Abstract
Probiotics confer immunological protection to the host through the regulation, stimulation, and modulation of immune responses. Researchers have shifted their attention to better understand the immunomodulatory effects of probiotics, which have the potential to prevent or alleviate certain pathologies for which proper medical treatment is as yet unavailable. It has been scientifically established that immune cells (T- and B-cells) mediate adaptive immunity and confer immunological protection by developing pathogen-specific memory. However, this review is intended to present the recent studies on immunomodulatory effects of probiotics. In the early section of this review, concepts of probiotics and common probiotic strains are focused on. On a priority basis, the immune system, along with mucosal immunity in the human body, is discussed in this study. It has been summarized that a number of species of Lactobacillus and Bifidobacterium exert vital roles in innate immunity by increasing the cytotoxicity of natural killer cells and phagocytosis of macrophages and mediate adaptive immunity by interacting with enterocytes and dendritic, Th1, Th2, and Treg cells. Finally, immunomodulatory effects of probiotics on proinflammatory and anti-inflammatory cytokine production in different animal models have been extensively reviewed in this paper. Therefore, isolating new probiotic strains and investigating their immunomodulatory effects on cytokine profiles in humans remain a topical issue.
1. Introduction
Probiotics are living microorganisms that confer several health benefits when administrated in adequate amounts to the host [1, 2]. Adhering to human intestines, probiotics stimulate, regulate, and modulate various different functions, including digestion, metabolism, epithelial innate immunity, competitive exclusion of pathogens, and brain-gut communication [3, 4]. Gut microorganisms produce several nontoxic metabolites and play important roles in nutritional and clinical applications [5–7]. Therefore, the microecology of the gastrointestinal tract, consisting of intestine, microbiota, and nondigestible food within the tract, is crucial for probiotic action in the host.
Probiotics act symbiotically by fermenting nondigestible food, known as prebiotics, for their energy and exert elite properties including antipathogenicity, antiobesity, and diabetic, antidiabetic, anti-inflammatory, anticancer, and angiogenic activities and efficacy on the brain and central nervous system [8]. However, the functions of probiotics can be classified as metabolic, protective, and trophic [9], since the trophic role has garnered attention in studies of immunomodulation. Typically, the immune system in vertebrates can be divided into innate and adaptive immunity. Innate immunity is a nonspecific defense mechanism exerting immediate or near-immediate responses to the presence of pathogens in body. On the other hand, adaptive immunity is highly specific and is able to destroy individual invading pathogens in vertebrates. Besides, a pathogen-specific long-lasting protective memory enables the adaptive immune system to attack and destroy pathogens when reencountered [10]. Lymphocytes, especially B cells and T cells, exert adaptive immune responses by recognizing antigens with their specific receptors.
In the last few years, probiotics have been extensively studied and reported, with humoral, cellular, and nonspecific immunity modulation, as well as promoting the immunological barrier [11, 12]. Probiotics have been evaluated for in vivo effects, such as increased peripheral immunoglobin production, stimulation of IgA secretion, and decreased proinflammatory cytokine production [13]. It has been reported that homogenates prepared from several probiotics, including Lactobacillus rhamnosus GG, Lactobacillus acidophilus, Lactobacillus delbrueckii sub sp. bulgaricus, Bifidobacterium lactis, and Streptococcus thermophiles, have the ability to suppress the proliferation of mononuclear cells [14]. It has also been reviewed that Bifidobacterium bifidum has a significant effect in enhancing antibody responses to ovalbumin, whereas B. breve has an increased humoral immune response after stimulation with IgA [15].
However, published articles regarding the immune responses of probiotics are few in number, while a number of research articles have focused on the metabolic actions of probiotics. In the treatment of various diseases, including inflammation, intestinal bowel diseases, and colon cancer, there is an urgent need to study probiotic strains and their effects on immune modulation. In this review article, particular attention has been paid to probiotics and their immunomodulatory effects on cytokine profiles in terms of pro- and anti-inflammatory cytokines in the host.
2. Background and Concept of Probiotics
In scientific and clinical research, Lactobacillus (L. acidophilus, L. casei, L. salivarius, and L. lactis) and Bifidobacterium (B. bifidum and B. lactis) are commonly found in healthy intestines and are noted as being prominent probiotics [2, 15] having different culture conditions, variability, and diverse characteristics, including sensitivity to low pH, gastric juice, pancreatic and intestinal fluids, bile acid, intestinal or respiratory mucus, adherence to intestinal cells, and interactions with other pathogenic microorganisms in the intestine [7, 8, 16]. Probiotics have been reported to produce a number of nonviable metabolic byproducts, such as bacteriocins, organic acids, acetaldehydes, diacetyl, ethanol, and hydrogen peroxide, which are nontoxic, nonpathogenic, and resistant to enzyme systems in mammals, and have been remarked on as an alternative to antibiotics due to their biological activity and inhibitory properties toward pathogenic microbes in the host [17, 18].
In the gut, probiotic microorganisms act symbiotically and modulate immunity [5, 6, 19]. Several studies have reported that probiotics also produce antioxidants (glutathione) and stimulate activity in reducing oxidative stress. Probiotic microorganisms, including L. acidophilus NCDC14 and L. casei NCDC19, inhibit lipid peroxidation and decrease streptozotocin- (STZ-) induced oxidative damage in pancreatic tissues of rats [20]. According to Sengul et al. [21], probiotic strains L. delbrueckii subsp. bulgaricus A13 and L. delbrueckii subsp. Bulgaricus B3 have a significant effect in reducing oxidative stress in colitis. A probiotic supplement containing freeze-dried beneficial strains, namely, L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, B. breve, B. longum, and S. thermophiles, has been found to increase the plasma total glutathione (GSHt) significantly [22]. However, the health benefits of probiotic microorganisms include prevention of infectious diseases of the intestinal and urinary tracts, prevention of diarrhea, reductions in allergy symptoms, serum cholesterol concentration, blood pressure, stimulation and modulation of the immune system, modulation of gene expression (cytokines, in particular), regression of tumors, and reduction in cocarcinogen production [23]. Researchers are now shifted to discerning deeper understanding of whether probiotics exert health benefits; proposed reaction mechanisms include inhibitory effects of lactate on pathogens, production of short chain fatty acids, lowering the production of toxic substances contributing to pathologies such as inflammatory bowel disease (IBD), and adherence of microbes to the gut through controlling water flow from the blood serum to the intestinal lumen [24]. Most common probiotic microorganisms are presented in Table 1.
Table 1.
Most used probiotic microorganisms.
| Probiotic genera | Probiotic strains | References |
|---|---|---|
| Lactobacillus | L. acidophilus, L. amylovorus, L. bulgaricus, L. crispatus, L. casei, L. gasseri, L. helveticus, L. johnsonii, L. pentosus, L. reuteri, L. paracasei, L. plantarum, L. rhamnosus | [17, 18, 30] |
| Bifidobacterium | B. animalis, B. breve, B. infantis, B. bifidum, B. lactis, B. catenulatum, B. longum, B. adolescentis | [31–34] |
| Enterococcus | Enterococcus faecium | [35] |
| Streptococcus | Streptococcus thermophilu | [36] |
| Lactococcus | Lactococcus lactis, L. lactis, L. reuteri, L. rhamnosus, L. casei, L. acidophilus, L. curvatus, L. plantarum | [37] |
| Bacillus | Bacillus clausii, B. coagulans, B. subtilis, B. laterosporus | [38, 39] |
| Pediococcus | Pediococcus acidilactici, P. pentosaceus | [40] |
| Propionibacterium | P. jensenii, P. freudenreichii | [30] |
| Streptococcus | Streptococcus sanguis, S. oralis, S. mitis, S. thermophiles, S. salivarius | [36] |
| Bacteroides | Bacteroides uniformis | [41] |
| Enterococcus | Enterococcus faecium | [35] |
| Peptostreptococcus | Peptostreptococcus productus | [39] |
| Escherichia | Escherichia coli Nissle 1917 | [38] |
| Faecalibacterium | Faecalibacterium prausnitzii | [42] |
| Akkermansia | A. muciniphila | [41] |
| Saccharomyces | Saccharomyces cerevisiae, S. boulardi | [43] |
In the small intestine, probiotic microorganisms ferment nondigestible carbohydrates, such as fructooligosaccharide, oligofructose, inulin, galactose, and xylose-containing oligosaccharides from various natural sources, including vegetables, fruits, and grains, and readily fulfill their energy requirements [25]. Common prebiotics used in the preparation of synbiotics and their natural sources are summarized in Table 2. In vivo, Lactobacillus casei has been found to produce significant amounts of GSHt, GSH, and -SH free groups by fermenting inulin, whereas physiological stress results in lower GSH concentrations with large amounts of oxidative stress markers [26]. Verma and Shukla [27] revealed that L. rhamnosus and L. acidophilus lead to the formation of large quantities of antioxidants, mainly GSH, with inulin being a nondigestible carbohydrate in the small intestine. In another study by Kavitha et al. [28], increased GSH concentrations have been reported due to the combined effects of insulin, pioglitazone, and probiotics in STZ-induced diabetic rats. In a very recent study, the effects of Lactobacillus plantarum HII11 are remarkable in preventing adrenomedullin- (ADM-) mediated colon cancer in the rat [29].
Table 2.
A list of common prebiotics for the preparation of synbiotics∗.
| Prebiotics | Sources | References |
|---|---|---|
| Fructooligosaccharides | Fructooligosaccharides Onion, Leek, Asparagus, Chicory, Jerusalem artichoke, Garlic, Wheat, Oat | [44] |
| Inulin | Agave, banana, burdock camas, chicory, coneflower, costus, elecampane, globe artichoke, dandelion, Jerusalem artichoke, jicama, wild yam, mugwort root, yacon, garlic, onion | [45] |
| Isomaltooligosaccharides | Miso, soy, sauce, sake, honey | [46] |
| Lactulose | Skim milk | [47] |
| Lactosucrose | Milk sugar | [48] |
| Galactooligosaccharides | Lentil, human milk, chickpea/hummus, green pea, lima bean, kidney bean | [49, 50] |
| Soybean oligosaccharides | Soybean | [51] |
| Xylooligosaccharides | Bamboo shoot, milk, honey | [34] |
| Fructooligosaccharides | Onion, chicory, garlic, asparagus, banana, artichoke | [52] |
| Arabinoxylan | Bran of grasses | [53] |
| Arabinoxylan oligosaccharides | Cereals | [54] |
| Resistant starch-1,2,3,4 | Beans, legumes, starchy fruits and vegetables, whole grains | [55] |
∗This information was reproduced from Kerry et al. [8].
3. Human Immune System
In the human gut, M cells present in Peyer's patches are crucial due to their capacity to transport macromolecules, antigens, and microorganisms and inert particles from the lumen into the lymphoid tissue by adsorptive endocytosis. In addition, enterocytes and M cells may uptake such as macromolecules, antigens, and microorganisms through a transepithelial vesicular transport mechanism. When antigenic molecules cross the intestinal barrier, they stimulate the innate and adaptive immune systems in the body [56].
3.1. Innate and Adaptive Immunity
Humans may come into contact with millions of pathogenic organisms through ingestion, inhalation, and many other ways, while the innate immune system plays a vital role in preventing infection by specific pathogen. Innate immunity is recognized as a first-line defense system against pathogens and can remember previous encounters while attacking again. Phagocytic cells, including neutrophils, monocytes, macrophages, and NK cells, enable this first-line defense system against pathogenic microorganisms in the human body, which destroys pathogens and protects from the corresponding infection. These key players are not specific in recognizing their targets, unlike adaptive immune responses. However, this first-line defense system largely depends on the number of phagocytic cells and proteins, which then activate the adaptive immune response in vertebrates through the activation of antigen-presenting cells (APCs) [57, 58].
On the other hand, development of adaptive immune responses to a new pathogen in the body is comparatively slower than innate immune responses. Usually, lymphocytes (B and T) are key players in the adaptive immune response, which exerts more effective immune responses, having specific antigen receptors, namely, the B cell receptor (BCR) for B cells and T cell receptor (TCR) for T cells [59]. Furthermore, antigen receptors of each naive lymphocyte possess unique specificity. Interestingly, B cells contribute to adaptive immunity, through the secretion of antibodies, known as humoral immunity, while T cells contribute cell-mediated immunity through subdivision into T helper cells (CD4+, called Th) and cytotoxic T cells (CD8+) [60]. It has been well known that B cells recognize specific antigens via BCRs, whereas CD8+ cells recognize antigens as peptide/MHC class I complexes, and CD4+ cells recognize antigens as peptide/MHC class II complex [61]. Once ACPs are activated, T cells proliferate and differentiate into CD8+ T cells and CD4+ T helper cells. Furthermore, CD8+ T cells convert into cytotoxic T lymphocytes (CTLs), whereas CD4+ T helper cells activate and regulate macrophages and B cells to respond to the adaptive immune system.
3.2. Mucosal Immunity
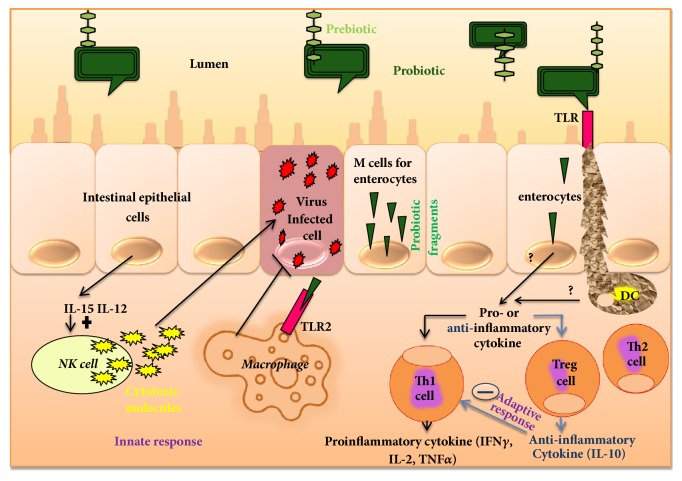
The mucosal immune system is very specific in protecting the whole inner surface, involving the oral-pharyngeal cavity, respiratory tract, gastrointestinal (GI) tract, and urogenital tract, as well as the exocrine glands in the human body. The mucosal immune system exerts similar features and anatomical organization for whole inner surface, although organs have different locations in the human body. The GI immune system can represent the mucosal immune system, as shown in Figure 1, while three major compartments, namely, the epithelial layer, lamina propria (LP), and mucosal-associated lymphoid tissue (MALT), are reported to be involved in the GI tract [62, 63]. Lymphoid tissue in the GI tract consists of Peyer's patches, which are characterized by follicle-associated epithelium (FAE) and are distributed in the intestinal epithelium and in secretory sites within the mucosa. However, the epithelial layer and lamina propria are battlefronts, while MALTs act as headquarters and initiate adaptive immune responses. APCs in Peyer's patches capture immunoglobulin A (IgA) antigen from epithelium and microfold cells. Usually, T cells become activated after antigen recognition, and, finally, APCs migrate antigen to lymphoid follicles, lymphoid tissue (LP), and mesenteric lymph nodes. IgA exerts first-line immune defense in the mucosal immune defense with two isotypes of IgA, one being IgA1 in the small intestine and the other being IgA2 in the colonic mucosa produced by B cells [64]. Nevertheless, activated T cells differentiate into effector cells and ensure the integrity of the mucosal barrier and GI environment. However, an application of Bifidobacterium bifidum R0071, Bifidobacterium infantis R0033, and Lactobacillus helveticus R0052 was reported to boost the immunity of infants with changes in salivary immunoglobulin A (SIgA) and the digestive system [65]. Probiotic fermented milk containing L. casei DN 114001was found to be effective for gut mucosal immunity in a BALC/c mouse model, with an increased number of T and IgA+B lymphocytes, macrophages, and cells from the nonspecific barrier (goblet cells), while IgA+B was also reported to activate the transcriptional factor NFAT, which is also a nuclear factor of activated T cells [66]. Dogi et al. [67] introduced nonpathogenic Gram-positive and Gram-negative bacteria in some animal models, while only Gram-positive strains, including L. acidophilus (strains CRL 1462 and A9) and L. casei (CRL 431), have been reported to increase TLR-9 expression.
Figure 1.
Immunomodulation of probiotics.
4. Immunomodulation of Cytokine Profiles
Immunomodulatory effects and clinical health benefits of probiotics have been attractive in the treatment of various degenerative diseases. Researchers are now concentrating on identifying the elite properties of probiotics, and some of these include effects on immunity, such as antipathogenicity, antiobesity and diabetic, anti-inflammatory, anticancer, antiallergic, and angiogenic activities and result in effects on the central nervous system (CNS), while efficacy largely depends on the mechanism of action. A number of studies have reported basic molecular mechanisms, such as enhanced IgA secretion, production of cytokines, production of antibacterial substances, enhanced tight junctions of the intestinal barrier against intercellular bacterial invasion, and competition with new pathogenic microorganisms for enterocyte adherence, by which probiotics regulate intestinal epithelial health, although the immunomodulatory effects of probiotics are not the same in every individual and largely depend on environment and epigenetic interactions with the host. In the immunomodulation, probiotic antigenic fragments, such as cell wall compounds, have the ability to cross the intestinal epithelial cells and M cells in Peyer's patches and to then modulate the innate and adaptive immune responses in the body, as illustrated in Figure 1 [68].
The immunomodulatory effect of probiotics is attributed to the release of cytokines, including interleukins (ILs), tumor necrosis factors (TNFs), interferons (IFNs), transforming growth factor (TGF), and chemokines from immune cells (lymphocytes, granulocytes, macrophages, mast cells, epithelial cells, and dendritic cells (DCs)) [69] which further regulate the innate and adaptive immune system [70]. It has been reported that cell wall components of Bifidobacteria and Lactobacilli, such as lipoteichoic acid, stimulate NO synthase, which is potential in pathogen-infected cell death mechanism (NO) presented by macrophages through TNF-α secretion. In addition, two surface phagocytosis receptors (FcγRIII and tool-like receptor (TLR)) are also upregulated by NO [61, 71]. Probiotics have been reported to interact with enterocytes and dendritic, Th1, Th2, and Treg cells in the intestine and to modulate the adaptive immunity into pro- and/or anti-inflammatory action. Studies with BALB/c (20–30 g) inbred mice and Fisher-344 inbred rats demonstrated that Lactobacillus paracasei subsp. Paracasei DC412 strain and L. acidophilus NCFB 1748 induced early innate immune responses and specific immune markers through phagocytosis, polymorphonuclear (PMN) cell recruitment, and TNF-alpha (TNF-α) production [72]. In another experimental animal model involving BALB/c mice, oral administration of L. casei favored rapid activation of immune cells and produced a higher number of specific markers such as CD-206 and TLR-2 cells [73], while TLRs improve the immunological defense mechanism in terms of pro- and anti-inflammatory cytokine production upon the detection of foreign objects [74].
4.1. Pro- and Anti-Inflammatory Cytokines
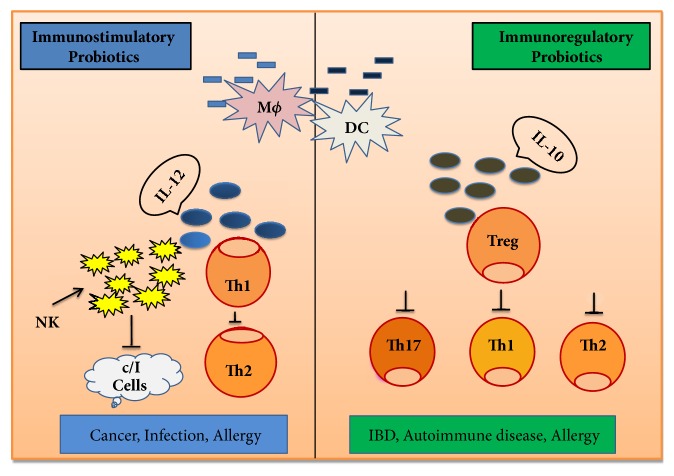
Probiotic strains have a significant influence on the gut barrier by stimulating B cells for the production of IgA. In in vitro studies with enterocyte cells (HT-29, caco-2, and dendritic cells derived from PBMC), probiotics have been reported to influence cytokine production by APCs, which initiates adaptive responses. Cytokines also enhance the defense system against invasion by bacterial, fungal, viral, and any pathogenic components. A number of researchers studying the immune system in animal models found that the importance of cytokines lies in binding to specific receptors on cell membrane and in triggering cellular cascades for the induction and improvement, as well as inhibition of several cytokine-regulated genes in the nucleus [75, 76]. The inflammatory process depends on proinflammatory and anti-inflammatory cytokines, where the anti-inflammatory cytokine, interleukin-10 (IL-10), is produced by monocytes, T cells, B cells, macrophages, natural killer cells, and dendritic cells, which inhibit proinflammatory cytokines, chemokines, and chemokine receptors, responsible for intestinal inflammation. A mechanism of immune regulation, involving two distinct categories probiotics (immunostimulatory and immunoregulatory), is shown in Figure 2. Immunostimulatory probiotics have the ability to act against infection and cancer cells, inducing IL-12 production, which activates NK cells and develops Th1 cells. These probiotics also act against allergy through a balance between Th1 and Th2. On the other hand, immunoregulatory probiotics have been characterized with IL-10 and Treg cell production, which results in decreases in allergy, IBD, autoimmune diseases, and inflammatory responses [77].
Figure 2.
Mechanism of immune regulation by probiotics.
In an in vitro study with Caco-2 cells [78], proinflammatory cytokines (IL-1β, IL-8, and TNF-α) were induced by Lactobacillus sakei, whereas Lactobacillus johnsonii influenced the production of TGF-β (anti-inflammatory). It has been revealed that IL-6 favors the clonal expansion of IgA B lymphocytes and stimulates the production of antibodies such as IgM, IgG, and reduced secretion of IgE [79]. In addition, anti-inflammatory cytokines, such as IL-4, IL-5, IL-6, IL-10, and IL-13, are produced by Th2 cells, DCs, monocytes, B cells, and Tregs and induce adaptive immune response in the body [80]. In an earlier study, Borruel et al. [81] cultured intestinal mucosa of Crohn's disease patient with nonpathogenic bacteria including Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus crispatus, and Escherichia coli to investigate bacterial modulating effects on cytokine responses. Considering a significant reduction in the proinflammatory cytokine TNF-α in inflamed mucosa cultured with L. casei and L. bulgaricus, the authors noted that probiotics interact with immunocompetent cells and modulate the production of proinflammatory cytokines. In an interleukin-10-deficient mouse model, Lactobacillus salivarius and Bifidobacterium infantis have been used to evaluate their impact on the immune system of hosts in terms of mucosal and systemic cytokine profiles [82]. Significant reductions in interferon-γ (INF-γ) and TNF-α by Peyer's patch lymphocytes and proinflammatory cytokine production by spleen cells were found in probiotic-treated mice. The administration of a mixture of L. paracasei and L. reuteri to IL-10-deficient mice infected with Helicobacter hepaticus resulted in reductions in mucosal proinflammatory cytokines and, consequently, reduced the development of colitis [83]. Yan and Polk [84] found that Lactobacillus rhamnosus GG plays a vital role in activating antiapoptotic Akt/protein kinase B and in the inhibition of proapoptotic factors through the p38 MAPK pathway. Recently, Karamese et al. [85] administrated a mixture of Lactobacillus and Bifidobacterium species to rats for evaluation of the immunomodulatory effects of probiotics, where modulation or regulation of immune responses is evident through the upregulation of IL-10 (an anti-inflammatory cytokine) and the downregulation of TNF-α and IL-6 (proinflammatory cytokines). It has also been reported that application of probiotics leads to significant increases in IgA and IgG concentrations in rats, although this is dose-dependent.
Probiotics also have potential as immunomodulators with the ability to interact with epithelium and DCs, monocytes/macrophages, and lymphocytes. Borruel et al. [81] cultured mucosal samples from Crohn's disease patients with Escherichia coli, Lactobacillus casei, Lactobacillus bulgaricus, and Lactobacillus crispatus and reported that Lactobacillus casei and Lactobacillus bulgaricus significantly reduced the production of the proinflammatory cytokine TNF-α through interaction with immunocompetent cells. The oral administration of lactic acid bacteria, such as Lactobacillus casei, L. acidophilus, L. rhamnosus, L. delbrueckii subsp. bulgaricus, L. plantarum, Lactococcus lactis, and Streptococcus thermophiles, increases the number of IgA-producing cells associated with lamina propria in mucosa and this effect is dose-dependent. It has been reported that most of the lactic acid bacteria assayed induced inflammatory immune responses, although none of them are unable to induce cytotoxicity mechanisms [86]. Livingston et al. [87] treated bone-marrow-derived dendritic cells (BMDCs) with Lactobacillus reuteri 100-23, which were then incubated with splenic T cells from ovalbumin T cell receptor transgenic mice. Anti-inflammatory cytokine IL-10, induced by BMDCs, resulted in lower IL-2 production and increased TGF-β production. Different Lactobacillus and Bifidobacterium strains demonstrate the potential to trigger epithelial cell expression of IL-10, TGF-β, and IL-6 and further stimulate the immunoglobulin production (IgA). Probiotic strains stimulate the immunoglobulin receptors of intestinal epithelial cells [88]. Various animal models suggest that the effects of probiotics on the immunomodulation of cytokines are strain-specific; therefore, combinations of different probiotic strains are beyond the scope of further study to treat inflammation-associated tissue damage and gastrointestinal inflammation in humans.
5. Conclusions
Probiotic treatment is a promising research arena in the medical sciences, since probiotics alone, or together with prebiotics, have potential in the modulation of gut microbiota and immune responses in the host. However, a number of scientific reports are identical in terms of the role of probiotics in preventing obesity, inflammatory diseases, and cancer. The immunomodulatory effects of probiotics have gained much attention for the treatment of degenerative and other diseases caused by pathogenic microorganisms. Probiotics have a positive influence on the innate immunity, exerting several antiviral properties. Furthermore, it has been established that probiotics increase gut barrier functions by stimulating B cells and by influencing cytokine production, which initiates adaptive responses in the host body, although there are insufficient research publications regarding how probiotics induce immunomodulatory effects in the treatment of inflammation. It is also urgent to understand cytokine secretion by Th2 cells, DCs, monocytes, B cells, and Tregs in order to establish new strains of probiotics. Further studies can be suggested to determine the precise action of probiotics on inflammation because these findings will be key routes in the medical sector and for better human health.
Acknowledgments
This work was supported by the National Key R&D Program of China (2016YFD0501201, 2016YFD0200900, and 2016YFD0500504) and the National Natural Science Foundation of China (31702127).
Disclosure
This review article does not contain any studies involving human participants or animals performed by any of the authors.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
Md. Abul Kalam Azad and Manobendro Sarker contributed equally to this manuscript.
References
- 1.Hill C., Guarner F., Reid G., et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Azad M. A. K., Sarker M., Li T., Yin J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Research International. 2018;2018:8. doi: 10.1155/2018/9478630.9478630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao S. C., Athalye-Jape G. K., Deshpande G. C., Simmer K. N., Patole S. K. Probiotic supplementation and late-onset sepsis in preterm infants: A meta-analysis. Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-3684.e20153684 [DOI] [PubMed] [Google Scholar]
- 4.Kristensen N. B., Bryrup T., Allin K. H., Nielsen T., Hansen T. H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Medicine. 2016;8(1, article no. 52) doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kau A. L., Ahern P. P., Griffin N. W., Goodman A. L., Gordon J. I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez-Brito M., Plaza-Díaz J., Muñoz-Quezada S., Gómez-Llorente C., Gil A. Probiotic mechanisms of action. Annals of Nutrition and Metabolism. 2012;61(2):160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 7.Bin P., Azad M. A. K., Liu G., Zhu D., Kim S. W., Yin Y. Effects of different levels of methionine on sow health and plasma metabolomics during late gestation. Food & Function. 2018;9(9):4979–4988. doi: 10.1039/C8FO01477A. [DOI] [PubMed] [Google Scholar]
- 8.George Kerry R., Patra J. K., Gouda S., Park Y., Shin H., Das G. Benefaction of probiotics for human health: A review. Journal of Food and Drug Analysis. 2018;26(3):927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Küskü-Kiraz Z., Genc S., Bekpınar S., et al. Effects of betaine supplementation on nitric oxide metabolism, atherosclerotic parameters, and fatty liver in guinea pigs fed a high cholesterol plus methionine diet. Nutrition Journal . 2018;45:41–48. doi: 10.1016/j.nut.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Tan C., Wei H., Sun H., et al. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Research International. 2015;2015:9. doi: 10.1155/2015/525218.525218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill H. S. Dietary probiotic supplementation to enhance cellular immunity in the elderly. British Journal of Biomedical Science. 2001;58:94–96. [PubMed] [Google Scholar]
- 12.Wood C., Keeling S., Bradley S., Johnson-Green P., Green-Johnson J. M. Interactions in the mucosal microenvironment: Vasoactive intestinal peptide modulates the down-regulatory action of Lactobacillus rhamnosus on LPS-induced interleukin-8 production by intestinal epithelial cells. Microbial Ecology in Health and Disease. 2007;19(3):191–200. doi: 10.1080/08910600701278722. [DOI] [Google Scholar]
- 13.Villena J., Medina M., Vintiñi E., Alvarez S. Stimulation of respiratory immunity by oral administration of Lactococcus lactis. Canadian Journal of Microbiology. 2008;54(8):630–638. doi: 10.1139/W08-052. [DOI] [PubMed] [Google Scholar]
- 14.Kankaanpää P., Sütas Y., Salminen S., Isolauri E. Homogenates derived from probiotic bacteria provide down-regulatory signals for peripheral blood mononuclear cells. Food Chemistry. 2003;83(2):269–277. doi: 10.1016/S0308-8146(03)00090-6. [DOI] [Google Scholar]
- 15.Bodera P., Chcialowski A. Immunomodulatory effect of probiotic bacteria. Recent Patents on Inflammation & Allergy Drug Discovery. 2009;3(1):58–64. doi: 10.2174/187221309787158461. [DOI] [PubMed] [Google Scholar]
- 16.Azad M. A. K., Bin P., Liu G., Fang J., Li T., Yin Y. Effects of different methionine level on offspring piglets during late gestation and lactation. Food & Function. 2018 doi: 10.1039/c8fo01343h. [DOI] [PubMed] [Google Scholar]
- 17.Islam S. U. Clinical Uses of Probiotics. Medicine. 2016;95(5):p. e2658. doi: 10.1097/MD.0000000000002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ooi M. F., Mazlan N., Foo H. L., et al. Effects of carbon and nitrogen sources on bacteriocin-inhibitory activity of postbiotic metabolites produced by Lactobacillus plantarum I-UL4. Malaysian Journal of Microbiology. 2015;11(2):176–184. [Google Scholar]
- 19.Topping D. L., Clifton P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiological Reviews. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 20.Yadav H., Jain S., Sinha P. R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. Journal of Dairy Research. 2008;75(2):189–195. doi: 10.1017/S0022029908003129. [DOI] [PubMed] [Google Scholar]
- 21.Şengul N., Isik S., Aslim B., Ucar G., Demirbag A. E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Digestive Diseases and Sciences. 2011;56(3):707–714. doi: 10.1007/s10620-010-1362-7. [DOI] [PubMed] [Google Scholar]
- 22.Asemi Z., Zare Z., Shakeri H., Sabihi S.-S., Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Annals of Nutrition and Metabolism. 2013;63(1-2):1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 23.Patel R. M., Denning P. W. Therapeutic Use of Prebiotics, Probiotics, and Postbiotics to Prevent Necrotizing Enterocolitis. What is the Current Evidence? Clinics in Perinatology. 2013;40(1):11–25. doi: 10.1016/j.clp.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saadatzadeh A. Biochemical and pathological evidences on the benefit of a new biodegradable nanoparticles of probiotic extract in murine colitis. Fundamental & Clinical Pharmacology. 2012;26(5):589–598. doi: 10.1111/j.1472-8206.2011.00966.x. [DOI] [PubMed] [Google Scholar]
- 25.Hutkins R. W., Krumbeck J. A., Bindels L. B., et al. Prebiotics: why definitions matter. Current Opinion in Biotechnology. 2016;37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleniewska P., Pawliczak R. Influence of Synbiotics on Selected Oxidative Stress Parameters. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/9315375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma A., Shukla G. Synbiotic (Lactobacillus rhamnosus+Lactobacillus acidophilus+inulin) attenuates oxidative stress and colonic damage in 1,2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague'Dawley rats: A long-term study. European Journal of Cancer Prevention. 2014;23(6):550–559. doi: 10.1097/CEJ.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 28.Kavitha K., Reddy A. G., Reddy K. K., Kumar C. S. V. S., Boobalan G., Jayakanth K. Hypoglycemic, hypolipidemic and antioxidant effects of pioglitazone, insulin and synbiotic in diabetic rats. Veterinary World. 2016;9(2):118–122. doi: 10.14202/vetworld.2016.118-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaiyasut C., Pattananandecha T., Sirilun S., Suwannalert P., Peerajan S., Sivamaruthi B. S. Synbiotic preparation with lactic acid bacteria and inulin as a functional food: In vivo evaluation of microbial activities, and preneoplastic aberrant crypt foci. Food Science and Technology. 2017;37(2):328–336. doi: 10.1590/1678-457x.26216. [DOI] [Google Scholar]
- 30.Dixit Y., Wagle A., Vakil B. Patents in the Field of Probiotics, Prebiotics, Synbiotics: A Review. Journal of Food: Microbiology Safety & Hygiene. 2016:01–02. [Google Scholar]
- 31.Westermann C., Gleinser M., Corr S. C., Riedel C. U. A Critical Evaluation of Bifidobacterial Adhesion to the Host Tissue. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartosch S., Woodmansey E. J., Paterson J. C. M., McMurdo M. E. T., Macfarlane G. T. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clinical Infectious Diseases. 2005;40(1):28–37. doi: 10.1086/426027. [DOI] [PubMed] [Google Scholar]
- 33.Macfarlane G. T., Steed H., Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. Journal of Applied Microbiology. 2008;104(2):305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 34.Aachary A. A., Prapulla S. G. Xylooligosaccharides (XOS) as an Emerging Prebiotic: Microbial Synthesis, Utilization, Structural Characterization, Bioactive Properties, and Applications. Comprehensive Reviews in Food Science and Food Safety. 2011;10(1):2–16. doi: 10.1111/j.1541-4337.2010.00135.x. [DOI] [Google Scholar]
- 35.Onyenweaku F., et al. Health benefits of probiotics. International Journal of Innovative and Applied Research. 2016;4(3):21–30. [Google Scholar]
- 36.Arora T., Singh S., Sharma R. K. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition Journal . 2013;29(4):591–596. doi: 10.1016/j.nut.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 37.El Jakee J., Eid R., Rashidy A. Potential Antimicrobial Activities of Probiotic Lactobacillus Strains Isolated from Raw Milk. Journal of Probiotics & Health. 2016:04–02. [Google Scholar]
- 38.European Food Safety Authority (EFSA) Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017 update) EFSA Journals. 2017;15:1–177. doi: 10.2903/j.efsa.2017.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen H.-T., Truong D.-H., Kouhoundé S., Ly S., Razafindralambo H., Delvigne F. Biochemical engineering approaches for increasing viability and functionality of probiotic bacteria. International Journal of Molecular Sciences. 2016;17(6, article no. 867) doi: 10.3390/ijms17060867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sornplang P., Piyadeatsoontorn S. Probiotic isolates from unconventional sources: a review. Journal of Animal Science and Technology. 2016;58(1) doi: 10.1186/s40781-016-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobyliak N., Conte C., Cammarota G., et al. Probiotics in prevention and treatment of obesity: a critical view. Nutrition & Metabolism. 2016;13(1) doi: 10.1186/s12986-016-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgetti G., Brandimarte G., Fabiocchi F., Ricci S., Flamini P., Sandri G., et al. Interactions between Innate Immunity, Microbiota, and Probiotics. Journal of Immunology Research. 2015;2015:7. doi: 10.1155/2015/501361.501361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Yang G., Song J. H., et al. Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064227.e64227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabater-Molina M., Larqué E., Torrella F., Zamora S. Dietary fructooligosaccharides and potential benefits on health. Journal of Physiology and Biochemistry. 2009;65(3):315–328. doi: 10.1007/bf03180584. [DOI] [PubMed] [Google Scholar]
- 45.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel S., Goyal A. The current trends and future perspectives of prebiotics research: a review. 3 Biotech. 2012;2(2):115–125. doi: 10.1007/s13205-012-0044-x. [DOI] [Google Scholar]
- 47.De Souza Oliveira R. P., Rodrigues Florence A. C., Perego P., De Oliveira M. N., Converti A. Use of lactulose as prebiotic and its influence on the growth, acidification profile and viable counts of different probiotics in fermented skim milk. International Journal of Food Microbiology. 2011;145(1):22–27. doi: 10.1016/j.ijfoodmicro.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X., Ruan Z., Huang X., Zhou Y., Liu S., Yin Y. The prebiotic lactosucrose modulates gut metabolites and microbiota in intestinal inflammatory rats. Food Science and Biotechnology. 2014;23(1):157–163. doi: 10.1007/s10068-014-0021-8. [DOI] [Google Scholar]
- 49.Torres D. P. M., Gonçalves M. D. P. F., Teixeira J. A., Rodrigues L. R. Galacto-Oligosaccharides: Production, properties, applications, and significance as prebiotics. Comprehensive Reviews in Food Science and Food Safety. 2010;9(5):438–454. doi: 10.1111/j.1541-4337.2010.00119.x. [DOI] [PubMed] [Google Scholar]
- 50.Vulevic J., Juric A., Tzortzis G., Gibson G. R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults1-3. Journal of Nutrition. 2013;143(3):324–331. doi: 10.3945/jn.112.166132. [DOI] [PubMed] [Google Scholar]
- 51.Rycroft C. E., Jones M. R., Gibson G. R., Rastall R. A. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. Journal of Applied Microbiology. 2001;91(5):878–887. doi: 10.1046/j.1365-2672.2001.01446.x. [DOI] [PubMed] [Google Scholar]
- 52.Moro G., Arslanoglu S., Stahl B., Jelinek J., Wahn U., Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Archives of Disease in Childhood. 2006;91(10):814–819. doi: 10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Barz M., Anhê F. F., Varin T. V., et al. Probiotics as Complementary Treatment for Metabolic Disorders. Diabetes & Metabolism Journal. 2015;39(4):p. 291. doi: 10.4093/dmj.2015.39.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grootaert C., van den Abbeele P., Marzorati M., et al. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiology Ecology. 2009;69(2):231–242. doi: 10.1111/j.1574-6941.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 55.Fuentes-Zaragoza E., Sánchez-Zapata E., Sendra E., et al. Resistant starch as prebiotic: A review. Starch - Stärke. 2011;63(7):406–415. doi: 10.1002/star.201000099. [DOI] [Google Scholar]
- 56.Snoeck V., Goddeeris B., Cox E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes and Infection. 2005;7(7-8):997–1004. doi: 10.1016/j.micinf.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Alberts B. Pathogens, infection, and innate immunity. In: Anderson M., Granum S., editors. Molecular Biology of the Cell. 5th. New York, NY, USA: Garland Science, Taylor & Francis Group; 2008. pp. 1485–1537. [Google Scholar]
- 58.Gourbeyre P., Denery S., Bodinier M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. Journal of Leukocyte Biology. 2011;89(5):685–695. doi: 10.1189/jlb.1109753. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Trincado J. L., Gomez-Perosanz M., Reche P. A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. Journal of Immunology Research. 2017;2017:14. doi: 10.1155/2017/2680160.2680160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molero-Abraham M., Glutting J.-P., Flower D. R., Lafuente E. M., Reche P. A. EPIPOX: Immunoinformatic Characterization of the Shared T-Cell Epitome between Variola Virus and Related Pathogenic Orthopoxviruses. Journal of Immunology Research. 2015;2015 doi: 10.1155/2015/738020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delcenserie V., et al. Immunomodulatory Effects of Probiotics in the Intestinal Tract. Current Issues in Molecular Biology. doi: 10.21775/cimb.010.037. [DOI] [PubMed] [Google Scholar]
- 62.van Wijk F., Cheroutre H. Intestinal T cells: Facing the mucosal immune dilemma with synergy and diversity. Seminars in Immunology. 2009;21(3):130–138. doi: 10.1016/j.smim.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy K. P. Janeway’s immunobiology. 8th. London/New York: Garland Science, Taylor & Francis Group; 2011. [Google Scholar]
- 64.Otten M. A., van Egmond M. The Fc receptor for IgA (FcαRI, CD89) Immunology Letters. 2004;92(1-2):23–31. doi: 10.1016/j.imlet.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Xiao L., Ding G., Ding Y., et al. Effect of probiotics on digestibility and immunity in infants. Medicine. 2017;96(14):p. e5953. doi: 10.1097/MD.0000000000005953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galdeano C. M., De Moreno De Leblanc A., Carmuega E., Weill R., Perdigón G. Mechanisms involved in the immunostimulation by probiotic fermented milk. Journal of Dairy Research. 2009;76(4):446–454. doi: 10.1017/S0022029909990021. [DOI] [PubMed] [Google Scholar]
- 67.Dogi C. A., Weill F., Perdigón G. Immune response of non-pathogenic Gram(+) and Gram(-) bacteria in inductive sites of the intestinal mucosa. Study of the pathway of signaling involved. Immunobiology. 2010;215(1):60–69. doi: 10.1016/j.imbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Galdeano C. M., Perdigón G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. Journal of Applied Microbiology. 2004;97(4):673–681. doi: 10.1111/j.1365-2672.2004.02353.x. [DOI] [PubMed] [Google Scholar]
- 69.Savan R., Sakai M. Genomics of fish cytokines. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2006;1(1):89–101. doi: 10.1016/j.cbd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Foligné B., Dewulf J., Breton J., Claisse O., Lonvaud-Funel A., Pot B. Probiotic properties of non-conventional lactic acid bacteria: Immunomodulation by Oenococcus oeni. International Journal of Food Microbiology. 2010;140(2-3):136–145. doi: 10.1016/j.ijfoodmicro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Schwandner R., Dziarski R., Wesche H., Rothe M., Kirschning C. J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. The Journal of Biological Chemistry. 1999;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 72.Kourelis A., Zinonos I., Kakagianni M., et al. Validation of the dorsal air pouch model to predict and examine immunostimulatory responses in the gut. Journal of Applied Microbiology. 2010;108(1):274–284. doi: 10.1111/j.1365-2672.2009.04421.x. [DOI] [PubMed] [Google Scholar]
- 73.Galdeano C. M., Perdigón G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clinical and Vaccine Immunology. 2006;13(2):219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson K. V. Toll signaling pathways in the innate immune response. Current Opinion in Immunology. 2000;12(1):13–19. doi: 10.1016/S0952-7915(99)00045-X. [DOI] [PubMed] [Google Scholar]
- 75.Mulder I. E., Wadsworth S., Secombes C. J. Cytokine expression in the intestine of rainbow trout (Oncorhynchus mykiss) during infection with Aeromonas salmonicida. Fish and Shellfish Immunology. 2007;23(4):747–759. doi: 10.1016/j.fsi.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Biswas G., Korenaga H., Nagamine R., et al. Elevated cytokine responses to vibrio harveyi infection in the japanese pufferfish (Takifugu rubripes) treated with lactobacillus paracasei spp. paracasei (06TCa22) isolated from the mongolian dairy product. Fish and Shellfish Immunology. 2013;35(3):756–765. doi: 10.1016/j.fsi.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Chiba Y., Shida K., Nagata S., et al. Well-controlled proinflammatory cytokine responses of Peyer's patch cells to probiotic Lactobacillus casei. The Journal of Immunology. 2010;130(3):352–362. doi: 10.1111/j.1365-2567.2009.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haller D., Bode C., Hammes W. P., Pfeifer A. M. A., Schiffrin E. J., Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47(1):79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galdeano C. M., De Moreno De Leblanc A., Vinderola G., Bibas Bonet M. E., Perdigón G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clinical and Vaccine Immunology. 2007;14(5):485–492. doi: 10.1128/cvi.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 81.Borruel N., Carol M., Casellas F., et al. Increased mucosal tumour necrosis factor α production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51(5):659–664. doi: 10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCarthy J., O'Mahony L., O'Callaghan L., et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52(7):975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peña J. A., Rogers A. B., Ge Z., et al. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infection and Immunity. 2005;73(2):912–920. doi: 10.1128/IAI.73.2.912-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan F., Polk D. B. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. The Journal of Biological Chemistry. 2002;277(52):50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karamese M., Aydin H., Sengul E., et al. The Immunostimulatory Effect of Lactic Acid Bacteria in a Rat Model. Iranian journal of immunology : IJI. 2016;13(3):220–228. [PubMed] [Google Scholar]
- 86.Vitiñi E., Alvarez S., Medina M., Medici M., de Budeguer M. V., Perdigón G. Gut mucosal immunostimulation by lactic acid bacteria. Biocell. 2000;24(3):223–232. [PubMed] [Google Scholar]
- 87.Livingston M., Loach D., Wilson M., Tannock G. W., Baird M. Gut commensal Lactobacillus reuteri 100–23 stimulates an immunoregulatory response. Immunology & Cell Biology. 2010;88(1):99–102. doi: 10.1038/icb.2009.71. [DOI] [PubMed] [Google Scholar]
- 88.Reséndiz-Albor A. A., Reina-Garfias H., Rojas-Hernández S., et al. Regionalization of pIgR expression in the mucosa of mouse small intestine. Immunology Letters. 2010;128(1):59–67. doi: 10.1016/j.imlet.2009.11.005. [DOI] [PubMed] [Google Scholar]