Introduction
Alopecia areata (AA) is a common and often emotionally devastating condition that may affect any hair-bearing site on the body. Therapeutic options are particularly limited when AA affects the eyelashes, as the use of traditional modalities such as topical corticosteroids and topical immunotherapy carry with them risk of serious adverse effects in this location.
Report of a case
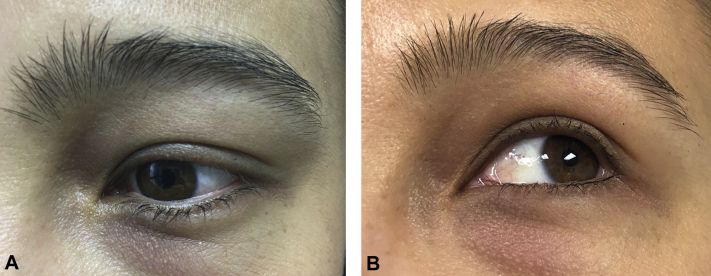
A patient in her late 20s presented for evaluation and management of loss of her left upper eyelashes of 11 months' duration. Multiple prior treatments were ineffective, including hydrocortisone 2.5% ointment twice daily for 4 weeks, 7 weekly sessions of excimer laser, and most recently tacrolimus 0.1% ointment twice daily for 8 months. She was otherwise healthy and took no medications, and a complete review of systems was negative. There was no family history of alopecia areata or other autoimmune disease. On examination, there was near-complete absence of the left upper eyelashes and subtle thinning of the left medial eyebrow (Fig 1, A). There were also 2 small (<1 cm) patches of relative thinning on the left frontal scalp. There was no pitting of the fingernails. A diagnosis of AA was suspected; however, given that the clinical presentation was somewhat atypical with prominent eyelash involvement but no patches of smooth alopecia elsewhere, a biopsy from one of the patches of thinning on the scalp was performed to aid in diagnosis. Histopathologic evaluation found peribulbar lymphocytic inflammation with miniaturization of hairs, consistent with AA.
Fig 1.
Left upper eyelashes before and after treatment with topical tofacitinib 2% solution. A, Left eyelashes at baseline. There are scant upper eyelashes present. B, After 7 months of treatment with tofacitinib 2% solution there has been near complete regrowth of the upper lashes.
The patient was extremely bothered by the lack of eyelashes and was interested in pursuing treatment with an oral Janus kinase (JAK) inhibitor; however, because of the limited involvement and potential risk for serious adverse events with systemic therapy, she was counseled that a trial of topical tofacitinib was advisable before considering this. Although not as efficacious as oral therapy, topical formulations of JAK inhibitors, including tofacitinib and ruxolitinib ointment and cream in concentrations of 1% to 2%, may have some efficacy in the treatment of AA.1, 2, 3, 4 In this case, a solution was chosen as the vehicle for tofacitinib, as it would provide greater ease of application to the eyelid margin and perhaps improved penetration.
The patient began treatment with topical tofacitinib 2% in solution (ChemistryRx, Philadelphia, PA) twice daily to the left upper eyelid. After 1 month of treatment, the patient reported noticing some regrowth of eyelashes. By 4 months, there was near complete regrowth of the left upper eyelashes. Once she had sustained regrowth for about a month, she decreased frequency of application to once daily, and at 7 months she continues to have near complete regrowth (Fig 1, B). The patient tolerated the medication without adverse effects, and ophthalmologic examination after 2 months of therapy showed no abnormalities. Given that the patches on the scalp were very subtle, she opted not to treat these sites.
Discussion
This case demonstrates successful treatment of localized AA with topical tofacitinib 2% solution. Although topical JAK inhibitors are impractical and unlikely to achieve cosmetically acceptable regrowth in cases of extensive AA, they may have a role for treatment of more localized disease, particularly in cases in which more traditional modalities have not been successful. Using a solution as a vehicle offers another method of delivery that may be favorable for treating AA in certain locations, in particular the eyebrow regions and eyelids. Of the 4 clinical trials investigating topical JAK inhibitors for AA, one was terminated early (clinicaltrials.gov identifier NCT02553330) and another has not yet published results (NCT02561585). The other 2 trials investigating the same topical (NCT03551821 for eyebrows; NCT03354637 for scalp) are currently recruiting. Of note, the investigational drug used in these trials (ATI-502) was recently granted Fast Track designation by the US Food and Drug Administration. The results of these studies will be important to further explore the safety and efficacy of topical JAK inhibitors for the treatment of AA.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Craiglow has received honoraria, served on an advisory board, and is a consultant for Pfizer. Her spouse has served on advisory boards for Pfizer and Aclaris Therapeutics; has received honoraria from Pfizer, Regneron, and Sanofi-Genzyme; and is a consultant to Pfizer, Concert Pharmaceuticals Inc, and Eli Lilly and Company.
References
- 1.Craiglow B.G., Tavares D., King B.A. Topical ruxolitinib for the treatment of alopecia universalis. JAMA Dermatol. 2016;152(4):490–491. doi: 10.1001/jamadermatol.2015.4445. [DOI] [PubMed] [Google Scholar]
- 2.Bayart C.B., DeNiro K.L., Brichta L., Craiglow B.G., Sidbury R. Topical Janus kinase inhibitors for the treatment of pediatric alopecia areata. J Am Acad Dermatol. 2017;77(1):167–170. doi: 10.1016/j.jaad.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Liu L.Y., Craiglow B.G., King B.A. Tofacitinib 2% ointment, a topical Janus kinase inhibitor, for the treatment of alopecia areata: A pilot study of 10 patients. J Am Acad Dermatol. 2018;78(2):403–404.e401. doi: 10.1016/j.jaad.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Putterman E., Castelo-Soccio L. Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J Am Acad Dermatol. 2018;78:1207–1209.e1. doi: 10.1016/j.jaad.2018.02.031. [DOI] [PubMed] [Google Scholar]