Abstract
Background
There has been increasing evidence in recent years that breast implants can, in rare cases, be associated with the development of an anaplastic large-cell lymphoma (ALCL).
Methods
This review is based on relevant publications retrieved by a selective search in PubMed for articles that appeared from the time of the initial description of breast-implant-associated ALCL onward (1997 to January 2018), and by a further search in German nationwide databases.
Results
516 pathologically confirmed cases of breast-implant-associated (BIA) ALCL were documented around the world until February 2018; seven of these arose in Germany and were reported to the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM). In approximately 80% of the affected women, the BIA-ALCL manifested itself as a late-developing seroma at the implant site; in the rest, as a solid tumor with or without an accompanying seroma. The mean implant exposure time ranged from 7 to 13 years on average. 16 fatalities have been reported worldwide. Among the 7 cases reported in Germany, four women had undergone breast reconstruction with implants after breast cancer surgery, and two had undergone breast augmentation surgery. In all patients, the entire capsule-and-implant unit was resected. One patient underwent chemotherapy and one further patient underwent chemotherapy and adjuvant radiotherapy.
Conclusion
The risk that a woman with breast implants will develop a primary anaplastic large-cell lymphoma is estimated at 0.35 to 1 case per million persons per year. The incidence of implant-associated ALCL is thus very low, yet nevertheless markedly higher than that of other primary lymphomas of the breast. Because of the low case numbers, recommendations for the diagnostic evaluation and treatment of this entity have not been adequately evaluated. Treatment with primary curative intent for BIA-ALCL confers a much better prognosis than when performed for a systemic ALCL. Whenever a patient with a breast implant presents with a late-developing seroma, BIA-ALCL should be included in the differential diagnosis. This diagnosis is reportable.
In February 2011 the United States Food and Drug Administration (FDA) confirmed on its website that breast implants can cause the rare tumor entity known as anaplastic large-cell lymphoma (ALCL) (1). This sparked growing interest both in professional journals and in the daily press. The first case of ALCL in a woman with breast implants was published in 1997 (2). The most recent publication from the FDA lists 414 implant-associated events (medical device reports, MDR) relating to breast implants, and the PROFILE database of the Plastic Surgery Foundation contains 516 cases (3, 4).
Owing to the low prevalence and as yet unknown incidence of breast implant-associated (BIA) ALCL, the existing treatment evaluation data come from case reports.The published guidelines are on the level of expert consensus, therefore treatment recommendations to date are not sufficiently evaluated and there is no standardized adjuvant therapy (5– 7). It is therefore vital to avoid confusing this special manifestation with the prognostically unfavorable systemic form of ALCL. Stage-related aggressive treatment is not indicated.
Method
So far seven cases of BIA-ALCL are known to have occurred in Germany. They were identified by personal inquiry to the Federal Institute for Drugs and Medical Devices (BfArM), the German Society of Plastic, Reconstructive, and Aesthetic Surgeons (Deutsche Gesellschaft der Plastischen, Rekonstruktiven und Ästhetischen Chirurgen, DGPRÄC), and the German Society for Gynecology and Obstetrics (Deutsche Gesellschaft für Gynäkologie und Geburtshilfe, DGGG). This article presents six of these cases with regard to the classification proposed in the literature and the treatments derived therefrom. The clinical symptoms and the diagnostic procedures that were used are described in detail to raise awareness of this new tumor entity among physicians.
Background and clinical findings
BIA-ALCL belongs to the group of T-cell lymphomas and arises in the implant bed following breast reconstruction in women with mammary carcinoma or after esthetic breast augmentation. In about 60% of cases the lymphoma becomes clinically evident as a delayed accumulation of fluid around the implant, in 17% as an intracapsular cell mass, and in circa 20% of cases both a seroma and solid tumor masses are found (5). The first symptom pointing to BIA-ALCL is almost always unilateral or bilateral late seroma at least a year after implantation, causing breast swelling, newly arising asymmetry, or pain. Skin symptoms (e.g., inflammation) and lymphadenopathy have been described. Most cases of BIA-ALCL have been detected 7 to 10 years after implantation, but in one patient the interval between surgery and diagnosis was only 2 years and in another woman it was 32 years (8– 10).
Lymphomas are the most frequently occurring hematological cancer and may arise from B or T-lymphocytes (11). ALCL are the third most common type of peripheral T-cell lymphoma, making up circa 2% of all lymphomas in adulthood. Any discussion of ALCL must take account of the WHO categorization of subtypes. Revised in 2016, this classification is based on clinical findings and molecular biological characteristics (12):
Primary systemic ALCL: anaplastic lymphoma kinase (ALK) positive
Primary systemic ALCL: ALK negative
Primary cutaneous ALCL (PC-ALCL)
BIA-ALCL: ALK negative, CD30 positive
Although ALCL is generally classed as clinically aggressive, the clinical course varies greatly according to subtype. For example, primary systemic ALCL shows rapid progression and spread. PC-ALCL is more benign than the systemic forms, with a 5-year survival rate of over 95%. Based on the experience of longer-term follow up to date, BIA-ALCL seems to follow the less aggressive trend of the PC-ALCL compared to the systemic forms, but the WHO nevertheless classifies it as a lymphoproliferative cancer type. Long-term studies will be necessary to achieve a better understanding of the tumor biology of BIA-ALCL (13). In a recently published report, the European Commission concludes that the current state of knowledge does not permit methodologically reliable risk assessment (14).
Pathogenesis
The pathogenesis of BIA-ALCL remains largely unknown. A connection with implant-induced chronic inflammation has been discussed (15, 16), as has genetic susceptibility in the sense of severe reactive dysplasia as a response to chronic inflammation (17). Other suspected causes include particle erosion of the implants, a subclinical biofilm, or chronic T-cell stimulation. It is striking that in almost 89% of the 272 FDA-registered MDR for which the implant surface properties were known, the implant was textured (3). In a collection presented by Clemens et al., all cases with full details of implant history involved a textured implant (9). In contrast with smooth-walled implants, the surface of textured implants is uneven. The depth and other characteristics of this structuring vary among manufacturers. The originally used smooth-surfaced prostheses had the disadvantage that the body could not hold the implant in position, resulting in rotation and sagging. Moreover, this seemed to be associated with a much higher rate of capsular fibrosis, as described in a large number of studies (e.g., [18]).
If round implants are used, rotation is largely unproblematic. In Germany, however, more natural-looking anatomical implants have long been in demand. Texturing became necessary to enable broadly stable integration by the recipient’s body.
The connection with implant surface properties is supported by an Australian animal experiment: in a porcine model, textured implants caused much greater lymphocyte immigration, with a predominance of T-cells, than smooth-walled implants. A total of 30 of the 414 MDR registered by the FDA definitely involved smooth-walled implants, and it cannot be excluded that in some of these cases an original textured implant was replaced with a smooth-walled implant, e.g., after the occurrence of capsular fibrosis. In the study by Brody et al. in 2015, 79 published cases and 94 previously unreported cases of ALCL were analyzed: every single one of these women currently had a textured implant or had previously had one (19).
With regard to the theory of biofilm formation and possible low-grade infection, Hu and Adams carried out two studies comparing the microbiological colonization of implant capsules between BIA-ALCL patients and women with capsular fibrosis. The BIA-ALCL groups had a higher bacterial burden and a significantly different distribution of bacteria, dominated by the gram-negative pathogen Ralstonia pickettii (20, 21).
Prevalence
The prevalence of BIA-ALCL cannot be quantified. It is estimated that there are currently well over 11 million women with breast implants worldwide, but the published data on BIA-ALCL vary widely (22). Since the recognition of BIA-ALCL, however, it has showed a marked increase in prevalence. Only 134 cases were detected between 1997 and 2016, but by 30 September 2017 the Manufacturer and User Facility Device Experience (MAUDE) database of the FDA contained 414 documented cases (3). An international study by Srinivas et al., who investigated 40 markets for breast implants, identified reports of 363 adverse event in the sense of BIA-ALCL to the nominated bodies (23).
However, none of the published studies permits accurate conclusions as to the prevalence of BIA-ALCL. The estimates range from 1 to 3 cases per million women with breast implants per year (24), as extrapolated in 2008 by Jung et al. from the data on only five cases in the Netherlands, to de Boer et al.’s 2018 figures of a cumulative risk of 1:35 000 for 50-year-old patients (29 cases per million women with breast implants up to age 50), 1:12 000 in 70-year-old patients (82 cases per million women with breast implants up to age 70), and 1:7000 in 75-year-old patients. The number of women with an implant needed to cause one case of ALCL has been stated as 6290 (25).
In a study published in 2017, Doren et al. gave a BIA-ALCL incidence of 1: 30 000 for women with a textured breast implant (26). Loch-Wilkinson et al. calculated the implant-specific risk of BIA-ALCL as 1: 3817 for so-called biocell implants and 1:7788 for polyurethane implants, based on all cases of BIA-ALCL in Australia and New Zealand (27).
This uncertainty is related to the limitations of the hitherto unstructured collection of data, potential duplicate registrations, unclear clinical and pathological data, the lack of information on the number of implants inserted as denominator, and, more than likely, undiagnosed cases (26, 27). Many treating physicians are unaware of the existence of BIA-ALCL as an independent lymphoma entity (28).
The occurrence of ALCL in association with other implantable medical devices has also been explored. B-cell lymphomas were found most frequently in patients with joint prostheses. There are single case reports of ALCL associated with a dental implant, a tibial implant, and a silicon port system for administration of chemotherapy (29– 31).
Diagnosis
BIA-ALCL must be considered among the differential diagnoses in every case of a seroma occurring a year or more after insertion of a breast implant that cannot be attributed to infection or trauma.
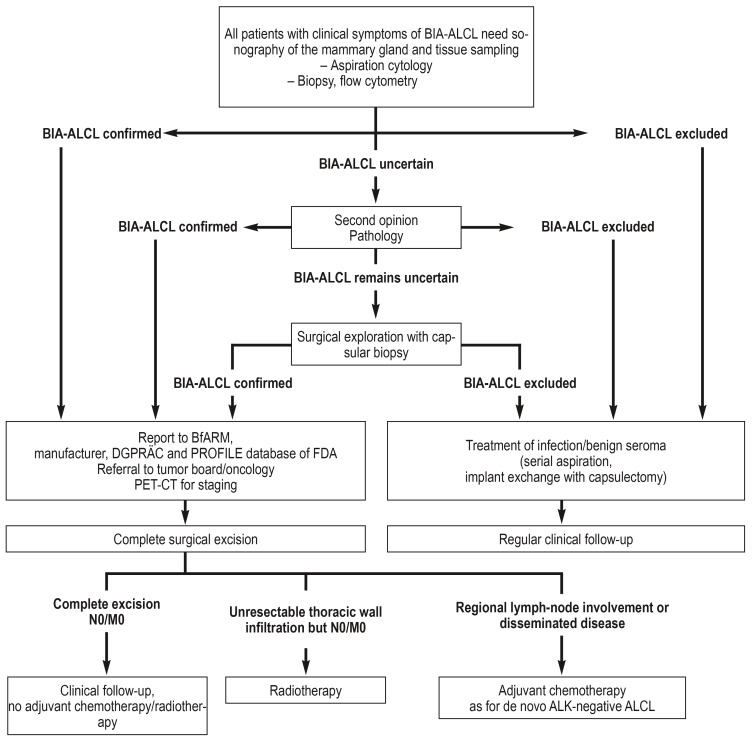
In March 2017 the National Comprehensive Cancer Network (NCCN), led by Clemens et al., published the first consensus guidelines on diagnosis and treatment (figure 1):
Figure 1.
Management of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) (modified from Clemens et al.: Clin Plast Surg 2015; 42: 605–13 [28])
ALK, Anaplastic lymphoma kinase; BfARM, Federal Institute for Drugs and Medical Devices; DGPRÄC, German Society of Plastic, Reconstructive, and Aesthetic Surgeons; FDA, Food and Drug Administration; PET-CT, positron-emission tomography/computed tomography
The enlarged breast should be investigated by sonography for fluid accumulation or a solid mass, the axillary, supraclavicular, and parasternal lymph tracts for suspicious changes. It must be borne in mind, however, that small periprosthetic fluid collections are a common occurrence. Any seroma that is identified should be aspirated under sonographic guidance. ALCL can be demonstrated by immunohistochemical detection of CD30. This surface protein from the TNF receptor family is not found in benign periprosthetic seromas. Postoperatively the diagnosis is histopathologically confirmed by cell-block cytology, which depicts the anaplastic large cells, together with flow cytometry to demonstrate the clonal T-cell proliferations (5).
Suspicious tumors must be biopsied. A further important criterion for BIA-ALCL is the absence of detection of anaplastic lymphoma kinase (ALK). All registered cases of BIA-ALCL are CD30 positive and ALK negative. Expression of other T-cell antigens, e.g., CD4 (84%), CD43 (80%), CD3 (30%), CD45 (36%), and CD2 (30%), is variable (32). The histopathological assessment should be validated at a reference center for lymph node diagnosis (efigure).
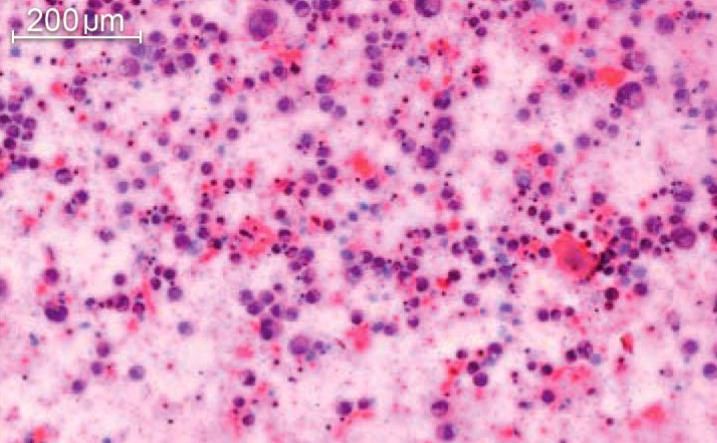
eFigure.
Histologic image of an anaplastic large-cell lymphoma (ALCL); HE staining. Morphologically, ALCL exhibits mostly large blastoid cells rich in cytoplasm with pleomorphic or sometimes multiple or hoof-shaped nuclei and prominent nucleoli.
Because the cardinal symptom in the majority of cases is effusion, the primary diagnostic imaging modality, due to its rapid availability and good sensitivity, should be sonography of the breast and axilla. In a cohort of 44 patients, Adrada et al. observed the following sensitivities/specificities for seroma:
Ultrasound: 84/75%
Computed tomography (CT): 55/83%
Magnetic resonance imaging (MRI): 82/33%
Positron emission tomography/CT (PET-CT): 38/83%
For the detection of solid tumors the sensitivities/specificities were:
Ultrasound: 46/100%
CT: 50/100%
MRI: 82/33%
PET-CT: 64/88%
Mammography was inferior to ultrasound and MRI in sensitivity and specificity, with no differences between seroma and solid findings (73/50%) (33).
MRI is generally carried out in cases where sonography yields unclear results and for staging, while PET-CT is not a suitable modality for local evaluation (33). In confirmed BIA-ALCL, however, PET-CT is useful for detection of tumor spread and for accurate staging (33). Bone marrow aspiration is rarely necessary, being reserved for isolated cases with suspected or confirmed systemic spread.
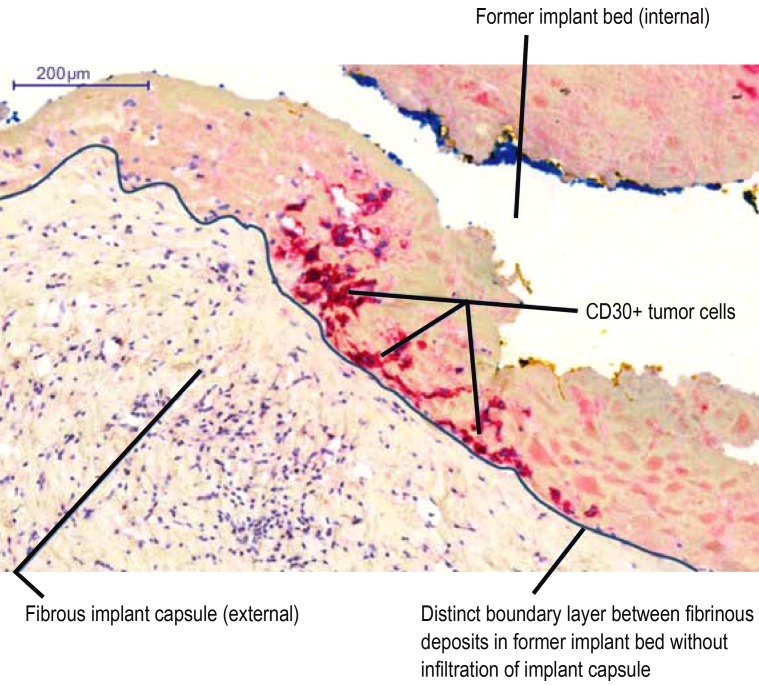
In the overwhelming majority of the patients diagnosed with malignant seroma to date, histopathological analysis has shown no involvement of the implant capsule (efigure). Occasionally there was benign lymphocytic hyperplasia of the capsule. Loch-Wilkinson et al. reported adhesion of tumor cells to the inner capsule in around 18% of cases, but without infiltration (Figures 2 and 3, implant capsule of the patient in case 1 in the Table) (27). All of these cases represent early stages. It has not yet been clarified whether serous manifestation alone represents an entity in itself or whether it is a premalignant phenomenon that always goes on to develop into a solid tumor.
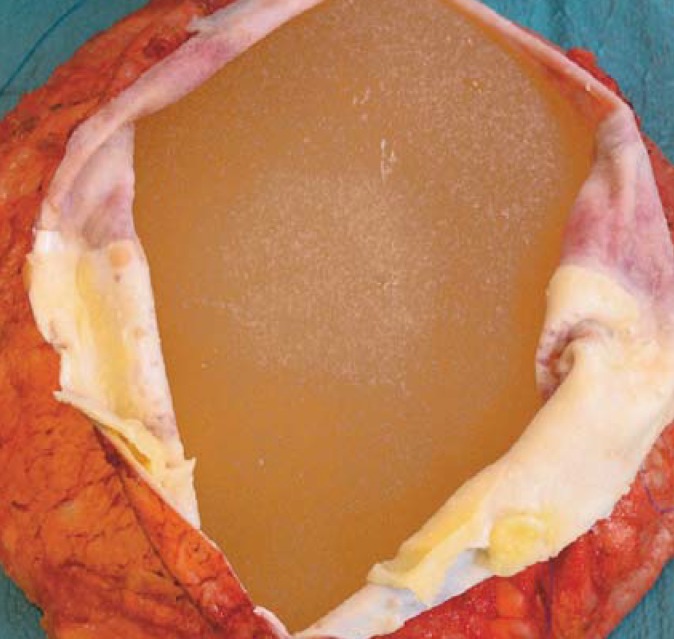
Figure 2.
Intraoperative view of opened capsule. Flap-like lymphocytic deposits on the acellular matrix and the capsule. Case 1
Figure 3.
CD30 immunohistology: CD30-positive tumor cells in the fibrin deposits on the internal wall of the fibrous implant capsule without infiltration of the capsule. Case 1
Table. Summary of cases of BIA-ALCL in Germany.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
| Indication | SSM after bilateral breast CA, right-sided radiotherapy | Esthetic augmentation | Bilateral NSM in right breast CA + DCIS and left LIN I | NSM in bilateral fibrocystic mastop‧athy with atypia | Esthetic augmentation | Breast ablation with delayed implant reconstruction 2 years later |
| Implant type and interval before BIA-ALCL manifestation | Initially bilateral expanders (manufacturer unknown), exchanged after 4 months for bilateral textured implants (Allergan), left implant exchanged (McGhan) with reinforced acellular dermal matrix (Strattice), left BIA-ALCL 5 years later, no abnormality to date on the right | Bilateral textured implants (Allergan), left BIA-ALCL 5 years later | Macrotextured implants (Polytech) + titanium mesh (TiMesh), right breast BIA-ALCL 8 years later | Initially expander implants (McGhan) left implant exchanged for Mentor CPG after 8 years, left BIA-ALCL 5 years later | Initially bilateral McGhan, exchanged for Allergan after 9 years, right BIA-ALCL 9 years later | Silimed implant (texture unspecified), BIA-ALCL 7 years later |
| Preoperative diagnostic procedures | In seroma of left breast: sonography with aspiration, CT, MRI | Palpation left axilla (tumor, not seroma), sonography, MRI, CT, bone punch biopsy | Seroma of right breast: MRI (finding: “spongious material”) | Left seroma and extensive tumor: sonography, CT | Right seroma: sonography | Left seroma: sonography |
| Pathology/ stage/TNM | CD30-positive seroma, capsule not involved/1A/ T1N0M0 | CD30-positive tumor, 1B (left)/T2aN0M0 | Aspirate initially not analyzed for CD30, histology initially inconspicuous, on second look demonstration of ALCL with infiltration of capsule/1C/T3N0M0 | CD30-positive ALCL,R1 resection to thoracic wall/2A/ T4N0M0 | CD30-positive ALCL, R0/1A / T1N0M0 | CD30-positive ALCL, R0/1A/ T1N0M0 |
| Treatment/ follow-up | Left complete capsulectomy / recurrence-free for 12 months, bilateral autologous conversion planned | Bilateral capsulectomy and implant removal + autologous conversion, recurrence-free for 12 months | Capsulectomy and implant exchange, chemotherapy (CHOP scheme), recurrence-free for 23 months | Capsulectomy and tumor resection (R1), chemotherapy (CHOP-14/G-CSF), radiotherapy, recurrence-free for 24 month, lost to follow-up 8 years ago | Capsulectomy, implant exchange, recurrence-free for 6 months | Capsulectomy and local flap repair, recurrence-free for 6 months |
We have included six of the seven German cases registered to date at the Federal Institute for Drugs and Medical Devices (BfArM).
BIA-ALCL, Breast implant-associated anaplastic large-cell lymphoma; CA, cancer; CHOP, cyclophosphamide, hydroxydauromycin, oncovin, prednisone;
CT, Computed tomography; DCIS, ductal carcinoma in situ; LIN, lobular intraepithelial neoplasia; MRI, magnetic resonance imaging; NSM, nipple-sparing mastectomy;
SSM, subcutaneous mastectomy; TNM, tumor, node, metastases (classification system)
Staging
Staging is crucial to distinguish localized, prognostically favorable stage I BIA-ALCL, which is confined to the capsule, from disease that has progressed beyond the capsule or metastasized. As mentioned above, PET-CT is the established imaging modality for this purpose. Clemens et al. have drawn up a staging system for BIA-ALCL (etable).
eTable. Staging of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
| Tumor extent (T) | T1 | T2 | T3 | T4 |
| Confined to seroma or a layer on luminal side of capsule | Early capsule infiltration | Cell aggregates or sheets infiltrating the capsule | Lymphoma infiltrates beyond the capsule | |
| Lymph nodes (N) | N0 | N1 | N2 | |
| No lymph node involvement | One regional lymph node positive | Multiple regional lymph nodes positive | ||
| Metastases (M) | M0 | M1 | ||
| No distant spread | Spread to other organs/distant sites | |||
| Stage: | Frequency: | |||
| IA | T(1)N(0)M(0) | 35.6% | ||
| IB | T(2)N(0)M(0) | 11.5% | ||
| IC | T(3)N(0)M(0) | 13.8% | ||
| IIA | T(4)N(0)M(0) | 25.3% | ||
| IIB | T(1–3)N(1)M(0) | 4.6% | ||
| III | T(4)N(1–2)M(0) | 9.2% | ||
| IV | T(any)N(any)M(1) | 0% | ||
Data modified from Clemens et al. J Clin Oncol 2016; 34: 160–8 (9)
Treatment
The treatment of BIA-ALCL should be oriented on the aforementioned, recently issued NCCN guidelines (5, 7). No prospective research into treatment success has yet been published, and the ongoing studies will probably take years to complete. The current consensus foresees complete surgical excision of the capsule and implant, accompanied by removal of solitary tumors with histological confirmation of negative resection margins (Figures 2 and 4 from our own patient cohort). Because the implant capsule drains into several different effluent lymph tracts, sentinel node biopsy is not recommended. Instead, enlarged lymph nodes detected by palpation should be excised in toto and not aspirated. In patients with implants in both breasts, removal of the contralateral prosthesis with its capsule can be considered, as around 5% of the registered cases of BIA-ALCL have been bilateral (34).
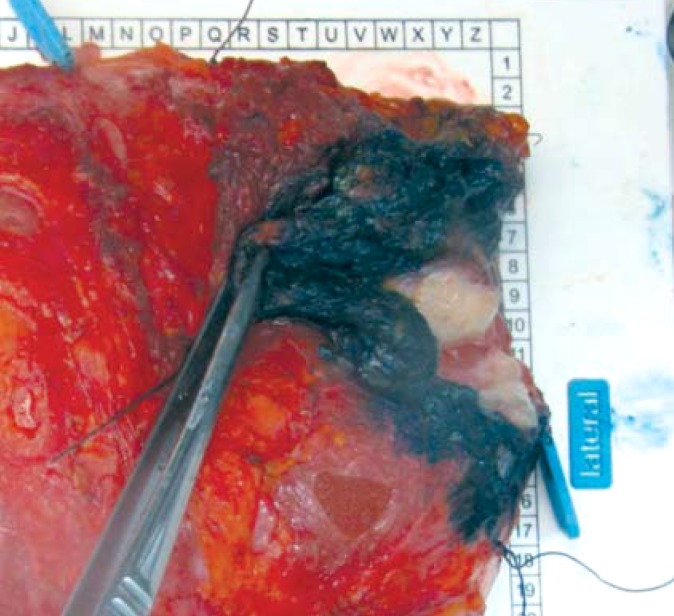
Figure 4.
En bloc resection specimen with solid foci and stained resection margins. Case 2
BIA-ALCL confined to the capsule (stage I A–C according to Clemens et al. [9]) takes a favorable course after surgical capsulectomy, even without adjuvant chemotherapy or radiotherapy: retrospective analysis of 87 patients with BIA-ALCL showed median overall survival of 13 years with a 3-year survival rate of 93% and a 5-year rate of 89% (9). The event-free survival rate at 1 year was 96% in the group with radical surgical resection (complete removal breast implant capsule), compared with 40% in patients with limited surgery, 82% for radiotherapy, and 76% for chemotherapy. The rate of tumor events after complete resection was 14.3% in stage T4 and 0% in stages T1 and T2.
The optimal management for patients with a tumor of stage II or greater (etable) has not yet been established (35). For locally advanced or disseminated tumors, Clemens et al. recommend an oncological policy analogous to that for systemic ALCL, i.e., anthracycline-based chemotherapy (CHOP: cyclophosphamide, doxorubicin, vincristine, prednisolone), with the addition of etoposide if needed.
However, ALK-negative lymphomas are known to respond to CHOP or similar chemotherapy regimens less favorably than their ALK-positive counterparts (5-year survival rates of 40–60%versus 70–90%) (36). Two thirds of patients with BIA-ALCL showed tumor progression after CHOP (4). In studies, the conjugated monoclonal anti-CD30 antibody brentuximab vedotin achieved overall response rates of 86% and complete response rates of 59% in ALK-negative ALCL. A positive effect can therefore be assumed for BIA-ALCL (37). An isolated case report from the UK described benefit in the form of complete remission after administration of brentuximab vedotin to a woman whose disease had progressed during conventional CHOP chemotherapy (38). The role of radiotherapy is currently under debate, and the decision for or against irradiation should be decided by an interdisciplinary tumor board on an individual basis.
With regard to follow-up, analogous to the procedure for lymphomas we advise clinical examination every 3 to 6 months and breast sonography every 6 months for 5 years. CT or PET-CT can also be carried out in the first 2 years if thought necessary. If an implant needs to be replaced, the new implant should have a smooth surface. Patients who receive a new implant (usually as a place holder to preserve the covering skin before autologous breast reconstruction) should be followed-up in more detail if required.
Key Messages.
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a peripheral T-cell lymphoma in the capsule of textured breast implants. With the appropriate treatment the prognosis of BIA-ALCL is excellent.
Most cases of BIA-ALCL manifest 7 to 10 years after implantation as a seroma or, less often, as a solid tumor. The findings to date indicate that radical extirpation is curative. Chemotherapy or radiotherapy should be considered only in the presence of disseminated disease or inoperability; the data are limited.
Patients planned for insertion of implants must be advised of the risk of BIA-ALCL.
The pathophysiology of BIA-ALCL development has not yet been elucidated. Implant surface properties and the presence of a biofilm seem to play a role.
The sparsity of data on BIA-ALCL underlines the need for legislation to introduce a mandatory register for insertion and removal of breast implants.
Acknowledgments
Translated from the original German by David Roseveare
Acknowledgments
We thank our collaborators Dr. med. J. de Grahl, City Breast Center, Saint Gertraud Hospital, Berlin; Prof. Dr. med. J. Liebau, Department of Plastic and Esthetic Surgery, Florence Nightingale Diakonie Hospital Kaiserwerth, Düsseldorf; Dr. med. V. Müller, Department of Gynecology and Gynecological Surgery, Diakonie Hospital Jung-Stilling, Siegen; Dr. med. R. Mett, Department of Plastic, Reconstructive, and Esthetic Surgery, Helios Hospital Schwerin, und Prof. Dr. med. K. Plogmeier, Plastic and Esthetic Surgery, Reconstructive Surgery, Berlin, for compilation and provision of clinical data. We are grateful to Dr. med. C. Nestle-Krämling, Düsseldorf Breast Center, Protestant Hospital Düsseldorf, for assistance with coordination.
Footnotes
Conflict of interest statement
Prof. Solbach has received lecture fees and reimbursement of congress attendance costs from Medtronic and Mentor. She is chair of the Study Group for Esthetic, Plastic, and Reconstructive Surgical Techniques in Gynecology (“Arbeitsgemeinschaft für ästhetische, plastische und wiederherstellende Operationsverfahren in der Gynäkologie”).
The remaining authors declare that no conflict of interest exists.
References
- 1.FDA. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/breastimplants/ucm239995.htm (last accessed on 2 July 2017) [Google Scholar]
- 2.Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 3.FDA. Medical device reports of breast implant-associated anaplastic large cell lymphoma. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm481899.htm (last accessed on 8 April 2018) [Google Scholar]
- 4.Clemens MW, Brody GS, Mahabir RC, Miranda RN. How to diagnose and treat breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2018;14:586e–599e. doi: 10.1097/PRS.0000000000004262. [DOI] [PubMed] [Google Scholar]
- 5.Clemens MW, Horwitz SM. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastical large cell lymphoma. Aesthet Surg J. 2017;37:285–289. doi: 10.1093/asj/sjw259. [DOI] [PubMed] [Google Scholar]
- 6.Kim B, Predmore ZS, Mattke S, et al. Breast-implant-associated anaplastic large cell lymphoma: updated results from a structured expert consultation process. Plast Reconstr Surg Glob Open. 2015;3 doi: 10.1097/GOX.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Non-Hodgkin’s Lymphomas; January 25, 2012. www.nccn.org/professionals/physician_gls/f_guidelines.asp#nhl (last accessed on 15 December 2017) [Google Scholar]
- 8.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Durg Pathol. 2012;36 doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 9.Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34:160–168. doi: 10.1200/JCO.2015.63.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson PA, Lade S, Webster H, et al. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct-pathological entity. Haematologica. 2010;95 doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIH. Adult non-hodgkin lymphoma treatment. (PDQ)-Patient Version. www.cancer.gov/types/lymphoma/patient/adult-nhl-treatment-pdq (last accessed on 1 March 2018) [Google Scholar]
- 12.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim B, Roth C, Chung KC, et al. Anaplastic large cell lymphoma and breast implants: a systematic review. Plast Reconstr Surg. 2011;127 doi: 10.1097/PRS.0b013e3182172418. [DOI] [PubMed] [Google Scholar]
- 14.European Comission Scientific Committee on Health Environmental and Emerging Risks SCHEER. Scientific advice on the state of scientific knowledge regarding a possible connection between breast implants and anaplastic large cell lymphoma. https://ec.europa.eu/health/scientific_committees/consultations/public_consultations/scheer_consultation_06_en (last accessed on 8 May 2018) 2017 [Google Scholar]
- 15.George EV, Pharm J, Houston C, et al. Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol. 2013;6 [PMC free article] [PubMed] [Google Scholar]
- 16.Bizjak M, Selmi C, Praprotnik S, et al. Silicone implants and lymphoma: the role of inflammation. J Autoimmun. 2015;65:64–73. doi: 10.1016/j.jaut.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Orciani M, Sorgentoni G, Torresetti M, et al. MSCs and inflammation: new insights into the potential association between ALCL and breast implants. Breast Cancer Res Treat. 2016;156:65–72. doi: 10.1007/s10549-016-3745-8. [DOI] [PubMed] [Google Scholar]
- 18.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 19.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants Analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. doi: 10.1097/PRS.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669. doi: 10.1097/PRS.0000000000002010. [DOI] [PubMed] [Google Scholar]
- 21.Adams WP Jr. Discussion: Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1670–1672. doi: 10.1097/PRS.0000000000002170. [DOI] [PubMed] [Google Scholar]
- 22.Lipworth L, Tarone RE, McLaughlin JK. Breast implants and lymphoma risk: a review of the epidemiologic evidence through 2008. Plast Reconstr Surg. 2009;123:790–793. doi: 10.1097/PRS.0b013e318199edeb. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasa DR, Miranda RN, Kaura A, et al. Global Adverse Event Reports of Breast Implant-Associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017;139:1029–1039. doi: 10.1097/PRS.0000000000003233. [DOI] [PubMed] [Google Scholar]
- 24.de Jong D, Vasel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 25.de Boer M, van Leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018;4:335–341. doi: 10.1001/jamaoncol.2017.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doren EL, Miranda RN, Selber JC, et al. US. epidemiology of breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2017;139:1042–1050. doi: 10.1097/PRS.0000000000003282. [DOI] [PubMed] [Google Scholar]
- 27.Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140:645–654. doi: 10.1097/PRS.0000000000003654. [DOI] [PubMed] [Google Scholar]
- 28.Clemens MW, Miranda RN. Coming of age: breast implant-associated anaplastic large cell lymphoma after 18 years of investigation. Clin Plast Surg. 2015;42:605–613. doi: 10.1016/j.cps.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Palraj B, Paturi A, Stone RG, et al. Soft tissue anaplastic large T-cell lymphoma associated with a metallic orthopedic implant: case report and review of current literature. J Foot Ankle Surg. 2010;49 doi: 10.1053/j.jfas.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Coleman MP. Cancer risk from orthopedic prostheses. Ann Clin Lab Sci. 1996;26:139–146. [PubMed] [Google Scholar]
- 31.Yoon HJ, Choe JY, Jeon YK. Mucosal CD30-positive T-cell lymphoproliferative disorder arising in the oral cavity following dental implants: report of the first case. Int J Surg Pathol. 2015;23:656–661. doi: 10.1177/1066896915599059. [DOI] [PubMed] [Google Scholar]
- 32.Taylor CR, Siddiqi IN, Brody GS. Anaplastic large cell lymphoma occurring in association with breast implants: review of pathologic and immunohistochemical features in 103 cases. Appl Immunohistochem Mol Morphol. 2013;21:13–20. doi: 10.1097/PAI.0b013e318266476c. [DOI] [PubMed] [Google Scholar]
- 33.Adrada BE, Miranda RN, Rauch GM, et al. Breast-implant associated anaplastic large cell lymphoma: sensitivity, specifitiy, and findings of imaging studies in 44 patients. Breast Cancer Res Treat. 2014;147:1–14. doi: 10.1007/s10549-014-3034-3. [DOI] [PubMed] [Google Scholar]
- 34.Bautista-Quach MA, Nademanee A, Weisenburger DD, Chen W, Kim YS. Implant-associated primary anaplastic large-cell lymphoma with simultaneous involvement of bilateral breast capsules. Clin Breast Cancer. 2013;13:492–495. doi: 10.1016/j.clbc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Clemens MW, Collins MS, Butler CE. Characteristics and treatment of patients with breast implant-associated anaplastic large cell lymphoma presenting with aggressive features. Plast Reconstr Surg. 2015;136 [Google Scholar]
- 36.Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17–25. doi: 10.1182/blood-2014-10-567461. [DOI] [PubMed] [Google Scholar]
- 37.Pro B, Advani R, Brice P, et al. Brentuximab vetodin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 38.Johnson L, O´Donoghue JM, McLean N, et al. Breast implant associated anaplastic large cell lymphoma: the UK experience Recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017;43:1393–1401. doi: 10.1016/j.ejso.2017.05.004. [DOI] [PubMed] [Google Scholar]