Abstract
Qi-deficiency (QX) is thought to promote the body's susceptibility to disease, but the underlying mechanism through which this occurs is not clear. We surveyed the traditional Chinese medicine constitution (TCMC) of healthy college students to identify those that were PH (balanced TCMC constitution) and QX (unbalanced TCMC constitution). We then used high-throughput sequencing of the 16SrRNA V3-4 region in fecal microbiota samples to identify differences between those obtained from PH and QX individuals. Our results demonstrated that the alpha diversity of QX samples was significantly lower than that of PH samples (p < 0.05) and that beta diversity was remarkably different in QX and PH samples. Four and 122 bacterial taxa were significantly overrepresented in QX and PH groups, respectively. The genera Sphingobium, Clostridium, and Comamonas were enriched in the QX group and had a certain pathogenic role. The QX group also showed a statistically significant lack of probiotics and anti-inflammatory bacteria such as Bifidobacterium and Bdellovibrio. The functional potential of QX bacterial taxa was reduced in fatty acid metabolism and butanoate metabolism. We contend that the imbalanced intestinal microbiota in QX and the following functional changes in metabolism influence immunity and energy metabolism, which could increase susceptibility to disease.
1. Introduction
Traditional Chinese medicine constitution (TCMC) refers to relatively stable physical and psychological characteristics and is based on epidemiological investigations [1], clinical manifestations [2], and genomic research among Han Chinese [3, 4]. There is one balanced constitution of TCMC, PH, and eight unbalanced constitutions, including Qi-deficiency (QX) [5]. TCMC theory digitizes empirical Traditional Chinese Medicine diagnosis and treatment and provides a standardized guideline which is particularly useful for clinics [6]. Previous findings show that QX is an early stage in cancer [7, 8], heart disease [9], hypertension [10], diabetes [11], chronic fatigue syndrome [12, 13], and depression [14] and that PH is a protective factor against these conditions [9, 15].
PH people are energetic and fit, are not susceptible to illness, and have stable and powerful pulses and quality sleep. QX people are characterized by fatigue, are lacking in strength, and have weak pulses. QX is related to overwork, working pressure, and aging [16]. QX could promote the body's susceptibility to disease, but its mechanism is not clear.
Studies have shown the downregulation of immune-related genes including those involved in major histocompatibility complex [17] and interleukin-1β binding [18] in QX people. Our previous studies revealed the presence of metabolic disorders in the plasma of healthy QX people, including the presence of betaine and alanine [19]. Previous reports indicated that betaine in plasma positively correlates with Clostridium in feces [20] and that Lactobacillus casei Shirota could improve plasma alanine-aminotransferase levels [21]. These cases revealed that the specific plasma metabolites of QX were associated with intestinal microbiota. However, visceral manifestation of QX has also indicated spleen- and lung-deficiencies and some results indicate that these deficiencies induce compositional and functional changes in intestinal microbiota [22, 23].
We hypothesize that QX-related increases in disease susceptibility are potentially mediated by intestinal microbiota. We performed pyrosequencing of the 16SrRNA gene in healthy QX and PH Han Chinese college students, to compare their intestinal microbiota, and predicted metabolic function to explore the biological mechanism of QX.
2. Method
2.1. Study Subjects
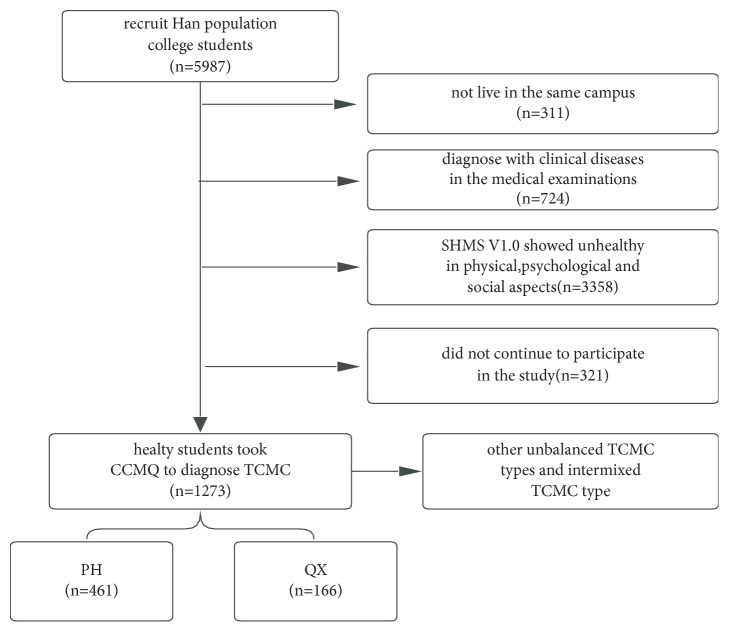
This study is reviewed and approved by China Ethics Committee of Registering Clinical Trials (No. ChiECRCT-20170064) and Chinese Clinical Trials Registry (No. ChiCTR-IOR-17012986). We screened 461 healthy PH students and 166 QX students from 5987 college students of southern Han ethnicity (Figure 1). The inclusion criteria were as follows: first, unremarkable physical and blood tests including urea, electrolytes, and liver function tests; second, healthy status according to the results of Subhealth Measurement Scale V1.0 [24]; third, results of PH/QX in Chinese Constitution Medicine Questionnaire [25, 26]. Random sampling was used to select 21 PH and 21 QX students for 16SrRNA sequencing (V3–4). These 42 students were prohibited to take antibiotics, probiotics, or Chinese herbal medicines for 16 weeks. We obtained 16 PH and 19 QX sequencing datasets.
Figure 1.
The sequence flow diagram of inclusion criteria for PH samples and QX samples.
2.2. Fecal Sample Collection and Sequencing
Fresh fecal samples were collected in sterile container, kept at 4°C (<0.5 h), and then frozen at −80°C. According to the manufacturer's instructions, total fecal DNA was extracted using DNA Stool mini kit (Shenzhen Bioeasy Biotechnologies, Co., Ltd.). We amplified the V3-4 region of the 16SrRNA gene by PCR using the universal primers V4F and V4R (Table S1). 25 μl reaction mixture was 0.25μl 5U/μl Premix Taq, 1 μl template DNA, 0.5 μl 10 μM barcodes forward primer, 0.5 μl 10 μM reverse primer, and 16.75 μl double-distilled H2O. The PCR cycle conditions were an initial denaturation at 94°C for 2 min, 30 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 40 s, and a final extension at 72°C for 5 min. PCR products were sequenced using Illumina MiSeq instrument (PE 300, Illumina, San Diego, California, USA) at Guangzhou RiboBio Co., Ltd.
Sequencing reads were clustered by Illumina paired barcoded-sequencing (end) (BIPES) (PE) process for preliminary analysis; the rest of the sequence was screened by UCHIME and the suspected chimeric sequence was removed. The two-stage clustering (TSC) was used for extracting the OTU, with sequences having greater than or equal to 97% similarity being assigned to the same OTU. Alpha diversity was performed with Chao1, observed species, Shannon and Simpson. Beta diversity was performed with principle coordinate analysis (PCoA) based on UniFrac distance [27]. The linear discriminant analysis (LDA) with effect size measurements (LefSe) [28] was used to identify statistically significant different bacterial species in QX group and PH group. PICRUSt [29] was used to predict the metabolic function of the 16SrRNA gene datasets with KEGG Orthologs classification (p<0.05, Welch's t-test) [30].
2.3. Statistical Analysis
Epidata 3.0 was used to establish a database. Enumeration data was tested by chi-square. quantitative data was tested by independent-samples t-test. Kruskal-Wallis, PCoA, LEfSe, and PICRUSt analyzed the sequencing data. Statistical analyses were performed with the SPSS 20.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be of statistical significance.
3. Results
3.1. Students and Samples
There was no statistical significance between the two groups in age, sex, BMI, and health status scores (Table 1, p>0.05). We obtained 119,909 sequences from 19 QX samples and 218,480 sequences from 16 PH samples (Table S2).
Table 1.
General situation.
| characteristics | QX (n = 19) | PH (n= 16) | p value |
|---|---|---|---|
| Age (years) | 20.75 ±1.47 | 20.13±1.31 | 0.2007 |
| Sex (male /female) | 7/12 | 5/11 | 0.728 |
| BMI (kg/m2) | 20.18±3.31 | 20.94±1.31 | 0.3951 |
| QX transformed score | 48.58±9.56 | 10.94±5.82 | — |
| Health Status Scores | 79.23±5.32 | 81.47±7.15 | 0.2962 |
No statistical significance between the two groups in age, sex, BMI, and health status scores (p>0.05).
3.2. Microbiota in QX Were Different from PH
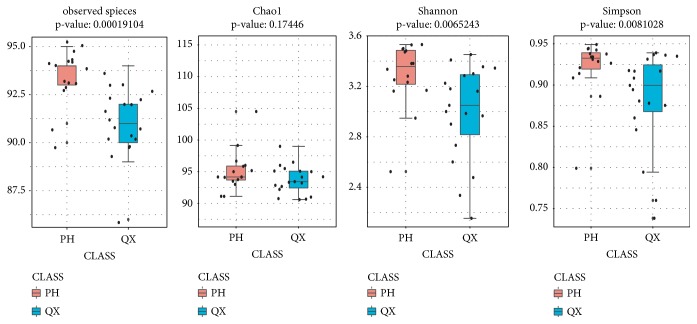
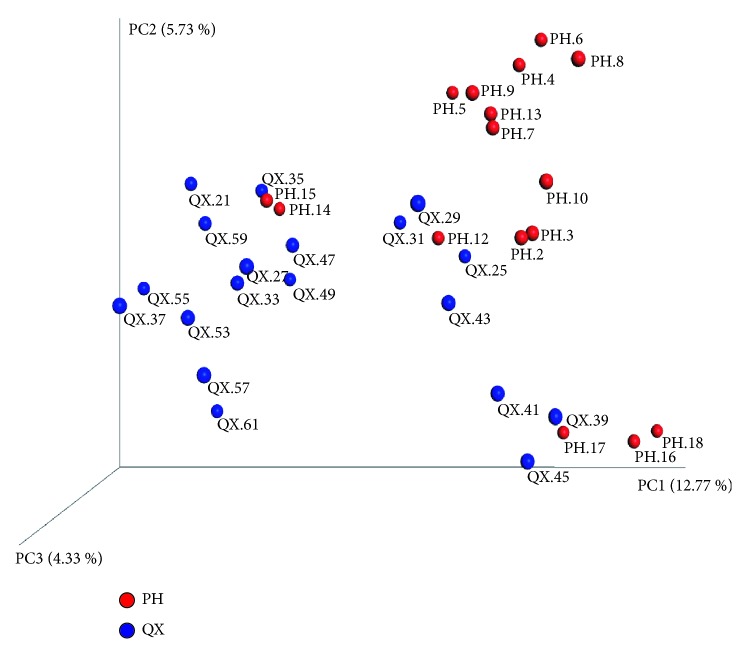
The numbers of observed species, Shannon and Simpson indices, were significantly lower in the QX group than in the PH group (p<0.05, Figure 2). PCoA (UniFrac) demonstrated a clear clustering of the microbial populations of the QX group, which were separated from the PH group (Figure 3).
Figure 2.
Bacterial alpha diversity in QX (blue) and PH (red) populations. Observed species, Shannon and Simpson indices demonstrate that the diversity in QX people is significantly lower than that in PH people. Mann-Whitney, p < 0.01.
Figure 3.
PCoA (unweighted) of intestinal microbiota in QX (blue) and PH (red) populations. Principal coordinate axis 1 (12.99% variability) and principal coordinate axis 2 (5.68% variability) highlight a clear clustering.
3.3. Phylum, Family, and Genus Level Taxonomic Distribution of Intestinal Microbiota in QX Populations
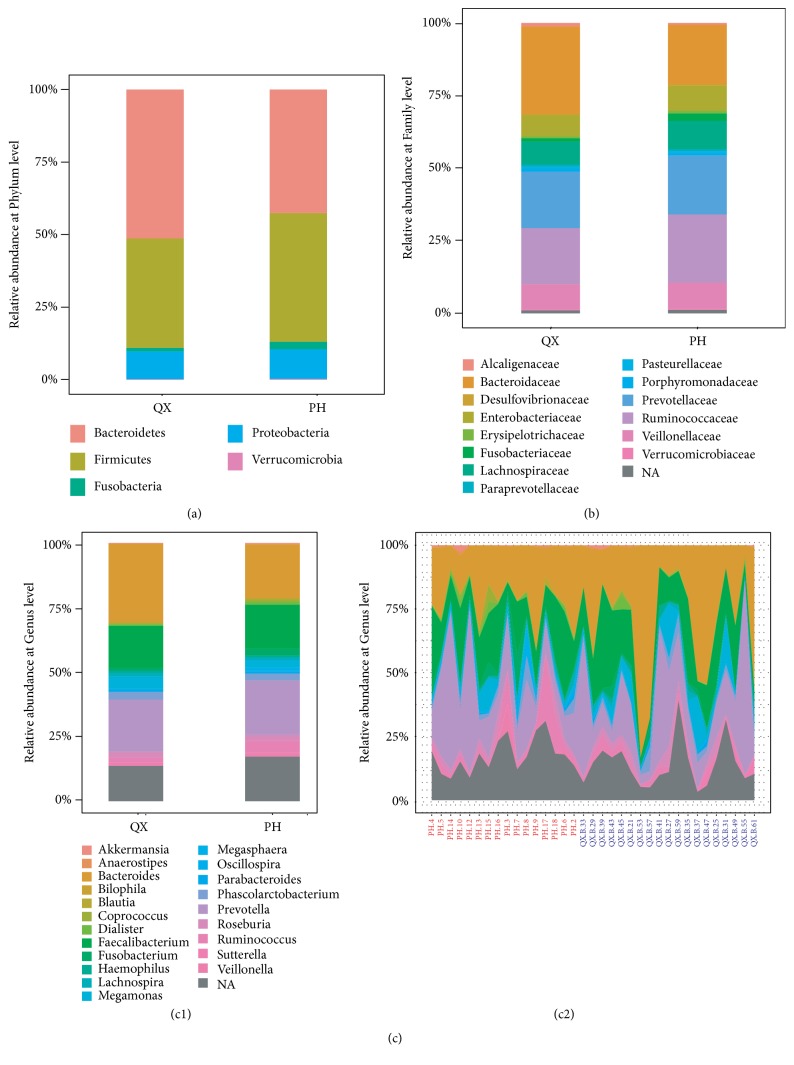
Bacteroidetes and Firmicutes were the top two phyla identified in QX samples and accounted for 47.64% and 39.23% of the total valid reads, respectively. In PH samples, Firmicutes and Bacteroidetes accounted for 43.99% and 38.52% (Figure 4(a)). At the family level, based on the average relative abundance, nine species were dominant (≥1%) in PH samples, and 10 species were dominant in QX samples. Bacteroidaceae and Alcaligenaceae were enriched in QX, whereas Ruminococcaceae, Fusobacteriaceae, and Bifidobacteriaceae were enriched in PH (Figure 4(b)). At the genera level, 12 genera were dominant in PH, and 11 genera were dominant in QX. Bacteroides, Megamonas, Lachnospira, and Sutterella were enriched in QX, and Ruminococcus, Fusobacterium, Megasphaera, and Coprococcus were more abundant in PH (Figure 4(c)). PH contained more Ruminococcus and Coprococcus which can produce butyrate [31], whereas Sutterella, present in QX samples, could give rise to unbalanced microflora by degrading secretory IgA and IgA itself [32].
Figure 4.
Relative abundance of intestinal microbiota at phylum (a), family (b), and genus (c) levels in PH and QX populations based on 16SrRNA sequencing. (b) shows that the taxa median relative abundance at the family level is ≥ 1% of total abundance. (c) shows that the taxa relative abundance at the genus level is ≥ 1% of the total abundance within groups (c1) and individuals (c2).
3.4. Decreased Bacteria Associated with Short-Chain Fatty Acid Production and Butanoate Metabolism Are Prominent Features of QX
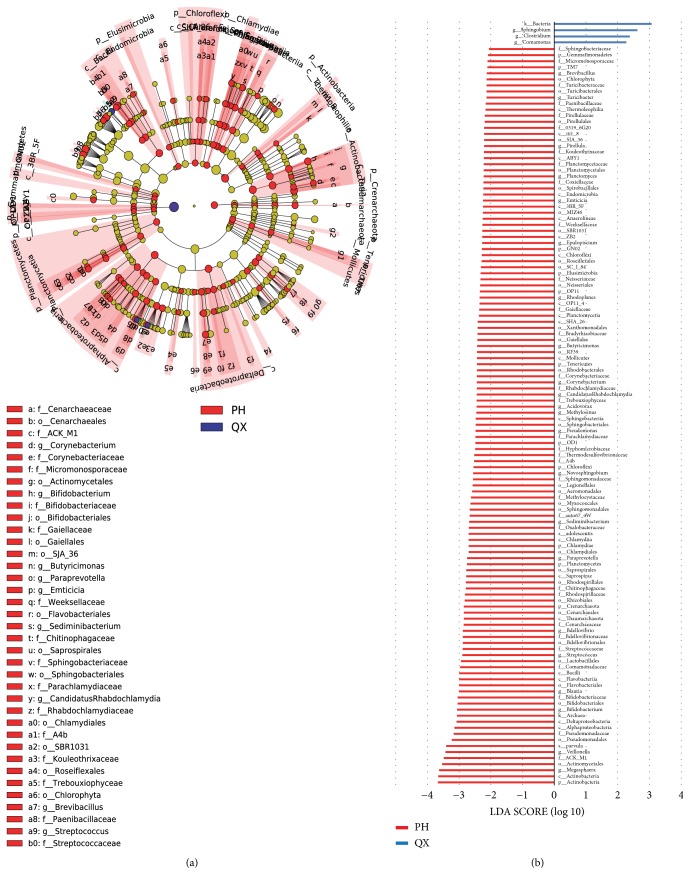
LefSe showed biomarkers for QX and PH (LDA>2, p<0.05). Four and 122 taxa were enriched in QX and PH samples, respectively (Figures 5(a) and 5(b)). At the genus level, Sphingobium, Clostridium, and Comamonas were significantly overrepresented in QX and Megasphaera, Veillonella, Veillonella parvula, Bifidobacterium, Blautia, Streptococcus, Bdellovibrio, and Paraprevotella were significantly overrepresented in PH. Moreover, at the family level, Veillonellaceae, Bifidobacteriaceae, Pseudomonadaceae, Lactobacillales, Lachnospiraceae, Streptococcaceae, and Bdellovibrionaceae were significantly overrepresented in PH.
Figure 5.
LefSe shows significantly discriminative features in QX (blue) and PH (red) populations. LDA > 2.0, p < 0.05. (a) Cladograms of the hierarchical structure. (b) Differential abundance bacterial taxa between the two groups presented as a histogram. The key microbiota taxa in QX were genus Sphingobium, Clostridium, and Comamonas.
Of the biomarkers for QX, Comamonas is a cellulolytic microbe [33] and Sphingobium [34] and Clostridium [35, 36] are associated with inflammation. Of the biomarkers PH for, Megasphaera [37], Veillonellaceae [38], Pseudomonadaceae [39], Streptococcaceae [40], and Bifidobacterium [41, 42] can produce and metabolize fat, starches, protein, and polysaccharides, which are significantly lacking in QX. PH biomarkers Bifidobacterium and lactobacilli can ferment inulin-type fructans to cross-feed the microbiota and significantly stimulate the production of n-butyrate [43, 44]. Additionally, PH samples were rich in anti-inflammatory bacteria, including Bifidobacterium [45, 46], Streptococcus [47], Lachnospiraceae [48], and Bdellovibrio [49]. Metabolites of Bifidobacterium, including acetic acid, lactic acid, ethanol, formic acid, vitamins, and amino acids, can also stimulate the immune response and enhance the function of NK cells and the proliferation of T lymphocytes[50, 51]. Bdellovibrio, considered as potential antibiotic substitutes, could also increase Coprococcus, which is reduced in irritable bowel syndrome and influences microbial balance [52].
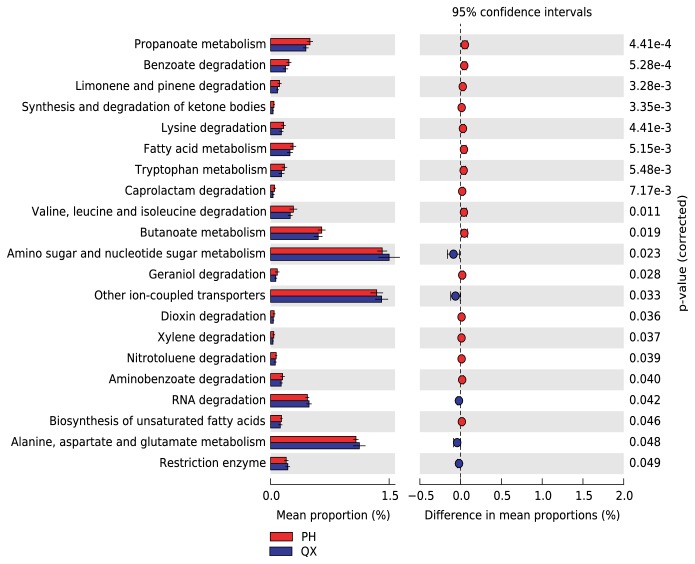
PICRUSt was used to investigate the potential function and metabolic pathways of the taxa, based on Kyoto Encyclopedia of Genes and Genomes (KEGG) modules. Of note, QX had a lower proportion of bacterial genes related to fatty acid metabolism and butanoate metabolism and a higher proportion of bacterial genes related to amino and nucleotide sugar metabolism and RNA degradation (Figure 6, p<0.05, Welch's inverted CI method effect size>0.01).
Figure 6.
PICRUSt shows that the 21 predicted KOs significantly differ in their distribution in healthy QX (blue) and PH (red) populations. Functional modules involved in amino and nucleotide sugar metabolism and in RNA degradation are more abundant in QX than they are in PH. P < 0.05, Welch's t-test, Welch's inverted CI method, and effect size > 0.01.
4. Discussion
Here, we described our investigation of intestinal microbiota characteristics among healthy QX and PH Han college students in Southern China. We performed 16SrRNA gene sequencing to examine the different microbiota in QX and PH groups and to explain the biological mechanism underlying susceptibility to disease in QX individuals. Our results show that the bacterial taxa data of healthy people were similar to that of Asian populations reported in previous studies [53, 54] but that the microbiota of QX group was statistically different from that of the PH group.
Functionally, QX microbiota have relatively decreased fatty acid and butanoate metabolism potentials and relatively enriched carbohydrate metabolism (amino sugar and nucleotide sugar metabolism) and RNA degradation potentials. The imbalance of energy metabolism caused by alterations in intestinal microbiota is associated with QX-related symptoms. QX people demonstrate significantly higher BMI scores and waist-to-hip ratios and significantly lower scores in back muscle strength [55]. QX symptoms include a reduction of resting metabolic rate, which results in flabby muscles, vulnerability to fatigue, feeble pulse, shallow breathing [56], and potential concern with disturbances in energy metabolism. Research has shown that GLUT4, a critical protein in glucose metabolism, increases following low-intensity exercise [57] and that moderate-intensity exercise increases fatty acid oxidation to adapt energy metabolism [58]. Per mole of oxygen consumed, the oxidation of carbohydrates produces 15% more ATP than the oxidation of lipids does [59]. However, lipid oxidation produces greater energy density compared to carbohydrate oxidation, and fatty acids can go straight to the muscles' mitochondria for energy. Therefore, we postulate that QX and PH people adopt different optimal-fuel strategies to acclimatize themselves to daily energy expenditure. Compared with PH people, QX people tend to adopt a carbohydrate-based metabolism to prolong the time for which energy is maintained, which makes it possible to induce the symptoms of lower metabolic rate. PH people tend to adopt fatty acid metabolism and butanoate metabolism. Lipid oxidation has a greater energy density than does carbohydrate oxidation, which efficiently elevates thermogenesis capacity. Higher rates of fat oxidation generally reflect well-trained body conditions, while lower fat oxidation rates may be related to obesity and insulin resistance [60].
The microbiota identified in the QX group had reduced levels of probiotics and decreased anti-inflammatory bacteria than did those identified in the PH group. The alteration of intestinal microbiota and unbalanced metabolism in QX, including reduction of fatty acid and butanoate metabolism, can increase disease susceptibility. Short-chain fatty acids (SCFAs), especially butyrate, play an important role in the regulation of host immunity. SCFAs directly contact intestinal epithelium cells (IECs), and SCFAs, especially butyrate, are used as ATP sources for energy metabolism. However, SCFAs also enhance IEC immune surveillance by increasing the expression of certain antimicrobial peptides and maintaining the integrity of intestinal mucosa [61–63]. For example, commensal microbe-derived butyrate induced the differentiation of colonic regulatory T cells, which have a central role in the suppression of inflammatory and allergic responses [64]. Furthermore, butyrate and valproic acid upregulated B cell microRNAs in human and mouse B cells to modulate antibody and autoantibody responses [65].
In conclusion, this study demonstrates that intestinal microbiota in the QX group showed lower biological diversity and possessed a different microbial signature than did that of the PH group. We contend that the QX's imbalanced intestinal microbiota and concomitant functional changes in metabolism potentially influence immunity and energy metabolism, which could increase susceptibility to disease.
Acknowledgments
The authors thank their study participants. This work was supported by the Key Project of National Natural Science Foundation of China (no. 81830117), the National Science Foundation of China (no. 81673840, no. 81703891, no. 81760821, and no. 81703952), Natural Science Foundation of Guangdong Province, China (no. 2017A030313791 and no. 2016A030310311), the Science & Technical Plan of Guangzhou, Guangdong, China (no. 2014Y2-00504), and TCM State Administration Foundation of Guangdong Province, China (20164001).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Xiaoshan Zhao and Ren Luo designed the experiments. Ke Ma, Jieyu Chen, Liuyan Kuang, Jianlu Bi, Jingru Cheng, Fei Li, and Xiaoli Nie performed the experiments. Jieyu Chen and Ke Ma analyzed the data. Ke Ma, Liuyan Kuang, and Xiaoshan Zhao prepared the manuscript. Xiaoshan Zhao and Xiaomin Sun were responsible for study supervision. All authors were involved in the formulation of the research questions. Ke Ma, Jieyu Chen, and Liuyan Kuang contributed equally to this work
Supplementary Materials
Table S1. The information of primers. Table S2. Samples sequencing and alpha diversity of the intestinal microbiota.
References
- 1.Qi W., Yan-bo Z. Epidemiological investigation of constitutional types of Chinese medicine in general population: Base on 21, 948 epidemiological investigation data of nine provinces in China. Journal of Traditional Chinese Medicine. 2009:p. 12. [Google Scholar]
- 2.Yumin H., Li W., Fengting S., Gengwu C., Dafeng Z., Yun X. Clustering study of TCM constitution. Chinese Journal of Basic Medicine in traditional chinese medicine. 1996:p. 10. [Google Scholar]
- 3.Wang Ji L., Bai Ming-Hua W. Genomics study on nine types of TCM constitution. China Journal of Traditional Chinese Medicine and Pharmacy. 2014:3871–3873. [Google Scholar]
- 4.Yu R., Liu D., Yang Y., et al. Expression profiling-based clustering of healthy subjects recapitulates classifications defined by clinical observation in Chinese medicine. Journal of Genetics and Genomics. 2017;44(4):191–197. doi: 10.1016/j.jgg.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Qi W. The Classification of Nine Kinds of Basic TCM Constitution and the Basis of Diagnosis. Journal of Beijing University of Traditional Chinese Medicine. 2005:1–8. [Google Scholar]
- 6.Wang J., Li Y., Ni C., Zhang H., Li L., Wang Q. Cognition research and constitutional classification in Chinese medicine. American Journal of Chinese Medicine. 2011;39(4):651–660. doi: 10.1142/s0192415x11009093. [DOI] [PubMed] [Google Scholar]
- 7.Xuedong C. A. A Preliminary Study on the Pathogenesis Underlying Nasopharyngeal Precancerous Lesion Induced by the Interactive Effeets of Qi-dificient Physique Status with N, N`-dinitrosopiperazine [Doctoral Dissertation]: HuNan university of traditional chinese medicine [Doctoral, thesis] HuNan university of traditional chinese medicine; 2005. [Google Scholar]
- 8.Tao F., Lü P., Xu C., et al. Metabolomics Analysis for Defining Serum Biochemical Markers in Colorectal Cancer Patients with Qi Deficiency Syndrome or Yin Deficiency Syndrome. Evidence-Based Complementary and Alternative Medicine. 2017;2017 doi: 10.1155/2017/7382752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y., Shi H., Wang Q., Wang Y., Yu X., Di J., et al. Association between Nine Types of TCM Constitution and Five Chronic Diseases: A Correspondence Analysis Based on a Sample of 2,660 Participants. Vol. 2017. Evid Based Complement Alternat Med; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y.-B., Wang Q., Deng Q.-W., Cai J., Song X.-H., Yan X. Relationships between constitutional types of traditional Chinese medicine and hypertension. Journal of Chinese Integrative Medicine. 2010;8(1):40–45. doi: 10.3736/jcim20100108. [DOI] [PubMed] [Google Scholar]
- 11.Juan Y. Traditional Chinese medical body constitution, its related clinical characteristics, and diabetes-associated chronic complications in patients with type 2 diabetes. DaLian Medical Universit; 2015. [Google Scholar]
- 12.Xinping L. Correlations between TCM Constitution and Chronic Fatigue Syndrome. World Chinese Medicine. 2017:1171–1174. [Google Scholar]
- 13.Yan H. The correlation of chronic fatigue symdrome and its impact factors and TCM constitution and clinical research [Doctoral Dissertation] GuangZhou university of chinese medicion; 2013. [Google Scholar]
- 14.Liao Y.-C., Chou C.-Y., Chang C.-T., et al. Qi deficiency is associated with depression in chronic hemodialysis patients. Complementary Therapies in Medicine. 2017;30:102–106. doi: 10.1016/j.ctim.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang T., Chen J., Sun X., et al. Effects of TCMC on transformation of good health status to suboptimal health status: a nested case-control study. Evidence-Based Complementary and Alternative Medicine. 2015;2015:8. doi: 10.1155/2015/259727.259727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang H. C., Yang S. T., Lee K. C., Huang P. Y., Hsu M., Chang H. H. From theory to clinic: key components of qi deficiency in traditional Chinese medicine. Altern Ther Health Med. 2012;18:28–36. [PubMed] [Google Scholar]
- 17.Ruilin W. Preliminary Research on Gene Expression Profile of Peripheral Blood of the constitution of Qi deficiency [Master Dissertation]: BeiJing university of chinese medicine [Master, thesis] BeiJing university of chinese medicine: Preliminary Research on Gene Expression Profile of Peripheral Blood of the constitution of Qi deficiency [Master Dissertation]; 2005. [Google Scholar]
- 18.Xiaojun Z., Daofa T., Shizhen W., Yan R. Genital Stable and Gene Expression with High Gastritis Cancer Family in Qi Deficiency. Information on Traditional Chinese Medicine. Genital Stable and Gene Expression with High Gastritis Cancer Family in Qi Deficiency. Information on Traditional Chinese Medicine. 2009:59–62. [Google Scholar]
- 19.Jianlu B., Jieyu C., Jingru C., Bingyan Y. The comparative plasma metabonomic analysis of qi-deficiency constitution and gentleness constitution. Journal of Tropical Medicine. 2014:717–720. [Google Scholar]
- 20.Ross A. B., Bruce S. J., Blondel-Lubrano A., et al. A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. British Journal of Nutrition. 2011;105(10):1492–1502. doi: 10.1017/S0007114510005209. [DOI] [PubMed] [Google Scholar]
- 21.Wagnerberger S., Spruss A., Kanuri G., et al. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. The Journal of Nutritional Biochemistry. 2013;24(3):531–538. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Liu Jia P. Y. Z. S. Investigation on intestinal microflora of elderly patients with spleen deficiency by 16S rDNA DGGE analysis. Journal of Traditional Chinese Medicine and Pharmacy. 2010:1566–1569. [Google Scholar]
- 23.Yanhua K. Structural Modulation of Gut Microbiota And Mechanism Research in Rats with Chronic Obstructive Pulmonary Disease Treated with Recuperating Lung Decoction. BeiJing university of chinese medicine; 2016. [Google Scholar]
- 24.Liyi F., Jun X., Ren L., Jincai Q, Jinhua Z. Analysis and Build-up for Sub-Health Evaluating Indicat or System. chinese general practice. 2011:37–40. [Google Scholar]
- 25.China Association of Traditional Chinese Medicine and Pharmacy. Classification and Judgment of TCM Constitution (ZYYXH/T157-2009) World Journal of Integrated Traditional and Western Medicine. 2009:303–304. [Google Scholar]
- 26.Qi W., Yanbo Z., Hesheng X., Shao L. primary compiling of constitution in chinese medicine questionnaire. chinese journal of clinical rehabilitation. 2006:p. 12. [Google Scholar]
- 27.Lozupone C., Hamady M., Knight R. UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7, article 371 doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segata N., Izard J., Waldron L., et al. Metagenomic biomarker discovery and explanation. Genome Biology. 2011;12(6, article R60) doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langille M. G. I., Zaneveld J., Caporaso J. G., et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M., Goto S., Sato Y., Kawashima M., Furumichi M., Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Research. 2014;42(1):D199–D205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryde S. E., Duncan S. H., Hold G. L., Stewart C. S., Flint H. J. The microbiology of butyrate formation in the human colon. FEMS Microbiology Letters. 2002;217(2):133–139. doi: 10.1016/s0378-1097(02)01106-0. [DOI] [PubMed] [Google Scholar]
- 32.Moon C., Baldridge M. T., Wallace M. A., Burnham C.-A. D., Virgin H. W., Stappenbeck T. S. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521(7550):90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanokratana P., Wongwilaiwalin S., Mhuantong W., Tangphatsornruang S., Eurwilaichitr L., Champreda V. Characterization of cellulolytic microbial consortium enriched on Napier grass using metagenomic approaches. Journal of Bioscience and Bioengineering. 2017 doi: 10.1016/j.jbiosc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Chen S., Tsai C., Lee Y., et al. Intestinal microbiome in children with severe and complicated acute viral gastroenteritis. Scientific Reports. 2017;7(1) doi: 10.1038/srep46130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arase S., Watanabe Y., Setoyama H., Nagaoka N., Kawai M., Matsumoto S. Disturbance in the mucosa-associated commensal bacteria is associated with the exacerbation of chronic colitis by repeated psychological stress; is that the new target of probiotics? PLoS ONE. 2016;11(8) doi: 10.1371/journal.pone.0160736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De La Cuesta-Zuluaga J., Mueller N. T., Corrales-Agudelo V., et al. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 37.Rychlik J. L., La Vera R., Russell J. B. Amino acid deamination by ruminal Megasphaera elsdenii strains. Current Microbiology. 2002;45(5):340–345. doi: 10.1007/s00284-002-3743-4. [DOI] [PubMed] [Google Scholar]
- 38.Bonder M. J., Tigchelaar E. F., Cai X., et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Medicine. 2016;8(1) doi: 10.1186/s13073-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Li J., Yu J., et al. Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. Journal of Pharmaceutical and Biomedical Analysis. 2018;149:425–435. doi: 10.1016/j.jpba.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Murphy K., Curley D., O’Callaghan T. F., et al. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Scientific Reports. 2017;7(1) doi: 10.1038/srep40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milani C., Andrea Lugli G., Duranti S., et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Scientific Reports. 2015;5 doi: 10.1038/srep15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milani C., Turroni F., Duranti S., et al. Genomics of the genus Bifidobacterium reveals species-specific adaptation to the glycan-rich gut environment. Applied and Environmental Microbiology. 2016;82(4):980–991. doi: 10.1128/AEM.03500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vuyst L., Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. International Journal of Food Microbiology. 2011;149(1):73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Tsukahara T., Hashizume K., Koyama H., Ushida K. Stimulation of butyrate production through the metabolic interaction among lactic acid bacteria, Lactobacillus acidophilus, and lactic acid-utilizing bacteria, Megasphaera elsdenii, in porcine cecal digesta. Animal Science Journal. 2006;77(4):454–461. doi: 10.1111/j.1740-0929.2006.00372.x. [DOI] [Google Scholar]
- 45.Markowiak P., Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9, article no. 1021) doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng Q., He X., Puthiyakunnon S., et al. Probiotic mixture golden Bifido prevents neonatal Escherichia coli K1 translocation via enhancing intestinal defense. Frontiers in Microbiology. 2017;8 doi: 10.3389/fmicb.2017.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wlodarska M., Luo C., Kolde R., et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host & Microbe. 2017;22(1):25–37.e6. doi: 10.1016/j.chom.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saresella M., Mendozzi L., Rossi V., et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: A pilot study. Frontiers in Immunology. 2017;8 doi: 10.3389/fimmu.2017.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shatzkes K., Tang C., Singleton E., et al. Effect of predatory bacteria on the gut bacterial microbiota in rats. Scientific Reports. 2017;7 doi: 10.1038/srep43483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H., Gong J., Wang W., et al. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maslowski K. M., MacKay C. R. Diet, gut microbiota and immune responses. Nature Immunology. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 52.Malinen E., Krogius-Kurikka L., Lyra A., et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World Journal of Gastroenterology. 2010;16(36):4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Guo Z., Xue Z., et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. The ISME Journal. 2015;9(9):1979–1990. doi: 10.1038/ismej.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nam Y., Jung M., Roh S. W., Kim M., Bae J., Dias-Neto E. Comparative Analysis of Korean Human Gut Microbiota by Barcoded Pyrosequencing. PLoS ONE. 2011;6(7):p. e22109. doi: 10.1371/journal.pone.0022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng D. Research of Characteristics of Shape and Function in Undergraduate Students with Pardal Constitution and Exercise Intervention to the People with Qi-insumciency Constitution [Doctoral Dissertation] ShanDong university of chinese medicien; 2008. [Google Scholar]
- 56.Mun S., Kim S., Bae K.-H., Lee S. Cold and Spleen-Qi Deficiency Patterns in Korean Medicine Are Associated with Low Resting Metabolic Rate. Evidence-Based Complementary and Alternative Medicine. 2017;2017 doi: 10.1155/2017/9532073.9532073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhen W. The effect of different intensity of treadmill exercise on mitochondrial glucose and fatty acid oxidation key factors in rats myocytes [Master Dissertation] HeBei Medical University; 2016. [Google Scholar]
- 58.Koonen D. P., Benton C. R., Arumugam Y., et al. Different mechanisms can alter fatty acid transport when muscle contractile activity is chronically altered. American Journal of Physiology-Endocrinology and Metabolism. 2004;286(6):E1042–E1049. doi: 10.1152/ajpendo.00531.2003. [DOI] [PubMed] [Google Scholar]
- 59.Brand M. The efficiency and plasticity of mitochondrial energy transduction. Biochemical Society Transactions. 2005;33(5):897–904. doi: 10.1042/BST20050897. doi: 10.1042/BST20050897. [DOI] [PubMed] [Google Scholar]
- 60.Randell R. K., Rollo I., Roberts T. J., Dalrymple K. J., Jeukendrup A. E., Carter J. M. Maximal Fat Oxidation Rates in an Athletic Population. Medicine & Science in Sports & Exercise. 2017;49(1):133–140. doi: 10.1249/MSS.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 61.Iraporda C., Errea A., Romanin D. E., et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220(10):1161–1169. doi: 10.1016/j.imbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Macia L., Tan J., Vieira A. T., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature Communications. 2015;6, article 7734 doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 63.Lin M. Y., De Zoete M. R., Van Putten J. P. M., Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Frontiers in Immunology. 2015;6 doi: 10.3389/fimmu.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furusawa Y., Obata Y., Fukuda S., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 65.White C. A., Pone E. J., Lam T., et al. Histone deacetylase inhibitors upregulate B cell microRNAs that silence AID and blimp-1 expression for epigenetic modulation of antibody and autoantibody responses. The Journal of Immunology. 2014;193(12):5933–5950. doi: 10.4049/jimmunol.1401702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The information of primers. Table S2. Samples sequencing and alpha diversity of the intestinal microbiota.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.