) were included [31].
Additional inclusion criteria were all human trials involving healthy individuals without any age limits who underwent treatment related to alveolar bone (ridge or socket preservation, sinus augmentation, and periodontal bony defect). Among them, only studies reporting at least five patients with at least 3 months of follow-up were analyzed. Studies using outcome measures related to bone regeneration (either clinical, radiographic/histologic, and histomorphometric) were included for further review. The exclusion criteria were (1) case reports, case series, and/or case control analyses; (2) studies without a control group; (3) studies without any comparison between the use of the alloplastic material and another treatment; and (4) in vitro, animal, and no clinical control studies (literature review/systematic review).
2.4. Data extraction and statistical analysis
Each study was evaluated independently by two readers (A.H.D. and I.D.A). Disagreements were resolved by discussion. The level of agreement between the reviewers was determined by κ value. The data were extracted based on general characteristics (treatment modality, study design, and outcome measure). Means and standard deviations (SD) from each study were used to calculate 95% confidence intervals (CI) [32]. Statistical analysis was performed with SPSS for Window v.15 (SPSS, Inc., Chicago, IL, USA). Furthermore, results of studies that used the same methods of evaluation and similar outcome measurements were combined and the data were presented in a statistical graph.
2.5. Quality assessment
To evaluate the methodologic quality and risk of bias, we used parameters combined from the Cochrane Collaboration and Consolidate Standards of Reporting Trials statement and previous studies [32, 33]. The following parameters were assessed in some studies, such as RCTs and CCTs: adequate sequence generation, allocation concealment, randomization method, masking, statement of eligibility criteria (inclusion and exclusion), follow-up, method of statistic (sample size calculation/power of statistic), and risk of bias category (low/moderate/high).
Randomization was accepted as adequate when the case allocation sequence was generated by referring to a random table (using a computer or tossing a coin or shuffling cards or envelopes). On the other hand, randomization was considered inadequate if the case allocation sequence was generated by odds or based on the date of birth, date of admission, or hospital/clinical record number. Adequate allocation concealment was further accepted if participant and investigator could not foresee assignments before assigning a subject to a group. Studies were considered as having adequate concealment before the intervention if the intervention was concealed using central telephone, web-based, pharmacy-controlled, and/or sequentially numbered drug containers in sealed opaque envelopes until the intervention was assigned.
Studies were considered qualified if they applied adequate statistical analysis and had low risk of bias. Statistical analysis was judged adequate if the reported group number, size of sample, normal distribution, parametric or nonparametric, and power of statistic (P value) data were available. The categories of risk of bias were divided into low, moderate, and high risk based on quality assessment. Low risk of bias was classified if the following criteria were clearly met in the study: adequate sequence generation with adequate allocation concealment as well as applied masking for participant and examiner with reported eligibility criteria and detailed report for the follow-up. Moderate and high risk of bias was considered if one and more than one criteria for risk of bias were lacking, respectively [31, 33].
3. Results
The MEDLINE literature search resulted in 504 articles. After the first step selection based on the title of the collected studies, 242 articles were included for further steps (inter-reader agreement, κ = 0.968). Step 2, based on abstract screening, resulted in 74 studies (inter-reader agreement, κ = 0.910). From these 74 studies, 42 completely fulfilled the inclusion criteria in the final step (inter-reader agreement, κ = 0.864), while 32 were excluded (Table 1) for reasons shown in Table 2. Table 3 shows the quality assessment for the overall treatment modalities (alveolar ridge or socket preservation, sinus augmentation, and periodontal bone defect) in the 42 included studies. Table 3 also presents the outcomes of the study quality assessment for RCTs and CCTs study designs. From the 42 studies, three, one, and 38 were classified as having low, moderate, and high risk of bias, respectively.
Table 1.
Selection of publications.
| Steps | Number of articles | Notes |
|---|---|---|
| Searched by MEDLINE Search via Pubmed EMBASE and Cochrane Database | 504 | Articles published by August 2015 |
| Applied first selection: Human trials | 241 | κ value: 0.968 |
| Screened by abstracts | 74 | κ value: 0.910 |
| Included by inclusion criteria | 42 | κ value: 0.864 |
| Excluded by exclusion criteria | 32 | Referred to Table 2 |
| Used for further analysis | 42 | |
| With similar outcome measures to be analyzed statistically | 17 | |
| Without similar outcome measures; Not allowing statistical analysis | 25 |
Table 2.
Reason for exclusion.
| No. | Study | Reason for exclusion | No. | Study | Reason for exclusion |
|---|---|---|---|---|---|
| 1 | Singh A et al., 2014 [34] | Only one patient | 17 | Cook DC et al., 2013 [35] | Not comparing a synthetic bone substitute |
| 2 | Coathup MJ et al., 2015 [36] | Treatment not for alveolar bone | 18 | Menezes LM et al., 2012 [37] | Not comparing a synthetic bone substitute |
| 3 | Kato E et al., 2015 [38] | Outcome measure not related to alveolar bone regeneration | 19 | Horvarth A et al., 2013 [39] | Not comparing a synthetic bone substitute |
| 4 | Gupta G et al., 2014 [40] | Not comparing a synthetic bone substitute | 20 | Ghanaati S et al., 2013 [25] | Control group not available |
| 5 | Troedan A et al., 2014 [41] | Outcome measure not related to alveolar bone regeneration | 21 | Pietruska M et al., 2012 [42] | Outcome measure not related to alveolar bone regeneration |
| 6 | Reichert C et al., 2014 [43] | Outcome measure not related to alveolar bone regeneration | 22 | Emam H et al., 2011 [44] | Not comparing a synthetic bone substitute and control group not available |
| 7 | Cosso MG et al., 2014 [3] | Not comparing a synthetic bone substitute | 23 | Lee CY et al., 2011 [45] | Not comparing a synthetic bone substitute |
| 8 | Ghanaati S et al., 2014 [46] | Outcome measure not related to alveolar bone regeneration | 24 | Mairoana C et al., 2011 [47] | Not comparing a synthetic bone substitute and control group not available |
| 9 | de Ruiter A et al., 2014 [4] | Control group not available | 25 | Caubet J et al., 2011 [48] | Control group not available |
| 10 | Traini T et al., 2015 [49] | Control group not available | 26 | Goene RJ et al., 2007 [50] | Outcome measure not related to alveolar bone regeneration |
| 11 | Gupta S et al., 2013 [51] | Not comparing a synthetic bone substitute | 27 | Pappalardo S et al., 2005 [52] | Only one patient |
| 12 | Cannizaro G et al., 2013 [53] | Outcome measure not related to alveolar bone regeneration | 28 | Nemcovsky CE et al., 2001 [54] | Not comparing a synthetic bone substitute |
| 13 | Peres MF et al., 2013 [55] | Not comparing a synthetic bone substitute | 29 | Fugazzotto PA et al., 1999 [56] | Only one patient |
| 14 | Pieri F et al, 2013 [57] | Control group not available | 30 | Wheeler SL et al., 1996 [58] | Not comparing a synthetic bone substitute |
| 15 | Kim DM et al., 2013 [59] | Not comparing a synthetic bone substitute | 31 | Galgut PN et al., 1992 [60] | Outcome measure not related to alveolar bone regeneration |
| 16 | Corinaldesi G et al., 2013 [61] | Not comparing a synthetic bone substitute | 32 | Hurzeler MB et al., 1996 [62] | Outcome measure not related to alveolar bone regeneration |
Table 3.
Quality assessment for all treatment modalities (modified from Plachokova et al., 2008 [32] and Vignoletti, 2011 [33]).
| Quality assessment items | Yes/no | Alveolar ridge or socket preservation | Sinus augmentation | Periodontal bony defect |
|---|---|---|---|---|
| Adequate sequence generation | Y | 1 | 4 | 7 |
| N | 5 | 12 | 13 | |
| Allocation concealment | Y | 1 | 4 | 4 |
| N | 5 | 12 | 16 | |
| Describe as randomized | Y | 3 | 12 | 16 |
| N | 3 | 4 | 4 | |
| Describe clear randomization methods | Y | 2 | 2 | 6 |
| N | 4 | 14 | 14 | |
| Describe control group | Y | 6 | 16 | 20 |
| N | 0 | 0 | 0 | |
| Blinding participant and/or examiner | Y | 2 | 3 | 5 |
| N | 4 | 13 | 15 | |
| Describe inclusion and exclusion criteria | Y | 2 | 12 | 14 |
| N | 4 | 4 | 6 | |
| Patient follow-up related to completed outcome data | Y | 5 | 11 | 16 |
| N | 1 | 5 | 4 | |
| Validity of statistical methods: sample calculation | Y | 1 | 8 | 4 |
| N | 5 | 8 | 16 | |
| Validity of statistical methods: power of statistic | Y | 4 | 12 | 18 |
| N | 2 | 4 | 2 | |
| Validity of outcome and estimation | Y | 6 | 16 | 20 |
| N | 0 | 0 | 0 | |
| Risk of bias: high | 5 | 15 | 18 | |
| Moderate | 0 | 0 | 1 | |
| Low | 1 | 1 | 1 | |
| Total | 6 | 16 | 20 |
3.1. Alveolar ridge or socket preservation
For alveolar ridge or socket preservation, six studies involved RCTs [1, 63] and prospective CCTs [2, 64, 65, 66] that reported parallel [65] or split mouth [1, 63] designs (Table 4). A number of participants (10–83 persons; age, 30–70 years) were divided into two to five study groups, with a follow-up of 4–24 months after grafting.
Table 4.
Alveolar ridge or socket preservation with primary outcome measure related to new bone formation (mean ± SD, 95% CI, and P value).
| Studies | Intervention group | N | Measurement methods | Follow-up (month) | Control group | Test group | Power of statistic |
|---|---|---|---|---|---|---|---|
| Shakibaie B 2013 (CCT) | T1: Bio-Oss + gelatin sponge T2: NanoBone + gelatin sponge C: Stypro gelatin sponge |
11 11 10 |
3D DVT (bone density) | 10 | 352 ± 29.3 | T1: 699 ± 13.3 T2:399 ± 15.6 |
NA |
| Sisti A et al., 2012 (RCT) | T: Mg-eHA + saline C: Guided bone regeneration |
10 10 |
CT (horizontal bone width) | 24 | 7.70 ± 0.92 | 9.48 ± 1.56 | Pval < 0.05 |
| Rebaudi A et al., 2003 (CCT) | T: Biostite (Containing HA) C: Naturally healing |
NA NA (83) |
Radiographic (radiopaque tissue over the implant) and histology | 4 6 |
12% | 20% | NA |
| Arenaz-búa J et al., 2010 (RCT) | C1: Any material-PRP Method 1 C2: Any material-PRP Method 2 C3: Autologous + PRP Method 1 C4: DBX + PRP Method 1 T: Novabone (HA) + PRP Method 1 |
NA NA NA NA NA (82) |
Digital panoramic | 3 6 |
Descriptive/No statistical analysis Bone formation after 3 months: groups C1, C2, C3 are higher and groups T and C4 are lower Bone formation after 6 months: groups C3, C4 are higher and group T is lower than the other group |
||
| Luczyszyn S M et al., 2005 (CCT) | T: Resorbable HA (Algipore) + Acellular dermal matrix graft (ADMG) C: ADMG |
NA NA (11) |
Histology (newly formed bone) | 6 | 46% | 1% | NA |
| De Coster P et al., 2011 (CCT) | T: Bone ceramic + Blood C: Natural healing (Untreated) |
14 14 |
Histology section | Descriptive/no statistical analysis | |||
The periodontal status of the extracted teeth was defined in one study [1]. The teeth extraction area was clearly reported in three studies [1, 2, 63]. Several kinds of HA bone substitutes, such as synthetic HA (NanoBone) [2], Mg-eHA [1], synthetic calcium HA (Novabone) [63], resorbable HA (Algipore) [65], and bone ceramic [64], were used in the test groups. The control group used materials such as gelatin sponge [2], acellular dermal matrix graft (ADMG) [65], and autologous grafts [63], in comparison with bone grafting material or extraction socket left untreated [64]. Radiographs with three-dimensional (3D) digital volume tomography (DVT) [2], cone beam computed tomographic (CT) scan [1], and digital panoramic X-rays [63] were used for analysis. Rebaudi et al. [66] performed radiograph evaluation but they did not mention the method and instrument used to perform the evaluation. In three studies, bone regeneration was evaluated histologically [64, 65, 66], but two of these studies [63, 64] were without statistical analysis. Based on the structural analysis, six studies reported that alveolar ridge or socket preservation was not allowed for further analysis. Thus, the effect of HA bone substitute on alveolar bone regeneration in the case of ridge preservation could not be determined.
3.2. Sinus augmentation
For sinus augmentation, 16 studies involved RCTs [67, 68, 69, 70, 71, 72] and prospective CCTs [5, 6, 7, 8, 22, 24, 73, 74, 75, 76] that reported a parallel [67, 69, 70] or split mouth [68, 75] design (Table 5). The study population included eight to 43 subjects (age, 18–80 years) divided in two to five study groups, with five to 12 months of follow-up after grafting. The outcome measures were analyzed by histomorphometry [5, 6, 8, 22, 67, 68, 69, 70, 72, 73, 74, 75], radiography by CT [24, 71, 76], and histology [7].
Table 5.
Sinus augmentation with primary outcome measure related to new bone formation (mean ± SD, 95% CI, and P value).
| Studies | Intervention group | N | Measurement methods | Follow-up (month) | Control group (C) | Test group (T) | Power of statistic |
|---|---|---|---|---|---|---|---|
| Schmitt CM et al., 2013 (RCT) | T: Biphasic calcium phosphate (BCP) with 60% HA | 14 | Histomorphometric (newly formed bone) | 5 | C1: 24.90 ± 5.67% C2: 35.41 ± 2.78% C3: 41.74 ± 2.10% |
T: 30.28 ± 2.16% | P = 0.000 between T & C3 |
| C: Inorganic bovine bone (ABB) | 15 | ||||||
| C2: Bone allograft (MCBA) | 12 | ||||||
| C3: Autologous bone (AB) | 12 | ||||||
| Ghanaati S et al., 2013 (RCT) | T: Synthetic HA (NanoBone) | 4 | Histomorphometric (newly formed bone) | 6 | C: 25.73 ± 7.94% | T: 21.85 ± 5.96% | P > 0.05 (Pval < 0.05) |
| C: Deproteinized bovine bone (Bio-Oss) | 4 | ||||||
| Lezzi G et al., 2012 (CCT) | T1: Phycogene HA (Algipore) | 6 | Histomorphometric (newly formed bone) | 6 | C1: 31.8 ± 2.9% C2: 32.9 ± 0.5% |
T1: 33.2 ± 1.2% T2: 30.5 ± 3.4% T3: 28.1 ± 3.9% |
P < 0.05 (Pval < 0.05) |
| T2: Macroporous biphasic CaPO4 (MBCP) | 6 | ||||||
| T3: Calcium carbonate (Biocoral) | 6 | ||||||
| C1: Collagenized porcine cortical/cancellous bone (Apatos) | 6 | ||||||
| C2: Inorganic bovine bone (ABB) | 6 | ||||||
| Kurkcu M et al., 2012 (CCT) | T: β-Tri calcium phosphate or β-TCP (Kasios) | 13 | Histomorphometric (newly formed bone) | 6.5 | 30.13 ± 3.45% | 21.09 ± 2.86% | P = 0.001 (Pval < 0.05) |
| C: Inorganic bovine derivate HA (Bone Plus-XS) | 13 | ||||||
| Lorenz J. et al., 2014 (CCT) | T: Synthetic HA (NanoBone) | 7 | Histomorphometric (nexwly formed bone) | 7 | 22.66 ± 7.97% | 19.02 ± 7.28% | P > 0.05 (Pval < 0.05) |
| C: Deproteinized bovine bone (Bio-Oss) | 7 | ||||||
| Lindrgen C et al., 2012 (RCT) | T: Biphasic calcium phosphate (BCP) | 5 | Histomorphometric (newly formed bone) | 8 | 31.7 ± 18.0% | 28.6 ± 14.3% | P = 0.67 (Pval < 0.05) |
| C: Deproteinized bovine bone (DBB) | 5 | ||||||
| Boeck-Neto RJ et al., 2001 (CCT) | T: Autogenous + HA | 5 | Histomorphometric (newly formed bone) | 10 | 50.46 ± 16.29% | 46.79 ± 8.56% | NA |
| C: Autogenous + DFDBA | 5 | ||||||
| Mangano C et al., 2007 (CCT) | T: Porous synthetic HA | 20 | Histomorphometric (newly formed bone) | 12 | 36.2 ± 1.4% | 34.7 ± 3.1% | P = 0.031 (Pval < 0.05) |
| C: Bovine derivate HA | 20 | ||||||
| Artzi Z et al., 2001 (RCT) | T: Synthetic nonceramic resorbable HA (NC-HA) | 14 | Histomorphometric (bone area fraction at 10 sites) | 12 | 42.1 ± 10.0% | 32.2 ± 8.15% | NA |
| C: Natural deproteinized bovine HA (BHA) | 14 | ||||||
| Baena RR et al., 2013 (RCT) | T: Polyacid-HA (PLGA/HA) | 4 | Radiographic by CT (bone density) | 6 | 946 ± 161.9 | 286 ± 134.4 | P = 0.002 (Pval < .05) |
| C: Deproteinized bovine bone | 4 | ||||||
| Tosta M et al., 2013 (RCT) | T: Biphasic calcium phosphate (with 60% HA and 40% β-TCP) + Membrane collagen C: Particulate autogenous + Membrane collagen |
15 15 |
Histomorphometric (area fraction of mineralized bone) | 9 | Area 1: 41.03 ± 4.62% Area 2: 38.63 ± 7.52% |
Area 1: 33.70 ± 8.08% Area 2: 26.68 ± 3.92% |
P1 = 0.008 P2 < 0.001 (Pval < 0.05) |
| Lindgren C et al., 2010 (CCT) | T: β-TCP + HA | 11 | Histological analysis EDS (Ca/P Ratio) |
8 | 1.72 ± 0.07 | 1.63 ± 0.02 | NA |
| C: Deproteinized Bovine Bone | 11 | ||||||
| Karabuda C et al., 2001 (CCT) | T: Porous HA (PHA) | 3 | Histomorphometric (newly formed bone) | 6 | C1: 70–75% C2: 50% |
20–35% | NA |
| C1: Demineralized freeze-dried bone powder (DFBP) | 1 | ||||||
| C2: Deproteinized bovine bone granule (DBBG) | 5 | ||||||
| Lorenzetti M et al., 1998 (CCT) | T: Autogenous from chin + PHA (Interpore 200) | 3 | Histomorphometric (newly formed bone) | 8&12 | C1: 53% C2: 69.3% C3: 62.6% |
T: 44.3% | NA |
| C1: Autogenous from iliac | 4 | ||||||
| C2: Autogenous from chin | 3 | ||||||
| C3: Particulate autogenous | 3 | ||||||
| Kuhl S et al., 2012 (CCT) | T: Blood + PAB + β-TCP | 10 | Radiographic by CT (bone density) | NA | #C: 0.5 | #T1: 0.08 #T2: 0.00031 |
P = 0.341 (Pval < 0.05) |
| T: Blood + PAB + β-TCP/HA | 10 | ||||||
| C: Blood + PAB | 10 | ||||||
| Kuhl S et al., 2013 (CCT) | T1: Blood + Autogenous Bone (PAB) + β-TCP | 10 | Radiographic by μ-CT (Volume fraction bone) | 5 | 18.5% | T1: 17.1% T2: 21.7% |
P = 0.578 |
| T2: Blood + PAB + β-TCP/HA | 10 | ||||||
| C: Blood + PAB | 10 |
In the test group, biphasic calcium phosphate (BCP) containing 60% HA [67, 69, 72, 73], synthetic HA (NanoBone) [68, 75], HA bone substitute [6], porous synthetic HA [5, 8, 22], synthetic nonceramic HA (NC-HA) [70], polyacid-HA (PLGA/HA) [71], composites containing HA [5, 6, 8, 22, 70, 71, 73, 75], and inorganic bovine-derived HA [7, 24, 74, 76] were used. The materials applied in the test group were used either alone or combined with other materials. The control group for the studies on sinus augmentation used inorganic bovine [7, 8, 22, 67, 68, 69, 70, 71, 73, 74, 75] and autologous [5, 6, 24, 72, 76] bone as a comparison.
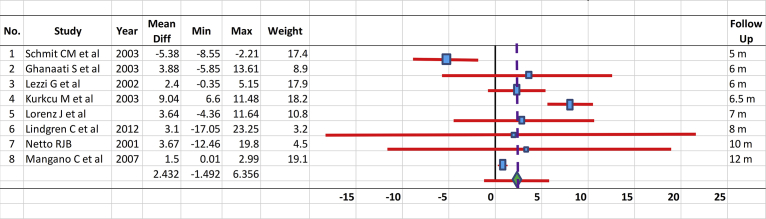
From 16 studies analyzing sinus augmentation-tested biomaterials containing HA, either alone or combined with another graft/autograft [5, 6, 76], membrane collagen [72], or the patient's own blood [24, 76], only eight could be compared to each other since they used the same measurement methods and reported mean, SD/standard error, or P values. Fig. 1 shows comparison of the mean values and 95% CIs for the test and control groups of the eight selected comparable studies performed for treatment of sinus augmentation. The analysis confirmed that HA had an effect in alveolar bone regeneration when used for sinus augmentation treatment. Only a study by Schmitt [67] shows no significant different between the treatments using biphasic calcium phosphate-containing HA compared to inorganic bovine bone.
Fig. 1.
Comparison of the mean values and 95% confidence intervals for the test and control groups of the selected studies performed dealing with the treatment of sinus augmentation.
3.3. Periodontal bone defect
For periodontal bone defects (Table 6), 20 studies involving RCTs [10, 77, 78, 79, 80, 81, 82, 83, 84, 85] and prospective CCTs [9, 86, 87, 88, 89, 90, 91, 92, 93, 94] reported a parallel [79, 84, 87] or split mouth [10, 81, 83, 86] design. The study population consisted of 12–263 persons (age, 14–75 years) divided into two to four study groups with a follow-up of 1–24 months after grafting. The outcome measures were analyzed radiographically by Intraoral Periapical (IOPA) [10, 78], CT [86], long cone-paralleling technique [80, 85, 91, 94], computer assisted densitometry image analysis (CADIA) [87], and other radiographic methods [9, 79, 81, 82, 83, 88, 89, 92, 93].
Table 6.
Periodontal bony pocket with primary outcome measure related to new bone formation (mean ± SD, 95% CI, and P value).
| Studies | Intervention group | N | Measurement methods | Follow-up (month) | Control group (C) | Test group (T) | Power of statistic |
|---|---|---|---|---|---|---|---|
| Pradeep AR et al., 2016 (RCT) | T1: Platelet-rich fibrin (PRF) + HA + Open flap debridement (OFD) | Radiographic by intra oral periapical/IOPA (bone defect fill) | 9 | 10.09 ± 4.28% | T1: 54.69 ± 1.93% T2: 61.94 ± 3.54% |
Pval < 0.001 | |
| T2: RSV gel + PRF + HA + OFD | 36 | ||||||
| C: OFD + Placebo gel | 37 | ||||||
| Debnath T et al., 2014 (RCT) | T1: HA-BG (bioactive glass) + biodegradable membrane | 10 | Radiographic by intra oral periapical/IOPA (bone defect fill) | 6 | 0.9 ± 0.7% | T1: 2.6 ± 0.66% T2: 1.6 ± 0.66% |
NA |
| T2: HAP + biodegradable membrane | 10 | ||||||
| C: OFD + biodegradable membrane | 10 | ||||||
| Kumar PG et al., 2011 (CCT) | T: Alloplast containing HA (Bonelike) | 10 | Radiograph CT (defect fill) | 6 | 37.41 ± 23.63 mm | 62.59 ± 31.58 mm | P = .04 |
| C: OFD | 10 | ||||||
| Chandrashekar KT & Saxena KT, 2009 (RCT) | T: Synthetic HA + β-TCP (Biograft HT) | 13 | Radiographic by the long cone-paralleling technique (defect fill) | 3 6 |
4.200 ± 0.978 3.800 ± 0.862 |
3.667 ± 1.029 2.633 ± 0.896 |
P = 0.212 P = 0.014 (Pval < 0.01) |
| C: OFD | 13 | ||||||
| Brown GD et al., 1998 (CCT) | T1 (2 defect): Flap & HAC | 8 | Radiographic by computer assisted densitometry image analysis/CADIA (defect fill) | 12 | Flap: 32.3 ± 23.8 | HAC: 29.2 ± 31.7 DFDB: 41.9 ± 42.9 |
(Pval < 0.05) |
| T2 (2 defect): DFDB + HAC | 16 | ||||||
| Leonardis DD et al., 2013 (RCT) | T: EMD (enamel matrix derivative) + HA/β-TCP | 36 | Radiographic (bone gain) | #0–12 #0–24 |
C1: 2.32 ± 0.40 C2: 0.13 ± 0.48 C1: 3.17 ± 0.69 C2: 0.23 ± 0.55 |
T: 2.61 ± 0.49 T: 3.35 ± 0.80 |
(Pval < 0.001) |
| C1: EMD | 36 | ||||||
| C2: OFD | 36 | ||||||
| Singhal et al., 2013 | T: OFD ± β-TCP/H + barrier membrane | 6 | Radiographic (bone defect area decreased) | #0–6 | 14.08 ± 12.7% | 48.88 ± 18.61 | P = 0.009 |
| C: OFD + barrier membrane | 6 | ||||||
| Singh VP et al., 2012 (RCT) | T: nanocrystal (Nc-HA) + collagen membrane | 10 | Radiographic (defect fill gain changes) | #0–6 | 0.91 ± 0.21 | 2.07 ± 0.67 |
Pcontrol = 0.008 Ptest = 0.007 |
| C: OFD | 10 | ||||||
| Mistry S et al., 2012 (CCT) | T1: OFD + HA | 8 | Radiographic (depth of the defect) | 6 #0–6 |
9.20 ± 0.44 3.00 ± 0.71 |
T1: 9.40 ± 1.51 T2: 8.80 ± 0.83 T3: 9.40 ± 1.51 T1: 3.00 ± 0.71 T2: 4.40 ± 1.14 T3: 4.20 ± 0.44 |
P < 0.01 |
| T2: OFD + BG (Bioactive Glass) | 8 | ||||||
| T3: OFD + BG + HA | 8 | ||||||
| C: OFD | 8 | ||||||
| Assche NV et al., 2013 (RCT) | T: Straumann bone ceramic | 14 | Radiographic (depth of defect changes) | #0–6 | 1.9 ± 1.2 | 1.5 ± 1.2 | P > 0.05 |
| C: Bio-Oss | 14 | ||||||
| Pietruska M et al., 2012 (CCT) | T: OFD + NHA | 15 | Radiographic (defect depth & defect width) | #0–6 | DD: 0.6 ± 0.9 DW: 0.7 ± 0.8 |
DD: 1.8 ± 1.5 DW: 0.7 ± 0.9 |
DD: Pval < 0.05 DW: Pval < 0.001 |
| C: OFD | 15 | ||||||
| Lekovic V et al., 1990 (CCT) | T: PHA + barrier membrane (Goretex) | NA | Acrylic stent (bone level) | 6 | Vertical bone level: 0.13 ± 0.26 Horizontal Bone level: 0.13 ± 0.09 |
Vertical bone level: 2.27 ± 0.18 Horizontal Bone level: 1.60 ± 0.021 |
Pval < 0.01 |
| C: Goretex | NA (15) |
| Studies | Intervention group | N | Measurement methods | Follow-up (month) | Control group | Test group | Power of statistic |
|---|---|---|---|---|---|---|---|
| Jepsen S et al., 2008 (RCT) | T: EMD ± synthetic bone Ceramic (SBC)-HA C: EMD |
29 29 |
Bone sounding (defect fill) | #0–6 | 2.07 ± 1.2 mm | 2.01 ± 2.1 mm | P < 0.001 |
| Bowen J A et al., 1989 (CCT) | T: particulate HA C: demineralized freeze cried bone allograft (DFDBA) |
6 6 |
Radiographic by paralleling device (bone defect fill) | 6 | 61% | 53% | Pval < 0.05 |
| Lai N & Dixit J 2012 (CCT) | T: OFD + HA + own blood C1: OFD + Cissus Quadrangu laris (CQ) + saline C2: Oxidized regenerated + OFD C3: OFD |
5 5 5 5 |
Radiographic (defect fill) | 1 3 6 |
C1: 1.6 mm3 C2: 3 mm3 C3: 0.60 mm3 |
T: 3 mm3 | NA |
| Tobon SI et al., 2002 (RCT) | T1: periradicular surgery + resorbable HA (OsteoGen) C1: periradicular surgery + nonabsorbable membrane (e-PTFE) C2: periradicular surgery with Conventional technique |
8 8 8 |
Radiographic by parallel technique (bone healing by size of lesion area) | 3 6 9 12 |
NA | NA |
PT2 = 0.0002 PC1 = 0.002 PC2 = 0.1 (Pval ≤ 0.05) |
| Harris RJ, 1998 (CCT) | T: DFDB + PHA + tetracyclin C: DFDB + tetracyclin |
50 50 |
Radiographic (descriptive) | 10 | NA | Horizontal probing depth reducing 7.0 mm–2.5 mm | NA |
| Kiliq AR, 1997 (CCT) | T1: e-PTFE membrane + HA + Collagen type 1 T2: HAC C1: e-PTFE membrane C2: Conventional flap (CF) |
10 10 10 10 |
Radiographic by the parallel technique (bone level) | 6 | C1: 36% C2:15% |
T1: 43% T2: 23% |
NA |
| Bowen JA et al., 1989 (CCT) | T: Particulate HA C: DFDBA |
6 6 |
Radiographic by customized paralleling (defect fill) | 6 | 61% | 53% | Pval < 0.05 |
| Queiros L A et al., 2015 (RCT) | T1: β-TCP/HA T2: Enamel matrix derivative (EMD) + β-TCP C: EMD |
13 13 13 |
Clinically (PI/GI/RGMP/RVCAL/RHCAL/PPD) for soft tissue | 12 | Outcome measure only for soft tissue | ||
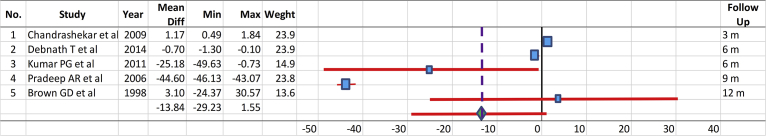
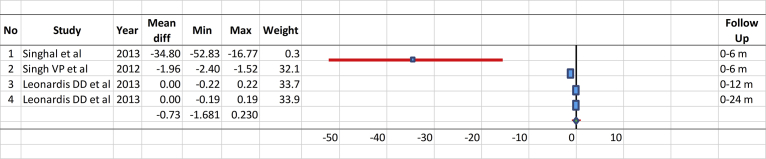
The control groups underwent open flap debridement (OFD) [9, 10, 78, 79, 80, 81, 82, 86, 88, 89], demineralized freeze-dried bone allografts (DFDBA) [87, 91], bovine bone [83], barrier membrane (Goretex) [90], enamel matrix derivative (EMD) [77, 84], nonabsorbable polytetrafluoroethylene membrane (e-PTFE) [85], and debridement only (DEBR) [93]. The test groups were alloplastic graft alone with β-tricalcium phosphate (β-TCP/HA) [77] and bonelike containing glass-reinforced HA [86], synthetic HA alone [93], porous HA (PHA) [90], resorbable HA (Osteogen) [66], or synthetic HA combined with OFD [9, 78, 88, 89], PRP [78], β-TCP [80, 81, 82], barrier membrane such as EMD [81, 84], collagen membrane [79], Goretex [90], e-PTFE membrane [94], bioactive glass [10], or DFDBA [92]. The mean values and 95% CIs for the test and control groups of the selected comparable studies performed for treatment of periodontal bone defect measured in certain follow-up periods (Fig. 2) and based on the difference between the baselines and certain follow-up periods (Δ in Fig. 3 were compared. There was no significant difference between the treatments of periodontal bony defect with HA compared to the controls.
Fig. 2.
Comparison of the mean values and 95% confidence intervals for the test and control groups of the selected studies performed dealing with the treatment of periodontal bone defects.
Fig. 3.
Comparison of the mean values and 95% confidence intervals for the test and control groups of the selected studies performed dealing with the treatment of periodontal bone defects.
4. Discussion
We reviewed studies on the effect of HA bone substitute on three treatment modalities (i.e., ridge or socket preservation, sinus augmentation, and periodontal bony defect). Besides autologous bone as a gold standard, other graft sources, such as allograft (DFDBA or mineralized freeze-dried bone allograft), xenogenic (organic bovine, porcine, caprine, or coral-derived HA), replicating (morphogenetic proteins), and alloplastic (bioglass, bioceramics) graft materials or combinations, are available.
The present systematic review was limited to RCTs and CCTs. The control groups included patients who had autogenous, allogenic, xenogenic, and barrier membrane, such as EMD, collagen, OFD, or an untreated socket. The test groups had synthetic HA, BCP-containing HA, or nanocrystalline HA (NC-HA). Several outcome measures related to healing of the hard or soft tissues after treatment were evaluated. The primary outcome measures were defined related to alveolar bone regeneration. These measurements involved a radiographic (IOPA/CADIA/parallel), histologic, and histomorphometric analysis. The amount of new bone formed and the changes in density from the baseline to several months of follow-up were evaluated. Several studies with the same methods and outcome measures were analyzed and displayed as a graph.
Compared to the previous systematic review [30, 31], our systematic review focused on evaluating HA bone substitute for the treatment of alveolar bone in cases of alveolar ridge or socket preservation, sinus augmentation, and periodontal bony defect compared to controls (without HA bone substitute) in relation to new bone regeneration on radiographic, histologic, and histomorphometric analysis.
The quality assessment of the included studies resulted in only three studies classified as having a low risk of bias. One study had a moderate and 38 a high risk of bias; thus, their results must be evaluated with caution. The reason for high risk of bias was because several studies lacked an adequate sequence generation, allocation concealment and patient masking, therapist, and statistician. Moreover, most studies did not perform sample size calculation, while definitive sample size calculation was conducted only in 13 of 42 studies. Consequently, insufficient reported data existed to be used further to determine the validity of outcome and estimation.
The new bone regeneration after HA bone substitute grafting was measured as the presence of newly formed bone (histomorphometric), bone density (radiographic), or bone defect fill (radiographic or bone sounding methods). Clinical measurements, such as PPD, CAL, PI, and GI, were performed to evaluate the soft tissue around the defect area. Histologic sections were assessed by descriptive analysis, without statistical analysis.
This systematic review aimed to evaluate the effectiveness of HA bone substitute alone or in combination compared to nonsynthetic graft or an untreated socket. The inter-reader agreement was high (i.e., initial step, κ = 0.968; first step, κ = 0.910, and second step, κ = 0.864), most probably because both readers were well experienced with the HA bone substitute and treatment evaluated in this study. The 95% CI was used to analyze the similar outcome measured data presented in the original studies with different treatment modalities and time to follow-up after treatment. Several methods were chosen in the control groups within the studies found. Control groups existed with an empty untreated socket and treatments with gelatin sponge, guided bone regeneration (GBR) methods, PRP, and EMD. This is the reason for the difficulties in comparing the studies since there was heterogeneity in the control groups. Thus, further evaluation could not be performed in some studies.
The systematic review confirmed that the same material was not always effective when applied in different cases. Some related studies showed that although NanoBone was effective in sinus augmentation, it was not suitable for socket preservation since the socket preservation deals with a special defect and exposure in the oral cavity [2]. In the case of the alveolar ridge, significantly better preservation was achieved with an inorganic bovine containing 10% collagen (Bio-Oss) than with a synthetic bone substitute composed of HA and silicone dioxide (NanoBone). Meanwhile, the autogenous grafts still were considered the golden standard for grafting procedures since they resulted in a high rate of new bone regeneration [63], followed by allogenic demineralized bone matrix [2, 63]. A particulate autogenous bone combination with β-TCP [23] or HA/β-TCP [76] as well autogenous graft alone for sinus maxillary augmentation resulted in a remarkable amount of newly formed bone after a 5-month healing period. However, the volume of newly formed bone was not significantly affected by adding synthetic bone ceramic to particulate autogenous bone but reduced the high risk of donor site morbidity and lowered the pain [76].
There were many RCTs that established PRP usefulness in the field of tissue regeneration [32, 63]. Arenaz-Búa et al. [63] found that PRP facilitated and accelerated bone formation in the 3 months postoperatively. The study showed that autograft or allograft combination with PRP resulted in a higher rate of newly formed bone than synthetic particulate HA-silicon dioxide (NovaBone) with PRP after 6 months of follow-up.
Inorganic bovine-derived HA [74] or deproteinized bovine bone (DBB) [7] appeared to be significantly more effective in osteoconduction compared to β-TCP alone or combined with synthetic HA [7] (P = 0.001). The histomorphometric analysis showed that new bone formation around the graft particles was similar for BCP and inorganic bovine bone (ABB) [67, 73] or DBB [69]. No statistical significant difference was noted between BCP and ABB or DBB with regard to newly formed bone in the defect site [7, 69]. Biphasic synthetic materials consisting of a mixture of HA and TCP were used for reconstruction in implant surgery. Artzi et al. (2008) [95] claimed that an optimum balance of the stable phase of HA and soluble phase of TCP induced increasing new bone regeneration. Synthetic or particulate HA compared to DBB (Bio-OSS) did not show a statistically significant difference to support new bone formation within the sinus cavity [8, 22, 68, 75]. A significantly higher new bone formation rate was found for natural deproteinized bovine HA (BHA) versus NC-HA after 12 months [70]. DFDBA and HA combined with autogenous bone were biocompatible and allowed osteoconduction. Their values for new bone formation in sinus augmentation were not significantly different. However, both materials were still present after 10 months [6].
The density of bone regeneration using PLGA/HA was significantly less than that of DBB. Clinical assessments demonstrated that PLGA/HA has sufficient characteristics to be used in sinus-lifting surgery [71]. On the contrary, PLGA synthetic polymer scaffolds brought fast resorption rates characteristically, making them difficult to provide adequate mechanical stability for osteoblasts delivered in the graft site [26]. Radiographic results showed that PLGA/HA was able to regenerate bone in the sinus-lift area, but with insufficient quantity and quality to insert endosseous implants. DBB provided greater bone density and equivalent vertical dimension with PLGA/HA grafted bone [71].
HA and β-TCP appear to be safe and may be clinically acceptable in various types of periodontal defects [87]. Combined HA and β/α TCP [7, 81, 82], whether or not with additional EMD or marginal pedicle periosteum, showed better results in bone defect filling compared to OFD alone or EMD alone. HA plus bioactive glass [10, 88] was able to ovoid the defect (significant difference, P < 0.05) compared to HA or OFD alone, but DFDBA did not have any statistical differences with HA bone graft at 6 months of follow-up [87]. DFDBA combined with PHA showed the same result as DFDBA [92] alone. DFDBA and synthetic or particulate HA had similar effects on periodontal bony defect treatment since their values were likewise not statistically different [87, 96]. When regeneration of the periodontium tissue was the desired goal, then DFDBA seemed to be an appropriate material. If only defect fill was the objective, then the choice appeared to be equivalent [96]. The NC-HA bone graft plus collagen membrane demonstrated clinical and radiographic advantages beyond the one achieved by OFD alone and showed a statistically significant difference for percentage of bone fill after 6 months of follow-up [79]. Additional use of NHA after OFD did not reduced the defect depth significantly [89].
There were small changes in the bone level when membrane alone was used. The combination of PHA with barrier membrane (Goretex) [90] or SBC (synthetic bone substitute composed of BCP with 60% HA and 40% β-TCP) with EMD or HA cement (HAC) with a nonbioabsorbable e-PTFE membrane [94] led to more attachment gain and bone fill than synthetic HA alone or a conventional flap [97]. They achieved increased bone fill in the defect site better than synthetic HA alone although the results were not significantly different after 6 months of follow-up.
5. Conclusions
Based on this systematic review, autograft is still considered the best bone grafting material for ridge or socket preservation, sinus augmentation, and periodontal bony defect. HA bone substitute is a good bone graft candidate to reduce the high risk of donor morbidity and evoke less pain, but no significant results were found in the studies. Thus, to overcome the problems in grafting procedures, superior bone substitute with ideal properties for the treatment of choice in alveolar ridge or socket preservation, sinus augmentation, and periodontal bony defect must be developed.
Declarations
Author contribution statement
Anne Handrini Dewi: performed the experiments.
Ika Dewi Ana: conceived and designed the experiments.
Anne Handrini Dewi, Ika Dewi Ana: analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Sisti A., Canullo L., Mottola M.P., Covani U., Barone A., Botticelli D. Clinical evaluation of a ridge augmentation procedure for the severely resorbed alveolar socket: multicenter randomized controlled trial, preliminary results. Clin. Oral Implants Res. 2012;23(5):526–535. doi: 10.1111/j.1600-0501.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- 2.Shakibaie M.B. Comparison of the effectiveness of two different bone substitute materials for socket preservation after tooth extraction: a controlled clinical study. Int. J. Periodontics Restor. Dent. 2013;33(2):223–228. doi: 10.11607/prd.0734. [DOI] [PubMed] [Google Scholar]
- 3.Cosso M.G., de Brito R.B., Jr., Piattelli A., Shibli J.A., Zenóbio E.G. Volumetric dimensional changes of autogenous bone and the mixture of hydroxyapatite and autogenous bone graft in humans maxillary sinus augmentation. A multislice tomographic study. Clin. Oral Implants Res. 2014;25(11):1251–1256. doi: 10.1111/clr.12261. [DOI] [PubMed] [Google Scholar]
- 4.De Ruiter A., Dik E., Van Es R., Van Der Bilt A., Janssen N., Meijer G., Koole R., Rosenberg A. Micro-structured calcium phosphate ceramic for donor site repair after harvesting chin bone for grafting alveolar clefts in children. J. Craniomaxillofac. Surg. 2014;42(5):460–468. doi: 10.1016/j.jcms.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzetti M., Mozzati M., Campanino P.P., Valente G. Bone augmentation of the inferior floor of the maxillary sinus with autogenous bone or composite bone grafts: a histologic-histomorphometric preliminary report. Int. J. Oral Maxillofac. Implants. 1998;13(1):69–76. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 6.Boëck-Neto R.J., Gabrielli M., Lia R., Marcantonio E., Shibli J.A., Marcantonio E., Jr. Histomorphometrical analysis of bone formed after maxillary sinus floor augmentation by grafting with a combination of autogenous bone and demineralized freeze-dried bone allograft or hydroxyapatite. J. Periodontol. 2002;73(3):266–270. doi: 10.1902/jop.2002.73.3.266. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren C., Hallman M., Sennerby L., Sammons R. Back-scattered electron imaging and elemental analysis of retrieved bone tissue following sinus augmentation with deproteinized bovine bone or biphasic calcium phosphate. Clin. Oral Implants Res. 2010;21(9):924–930. doi: 10.1111/j.1600-0501.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- 8.Karabuda C., Ozdemir O., Tosun T., Anil A., Olgaç V. Histological and clinical evaluation of 3 different grafting materials for sinus lifting procedure based on 8 cases. J. Periodontol. 2001;72(10):1436–1442. doi: 10.1902/jop.2001.72.10.1436. [DOI] [PubMed] [Google Scholar]
- 9.Lal N., Dixit J. Biomaterials in periodontal osseous defects. J. Oral Biol. Craniofac. Res. 2012;2(1):36–40. doi: 10.1016/S2212-4268(12)60009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debnath T., Chakraborty A., Pal T.K. A clinical study on the efficacy of hydroxyapatite – bioactive glass composite granules in the management of periodontal bony defects. J. Indian Soc. Periodontol. 2014;18(5):593–600. doi: 10.4103/0972-124X.142451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaccaro R. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002;25(11):1224. doi: 10.3928/0147-7447-20020502-05. [DOI] [PubMed] [Google Scholar]
- 12.Betz R.R. Limitation of autograft and allograft: new synthetic solutions. Orthopedics. 2002;25(2):5561–5570. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 13.Molly L., Vandromme H., Quirynen M., Schepers E., Adams J.L., Van Steenberghe D. Bone formation following implantation of bone biomaterials into extraction sites. J. Periodontol. 2008;79:1108–1115. doi: 10.1902/jop.2008.070476. [DOI] [PubMed] [Google Scholar]
- 14.Carson J.S., Bostrom M.P.G. Synthetic bone scaffolds and fracture repair. Inj. Int. J. Care Injured. 2007;3851:533–537. doi: 10.1016/j.injury.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Supova M. Problem of hydroxyapatite dispersion in polymer matrices: a review. J. Mater. Sci. Mater. Med. 2009;20:1201–1213. doi: 10.1007/s10856-009-3696-2. [DOI] [PubMed] [Google Scholar]
- 16.Ana I.D., Matsuya S., Ishikawa K. Engineering of carbonate apatite bone substitute based on composition-transformation of gypsum and calcium hydroxide. Engineering. 2010;2(05):344–349. [Google Scholar]
- 17.Martinetti R., Dolcini L., Mangano C. Physical and chemical aspects of a new porous hydroxyapatite. Anal. Bioanal. Chem. 2005;381:634–638. doi: 10.1007/s00216-004-2957-7. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento C., Issa J.P.M., Oliviera R.R., Iyosoma M.M., Siessere S., Regalo S.C.H. Biomaterials applied to the bone healing process. Int. J. Morphol. 2007;25(4):839–846. [Google Scholar]
- 19.Heilmann F., Standard O.C., Mϋller F.A., Hoffman M. Development of graded hydroxyapatite/CaCO3 composite structures for bone ingrowth. J. Mater. Sci. Mater. Med. 2007;18:1817–1824. doi: 10.1007/s10856-007-3028-3. [DOI] [PubMed] [Google Scholar]
- 20.Frayssinet P., Fages J., Bonel Rouquet N. Biotechnology, material sciences and bone repair. Eur. J. Orthop. Surg. Traumatol. 1998;8:17–25. [Google Scholar]
- 21.Hench L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991;74:1485–1510. [Google Scholar]
- 22.Mangano C., Scarano A., Perroti V., Lezzi G., Piatelli A maxillary sinus augmentation with a porous synthetic hydroxyapatite and bovine-derived hydroxyapatite: a comparative clinical and histologic study. Int. J. Oral Maxillofac. Implants. 2007;22(6):980–986. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 23.Kasaj A., Rohrig B., Zafiropoulos G.G., Willershausen B. Clinical evaluation of nanocrystalline hydroxyapatite paste in the treatment of human periodontal bony defect – a randomized controlled clinical trial: 6-month results. J. Periodontol. 2008;79(3):394–400. doi: 10.1902/jop.2008.070378. [DOI] [PubMed] [Google Scholar]
- 24.Kühl S., Götz H., Brochhausen C., Jakse N., Filippi A., d'Hoedt B., Kreisler M. The influence of substitute materials on bone density after maxillary sinus augmentation: a microcomputed tomography study. Int. J. Oral Maxillofac. Implants. 2012;27(6):1541–1546. [PubMed] [Google Scholar]
- 25.Ghanaati S., Barbeck M., Willershausen I., Thimm B., Stuebinger S., Korzinskas T., Obreja K., Landes C., Kirkpatrick C.J., Sader R.A. Nanocrystalline hydroxyapatite bone substitute leads to sufficient bone tissue formation already after 3 months: histological and histomorphometrical analysis 3 and 6 months following human sinus cavity augmentation. Clin. Implant Dent. Relat. Res. 2013;15(6):883–892. doi: 10.1111/j.1708-8208.2011.00433.x. [DOI] [PubMed] [Google Scholar]
- 26.Mangano C., Piattelli A., Mangano A., Mangano F., Mangano A., Iezzi G., Borges F.L., d'Avila S., Shibli J.A. Combining scaffolds and osteogenic cells in regenerative bone surgery: a preliminary histological report in human maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2009;11(Suppl. 1):e92–e102. doi: 10.1111/j.1708-8208.2009.00227.x. [DOI] [PubMed] [Google Scholar]
- 27.Bundela H., Bajpai A.K. Design of hydroxyapatite-gelatin based porous matrix as bone substitute: correlation with biocompatibility aspects. Express Polym. Lett. 2008;2(3):201–213. [Google Scholar]
- 28.Reynolds M.A., Aichelmann-Reidy M.E., Branch-Mays G.L., Gunsolley J.C. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann. Periodontol. 2003;8(1):227–265. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 29.Dutta S.R., Passi D., Singh P., Bhuibhar A. Ceramic and nonceramic hydroxyapatite as a bone graft material: a brief review. Ir. J. Med. Sci. 2015;184:101–106. doi: 10.1007/s11845-014-1199-8. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz G.A., De Buitrago J.G., Reddy M.S. Periodontal regeneration-furcation defects: a systematic review from the AAP regeneration workshop. J. Periodontol. 2015;86(Suppl.):S108–S130. doi: 10.1902/jop.2015.130677. [DOI] [PubMed] [Google Scholar]
- 31.Horvath A., Mardas N., Mezzomo L.A., Needleman I.G., Donos N. Alveolar ridge preservation: a systematic review. Clin. Oral Invest. 2013;17:341–363. doi: 10.1007/s00784-012-0758-5. [DOI] [PubMed] [Google Scholar]
- 32.Plachokova A.S., Nikolidakis D., Mulder J., Jansen J.A., Creugers N.H.H. Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin. Oral Implant Res. 2008;19:539–544. doi: 10.1111/j.1600-0501.2008.01525.x. [DOI] [PubMed] [Google Scholar]
- 33.Vignoletti F., Matesanz P., Rodrigo D., Figuero E., Martin C., Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implant Res. 2012;23(Suppl. 5):22–38. doi: 10.1111/j.1600-0501.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 34.Singh A., Daing A., Anand V., Dixit J. Two dimensional alveolar ridge augmentation using particulate hydroxyapatite and collagen membrane: a case report. J. Oral Biol. Craniofac. Res. 2014;4(2):151–154. doi: 10.1016/j.jobcr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook D.C., Mealey B.L. Histologic comparison of healing following tooth extraction with ridge preservation using two different xenograft protocols. J. Periodontol. 2013;84(5):585–594. doi: 10.1902/jop.2012.120219. [DOI] [PubMed] [Google Scholar]
- 36.Coathup M.J., Sanghrajka A., Aston W.J., Gikas P.D., Pollock R.C., Cannon S.R., Skinner J.A., Briggs T.W., Blunn G.W. Hydroxyapatite-coated collars reduce radiolucent line progression in cemented distal femoral bone tumor implants. Clin. Orthop. Relat. Res. 2015;473(4):1505–1514. doi: 10.1007/s11999-014-4116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menezes L.M., Rao J. Long-term clinical evaluation of platelet-rich plasma in the treatment of human periodontal intraosseous defects: a comparative clinical trial. Quintessence Int. 2012;43(7):571–582. www.quintpub.com/Journals Retrieved from. [PubMed] [Google Scholar]
- 38.Kato E., Yamada M., Sakurai K. Retrospective clinical outcome of nanopolymorphic crystalline hydroxyapatite-coated and anodic oxidized titanium implants for 10 years. J. Prosthodont. Res. 2015;59(1):62–70. doi: 10.1016/j.jpor.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Horváth A., Stavropoulos A., Windisch P., Lukács L., Gera I., Sculean A. Histological evaluation of human intrabony periodontal defects treated with an unsintered nanocrystalline hydroxyapatite paste. Clin. Oral Investig. 2013;17(2):423–430. doi: 10.1007/s00784-012-0739-8. [DOI] [PubMed] [Google Scholar]
- 40.Gupta G. Clinical and radiographic evaluation of intra-bony defects in localized aggressive periodontitis patients with platelet rich plasma/hydroxyapatite graft: a comparative controlled clinical trial. Contemp. Clin. Dent. 2014;5(4):445–451. doi: 10.4103/0976-237X.142806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troedhan A., Schlichting I., Kurrek A., Wainwright M. Primary implant stability in augmented sinuslift-sites after completed bone regeneration: a randomized controlled clinical study comparing four subantrally inserted biomaterials. Sci. Rep. 2014;30(4):5877. doi: 10.1038/srep05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietruska M., Pietruski J., Nagy K., Brecx M., Arweiler N.B., Sculean A four-year results following treatment of intrabony periodontal defects with an enamel matrix derivative alone or combined with a biphasic calcium phosphate. Clin. Oral Investig. 2012;16(4):1191–1197. doi: 10.1007/s00784-011-0611-2. [DOI] [PubMed] [Google Scholar]
- 43.Reichert C., Wenghoefer M., Kutschera E., Götz W., Jäger A. Ridge preservation with synthetic nanocrystalline hydroxyapatite reduces the severity of gingival invaginations – a prospective clinical study. [Article in German] J. Orofac. Orthop. 2014;75(1):7–15. doi: 10.1007/s00056-013-0175-7. [DOI] [PubMed] [Google Scholar]
- 44.Emam H., Beheiri G., Elsalanty M., Sharawy M. Microcomputed tomographic and histologic analysis of anorganic bone matrix coupled with cell-binding peptide suspended in sodium hyaluronate carrier after sinus augmentation: a clinical study. Int. J. Oral Maxillofac. Implants. 2011;26(3):561–570. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 45.Lee C.Y., Prasad H.S., Suzuki J.B., Stover J.D., Rohrer M.D. The correlation of bone mineral density and histologic data in the early grafted maxillary sinus: a preliminary report. Implant Dent. 2011;20(3):202–214. doi: 10.1097/ID.0b013e318211f72e. [DOI] [PubMed] [Google Scholar]
- 46.Ghanaati S., Lorenz J., Obreja K., Choukroun J., Landes C., Sader R.A. Nanocrystalline hydroxyapatite-based material already contributes to implant stability after 3 months: a clinical and radiologic 3-year follow-up investigation. J. Oral Implantol. 2014;40(1):103–109. doi: 10.1563/AAID-JOI-D-13-00232. [DOI] [PubMed] [Google Scholar]
- 47.Maiorana C., Beretta M., Battista Grossi G., Santoro F., Scott Herford A., Nagursky H., Cicciù M. Histomorphometric evaluation of anorganic bovine bone coverage to reduce autogenous grafts resorption: preliminary results. Open Dent. J. 2011;25(5):71–78. doi: 10.2174/1874210601105010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caubet J., Petzold C., Sáez-Torres C., Morey M., Iriarte J.I., Sánchez J., Torres J.J., Ramis J.M., Monjo M. Sinus graft with safescraper: 5-year results. J. Oral Maxillofac. Surg. 2011;69(2):482–490. doi: 10.1016/j.joms.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 49.Traini T., Piattelli A., Caputi S., Degidi M., Mangano C., Scarano A., Perrotti V., Iezzi G. Regeneration of human bone using different bone substitute biomaterials. Clin. Implant Dent. Relat. Res. 2015;17(1):150–162. doi: 10.1111/cid.12089. [DOI] [PubMed] [Google Scholar]
- 50.Goené R.J., Testori T., Trisi P. Influence of a nanometer-scale surface enhancement on de novo bone formation on titanium implants: a histomorphometric study in human maxillae. Int. J. Periodontics Restor. Dent. 2007;27(3):211–219. [PubMed] [Google Scholar]
- 51.Gupta S., Vandana K.L. Evaluation of hydroxyapatite (Periobone-G) as a bone graft material and calcium sulfate barrier (Capset) in treatment of interproximal vertical defects: a clinical and radiologic study. J. Indian Soc. Periodontol. 2013;17(1):96–103. doi: 10.4103/0972-124X.107483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pappalardo S., Baglio O.A., Grassi R.F., Grivetto F., Mortellaro C. Mandibular bone deficit with a histologic study in man. J. Craniofac. Surg. 2005;16(1):174–180. doi: 10.1097/00001665-200501000-00037. https://journals.lww.com/jcraniofacialsurgery/Pages/default.aspx Retrieved from. [DOI] [PubMed] [Google Scholar]
- 53.Cannizzaro G., Felice P., Minciarelli A.F., Leone M., Viola P., Esposito M. Early implant loading in the atrophic posterior maxilla: 1-stage lateral versus crestal sinus lift and 8 mm hydroxyapatite-coated implants. A 5-year randomised controlled trial. Eur. J. Oral Implantol. 2013;6(1):13–25. www.quintpub.com/Journals/EJOI Retrieved from. [PubMed] [Google Scholar]
- 54.Nemcovsky C.E., Artzi Z., Moses O., Gelernter I. Healing of marginal defects at implants placed in fresh extraction sockets or after 4–6 weeks of healing. A comparative study. Clin. Oral Implants Res. 2002;13(4):410–419. doi: 10.1034/j.1600-0501.2002.130410.x. [DOI] [PubMed] [Google Scholar]
- 55.Peres M.F., Ribeiro E.D., Casarin R.C., Ruiz K.G., Junior F.H., Sallum E.A., Casati M.Z. Hydroxyapatite/β-tricalcium phosphate and enamel matrix derivative for treatment of proximal class II furcation defects: a randomized clinical trial. J. Clin. Periodontol. 2013;40(3):252–259. doi: 10.1111/jcpe.12054. [DOI] [PubMed] [Google Scholar]
- 56.Fugazzotto P.A. Immediate implant placement and GBR in humans: a case report and histologic evaluation. Int. J. Periodontics Restor. Dent. 1999;19(5):457–463. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 57.Pieri F., Aldini N.N., Marchetti C., Corinaldesi G. Esthetic outcome and tissue stability of maxillary anterior single-tooth implants following reconstruction with mandibular block grafts: a 5-year prospective study. Int. J. Oral Maxillofac. Implants. 2013;28(1):270–280. doi: 10.11607/jomi.2560. [DOI] [PubMed] [Google Scholar]
- 58.Wheeler S.L., Holmes R.E., Calhoun C.J. Six-year clinical and histologic study of sinus-lift grafts. Int. J. Oral Maxillofac. Implants. 1996;11(1):26–34. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 59.Kim D.M., De Angelis N., Camelo M., Nevins M.L., Schupbach P., Nevins M. Ridge preservation with and without primary wound closure: a case series. Int. J. Periodontics Restor. Dent. 2013;33(1):71–78. doi: 10.11607/prd.1463. [DOI] [PubMed] [Google Scholar]
- 60.Galgut P.N., Waite I.M., Brookshaw J.D., Kingston C.P. A 4-year controlled clinical study into the use of a ceramic hydroxylapatite implant material for the treatment of periodontal bone defects. J. Clin. Periodontol. 1992;19(8):570–577. doi: 10.1111/j.1600-051x.1992.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 61.Corinaldesi G., Piersanti L., Piattelli A., Iezzi G., Pieri F., Marchetti C. Augmentation of the floor of the maxillary sinus with recombinant human bone morphogenetic protein-7: a pilot radiological and histological study in humans. Br. J. Oral Maxillofac. Surg. 2013;51(3):247–252. doi: 10.1016/j.bjoms.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Hürzeler M.B., Kirsch A., Ackermann K.L. Reconstruction of the severely resorbed maxilla with dental implants in the augmented maxillary sinus: a 5-year clinical investigation. Oral Maxillofac. Implants. 1996;11(4):466–475. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 63.Arenaz-Búa J., Luaces-Rey R., Sironvalle-Soliva S., Otero-Rico A., Charro-Huerga E., Patiño-Seijas B., García-Rozado A., Ferreras-Granados J., Vázquez-Mahía I., Lorenzo-Franco F., Martín-Sastre R., López-Cedrún J.L. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regeneration after mandibular impacted third molar extraction. Med. Oral Patol. Oral Cir. Bucal. 2010;15(3):e483–e489. doi: 10.4317/medoral.15.e483. [DOI] [PubMed] [Google Scholar]
- 64.De Coster P., Browaeys H., De Bruyn H. Healing of extraction sockets filled with BoneCeramic® before implant placement: preliminary histological findings. Clin. Implant Dent. Relat. Res. 2011;13(1):34–45. doi: 10.1111/j.1708-8208.2009.00184.x. [DOI] [PubMed] [Google Scholar]
- 65.Luczyszyn S.M., Papalexiou V., Novaes A.B., Jr., Grisi M.F., Souza S.L., Taba M., Jr. Acellular dermal matrix and hydroxyapatite in prevention of ridge deformities after tooth extraction. Implant Dent. 2005;14(2):176–184. doi: 10.1097/01.id.0000165082.77499.41. [DOI] [PubMed] [Google Scholar]
- 66.Rebaudi A., Silvestrini P., Trisi P. Use of a resorbable hydroxyapatite-collagen chondroitin sulfate material on immediate postextraction sites: a clinical and histologic study. Int. J. Periodontics Restor. Dent. 2003;23(4):371–379. www.quintpub.com/journals/prd/ Retrieved from. [PubMed] [Google Scholar]
- 67.Schmitt C.M., Doering H., Schmidt T., Lutz R., Neukam F.W., Schlegel K.A. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin. Oral Implants Res. 2013;24(5):576–585. doi: 10.1111/j.1600-0501.2012.02431.x. [DOI] [PubMed] [Google Scholar]
- 68.Ghanaati S., Barbeck M., Lorenz J., Stuebinger S., Seitz O., Landes C., Kovács A.F., Kirkpatrick C.J., Sader R.A. Synthetic bone substitute material comparable with xenogeneic material for bone tissue regeneration in oral cancer patients: first and preliminary histological, histomorphometrical and clinical results. Ann. Maxillofac. Surg. 2013;3(2):126–138. doi: 10.4103/2231-0746.119221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindgren C., Mordenfeld A., Johansson C.B., Hallman M. A 3-year clinical follow-up of implants placed in two different biomaterials used for sinus augmentation. Int. J. Oral Maxillofac. Implants. 2012;27(5):1151–1162. www.quintpub.com/journals/omi/ Retrieved from. [PubMed] [Google Scholar]
- 70.Artzi Z., Nemcovsky C.E., Tal H., Dayan D. Histopathological morphometric evaluation of 2 different hydroxyapatite-bone derivatives in sinus augmentation procedures: a comparative study in humans. J. Periodontol. 2001;72(7):911–920. doi: 10.1902/jop.2001.72.7.911. [DOI] [PubMed] [Google Scholar]
- 71.Baena R.R., Lupi S.M., Pastorino R., Maiorana C., Lucchese A., Rizzo S. Radiographic evaluation of regenerated bone following poly(lactic-co-glycolic) acid/hydroxyapatite and deproteinized bovine bone graft in sinus lifting. J. Craniofac. Surg. 2013;24(3):845–848. doi: 10.1097/SCS.0b013e31827ca01a. [DOI] [PubMed] [Google Scholar]
- 72.Tosta M., Cortes A.R., Corrêa L., Pinto Ddos S., Jr., Tumenas I., Katchburian E. Histologic and histomorphometric evaluation of a synthetic bone substitute for maxillary sinus grafting in humans. Clin. Oral Implants Res. 2013;24(8):866–870. doi: 10.1111/j.1600-0501.2011.02384.x. [DOI] [PubMed] [Google Scholar]
- 73.Lezzi G., Degidi M., Piattelli A., Mangano C., Scarano A., Shibli J.A., Perrotti V. Comparative histological results of different biomaterials used in sinus augmentation procedures: a human study at 6 months. Clin. Oral Implants Res. 2012;23(12):1369–1376. doi: 10.1111/j.1600-0501.2011.02308.x. [DOI] [PubMed] [Google Scholar]
- 74.Kurkcu M., Benlidayi M.E., Cam B., Sertdemir Y. Anorganic bovine-derived hydroxyapatite vs Β-tricalcium phosphate in sinus augmentation: a comparative histomorphometric study. J. Oral Implantol. 2012;38:519–526. doi: 10.1563/AAID-JOI-D-11-00061. [DOI] [PubMed] [Google Scholar]
- 75.Lorenz J., Kubesch A., Korzinskas T., Barbeck M., Landes C., Sader R., Kirkpatrick C.J., Ghanaati S. TRAP-positive multinucleated giant cells are foreign body giant cells rather than osteoclasts: results from a split-mouth study in humans. J. Oral Implantol. 2014;9:e257–e266. doi: 10.1563/aaid-joi-D-14-00273. [DOI] [PubMed] [Google Scholar]
- 76.Kühl S., Brochhausen C., Götz H., Filippi A., Payer M., d'Hoedt B., Kreisler M. The influence of bone substitute materials on the bone volume after maxillary sinus augmentation: a microcomputerized tomography study. Clin. Oral Investig. 2013;17(2):543–551. doi: 10.1007/s00784-012-0732-2. [DOI] [PubMed] [Google Scholar]
- 77.Queiroz L.A., Santamaria M., Casati M., Silverio K., Nociti-Junior F., Sallum E. Enamel matrix protein derivative plus synthetic bone substitute for the treatment of mandibular class II furcation defects: a case series. Quintessence Int. 2015;46(3):199–205. doi: 10.3290/j.qi.a32988. [DOI] [PubMed] [Google Scholar]
- 78.Pradeep A.R., Karvekar S., Nagpal K., Patnaik K., Raju A., Singh P. Rosuvastatin 1.2 mg in situ gel combined with 1:1 mixture of autologous platelet-rich fibrin and porus- hydroxyappatite bone graft in surgical treatment of mandibular degree II furcation defects: a randomized clinical control trial. J. Periodontol. 2016;87(1):5–13. doi: 10.1902/jop.2015.150131. [DOI] [PubMed] [Google Scholar]
- 79.Singh V.P., Nayak D.G., Uppoor A.S., Shah D. Clinical and radiographic evaluation of nano-crystalline hydroxyapatite bone graft (Sybograf) combined with bioresorbable collagen membrane (Periocol) in periodontal intrabony defects. Dent. Res. J. 2012;9(1):60–67. doi: 10.4103/1735-3327.92945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chandrashekar K.T., Saxena C. Biograft-HT® as a bone graft material in the treatment of periodontal vertical defects and its clinical and radiological evaluation: clinical study. J. Indian Soc. Periodontol. 2009;13(3):138–144. doi: 10.4103/0972-124X.60226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Leonardis D., Paolantonio M. Enamel matrix derivative, alone or associated with a synthetic bone substitute, in the treatment of 1- to 2-wall periodontal defects. J. Periodontol. 2013;84(4):444–455. doi: 10.1902/jop.2012.110656. [DOI] [PubMed] [Google Scholar]
- 82.Singhal R., Nandlal Kumar A., Rastogi P. Role of space provision in regeneration of localized two-wall intrabony defects using periosteal pedicle graft as an autogenous guided tissue membrane. J. Periodontol. 2013;84(3):316–324. doi: 10.1902/jop.2012.110734. [DOI] [PubMed] [Google Scholar]
- 83.Assche V.N., Michels S., Naert I., Quirynen M. Randomized controlled trial to compare two bone substitutes in the treatment of bony dehiscences. Clin. Implant Dent. Relat. Res. 2013;15(4):558–568. doi: 10.1111/j.1708-8208.2011.00408.x. [DOI] [PubMed] [Google Scholar]
- 84.Jepsen S., Topoll H., Rengers H., Heinz B., Teich M., Hoffmann T., Al-Machot E., Meyle J., Jervøe-Storm P.M. Clinical outcomes after treatment of intra-bony defects with an EMD/synthetic bone graft or EMD alone: a multicentre randomized-controlled clinical trial. J. Clin. Periodontol. 2008;35(5):420–428. doi: 10.1111/j.1600-051X.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 85.Tobón S.I., Arismendi J.A., Marín M.L., Mesa A.L., Valencia J.A. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int. Endod. J. 2002;35(7):635–641. doi: 10.1046/j.1365-2591.2002.00523.x. [DOI] [PubMed] [Google Scholar]
- 86.Kumar P.G., Kumar J.A., Anumala N., Reddy K.P., Avula H., Hussain S.N. Volumetric analysis of intrabony defects in aggressive periodontitis patients following use of a novel composite alloplast: a pilot study. Quintessence Int. 2011;42(5):375–384. www.quintpub.com/Journals Retrieved from. [PubMed] [Google Scholar]
- 87.Brown G.D., Mealey B.L., Nummikoski P.V., Bifano S.L., Waldrop T.C. Hydroxyapatite cement implant for regeneration of periodontal osseous defects in humans. J. Periodontol. 1998;69(2):146–157. doi: 10.1902/jop.1998.69.2.146. [DOI] [PubMed] [Google Scholar]
- 88.Mistry S., Kundu D., Datta S., Basu D. Effects of bioactive glass, hydroxyapatite and bioactive glass – hydroxyapatite composite graft particles in the treatment of infrabony defects. J. Indian Soc. Periodontol. 2012;16(2):241–246. doi: 10.4103/0972-124X.99269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pietruska M., Skurska A., Pietruski J., Dolińska E., Arweiler N., Milewski R., Duraj E., Sculean A. Clinical and radiographic evaluation of intrabony periodontal defect treatment by open flap debridement alone or combined with nanocrystalline hydroxyapatite bone substitute. Ann. Anat. 2012;194(6):533–537. doi: 10.1016/j.aanat.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Lekovic V., Kenney E.B., Carranza F.A., Jr., Danilovic V. Treatment of class II furcation defects using porous hydroxylapatite in conjunction with a polytetrafluoroethylene membrane. J. Periodontol. 1990;61(9):575–578. doi: 10.1902/jop.1990.61.9.575. [DOI] [PubMed] [Google Scholar]
- 91.Bowen J.A., Mellonig J.T., Gray J.L., Towle H.T. Comparison of decalcified freeze dried bone allograft and porous particulate hydroxyapatite in human periodontal osseous defect. J. Periodontol. 1989;60:647–654. doi: 10.1902/jop.1989.60.12.647. [DOI] [PubMed] [Google Scholar]
- 92.Harris R.J. A clinical evaluation of an allograft combined with a bioabsorbable membrane versus an alloplast/allograft composite graft combined with a bioabsorbable membrane. 100 consecutively treated cases. J. Periodontol. 1998;69(5):536–546. doi: 10.1902/jop.1998.69.5.536. [DOI] [PubMed] [Google Scholar]
- 93.Yukna R.A. Osseous defect responses to hydroxylapatite grafting versus open flap debridement. J. Clin. Periodontol. 1989;16(7):398–402. doi: 10.1111/j.1600-051x.1989.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 94.Kiliç A.R., Efeoğlu E., Yilmaz S. Guided tissue regeneration in conjunction with hydroxyapatite-collagen grafts for intrabony defects. A clinical and radiological evaluation. J. Clin. Periodontol. 1997;24(6):372–383. doi: 10.1111/j.1600-051x.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 95.Artzi Z., Weinreb M., Carmeli G., Lev-Dor R., Dard M., Nemcovsky C.E. Histomorphometric assessment of bone formation in sinus augmentation utilizing a combination of autogenous and hydroxyapatite/biphasic tricalcium phosphate graft materials: at 6 and 9 months in humans. Clin. Oral Implants Res. 2008;19:686–692. doi: 10.1111/j.1600-0501.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- 96.Barnett J.D., Mellonig J.T., Gray J.L., Towle H.J. Comparison of freeze-dried bone allograft and porous hydroxylapatite in human periodontal defects. J. Periodontol. 1989;60(5):231–237. doi: 10.1902/jop.1989.60.5.231. [DOI] [PubMed] [Google Scholar]
- 97.Scropp L., Wenzel A., Kostopoulos L., Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int. J. Periodontics Resor. Dent. 2003;23:313–323. [PubMed] [Google Scholar]