Highlights
-
•
We expressed in E. coli, refolded and purified keratin K31, which is known to be a major protein degraded and eluted in hair damage.
-
•
Analysis of the recombinant protein by MALDI-TOF, CD spectroscopy and molecular modelling showed its similarity to the native protein.
-
•
Marked improvements in thickness, strength and smoothness on application of this protein on damaged hair has been shown for the first time.
Keywords: Keratin K31, Escherichia coli, Hair cosmetics, Hair treatment, Computer modeling
Abstract
Hair, being one of the most important components of the beauty care processes, attracts the use of a variety of hair treating cosmetics. Conventional hair treating cosmetics are not satisfactory for one reason or the other. Commercially used keratins are isolated from wool or chicken feathers. As these lack complete sequence identity with human hair keratin, are likely to be less efficient than the human hair keratin. K31, a type I acidic keratin, is a major protein of human hair keratin complex and it is essential for maintaining the hair tensile strength. In this study keratin K31 (46 kDa) gene was expressed in Escherichia coli at a level of approximately 35% of the total cell proteins. The protein, however, was expressed as insoluble inclusion bodies. The expressed protein was refolded and purified by FPLC using an anion-exchange column. The CD analysis results showed that the K31 was properly refolded. MALDI-TOF mass spectroscopy showed the characteristics expected for human K31 keratin. The refolded and partially purified K31 protein, when applied on chemically damaged hairs, increased the diameter of the hair up to 49%. The mechanical strength of the bleached hair increased by almost 2 fold after a single treatment of K31. The protein also straightened curly hair efficiently on a single treatment for one hour. Application of K31 resulted in marked improvements in smoothness, diameter and mechanical strength of the damaged hair.
1. Introduction
Keratin, a basic building block of the complex morphological structure of hair and feathers, has been commonly used in hair improvement cosmetics. However, at industrial level the keratins used are obtained mostly from non-human sources like chicken feather, wool, etc. [1,2]. Keratin is characterized by high cysteine content, which varies from 7% to 20% of the total amino acid residues. Formation of intra and intermolecular disulfide linkages largely accounts for toughness and mechanical strength of hair [3,4].
Two types of keratin compound the human hair. i.e. type I (acidic) and type II (basic). Type II keratin is differentiated into six groups, which are Keratin K81-K86 (Hhb1-Hshb2). The molecular weight of these keratins ranges from 54 to 57 kDa. Type I keratins are differentiated into 10 groups i.e. keratin K31-K40. The molecular weight of these keratins range from 46 to 55 kDa. Type I acidic keratins are characterized by a large number of cysteine as well as proline residues [5].
Hair fibers have three basic morphological components [6], which are cuticle [7], cortex [8], and medulla [9]. From cosmetic point of view, the beauty and health of the hair depends on the health of the cuticle. Most hair cosmetic products and hair treatments initially affect the cuticle, changing the appearance, softness and even texture of hair [10].
Keratin K31, a major component of hair cortex, contributes to the strength of the hair shaft [11]. Chemical treatment of hair, such as bleaching, causes damage by penetrating deep within the hair fiber and cleaving keratin K31, which results in decreased tensile strength.
Application of keratin to restore the natural characteristics and youthfulness of hair has been a growing trend [12]. Keratin from chicken feather or other animal sources extracted using chemical methods, either in the intact form or its hydrolyzed product have been used in the commercial cosmetic products [1]. However, the intact protein has important implications in restoring or improving the mechanical strength of the damaged hair fibers [13,14].
Human hair keratin K31, selected in this study, is type I acidic keratin of 46 kDa. Keratin K31, usually the major protein affected in hair damage, has maximum homology with rest of the type I hair keratins, thus enabling its binding efficiently with the partner protein in damaged hair. The objective of this study was to produce recombinant K31 in a native form and study its role in the treatment of naturally and chemically damaged hairs.
2. Material and methods
2.1. Bacterial strains, vectors and culture media
E. coli DH5α was used for cloning and vector propagation and E. coli BL21 CodonPlus (RIL) was used for the gene expression. DNA encoding Keratin K31 (Gene ID: 3881) was synthesized in pEX-K cloning vector (Eurofins Genomics, Wolverhampton, UK) along with NdeI and EcoRI restriction sites at the 5′ and 3′ end, respectively. T7 promoter based pET-22b (+) was used as the expression vector (Novagen, Madison, USA). LB medium was used for growth of E. coli cells as initial culture. Agar plates supplemented with 100 μg ml−1 ampicillin were used for selection of transformed colonies. M9NG medium [15] was used for expression of keratin K31.
2.2. Cloning of keratin K31 gene
The recombinant cloning vector pEX-K was double digested with NdeI and EcoRI and ligated into linearized pET-22b (+) using T4 DNA ligase followed by transformation initially into E. coli DH5α cells using the standard protocol [16]. The transformants were analyzed by colony PCR to confirm correctness of the inserts, using the forward primer 5ʹ-CATATGCCCTACAACTTTTGCCTGC-3ʹ and the reverse primer 5ʹ-GGAGCTCGAATTCCTAGCGCAC-3ʹ.
2.3. Protein expression and purification
The inoculum was prepared by cultivation of the selected transformant in LB broth containing 100 μg ml−1 ampicillin to an OD600 ∼1.0. After inoculation in M9NG medium, containing lactose as inducer, the culture was incubated overnight in an orbital shaker at 37 °C, achieving an OD600nm 10.0. The cells were harvested by centrifugation, re-suspended in 0.05 M Tris buffer (pH 6.0) and lysed by sonication using a UP400S Ultraschall prozessor (Dr. Heilscher GmbH, Teltow, Germany) [17,18]. The sonicated mixture was centrifuged at 7000 rpm (8983 x G) for 30 min K31 expressed as inclusion bodies were washed thrice with wash buffer (pH 7.5) containing 0.05 M Tris-Cl, 5 mM EDTA and 0.5% Triton-X100 to remove the cell debris. The washed inclusion bodies were solubilized in a solution containing 1 M urea and 2 mM DTT in 0.05 M Tris buffer (pH 12.0). Urea and other reagents were removed by dialysis against 0.05 M Tris buffer, lowering the pH to 7.5 in a step-wise manner [19].
The refolded K31protein was purified by anion exchange chromatography using Hi Trap QFF- 1 ml column on fast performance liquid chromatography (FPLC) system, AKTA purifier (GE healthcare, United Kingdom). The column was equilibrated with 2 column volume of 0.05 M Tris-Cl (pH 7.5). The bound protein was eluted with a continuous NaCl gradient (0.1–1 M) prepared in Tris buffer at a flow rate of 1 ml per minute. The eluted fractions containing the desired protein, as shown by SDS-PAGE analysis, were pooled.
2.4. Circular dichroism analysis
Secondary structure analysis of the refolded and purified K31 was done by circular dichorism spectroscopy using Chirascan plus CD spectrophotometer (Applied Photophysics, Leatherhead surrey, UK). The CD was calibrated with 0.05 M Tris-Cl (pH 7.5) before loading the sample. The K31 protein solution with protein concentration of 0.27 mg ml−1 was scanned at wavelength range 190–260 nm at 37 °C using a quartz cell of 0.5 mm path length. Each spectrum obtained was the average of two consecutive scans with a 1 nm bandwidth. The secondary structure composition was calculated from CD spectrum analysis using deconvolution software CDNN [20]. The CD spectroscopic data was compared with that obtained from the 3D structure produced through a computer based modelling tool, I-TASSER [21] as well as the secondary structure data obtained using on-line tool 2Struc [22].
2.5. Mass spectroscopic analysis
Tryptic digest of the purified keratin K31 was prepared by treatment with sequencing grade trypsin (Promega, V511 A) by the previously reported protocol with some modifications [23]. The Tryptic digest thus obtained was analyzed by MALDI-TOF mass spectrometry. The peptide mass thus obtained were searched against the SWISS PROT and NCBI protein database using the MASCOT peptide mass fingerprint program from Matrix Science (http://www.matrixscience.com/wizard.html) and were further confirmed by MS-Fit program from Protein Prospector (http://prospector.ucsf.edu). A MASCOT score greater than 67 is significant (p < 0.05), whereas protein identification with scores lower than 67 are considered positive if they also showed a minimum of six matched peptides or a sequence coverage of over 20%.
2.6. Hair treatment with K31
Strands of hairs were damaged chemically by treating with commonly used chemical for hairdressing such as bleach. The hair sample after treating with 10% bleach solution [24] for 1 h in shaking incubator was rinsed with water and then air dried. For comparison, unbleached hairs were used as a control.
Purified K31 was applied on both the bleach-treated and the untreated hairs. Five fibers from each of the hair tress were randomly selected and incubated in 15 ml 0.05 M Tris-Cl buffer (pH 7.5) containing 0.2 mg ml−1 K31protein in a petri plate for 1 h in an orbital shaker at 120 rpm. The hairs were then rinsed three times with water and air-dried.
To determine the straightening ability of K31, 60–80 hairs from a curly tress of average 10 cm length was placed in 100 ml of 0.75 M NaOH for 30 min in orbital shaker at 120 rpm. The alkali was washed out and the hairs were extended into straight configuration with the help of a comb. The hairs were then placed in 30 ml K31 solution for 1 h followed by washing with 0.1 M acetic acid solution. The neutralizing agent was then washed out using a commercial shampoo [25].
Diameter of the hairs before and after treatment with K31 was measured by inverted microscope (Nikon Eclipse TS 100). Tensile property of the hairs was determined using guidelines outlined in ASTM D1445-95 for fibre tensile testing. Five hairs of each sample including unbleached, bleach-treated and bleached hair treated with keratin K31, each of approximately 90 mm in length, were subjected for tensile strength measurement using an instron 4505 testometric material testing machine (Rochdale, Lancashire, England). The hair samples were attached into a pair of cardboard frames Centre with internal rectangular cut frame of 90 × 45 mm following the longest direction [24]. For statistical analysis the standard error and t-test (P ≤ 0.05) was used to differentiate the mean effect of treatments on the studied parameters (hair thickness, Young’s modulus and breaking force).
3. Results and discussion
3.1. Cloning and expression of K31
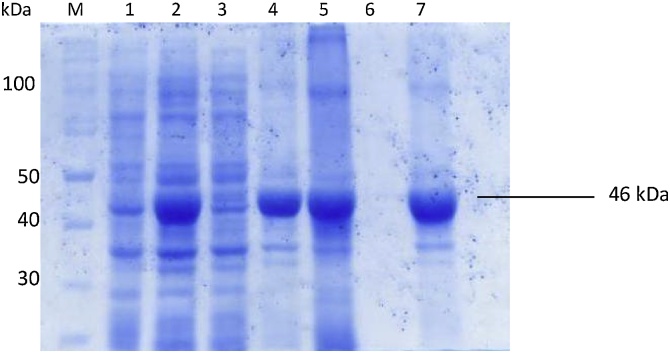
The selection of K31 was influenced on the basis of the 2D-gel analysis of proteins eluted from the hair damaged by conventional chemical cosmetic treatment, which showed that K31 was the its major component [11]. Thus in this study E. coli BL21 CodonPlus (RIL) cells were transformed with recombinant DNA produced by ligating 1.2 kb DNA fragment encoding K31 into pET-22b (+).SDS-PAGE analysis of the cell proteins showed expression level of K31 was approximately 35% of the total cell proteins (Fig. 1). As the medium have both glucose and lactose as carbon sources, E. coli shall utilize glucose first through carbon catabolite repression mechanism until it is exhausted followed by utilization of lactose as the carbon source [26]. This would allow induction of the expression system, resulting in heterologous expression of the recombinant protein [27].
Fig. 1.
SDS PAGE analysis of proteins in E. coli cells expressing keratin K31. M: size marker, 1: uninduced cells; 2: induced cells; 3: cell lysate supernatant; 4: cell lysate pellet; 5: washed inclusion bodies; 6: washings; 7: refolded keratin K31.
3.2. Refolding and purification
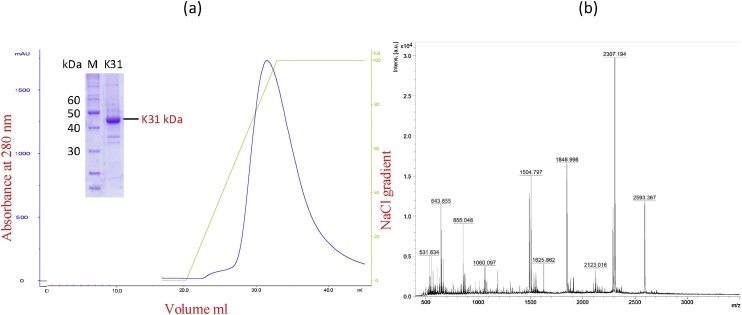
Most eukaryotic proteins are expressed as inclusion bodies in prokaryotes due to different physiological conditions in prokaryotic cellular environment [28]. The insoluble expression of K31 in E. coli is in agreement with the previously reported insoluble expression of human hair keratin K35 and K85 in E. coli [29]. The SDS-PAGE analysis of the refolded keratin K31 solution is shown in Fig. 1. The refolded K31 protein after anion exchange chromatography was purified up-to ∼85% level, as determined from SDS-PAGE analysis (Fig. 2a).
Fig. 2.
(a) FPLC elution profile of the sample containing refolded K31, with SDS-PAGE analysis of the major fraction obtained after elution. (b) Mass spectroscopic analysis of the tryptic digest of keratin K31.
Analysis of tryptic digests of keratin K31 by Mass Spectroscopy produced a MASCOT score of 76, while 8 peptides were matched and the protein sequence coverage was 28%. The purified protein was thus confirmed to be keratin K31 (Fig. 2b).
3.3. Secondary structure analysis
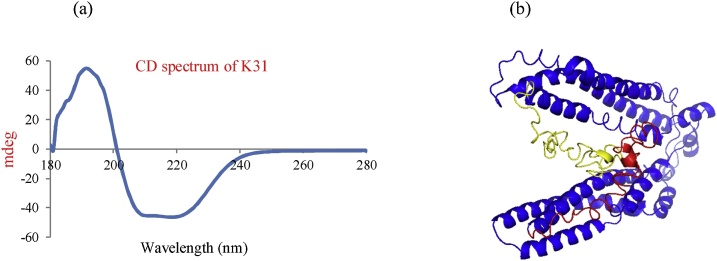
Keratin K31 when analyzed by CD spectrum at 37 °C showed a positive peak at 193 nm and 2 negative peaks at 212 nm and 223 nm (Fig. 3a). CDNN software analysis of the CD spectrum showed that K31 consisted of 64% α-helices, 0.7% antiparallel β-sheets, 3.3% parallel β-sheets, 12.2% β-turns and 11.7% random coils. The structure of keratin K31 produced by I-TASSER was submitted to online secondary structure server (2Struc) for secondary structure prediction, which showed 64.7% α-helices, 0.7% antiparallel β-sheets, 15.7% beta turns and 15.1% random coils (Fig. 3b). The similarity in structural data obtained by CD spectrum analysis to that obtained by in silico analysis suggests that the keratin produced recombinantly was properly refolded into its native state.
Fig. 3.
(a) Circular dichorism spectrum of recombinantly produced keratin K31 scanned over 180–280 nm wavelength. (b) 3-D structure of keratin K31 obtained through molecular modeling. The rod domain (blue) shown is largely α-helices, while the head (yellow) and the tail domains (red) are largely non-helical regions.
3.4. Application of K31 in hair treatment
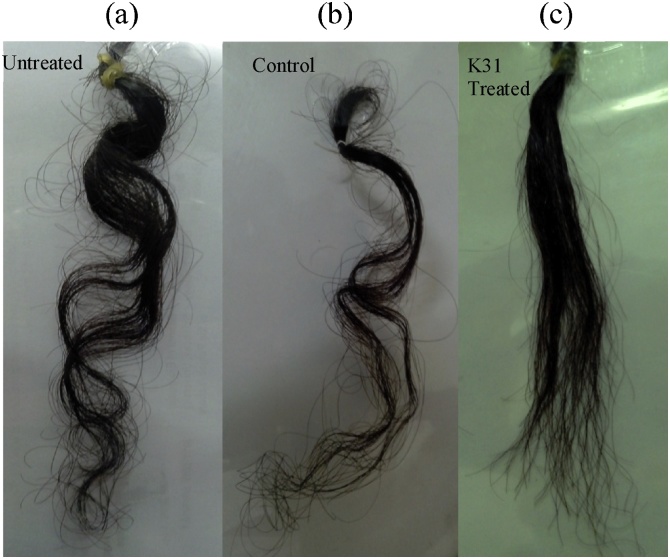
The ability of K31 to straighten the curly hair and repairing the chemically damaged hair was investigated. The alkali treated hair on K31 treatment efficiently straightened the curly hair (Fig. 4). The alkali treated hair sample, however, when washed with plain water retained its curly form. Previously a preparation of keratin peptides of thirteen amino acids in an organic solvent was reported to straighten the curly hair, with improved diameter and tensile strength [25].
Fig. 4.
Application of keratin K31 in straightening curly hair. (a) Untreated curly hair; (b) curly hair after alkali treatment and washing; (c) alkali treated and washed hair after keratin K31 treatment.
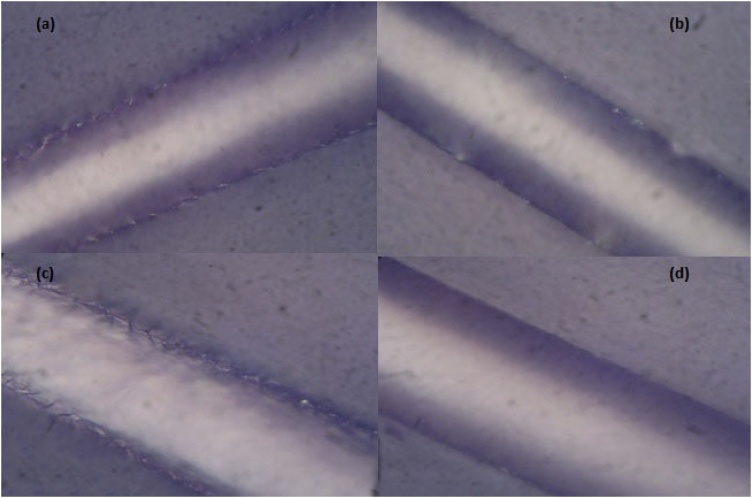
Both the naturally damaged hair and those partially damaged by bleach treatment showed gain in their diameter after keratin treatment. The hair sample with an average diameter 101 μm, on bleach treatment was reduced to an average of 94 μm. Application of bleach is known to damage the hair fiber by eluting out the protein [[30], [31], [32]]. After keratin treatment the diameter of the bleach treated hairs increased to an average of 139 μm, a gain of nearly 49% (Table. 1). These hair samples before and after K31 treatments observed by confocal microscopy at 100X showed a significant improvement in the smoothness of the hair surface after the treatment (Fig. 5). Increased smoothness of the hair surfaces should make a significant contribution in not only improving their look but also in eliminating entanglement, if any, more easily.
Table 1.
Hair thickness, Young’s modulus, and breaking force values for the undamaged, chemically damaged and the keratin treated hairs.
| Hair sample | Hair thickness |
Young’s modulus |
Breaking force |
|||
|---|---|---|---|---|---|---|
| μm | % changea | N/mm2 | % change | N | % change | |
| Undamaged | 101 ± 1.50 | ---- | 10,486 ± 118.6 | ---- | 20 ± 0.58 | ---- |
| Chemically damaged | 94*±0.58 | −7 | 6595**±60.20 | −37 | 11**±0.58 | −45 |
| Keratin treated | 140**±1.20 | +49 | 12,256**±27.30 | +86 | 26**±1.20 | +136 |
Percentage changes for the chemically damaged hairs are with respect to the undamaged hair, while those for the keratin treated hairs are with respect to the chemically damaged hairs.
Significant at P ≤ 0.05.
Significant at P ≤ 0.001 according to t -test comparing the mean difference of applied treatments.
Fig. 5.
Confocal microscopy of damaged hair before and after keratin K31 treatment. Natural hair before (a) and after keratin treatment (b); chemically damaged (bleached) hair before (c) and after keratin treatment (d).
Tensile strength of the naturally damaged and the bleach-treated hair showed marked improvement after keratin treatment. Mean values of the stress (the force required to break the hair) before and after keratin treatment are given in Table 1. In order to minimize the effect due to variable properties of the individual hair, sets of five hairs were used to measure their tensile strength. The untreated hair sample selected for tensile properties showed Young’s modulus 10,486 N/mm2, which was reduced to 6595 N/mm2 after the chemical treatment. The K31 treatment of the damaged hairs showed young modulus score of 12,256 N/mm2, which is almost 2 fold higher as compared to that of the chemically damaged hair. This increase is significantly higher than the previously reported increase in Young’s modulus gained after treatment with keratin peptides [33]. Similarly, the force required to break the hair before and after the chemical treatment was 20 N and 11 N, respectively. Application of K31 to the chemically damaged hairs resulted in increase of mechanical strength more than twice to 26 N.
It appears that keratin K31, the major protein lost in the damaged hair [11], gets integrated in to the damaged hair filling the gaps and thus enhancing their elasticity and mechanical strength. Hydrolyzed keratin peptides isolated from wool or chicken feathers have been used mostly in hair treatment [[25], [26], [27], [28], [29], [30], [31]]. However, the intact protein in its native state can be more effective in forming a cohesive film by filling the gaps produced in the damaged hair [24]. The human keratin K31 being a major constituent of human hair and having high level of similarity with the rest of the human hair keratin types [34,35], would make it more effective in hair improvement.
Previous studies have reported that cortex of the hair are mainly responsible for the mechanical strength of the hair and this strength is weakened by bleaching [36,37]. However, our study shows that bleaching decreases the strength as well as the diameter of the hair and this strength and diameter is regained and increased after treatment with intact keratin K31, produced in E. coli. K31 would integrate into the hair intermediate filament protein by making disulfide bonds with the damaged keratin, filling the gaps and rebuilding the damaged hair protein profile, resulting in increased mechanical strength and recovery of the properties of damaged hair [38].
4. Conclusion
Keratin K31, the major component of the hair structure, has an important role in maintenance of hair in a healthy state. We produced recombinant Keratin K31, refolded and purified for studying its role in reshaping hair and treatment of damaged hair. The alkali treated curly hair were straightened after treatment with K31. Application of K31 to the chemically damaged hair also resulted in significant increase in their diameter, tensile strength and surface smoothness. These findings show the potential of K31 in the development of hair care and styling products.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
This work was carried out in the laboratories of School of Biological Sciences, University of the Punjab, Pakistan. The authors gracefully acknowledge Ayesha aslam for providing curly hairs and Sana batool for providing hairs used in testing the mechanical properties of hair.
Contributor Information
Abdul Basit, Email: basitbch@gmail.com.
Faiza asghar, Email: faizafarhan.malhi@gmail.com.
Saima Sadaf, Email: saima.ibb@pu.edu.pk.
M. Waheed Akhtar, Email: mwa.sbs@pu.edu.pk.
References
- 1.Nakamura A., Arimoto M., Takeuchi K., Fujii T. A rapid extraction procedure of human hair proteins and identification of phosphorylated species. Biol. Pharm. Bull. 2002;25(5):569–572. doi: 10.1248/bpb.25.569. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A., Kamarudin N.B., Kee C.Y.G., Yunus R.B.M. Extraction of keratin protein from chicken feather. J. Chem. Chem. Eng. 2012;6(8):732. [Google Scholar]
- 3.Wilson R.H., Lewis H.B. The cystine content of hair and other epidermal tissues. J. Biol. Chem. 1927;73(2):543–553. [Google Scholar]
- 4.Ziegler K. Chemistry of Natural Protein Fibers. Springer; 1977. Crosslinking and self-crosslinking in keratin fibers; pp. 267–300. [Google Scholar]
- 5.Langbein L., Rogers M.A., Praetzel-Wunder S., Böckler D., Schirmacher P., Schweizer J. Novel type I hair keratins K39 and K40 are the last to be expressed in differentiation of the hair: completion of the human hair keratin catalog. J. Invest. Dermatol. 2007;127(6):1532–1535. doi: 10.1038/sj.jid.5700734. [DOI] [PubMed] [Google Scholar]
- 6.Franbourg A., Hallegot P., Baltenneck F., Toutaina C., Leroy F. Current research on ethnic hair. J. Am. Acad. Dermatol. 2003;48(6):S115–S119. doi: 10.1067/mjd.2003.277. [DOI] [PubMed] [Google Scholar]
- 7.Swift J. Human hair cuticle: biologically conspired to the owner’s advantage. J. Soc. Cosmet. Chem. 1999;50(1):23–47. [Google Scholar]
- 8.Plowman J.E. The proteomics of keratin proteins. J. Chromatogr. B. 2007;849(1):181–189. doi: 10.1016/j.jchromb.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Feughelman M. UNSW press; 1997. Mechanical Properties and Structure of Alpha-keratin Fibres: Wool, Human Hair and Related Fibres. [Google Scholar]
- 10.Miranda‐Vilela A., Botelho A., Muehlmann L. An overview of chemical straightening of human hair: technical aspects, potential risks to hair fibre and health and legal issues. Int. J. Cosmet. Sci. 2014;36(1):2–11. doi: 10.1111/ics.12093. [DOI] [PubMed] [Google Scholar]
- 11.Sinclair R., Flagler M., Jones L., Rufaut N., Davis M. The proteomic profile of hair damage. Br. J. Dermatol. 2012;166(s2):27–32. doi: 10.1111/j.1365-2133.2012.10862.x. [DOI] [PubMed] [Google Scholar]
- 12.Roddick-Lanzilotta A., Kelly R., Scott S., Chahal S. New keratin isolates: actives for natural hair protection. J. Cosmet. Sci. 2006;58(4):405–411. [PubMed] [Google Scholar]
- 13.Barba C., Martí M., Roddick-Lanzilotta A., Manich A., Carilla J., Parra J.L., Coderch L. Effect of wool keratin proteins and peptides on hair water sorption kinetics. J. Therm. Anal. Calorim. 2010;102(1):43–48. [Google Scholar]
- 14.Roddick-Lanzilotta A., Kelly R., Scott S., Chahal S. New keratin isolates: actives for natural hair protection. J. Cosmet. Sci. 2007;58(4):405–411. [PubMed] [Google Scholar]
- 15.Sadaf S., Khan M.A., Akhtar M.W. Production of bubaline somatotropin by auto-induction in Escherichia coli. Biotechnol. Appl. Biochem. 2007;47(Pt 1):21–26. doi: 10.1042/BA20060154. [DOI] [PubMed] [Google Scholar]
- 16.Russell Sambrook, J., Molecular cloning: a laboratory manual, 3rd edn. Coldspring Harbour. In Coldspring Laboratory Press, NY Google Scholar: 2001.
- 17.Sajjad M., Khan M.I.M., Akbar N.S., Ahmad S., Ali I., Akhtar M.W. Enhanced expression and activity yields of Clostridium thermocellum xylanases without non-catalytic domains. J. Biotechnol. 2010;145(1):38–42. doi: 10.1016/j.jbiotec.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Abdul, B.; W., A. M., Truncation of the processive Cel5A of Thermotoga maritima results in soluble expression and several fold increase in activity. Biotechnology and Bioengineering 2018, 115, (7), 1675-1684. [DOI] [PubMed]
- 19.Yamaguchi H., Miyazaki M. Refolding techniques for recovering biologically active recombinant proteins from inclusion bodies. Biomolecules. 2014;4(1):235–251. doi: 10.3390/biom4010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böhm G., Muhr R., Jaenicke R. Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng. 1992;5(3):191–195. doi: 10.1093/protein/5.3.191. [DOI] [PubMed] [Google Scholar]
- 21.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat. Methods. 2015;12(1):7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klose D.P., Wallace B.A., Janes R.W. 2Struc: the secondary structure server. Bioinformatics. 2010;26(20):2624–2625. doi: 10.1093/bioinformatics/btq480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fatima I., Sadaf S., Musharraf S.G., Hashmi N., Akhtar M.W. CD5 molecule-like and transthyretin as putative biomarkers of chronic myeloid leukemia-an insight from the proteomic analysis of human plasma. Sci. Rep. 2017;7:40943. doi: 10.1038/srep40943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barba C., Scott S., Roddick-Lanzilotta A., Kelly R., Manich A.M., Parra J.L., Coderch L. Restoring important hair properties with wool keratin proteins and peptides. Fibers Polym. 2010;11(7):1055–1061. [Google Scholar]
- 25.Fernandes M.M., Lima C.F., Loureiro A., Gomes A., Cavaco‐Paulo A. Keratin‐based peptide: biological evaluation and strengthening properties on relaxed hair. Int. J. Cosmet. Sci. 2012;34(4):338–346. doi: 10.1111/j.1468-2494.2012.00727.x. [DOI] [PubMed] [Google Scholar]
- 26.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2008;11(2):87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Studier F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005;41(1):207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Fischer B., Perry B., Sumner I., Goodenough P. A novel sequential procedure to enhance the renaturation of recombinant protein from Escherichia coil inclusion bodies. Protein Eng. 1992;5(6):593–596. doi: 10.1093/protein/5.6.593. [DOI] [PubMed] [Google Scholar]
- 29.Ishii D., Abe R., Watanabe S.-i., Tsuchiya M., Nöcker B., Tsumoto K. Stepwise characterization of the thermodynamics of trichocyte intermediate filament protein supramolecular assembly. J. Mol. Biol. 2011;408(5):832–838. doi: 10.1016/j.jmb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Gray J. Hair care and hair care products. Clin. Dermatol. 2001;19(2):227–236. doi: 10.1016/s0738-081x(00)00133-4. [DOI] [PubMed] [Google Scholar]
- 31.Bolduc C., Shapiro J. Hair care products: waving, straightening, conditioning, and coloring. Clin. Dermatol. 2001;19(4):431–436. doi: 10.1016/s0738-081x(01)00201-2. [DOI] [PubMed] [Google Scholar]
- 32.Dawber R. Hair: its structure and response to cosmetic preparations. Clin. Dermatol. 1996;14(1):105–112. doi: 10.1016/0738-081x(95)00117-x. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes M., Cavaco-Paulo A. Protein disulphide isomerase-mediated grafting of cysteine-containing peptides onto over-bleached hair. Biocatal. Biotransformation. 2012;30(1):10–19. [Google Scholar]
- 34.Schweizer J., Bowden P.E., Coulombe P.A., Langbein L., Lane E.B., Magin T.M., Maltais L., Omary M.B., Parry D.A.D., Rogers M.A., Wright M.W. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006;174(2):169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweizer J., Langbein L., Rogers M.A., Winter H. Hair follicle-specific keratins and their diseases. Exp. Cell Res. 2007;313(10):2010–2020. doi: 10.1016/j.yexcr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Robbins, C.; Crawford, R., Cuticle damage and the tensile properties of human hair. J Soc Cosmet Chem 42, 59-67.
- 37.Langbein L., Yoshida H., Praetzel-Wunder S., Parry D.A., Schweizer J. The keratins of the human beard hair medulla: the riddle in the middle. J. Invest. Dermatol. 2010;130(1):55–73. doi: 10.1038/jid.2009.192. [DOI] [PubMed] [Google Scholar]
- 38.Moll R., Divo M., Langbein L. The human keratins: biology and pathology. Histochem. Cell Biol. 2008;129(6):705. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]