IgA nephropathy is the most common form of primary GN worldwide. It is characterized by the deposition of IgA1 (in particular, galactose-deficient IgA1) in the mesangial area of the glomeruli. In the circulation of most patients with IgA nephropathy, galactose-deficient IgA1 and its corresponding IgG and/or IgA autoantibodies are elevated and correlated with increased risk of disease progression. Although the exact pathogenesis of IgA nephropathy remains unclear, targeting the production of galactose-deficient IgA1 and its autoantibodies seems to be a promising specific therapy for IgA nephropathy.
Recently, a number of large clinical trials on immunosuppressive therapy in IgA nephropathy have been reported, such as the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy trial (NCT00554502), the Targeted-release Budesonide Versus Placebo in Patients with IgA Nephropathy (NEFIGAN) Study (NCT01738035), and the Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study (NCT01560052). Currently, the use of systemic corticosteroids is being questioned due to questionable efficacy and significant side effects. Importantly, with a deeper understanding of the role of mucosal immunity, B cell activation, and complement activation in IgA nephropathy, several clinical trials of targeted therapies are now in progress. Especially because B cells may be involved in the production of galactose-deficient IgA1 and its antibodies in IgA nephropathy, B cell–depleting therapy using rituximab, originally developed for the treatment of rheumatoid arthritis and B cell malignancies, is an appealing therapeutic option. In a recent study, Lafayette et al. (1) showed results from a randomized, controlled trial that was designed to determine the efficacy of rituximab in IgA nephropathy. Although rituximab achieved effective depletion of CD19+ B cells, it failed to improve eGFR decline and lead to proteinuria reduction compared with supportive treatment. Furthermore, neither serum levels of galactose-deficient IgA1 nor its antibodies were reduced. More importantly, similar to the result in IgA nephropathy, rituximab showed no positive effects in patients with ulcerative colitis (2). Because mucosal immunity has been reported to play an important role in the pathogenesis of IgA nephropathy and ulcerative colitis, the authors proposed that the significant role of mucosal immunity might render B cell depletion therapy less effective.
Previously, the gut-kidney axis in IgA nephropathy was comprehensively reviewed by Coppo (3,4), who indicated that genetic background, B cell activity, IgA synthesis, gut-associated lymphoid tissue intestinal immunity, and diet may interact in the development and progression of IgA nephropathy. Peyer patches are essential lymphoid organs for maintenance of gut homeostasis. In genetically predisposed individuals, dendritic cells could process and present microbiota or dietary antigens to T cells in Peyer patches that activate B cells and result in increasing IgA1 production through IgA class switching recombination in the context of TNF ligand superfamily member 13 (APRIL), TNF ligand superfamily member 13B (BAFF), and TGFβ. Because Peyer patches are supposed to be primed to produce galactose-deficient IgA1, the initial step in the pathogenesis of IgA nephropathy, focusing on a drug targeted to the mucosal B lymphocytes in Peyer patches seems to be a promising treatment closer to the source (3,4). However, the germinal center B cells within Peyer patches have been reported to be resistant to rituximab in mouse models (5). In patients treated with rituximab, it has also been implied that CD20−CD19+CD27high plasmablast/plasma cell counts were stably maintained during the long-term depletion of circulating CD20+ B cells, which expressed IgA, the mucosal cell adhesion molecule β7 integrin, and the mucosal chemokine receptor CCR10 (6,7). Furthermore, this rituximab-resistant IgA+ B cell subset is not abrogated by splenectomy and expressed HLA-DRhigh and Ki-67. Thus, the rituximab-resistant mucosal B cells may account for the failure of rituximab to reduce the serum galactose-deficient IgA1 and thus, abrogate any beneficial effect of rituximab on the course of IgA nephropathy. Furthermore, the persisting anti-IgA1 IgG autoantibody titers observed during rituximab treatment seem to be provided by the CD19−CD20− long-lived plasma cells in bone marrow (1,8); a similar finding was observed in rheumatoid arthritis and ANCA-associated vasculitis, where treatment with rituximab might preserve protective humoral immunity (9).
Interestingly, the recent double-blind, randomized, controlled clinical trial, the NEFIGAN Study, added additional information on this concept. It was designed to evaluate the effectiveness and safety of Nefecon, an oral formulation that releases the glucocorticosteroid budesonide in the lower ileum and ascending colon, which have a high density of Peyer patches (10). Thus, local lymphoid tissue exposure to corticosteroids could theoretically alter mucosal immune responses to intestinal antigens, which in turn, mediate B cell activation and Ig class switch. It was shown that Nefecon significantly decreased the level of proteinuria and maintained stable kidney function in patients with IgA nephropathy. More importantly, compared with systemic use of corticosteroids, the targeted release corticosteroids for IgA nephropathy were well tolerated. Although the changes of serum levels of galactose-deficient IgA1 and its autoantibodies have not been reported yet from the NEFIGAN Study, targeted release corticosteroids for IgA nephropathy seem to be a promising treatment that targets relevant pathophysiology. In the future, multicenter and multinational studies are necessary to confirm these results and evaluate the long-term effect on kidney function, especially on galactose-deficient IgA1 and its autoantibodies levels.
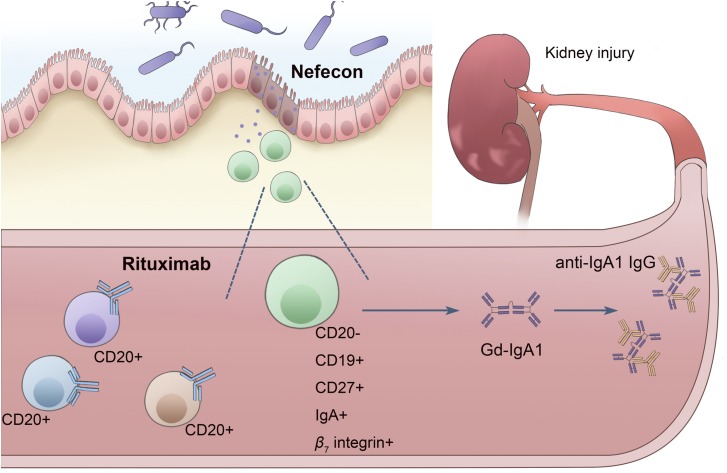
Taken together, the possible mechanisms for the failure of rituximab and the clinical success of Nefecon in IgA nephropathy are summarized in Figure 1. This implies a possible link between rituximab-resistant mucosal B cell and disease activity and the persistent generation of IgA1 and further supports the role of mucosal immunity in IgA nephropathy. However, the scientific and clinical implications require further research. First, exploring the detailed characteristics of the treatment-resistant IgA-secreting plasmablasts/plasma cells may help develop targeted therapeutic strategies in IgA nephropathy. Second, mucosal B cells are thought to be primed in the generation of galactose-deficient IgA1, and thus, targeted immunosuppression to sites of mucosal B cell induction may provide an alternative regimen rather than relying on systemic immunosuppression, which is associated with significant side effects. Third, promising agents that target increasing levels of circulating IgA, including blisibimod (a BAFF inhibitor) and atacicept (a humanized recombinant TACI-IgGFc fusion protein with anti-APRIL and anti-BAFF activity), provide more specific therapy than what is currently available. In the future, a deeper understanding of the pathogenesis of IgA nephropathy will help us to identify more potential biochemical pathways that might be amenable to therapeutic strategies.
Figure 1.
Plasmablasts with a mucosal phenotype may contribute to the failure of rituximab and the clinical success of Nefecon in IgA nephropathy. Peyer patches are essential lymphoid organs for maintenance of gut homeostasis that are supposed to be primed to produce galactose-deficient IgA1 through IgA class switching recombination (CSR) in the context of TNF ligand superfamily member 13 (APRIL), TNF ligand superfamily member 13B (BAFF), and TGFβ (represented by the colored squares, triangles, and circles). The stable presence of apparently rituximab-resistant CD20−CD19+CD27high IgA-secreting cells of mucosal origin during the long-term depletion of circulating CD20+ B cells and the beneficial effects of Nefecon releasing budesonide in the lower ileum and ascending colon, which have a high density of Peyer patches, indicate the role of mucosal immunity in pathogenesis of IgA nephropathy. The two recent randomized, controlled clinical trials, the rituximab trial (NCT00498368) and the Targeted-release Budesonide Versus Placebo in Patients with IgA Nephropathy (NEFIGAN) Study (NCT01738035), provide tantalizing support for the possibility that targeting mucosal immunity would be closer to the source in treatments for IgA nephropathy.
Disclosures
None.
Acknowledgments
This work was supported by Training Program of the Major Research Plan of the National Natural Science Foundation of China grant 91642120, National Key Research and Development Program of China grant 2016YFC0904102 and the Fund for Fostering Young Scholars of Peking University Health Science Center BMU2017PY007.
The content of this article does not reflect the views or opinions of the American Society of Nephrology (ASN) or the Clinical Journal of the American Society of Nephrology (CJASN). Responsibility for the information and views expressed therein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC: A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 28: 1306–1313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiper K, Martin K, Ellis A, Subramanian S, Watson AJ, Christmas SE, Howarth D, Campbell F, Rhodes JM: Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut 60: 1520–1526, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Coppo R: The intestine-renal connection in IgA nephropathy. Nephrol Dial Transplant 30: 360–366, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Coppo R: The gut-kidney axis in IgA nephropathy: Role of microbiota and diet on genetic predisposition. Pediatr Nephrol 33: 53–61, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC: Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174: 817–826, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Mei HE, Frölich D, Giesecke C, Loddenkemper C, Reiter K, Schmidt S, Feist E, Daridon C, Tony HP, Radbruch A, Dörner T: Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood 116: 5181–5190, 2010 [DOI] [PubMed] [Google Scholar]
- 7.He Y, Shimoda M, Ono Y, Villalobos IB, Mitra A, Konia T, Grando SA, Zone JJ, Maverakis E: Persistence of autoreactive IgA-secreting B cells despite multiple immunosuppressive medications including rituximab. JAMA Dermatol 151: 646–650, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang KY, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE: Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity 43: 132–145, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortazar FB, Pendergraft WF 3rd, Wenger J, Owens CT, Laliberte K, Niles JL: Effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 69: 1045–1053, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, Floege J, Hetzel G, Jardine AG, Locatelli F, Maes BD, Mercer A, Ortiz F, Praga M, Sørensen SS, Tesar V, Del Vecchio L; NEFIGAN Trial Investigators: Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117–2127, 2017 [DOI] [PubMed] [Google Scholar]