Abstract
Objective
There are limited evidence-based published heart rate ranges for premature neonates. We determined heart rate ranges in premature neonates based on gestational and post-menstrual age.
Study Design
Retrospective observational study of premature neonates admitted to the neonatal intensive care unit at the University of Virginia between January 2009 and October 2015. We included gestational ages between 23 0/7 weeks and 34 6/7 weeks. We stratified data by gestational and post-menstrual age groups.
Results
Over two billion heart rate values in 1703 neonates were included in our study. We established percentile-based reference ranges based on gestational and post-menstrual age. Our results demonstrate a slight increase in the initial weeks after birth, followed by a gradual decline with age. The baseline heart rate is lower with advancing gestational age.
Conclusions
Knowing heart rate reference ranges in the premature neonatal population can be helpful in the bedside assessment of the neonate.
Introduction
In 2015, 383,128 premature neonates (< 37 weeks gestation) were born in the United States [1]. Premature neonates often require care in a neonatal intensive care unit (NICU) where routine measurement of heart rate (HR) is performed. Published guidelines exist for normal reference ranges in healthy term neonates [2–4], but not in premature neonates.
Clinical decision-making is often influenced by the presence of abnormal vital signs, so knowing what is truly normal is paramount. Identifying and using accurate, evidenced-based ranges can appropriately guide clinical care decisions and perhaps improve management strategies and avoid unnecessary treatments. We sought to establish HR reference ranges in premature neonates based on gestational age (GA) and post-menstrual age (PMA) in a large cohort of neonates.
Patients and methods
The Institutional Review Board at the University of Virginia School of Medicine approved this study. We performed a retrospective observational cohort study of all neonates admitted to the NICU at the University of Virginia Children’s Hospital between January 2009 and October 2015. We included neonates with GAs between 23 0/7 weeks and 34 6/7 weeks. There were no exclusion criteria. We collected HR data on a dedicated computer cluster. HR values were captured every 2 seconds from the bedside monitor, and analyzed in all neonates until a PMA of 38 6/7 weeks.
Values of HR were stratified by both GA and PMA. Average values for each stratum were calculated and used to generate a heat map. We further sub-divided the data into four clinically relevant GA groups: 23 0/7–25 6/7 weeks (group 1), 26 0/7–28 6/7 weeks (group 2), 29 0/7–31 6/7 weeks (group 3), and 32–34 6/7 weeks (group 4). Percentile curves and tables were constructed for each group.
Results
A total of 2,002,756,757 data points from 1703 premature neonates were included in our study. The median GA was 31 weeks (interquartile range 28–33 weeks). Table 1 shows patient demographics including GA, sex, birth weight, size, race/ethnicity, and delivery type.
Table 1.
Patient demographics are shown
| Characteristic | Number (%) |
|---|---|
| Gestational age | |
| 23 0/7–25 6/7 weeks | 212 (12%) |
| 26 0/7–28 6/7 weeks | 259 (15%) |
| 29 0/7–31 6/7 weeks | 392 (23%) |
| 32–34 6/7 weeks | 840 (49%) |
| Total | 1703 |
| Sex | |
| Male | 927 (54%) |
| Female | 776 (46%) |
| Birth weight | |
| ELBW (< 1000 g) | 349 (20%) |
| VLBW (< 1500 g) | 388 (23%) |
| LBW (< 2500 g) | 849 (50%) |
| BW> = 2500 g | 117 (7%) |
| Birth size | |
| AGA | 957 (56%) |
| LGA | 36 (2%) |
| SGA | 161 (10%) |
| Unknown | 549 (32%) |
| Race/ethnicity | |
| Caucasian | 1145 (67%) |
| African American | 348 (21%) |
| Hispanic | 89 (5%) |
| Asian | 18 (1%) |
| Other | 53 (3%) |
| Unknown | 50 (3%) |
| Delivery type | |
| Vaginal delivery | 670 (39%) |
| Elective Cesarean Section | 114 (7%) |
| Urgent Cesarean Section | 630 (37%) |
| Emergent Cesarean Section | 230 (14%) |
| Unknown | 59 (3%) |
SGA defined as birth weight < 10th percentile, AGA defined as birth weight between 10–90th percentile, and LGA defined as birth weight > 10th percentile
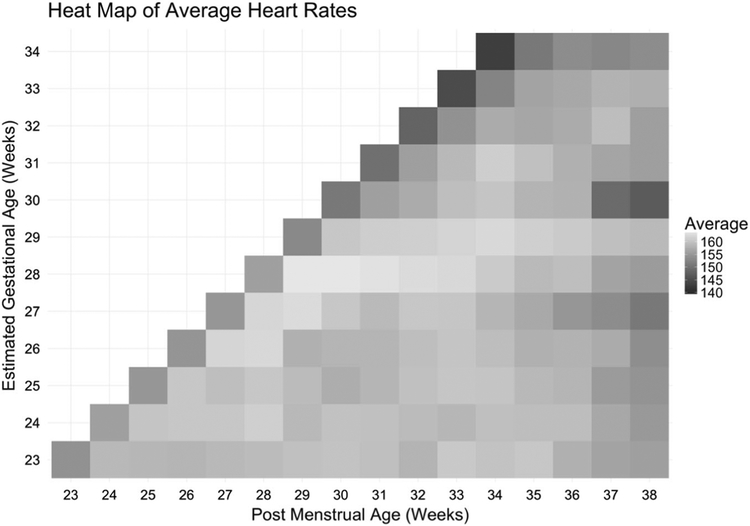
We plotted average HR values for each GA/PMA strata to create a heat map (Fig. 1). This demonstrates a slight increase in HR after birth followed by a small and gradual decline with PMA. It also demonstrates a lower baseline HR with advancing GA.
Fig. 1.
Heat map of average heart rates based on gestational age and post-menstrual age
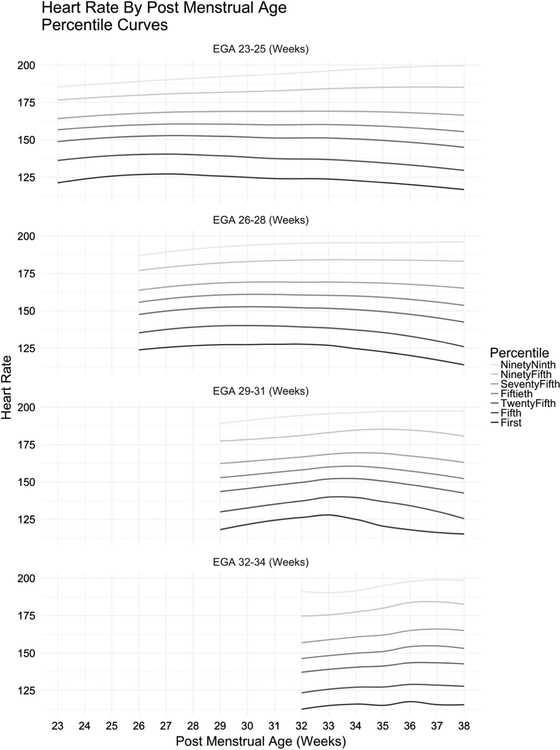
Figure 2 shows the HR percentiles for each GA group with the 5th–95th percentiles displayed as a function of PMA, demonstrating an overall HR increase during the initial weeks after birth followed by a gradual decline with age. Table 2 shows a HR reference table with 5th and 95th percentile data points listed.
Fig. 2.
Heart rate percentiles for each gestational age group displayed as a function of post-menstrual age
Table 2.
Heart rate percentile reference table is shown
| Post-menstrual age | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational age | HR percentile | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 |
| 23 | 5th | 135 | 139 | 139 | 140 | 140 | 141 | 141 | 139 | 137 | 137 | 139 | 139 | 136 | 133 | 130 | 129 |
| 95th | 177 | 179 | 180 | 180 | 181 | 180 | 181 | 184 | 183 | 182 | 186 | 185 | 187 | 188 | 187 | 189 | |
| 24 | 5th | 139 | 140 | 141 | 141 | 141 | 138 | 138 | 138 | 137 | 135 | 135 | 132 | 133 | 131 | 130 | |
| 95th | 175 | 181 | 181 | 181 | 183 | 182 | 182 | 184 | 185 | 184 | 184 | 185 | 186 | 184 | 185 | ||
| 25 | 5th | 137 | 142 | 141 | 141 | 138 | 136 | 137 | 138 | 135 | 137 | 135 | 132 | 132 | 131 | ||
| 95th | 176 | 180 | 180 | 181 | 181 | 181 | 181 | 182 | 184 | 184 | 184 | 184 | 182 | 182 | |||
| 26 | 5th | 134 | 143 | 143 | 137 | 140 | 137 | 137 | 139 | 139 | 135 | 135 | 134 | 127 | |||
| 95th | 176 | 182 | 183 | 181 | 181 | 183 | 184 | 184 | 183 | 182 | 182 | 184 | 182 | ||||
| 27 | 5th | 134 | 143 | 144 | 141 | 139 | 140 | 138 | 135 | 134 | 131 | 131 | 124 | ||||
| 95th | 178 | 182 | 184 | 182 | 183 | 184 | 183 | 184 | 185 | 183 | 184 | 184 | |||||
| 28 | 5th | 131 | 140 | 141 | 142 | 140 | 140 | 138 | 136 | 133 | 128 | 125 | |||||
| 95th | 176 | 184 | 184 | 184 | 185 | 185 | 185 | 186 | 185 | 184 | 183 | ||||||
| 29 | 5th | 130 | 141 | 142 | 141 | 143 | 142 | 139 | 137 | 136 | 134 | ||||||
| 95th | 177 | 182 | 182 | 183 | 185 | 185 | 186 | 186 | 185 | 185 | |||||||
| 30 | 5th | 126 | 135 | 137 | 139 | 140 | 135 | 134 | 132 | 128 | |||||||
| 95th | 176 | 179 | 180 | 182 | 184 | 184 | 183 | 176 | 172 | ||||||||
| 31 | 5th | 123 | 134 | 137 | 139 | 135 | 130 | 126 | 113 | ||||||||
| 95th | 176 | 180 | 182 | 185 | 186 | 185 | 188 | 185 | |||||||||
| 32 | 5th | 123 | 133 | 136 | 135 | 133 | 130 | 127 | |||||||||
| 95th | 175 | 178 | 182 | 183 | 185 | 184 | 178 | ||||||||||
| 33 | 5th | 119 | 130 | 133 | 133 | 132 | 131 | ||||||||||
| 95th | 172 | 179 | 184 | 186 | 185 | 182 | |||||||||||
| 34 | 5th | 117 | 127 | 128 | 128 | 129 | |||||||||||
| 95th | 172 | 179 | 183 | 184 | 185 | ||||||||||||
Gestational age and post-menstrual age are shown in weeks.
Discussion
There are limited evidenced-based HR reference ranges available for the premature neonatal population. By providing a heat map, percentile curves, and a reference table for this patient population, clinicians will be more informed of appropriate HR ranges, which can potentially lead to improvement in management strategies and more prompt identification of patients in need of immediate medical attention.
Considerable attention has been placed in recent years on the issue of alarm fatigue. Although determining appropriate lower limits for alarm settings in the neonatal population was outside the scope of this project, our data provides some interesting insights that may inform future study. For example, among neonates with PMA of 38 weeks, irrespective of GA, we found the 1st percentile and 0.1st percentile of HRs to be 95 and 72, respectively. Efforts to evaluate the impact of different lower limits on alarm fatigue and response to clinically significant events are needed.
This study has substantial strengths. We have included over two billion data points in our analysis and our patient population includes GAs typically seen in NICUs. There are some additional limitations as well. Our patient demographics, such as race, are not representative of all other institutions. It is possible some of these demographic factors unknowingly influence HR values. It is also possible that confounders other than vasoactive use and supplemental oxygen exist in this dataset.
Analysis of HR values from a large cohort of premature neonates has provided a useful heat map, percentile curves, and a reference tables in this vulnerable population. These results can be used at the bedside while assessing the critically ill neonate. Additional research efforts will focus on the development of an interactive website that will be easily accessible to all care providers.
Acknowledgements
We acknowledge Karen Fairchild and Robert Sinkin for their helpful insights into the completion of this study.
Footnotes
Compliance with ethical standards
Conflict of interest Randall Moorman, MD, is the Chief Medical Officer of Advanced Medical Predictive Devices, Diagnostics, and Displays. All other authors declare that they have no conflict of interest.
References
- 1.Martin JA, Hamilton BE, Osterman MJKS, Driscoll AK, Mathews TJ. National Vital Statistics Reports. 2017;66. [PubMed] [Google Scholar]
- 2.Organization WH. WHO Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. Department of Maternal, Newborn, Child, and Adolescent Health (MCA) Switzerland; World Health Organization; 1991;1–24. [Google Scholar]
- 3.Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet (Lond, Engl). 2011;377:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chameides L, Samson RA, Schexnayder SM, Hazinski MF. Pediatric advanced life support: provider manual. Profession. American Heart Association; Dallas: 2012. [Google Scholar]