Introduction
Scabies is a contagious skin parasitosis caused by the mite Sarcoptes scabiei variety hominis and is transmitted by person-to-person contact. Adult female scabies mites are around 0.3 to 0.5 mm in diameter and males are smaller.1 The female mite lays 2 to 4 eggs per day in skin burrows in the superficial epidermis.2 Eggs take 3 to 4 days to hatch and, after hatching, go through various larval stages before becoming adults.1 The average infested human has 10 to 12 adult female mites on their body at a given time.2
Scabies can be a difficult skin condition to diagnose, as it requires a skin scraping and microscopic detection of the mite, ova, or feces, and the sensitivity of this is low.2 Multiphoton microscopy (MPM) is a laser scanning microscopy technique that uses label-free contrast based on optical signals generated through nonlinear light-matter interactions. Recent applications of this technology in a clinical setting include detection and diagnosis of skin cancer,3 and assessing the effects of imaging of cutaneous laser therapy.4 In these applications, the contrast mechanisms were generally based on second-harmonic generation from collagen fibers and 2-photon–excited fluorescence (TPEF) from nicotinamide adenine dinucleotide + hydrogen, flavin adenine dinucleotide, keratin, melanin, and elastin fibers. In this report, we exploit the fluorescent property of chitin in mites5 to investigate the ability of MPM to provide a noninvasive diagnosis of human skin affected by scabies.
Case report
A 60-year-old white male home health nurse presented with an itchy rash on his hand and groin region. Based on the patient's history and clinical examination using dermoscopy (delta sign), we suspected the patient had scabies. The area imaged was on top of the fourth finger of the patient's right hand. In this study, we used an MPM-based clinical tomograph (MPTflex; JenLab GmbH, Jena, Germany) for in vivo imaging of the scabies. We used 100 femtoseconds (fs) of near infrared light (790 nm) to generate TPEF signals from chitin, the predominant component of the exoskeleton of the scabies mite, an arthropod of the class arachnida. Chitin is found to provide endogenous TPEF signal in our detection range (410 nm-650 nm) when excited by near infrared femtosecond pulses. Optical sections of up to 300 × 300 μm2 were acquired en-face at different depths ranging from 0 to about 100 μm (5-μm steps for optical sections). The excitation power used was approximately 10 to 20 mW.
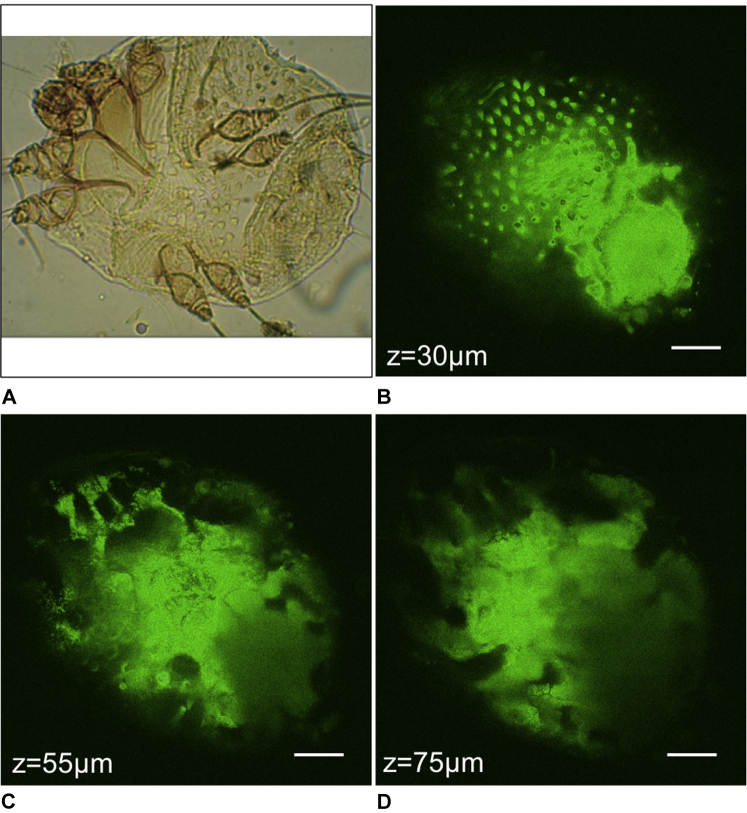
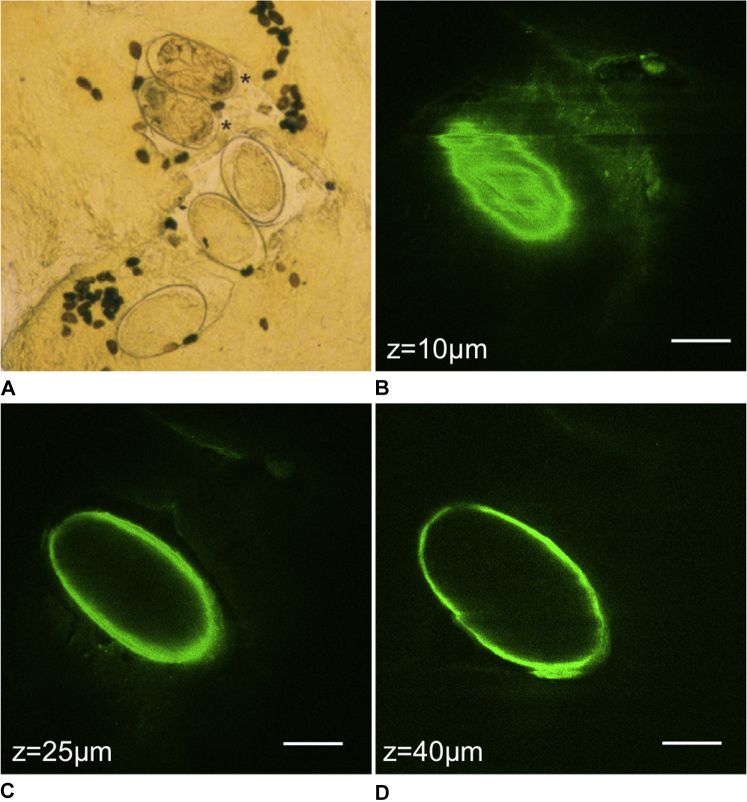
Fig 1, A shows a photomicrograph of a scabies mite.6 According to MPM images (Fig 1, B-D) the scabies mite appeared in the superficial epidermis as an oval body with short legs. The submicron resolution of the technique allowed the visualization of spikes on the top of the mite's body (Fig 1, B), and of its short legs (Fig 1, C and D). Fig 2 shows a photomicrograph image of mite eggs (Fig 2, A)6 and MPM images of an egg we acquired in close proximity to the mite (Fig 2, B-D).
Fig 1.
The scabies mite. A, Photomicrograph of the scabies mite.6 MPM images show the spikes on top of the mite's body (B), and the appearance of legs in the upper left portion of the image (C and D). Scale bar is 40 μm in all MPM images, and z is the approximate depth of the image below the skin surface.
Fig 2.
The scabies egg. A, photomicrograph of scabies eggs (large, light, ovals) and feces (small, dark, ovals).6B, MPM image of the very top portion of the egg and 2 images deeper through the middle of the egg (C and D). Scale bar is 40 μm in all MPM images, and z is the approximate depth of the image below the skin surface.
Discussion
We found that MPM is capable of imaging the scabies mite and its eggs in vivo, label free, with submicron resolution. In vivo, high-resolution visualization of the mite is helpful to support a correct diagnosis of the condition, especially as clear diagnosis of scabies can be difficult because it can often mimic other skin conditions.7
Other optical imaging techniques such as reflectance confocal microscopy8 and optical coherence tomography9 have proved successful for in vivo imaging of scabies mites and their eggs in human skin. Although a higher cost solution compared with these techniques, if available in a clinical setting, MPM can be used successfully for accurate, noninvasive diagnosis of scabies. Besides its high cost, the reduced scanning area currently provided by clinical MPM (300 × 300 μm2) represents a significant limitation. In this particular application, an increase in scanning area, while maintaining the submicron resolution, would allow imaging of multiple mites and eggs and a better assessment of the overall severity of the infestation. The MPM technology's superior contrast and resolution compared with alternative optical imaging techniques currently used in the clinic have prompted efforts to address these technical limitations by providing solutions that can be implemented in future designs to enhance the clinical value of this technology.10
Footnotes
Funding sources: This study was supported in part by the following grants: National Institutes of Health (NIH) NIBIB Laser Microbeam and Medical Program (LAMMP,P41-EB015890) and by the Beckman Laser Institute programmatic support from the Arnold and Mabel Beckman Foundation.
Conflicts of interest: Dr Koenig is the cofounder of Jenlab, GmbH. Drs Tromberg and Balu report a pending patent, which is owned by the University of California, that is related to the technology described in this study. The rest of the authors have no conflicts to disclose.
References
- 1.McCarthy J.S., Kemp D.J., Walton S.F., Currie B.J. Scabies: more than just an irritation. Postgrad Med J. 2004;80(945):382. doi: 10.1136/pgmj.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heukelbach J., Feldmeier H. Scabies. Lancet. 2006;367(9524):1767–1774. doi: 10.1016/S0140-6736(06)68772-2. [DOI] [PubMed] [Google Scholar]
- 3.Balu M., Zachary C.B., Harris R.M. In vivo multiphoton microscopy of basal cell carcinoma. JAMA Dermatol. 2015;151(10):1068–1074. doi: 10.1001/jamadermatol.2015.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balu M., Lentsch G., Korta D.Z. In vivo multiphoton-microscopy of picosecond-laser-induced optical breakdown in human skin. Laser Surg Med. 2017;49(6):555–562. doi: 10.1002/lsm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabasović M.D., Pantelić D.V., Jelenković B.M. Nonlinear microscopy of chitin and chitinous structures: a case study of two cave-dwelling insects. J Biomed Opt. 2015;20(1):016010. doi: 10.1117/1.JBO.20.1.016010. [DOI] [PubMed] [Google Scholar]
- 6.Hengge U.R., Currie B.J., Jäger G., Lupi O., Schwartz R.A. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6(12):769–779. doi: 10.1016/S1473-3099(06)70654-5. [DOI] [PubMed] [Google Scholar]
- 7.Kutlu N.S., Turan E., Erdemir A., Gürel M.S., Bozkurt E. Eleven years of itching: a case report of crusted scabies. Cutis. 2014;94(2):86–88. 95. [PubMed] [Google Scholar]
- 8.Gürel M.S., Turgut Erdemir A.V., Tekin B. A case report of real-time in vivo demonstration of sarcoptes scabiei. Turkiye Parazitol Derg. 2017;41(4):229–232. doi: 10.5152/tpd.2017.5408. [DOI] [PubMed] [Google Scholar]
- 9.Banzhaf C.A., Themstrup L., Ring H.C., Welzel J., Mogensen M., Jemec G.B. In vivo imaging of sarcoptes scabiei infestation using optical coherence tomography. Case Rep Dermatol. 2013;5(2):156–162. doi: 10.1159/000352066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balu M., Mikami H., Hou J., Potma E.O., Tromberg B.J. Rapid mesoscale multiphoton microscopy of human skin. Biomed Opt Express. 2016;7(11):4375–4387. doi: 10.1364/BOE.7.004375. [DOI] [PMC free article] [PubMed] [Google Scholar]