Abstract
Background and objectives
Claudin-16 and -19 are proteins forming pores for the paracellular reabsorption of divalent cations in the ascending limb of Henle loop; conversely, claudin-14 decreases ion permeability of these pores. Single-nucleotide polymorphisms in gene coding for claudin-14 were associated with kidney stones and calcium excretion. This study aimed to explore the association of claudin-14, claudin-16, and claudin-19 single-nucleotide polymorphisms with calcium excretion.
Design, setting, participants, & measurements
We performed a retrospective observational study of 393 patients with hypertension who were naïve to antihypertensive drugs, in whom we measured 24-hour urine calcium excretion; history of kidney stones was ascertained by interview; 370 of these patients underwent an intravenous 0.9% sodium chloride infusion (2 L in 2 hours) to evaluate the response of calcium excretion in three different 2-hour urine samples collected before, during, and after saline infusion. Genotypes of claudin-14, claudin-16, and claudin-19 were obtained from data of a previous genome-wide association study in the same patients.
Results
Thirty-one single-nucleotide polymorphisms of the 3′ region of the claudin-14 gene were significantly associated with 24-hour calcium excretion and calcium excretion after saline infusion. The most significant associated single-nucleotide polymorphism was rs219755 (24-hour calcium excretion in GG, 225±124 mg/24 hours; 24-hour calcium excretion in GA, 194±100 mg/24 hours; 24-hour calcium excretion in AA, 124±73 mg/24 hours; P<0.001; calcium excretion during saline infusion in GG, 30±21 mg/2 hours; calcium excretion during saline infusion in GA, 29±18 mg/2 hours; calcium excretion during saline infusion in AA, 17±11 mg/2 hours; P=0.03). No significant associations were found among claudin-16 and claudin-19 single-nucleotide polymorphisms and calcium excretion and between claudin-14, claudin-16, and claudin-19 single-nucleotide polymorphisms and stones. Bioinformatic analysis showed that one single-nucleotide polymorphism at claudin-14 among those associated with calcium excretion may potentially influence splicing of transcript.
Conclusions
Claudin-14 genotype at the 3′ region is associated with calcium excretion in 24-hour urine and after the calciuretic stimulus of saline infusion.
Keywords: claudin-14; hypercalciuria; claudin-16; claudin-19; Polymorphism, Single Nucleotide; Antihypertensive Agents; Genome-Wide Association Study; Cations, Divalent; Sodium Chloride; Computational Biology; Retrospective Studies; Claudins; Kidney Calculi; Calcium, Dietary; Genotype; Permeability
Visual Abstract
Introduction
Claudins (CLDNs) are a family of transmembrane proteins that interact with each other and other proteins to form barriers or pores regulating paracellular solute transport in tight junctions (1–4). Different CLDNs are expressed in specific segments of the kidney tubule and contribute to the development of distinctive properties of each tubular portion (2,3). The thick ascending limb of the Henle loop reabsorbs 25% of filtered calcium through a passive paracellular pathway involving CLDN14, CLDN16, and CLDN19 (2,5). CLDN16 and CLDN19 interact to form pores for calcium and magnesium reabsorption in tight junctions. They are distributed along the ascending limb according to a mosaic pattern with CLDN10, which is involved in paracellular sodium reabsorption. Therefore, they may mediate calcium magnesium reabsorption through specific tight junction pores in the cortex and the outer stripe of outer medulla (6). Conversely, CLDN14 may interact with CLDN16 to inhibit permeability of tight junctions to calcium and magnesium (2,5,7). Interestingly, dietary calcium load and extracellular calcium may stimulate CLDN14 expression in the thick ascending limb through the activation of a calcium-sensing receptor (7,8), which may inhibit the transcription of two microRNAs (miRs; miR-9 and miR-374) suppressing CLDN14 gene (21q22) expression (9,10). Calcium-sensing receptor may also slow down CLDN16 phosphorylation necessary for its location in tight junctions (11,12). Therefore, extracellular calcium may inhibit calcium reabsorption in the ascending limb by increasing CLDN14 expression and decreasing CLDN16 activity in tight junctions (2,7,8). In genetically modified mice, CLDN14 expression in the ascending limb was inhibited by the parathyroid hormone (PTH) and appeared as the main target in the pathway used by PTH to increase calcium reabsorption in this tubular segment (13). These findings attribute to CLDN14 and CLDN16 a crucial role in the regulation of calcium excretion (2,5).
Single-nucleotide polymorphisms (SNPs) rs219778, rs219779, rs219780, and rs219781 of CLDN14 were associated with kidney stones, calcium excretion, and bone mineral density in a genome-wide association study in an Icelandic population (14). These SNPs were located in the 3′ region of CLDN14 (last exon/intron) and in a unique haplotype block. These findings were replicated in another Icelandic population, in which rs199565725 in CLDN14 showed the strongest association with kidney stones (15). This SNP was located in the intron 1, and it was in linkage with the SNPs associated with stones in the previous study. CLDN14 SNPs rs219778 and rs219780 were also associated with kidney stones in patients from the eastern part of India (16).
Epidemiologic studies showed a disorder of calcium handling in patients with hypertension and their offspring, which was characterized by low calcium intake and increased excretion of calcium (17–19) that may be promoted by an excessive sodium chloride (NaCl) load (20–22). Therefore, we assessed the association of CLDN14, CLDN16, and CLDN19 SNPs with calcium excretion in 24-hour urine and urine collected after an acute saline infusion in an Italian population of never-treated patients with hypertension.
Materials and Methods
Patients
We enrolled 393 never-treated, recently discovered patients with essential hypertension with high/normal BP level or grade 1 or 2 hypertension (diastolic BP range, 90–110 mm Hg; systolic BP range, 110–180 mm Hg) according to the guidelines for management of arterial hypertension (17). All patients were recruited at the Outpatient Clinic for Hypertension of San Raffaele Hospital (Milan, Italy), and they were enrolled in the HYPERGENES-InterOmics Project studying genetic determinants of arterial hypertension (23). Patients with secondary causes of hypertension (by biochemical and instrumental tests), endocrine disorders, body mass index >32 kg/m2, or chronic and acute concomitant diseases (cardiocerebrovascular diseases, diabetes mellitus, or hepatic and kidney diseases) and women taking contraceptive pills were excluded from the study. Participants could not have taken thiazide, vitamin D, calcium supplements, or drugs for osteoporosis or mineral and electrolyte metabolism. Glomerular filtration (eGFR) was estimated with the Chronic Kidney Disease Epidemiology Collaboration equation (24) and had to be >60 ml/min per 1.73 m2. All patients were studied during a free diet. Idiopathic hypercalciuria was diagnosed as 24-hour calcium excretion >7.5 mmol in men or 6.25 mmol in women or 100 μmol/kg body wt in both men and women. History of kidney stones was ascertained according to self-reported data.
The Ethics Committee of the San Raffaele Hospital and the Steering Committee of the HYPERGENES-InterOmics Project approved the study, which was conducted in adherence with the Helsinki Declaration principles. Informed consent was obtained from each participant.
Saline Load Test
Three hundred seventy subjects underwent a saline infusion test according to the reported protocol (25). An intravenous infusion of 2 L of 0.9% NaCl solution (308 mmol of NaCl) was administered in 2 hours. Blood and 24-hour urine were collected before the test (24-hour urine was defined as baseline urine collection). Two-hour urine samples were collected immediately before the saline infusion (from −120 to 0 minutes of the test; defined as equilibration time), during the whole infusion (from 0 to 120 minutes; defined as infusion time), and after the infusion (from 120 to 240 minutes; defined as recovery time). Blood and urine samples were stored at −20°C to consent electrolyte determination. Calcium excretion in these three samples was expressed as calcium excreted in 2 hours (milligrams per 2 hours or micrograms per minute). BP was measured with a standardized procedure before the beginning of saline infusion, at the end of infusion, and at the end of test. Each measurement consisted of three readings taken at 5-minute intervals. BP values used in the analysis were the averages of the three readings.
Genotyping
Genomic DNA was extracted from the peripheral blood. All samples were genotyped using the Illumina Human1M-Duo array (n=552) within the HYPERGENES project (23) and the Illumina HumanOmniExpress array (n=119) (Illumina Inc., San Diego, CA) within the InterOmics Project as previously reported (http://www.interomics.eu/). We selected the whole genetic variants of CLDN14, CLND16, and CLDN19 genes from these data. We excluded SNPs with a minor allele frequency <5% and call rate <99%, leaving a total of 616 SNPs for analysis: 482 for CLDN14, 97 for CLDN16, and 37 for CLDN19.
Bioinformatic Analyses for CLDN14
The genomic context surrounding SNPs in CLDN14 associated with 24-hour calcium excretion was analyzed by using Ensembl 91 annotation (http://www.ensembl.org/Homo_sapiens/Info/Annotation) and GENCODE 27 (http://www.gencodegenes.org/releases/27.html). Transcript expression was examined using the GTEx RNA-seq portal (https://www.gtexportal.org). Transcription factor binding data from a large collection of chromatin immunoprecipitation and DNA sequencing experiments performed by the ENCODE project (http://www.genome.gov/encode) were also retrieved for these SNPs.
We used ESEfinder with default parameters (http://krainer01.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi?process=home) (26) to explore the potential effect of SNPs on possible exonic splicing enhancer motifs created or removed by specific variants. Variant alleles were analyzed for matrix libraries of SR proteins (SRSF1, SRSF1 [IgM-BRCA1], SRSF2, SRSF5, and SRSF6) and splice sites (5′ donor and 3′ acceptor and branch site), and changes were considered significant when an enhancer motif was either destroyed or generated.
Statistical Analyses
Continuous variables are expressed as means±SD. Dichotomous variables are presented as percentages. One-way ANOVA with Tukey test for multiple comparisons was used to compare continuous variables. Linear association was used to assess the genotype to phenotype association. Chi-squared test and multinomial logistic regression were used to evaluate the association of genotypes with discrete variables. Odds ratio (ORs) and 95% confidence interval (95% CIs) were computed. Calcium excretion before and during the saline infusion test was compared in patients with different genotypes using ANOVA for repeated measures. Linkage disequilibrium between SNPs was evaluated by calculating r2, and it was defined as r2>0.8; this threshold was used to identify haplotype blocks, including linked SNPs (27). For each haplotype block, we selected the relative tag SNP with the most significant association with calcium excretion.
A two-sided P value of <0.05 was considered to indicate statistical significance. False discovery rate was used for multiple test corrections of significance: for a false discovery rate of 5%, threshold value was P<0.01 for CLDN14, P<0.01 for CLDN16, and P<0.01 for CLDN19 (28). All analyses were performed with SPSS 24.0 software (IBM, Inc., Armonk, NY) and PLINK 1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) (29).
Results
The study population (n=393 patients) is detailed in Table 1. History of kidney stones was present in 9% of patients. Idiopathic hypercalciuria was found in 26% of patients. No significant associations were observed between SNPs of CLDN14, CLDN16, and CLDN19 and serum calcium, kidney stone disease, and arterial BP measured at different times during the NaCl infusion test.
Table 1.
Characteristics of population under study
| Variable | Values |
|---|---|
| N (men/women) | 393 (317/76) |
| Age, yr | 45±10 |
| Body mass index, kg/m2 | 25.8±3 |
| History of kidney stones, n (%) | 33 (9) |
| Hypercalciuric subjects, n (%) | 102 (26) |
| eGFR, ml/min per 1.73 m2 | 94±18 |
| Serum creatinine, mg/dl | 0.9±0.2 |
| Serum sodium, mmol/L | 142±2 |
| Serum calcium, mg/dl | 9.2±0.4 |
| Urinary sodium, mmol/24 h | 146±67 |
| Urinary calcium, mg/24 h | 207±115 |
| Basal systolic BP, mm Hg | 142±15 |
| Basal diastolic BP, mm Hg | 91±11 |
| Basal mean BP, mm Hg | 108±11 |
| N (men/women) | 370 (301/69) |
| Urinary sodium before NaCl infusion from −120 to 0 min, mmol/2 h | 15.4±13.6 |
| Urinary sodium during NaCl infusion from 0 to 120 min, mmol/2 h | 52.4±29.9 |
| Urinary sodium after NaCl infusion from 120 to 240 min, mmol/2 h | 51.5±29.0 |
| Urinary calcium before NaCl infusion from −120 to 0 min, mg/2 h | 21.7±18.8 |
| Urinary calcium during NaCl infusion from 0 to 120 min, mg/2 h | 28.9±19.4 |
| Urinary calcium after NaCl infusion from 120 to 240 min, mg/2 h | 18.4±15.2 |
| Mean BP increment at the end of NaCl infusion, mm Hg | 2.3±6.6 |
| Mean BP increment at the end of test, mm Hg | 1.9±6.9 |
Calcium excretion was measured in 24-hour urine in 393 patients and 2-hour urine collections during the NaCl infusion test in 370 patients. The NaCl infusion test lasted 6 hours and consisted of three 2-h periods during which urine was collected: an equilibration period followed by an infusion period (in which patients were infused with 2 L of 0.9% NaCl solution) and a recovery period after the infusion.
Twenty-Four–Hour Calcium Excretion and CLDN14 SNPs
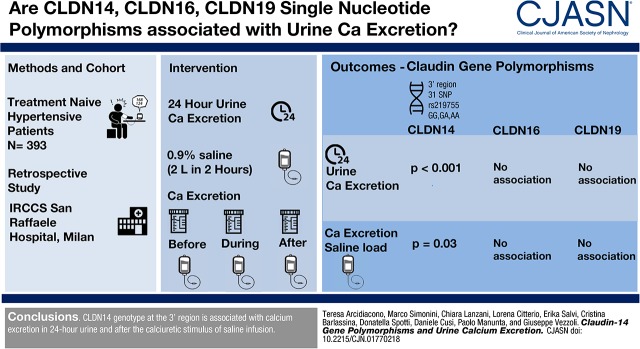
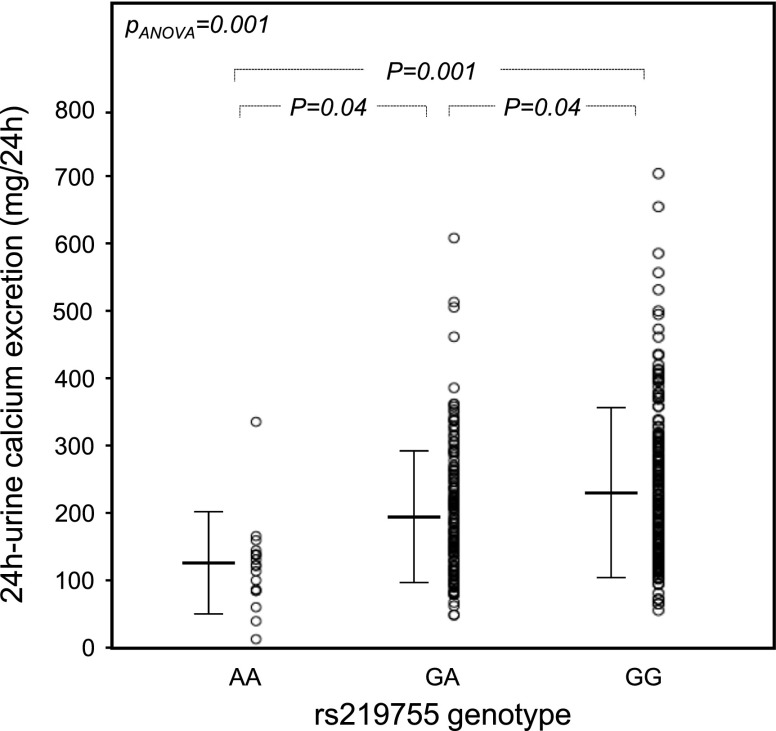
A strong association was observed between 78 SNPs of CLND14 and basal 24-hour calcium excretion (Supplemental Table 1), but only the 31 SNPs summarized in Table 2 passed the multiple comparison test (significance threshold defined by the multiple comparison test: P<0.01). All of these SNPs mapped the 3′ region of CLND14 (Supplemental Figure 1). rs219755 showed the strongest association with 24-hour calcium excretion that significantly decreased from homozygous patients for the major G allele to heterozygous patients and homozygous patients for the minor A allele with an apparent additive effect (Figure 1, Table 3). This result remained significant even after adjustment for age, sex, body mass index, basal level of BP, history of kidney stones, eGFR, and basal sodium excretion (P=0.004). The proportion of patients who were hypercalciuric decreased with the number of copies of the minor allele A at rs219755 (Table 3). The probability of being hypercalciuric was decreased in patients carrying genotype AA (OR, 0.22; 95% CI, 0.05 to 0.99; P=0.05) or GA (OR, 0.62; 95% CI, 0.38 to 1; P=0.05) compared with the GG genotype. Similar findings were observed for the majority of the SNPs significantly associated with calcium excretion.
Table 2.
Thirty-one single-nucleotide polymorphisms of the claudin-14 3′ regions significantly associated with 24-hour calcium excretion
| SNP | Chromosome 21 Position (Ch37.p13) | Domain | Alleles | MAF | P Value | r2 with rs219755 | SNP Blocks r2>0.8 |
|---|---|---|---|---|---|---|---|
| rs219738 | 37856682 | Intron | A>G | 0.25 | <0.01 | 0.45 | Block A |
| rs219739 | 37856536 | Intron | C>T | 0.25 | <0.01 | 0.45 | Block A |
| rs9636632 | 37855760 | Intron | A>G | 0.43 | <0.01 | 0.14 | — |
| rs2633324 | 37854906 | Intron | C>T | 0.25 | <0.01 | 0.45 | Block A |
| rs2850112 | 37854507 | Intron | A>G | 0.25 | <0.01 | 0.45 | Block A |
| rs2845768 | 37854364 | Intron | C>Ta | 0.25 | <0.01 | 0.45 | Block A |
| rs219755 | 37847170 | Intron | G>A | 0.26 | <0.001 | — | Block B |
| rs56183874 | 37841692 | Intron | C>T | 0.08 | <0.01 | 0.25 | Block C |
| 21:37841612b | 37841612 | — | R>D | 0.29 | <0.001 | 0.86 | Block B |
| 21:37841064b | 37841064 | — | R>D | 0.28 | <0.01 | 0.83 | Block B |
| 21:37840919b | 37840919 | — | R>D | 0.29 | 0.002 | 0.85 | Block B |
| rs219758 | 37840173 | Intron | A>T | 0.29 | <0.001 | 0.84 | Block B |
| rs219763 | 37838421 | Intron | A>A | 0.29 | 0.002 | 0.84 | Block B |
| rs2835364 | 37838111 | Intron | G>A | 0.08 | <0.01 | 0.24 | Block C |
| rs219764 | 37837930 | Intron | C>T | 0.26 | 0.003 | 0.95 | Block B |
| rs7277076 | 37836973 | Intron | C>T | 0.41 | 0.002 | 0.47 | Block D |
| rs12626330 | 37835982 | Intron | G>C | 0.48 | 0.002 | 0.36 | Block D |
| rs219768c | 37835764 | Intron | C>G | 0.23 | 0.004 | 0.02 | Block E |
| rs219769c | 37835675 | Intron | C>A | 0.23 | 0.004 | 0.02 | Block E |
| rs219770c | 37835648 | Intron | A>G | 0.23 | 0.004 | 0.02 | Block E |
| rs219771c | 37835501 | Intron | C>T | 0.23 | 0.004 | 0.02 | Block E |
| rs219772c | 37835347 | Intron | A>T | 0.23 | 0.004 | 0.02 | Block E |
| rs219773c | 37835333 | Intron | G>A | 0.23 | 0.004 | 0.02 | Block E |
| 21:37835239b,c | 37835239 | — | R>D | 0.23 | <0.01 | 0.02 | Block E |
| 21:37835238b,e | 37835238 | Intron | R>D | 0.21 | <0.01 | 0.03 | Block E |
| 21:37835184b | 37835184 | — | D>R | 0.42 | 0.002 | 0.47 | — |
| rs219775c | 37834944 | Intron | A>G | 0.23 | 0.004 | 0.02 | Block E |
| rs2835363c | 37834914 | Intron | A>T | 0.23 | 0.004 | 0.02 | Block E |
| rs219776c | 37834835 | Intron | T>C | 0.23 | 0.004 | 0.02 | Block E |
| rs219778c,d,e | 37834641 | Intron | A>G | 0.23 | 0.004 | 0.02 | Block E |
| rs219779c | 37833751 | Exon | G>Af | 0.23 | <0.01 | 0.02 | Block E |
| rs219780c | 37833307 | Exon | C>Tf | 0.18 | 0.04 | 0.04 | Block E |
Findings reported here passed a false discovery rate of 5% (P<0.01). rs219780 was further included, because it was associated with calcium excretion in a previous work (14), even if multiple comparison failed. P values are for unadjusted association with 24-hour calcium excretion estimated with linear regression. The r2 column reports linkage disequilibrium between SNP and rs219755 (the strongest associated SNP). SNP, single-nucleotide polymorphism; MAF, minor allele frequency; —, SNP not included in any blocks.
Noncoding transcript variant.
Internal tag SNP in GWA Illumina array: not yet codified in international database; R and D are unknown bases of SNP.
r2=0.99.
r2=0.86 inside haplotype block E, r2=0.75 inside block D, r2=0.99 inside block C, and r2=1.00 inside block A.
r2=0.71.
Synonymous change.
Figure 1.
Twenty-four–hour urinary excretion of calcium in 393 patients according to claudin-14 rs219755 (G>A) genotype. Data were corrected for age, sex, body mass index, and basal sodium excretion. Sample size: AA, n=22; GA, n=157; GG, n=214.
Table 3.
Characteristics of patients according to genotype at rs219755, located in block B, in all 393 patients and 370 patients undergoing the sodium chloride infusion test
| Variables | AA | GA | GG | P Value |
|---|---|---|---|---|
| N (men/women) | 22 (18/4) | 157 (125/32) | 214 (174/40) | 0.91 |
| Age, yr | 44±10 | 44±11 | 44±9 | 0.96 |
| Body mass index, kg/m2 | 24.2±2.7 | 25.7±2.7 | 25.5±3.0 | 0.47 |
| History of stones, n (%) | 1 (5) | 15 (10) | 17 (8) | 0.68 |
| Patients who were hypercalciuric, n (%) | 2 (9) | 34 (22) | 66 (31)a | 0.02 |
| eGFR, ml/min per 1.73 m2 | 89±18 | 92±16 | 92±16 | 0.88 |
| Serum calcium, mg/dl | 9.2±0.4 | 9.2±0.4 | 9.2±0.4 | 0.73 |
| Urine calcium excretion, mg/24 h | 124±73b | 194±100c | 225±124 | <0.001 |
| Urine sodium excretion, mmol/24 h | 129±59 | 141±65 | 151±68 | 0.05 |
| Basal mean BP, mm Hg | 109±12 | 106±11 | 108±12 | 0.35 |
| N (men/women) | 20 (17/3) | 148 (117/31) | 202 (167/35) | 0.63 |
| Urinary calcium before NaCl infusion from −120 to 0 min, mg/2 h | 14±17 | 20±17 | 22±20 | 0.12 |
| Urinary calcium during NaCl infusion from 0 to 120 min, mg/2 h | 17±11d | 29±18 | 30±21 | 0.03 |
| Urinary calcium after NaCl infusion from 120 to 240 min, mg/2 h | 15±13 | 18±14 | 19±17 | 0.36 |
| Serum calcium at the end of NaCl infusion, mg/dl | 8.2±0.5 | 8.2±0.6 | 8.1±0.7 | 0.60 |
| Serum calcium at the end of test, mg/dl | 8.4±0.4 | 8.5±0.4 | 8.4±0.5 | 0.50 |
| Urinary sodium before NaCl infusion from −120 to 0 min, μmol/2 h | 13±22 | 15±13 | 16±13 | 0.61 |
| Urinary sodium during NaCl infusion from 0 to 120 min, μmol/2 h | 41±29 | 55±31 | 52±28 | 0.65 |
| Urinary sodium after NaCl infusion from 120 to 240 min, μmol/2 h | 43±21 | 55±31 | 59±28 | 0.78 |
| Mean BP increment at the end of NaCl infusion, mm Hg | 0.4±6.7 | 1.8±5.9 | 2.1±7.1 | 0.50 |
| Mean BP increment at the end of test, mm Hg | 3.5±9.1 | 3.0±9.8 | 3.1±9.5 | 0.78 |
P value for genotype-phenotype association was estimated with linear regression. Comparisons between groups were performed with one-way ANOVA and Tukey test.
P=0.05 versus patients with genotype GA and P=0.05 versus patients with genotype AA.
P=0.04 versus patients with genotype GA and P=0.001 versus patients with genotype GG.
P=0.04 versus patients with genotype AA and P=0.04 versus patients with genotype GG.
P=0.04 versus patients with genotype GA and P=0.01 versus patients with genotype GG.
Six SNPs were in linkage disequilibrium (r2>0.80) with the tag SNP rs219755, and they were included in the same haplotype block reported as block B in Table 2. Fourteen other SNPs were located in another haplotype block (not including rs219755) and reported as block E (Table 2). Similarly, two SNPs were included in the block D, another two SNPs were included in block C and five SNPs were included in block A (Table 2). For each of these five blocks, we identified the tag SNP showing the most significant association with 24-hour calcium excretion: namely, rs219755, rs7277076, rs219778, rs2835364, and rs219739. We also identified two SNPs (21:37835184 and rs9636632) associated with 24-hour calcium excretion that were not included in any blocks. The minor allele at these SNPs was significantly associated with lower 24-hour calcium excretion (Table 4). We also considered SNPs rs219778, rs219779, and rs219780, which are known to be associated with kidney stones and calcium excretion in previous works (13,14). These SNPs were associated with 24-hour calcium excretion in our cohort, but only rs219778 and rs219779 passed the multiple comparison test (P=0.004 and P<0.01, respectively; linear regression analysis) (Table 2). Because of its historical relevance, calcium excretion in patients with different genotypes at rs219780 was reported in Table 4 (14–16).
Table 4.
Association between the genotype at claudin-14 and calcium excretion in 24-hour urine (n=393) and 2-hour urine collected during sodium chloride infusion (n=370)
| Variables | Homozygotes for the Minor Allele | Heterozygotes | Homozygotes for the Major Allele | P Value |
|---|---|---|---|---|
| rs219739 (C>T) in block A calcium excretion, mg/24 h | 178±85 (n=28) | 191±108a (n=151) | 222±121 (n=214) | <0.01 |
| calcium excretion during NaCl infusion, mg/2 h | 22±14 (n=26) | 28±21 (n=144) | 30±18 (n=200) | 0.03 |
| rs9636632 (A>G), no block calcium excretion, mg/24 h | 174±88 (n=72) | 208±118a (n=191) | 224±120 (n=130) | <0.01 |
| calcium excretion during NaCl infusion, mg/2 h | 26±18 (n=65) | 29±20 (n=185) | 31±19 (n=121) | 0.15 |
| rs2835364 (G>A) in block C calcium excretion, mg/24 h | 87±36 (n=2) | 171±86a (n=63) | 215±118 (n=328) | <0.01 |
| calcium excretion during NaCl infusion, mg/2 h | 14±8 (n=2) | 25±17 (n=61) | 30±20 (n=307) | 0.05 |
| rs7277076 (T>C) in block D calcium excretion, mg/24 h | 164±112a (n=51) | 206±107 (n=198) | 226±123 (n=144) | 0.002 |
| calcium excretion during NaCl infusion, mg/2 h | 27±20 (n=48) | 28±16 (n=189) | 31±23 (n=135) | 0.08 |
| 21:37835184 (D>R), no block calcium excretion, mg/24 h | 159±110b (n=54) | 208±104 (n=200) | 224±127 (n=139) | 0.002 |
| calcium excretion during NaCl infusion, mg/2 h | 23±19a (n=53) | 29±17 (n=189) | 31±23 (n=128) | 0.02 |
| rs219778 (A>G) in block E calcium excretion, mg/24 h | 142±84a (n=23) | 198±106 (n=127) | 218±120 (n=243) | 0.004 |
| calcium excretion during NaCl infusion, mg/2 h | 28±19 (n=23) | 26±15 (n=115) | 30±21 (n=232) | 0.10 |
| rs219780 (C>T) in block E calcium excretion, mg/24 h | 150±77 (n=9) | 193±111 (n=107) | 214±117 (n=277) | 0.04 |
| calcium excretion during NaCl infusion, mg/2 h | 40±24.2 (n=9) | 26±15 (n=99) | 30±20 (n=262) | 0.04 |
Findings about rs219755 located in the block B are reported in Table 3. Calcium excretion in 24-hour urine and 2-hour urine collected at the end of the NaCl infusion test (intravenous infusion of 2 L of 0.9% NaCl in 120 minutes) was tested in patients with different genotypes at the single-nucleotide polymorphisms with the most significant association with calcium excretion among those of each 3′ region haplotype block of claudin-14. D is an unknown base of 21:37835184 single-nucleotide polymorphism, which is not yet codified within international databases. P value for genotype-phenotype association was estimated with linear regression. Comparison between groups was performed with one-way ANOVA and Tukey test. NaCl, sodium chloride.
P<0.05 versus homozygotes for the major allele.
P<0.05 versus heterozygotes and P<0.01 versus homozygotes for the major allele.
Calcium Excretion after NaCl Infusion and CLDN14 SNPs
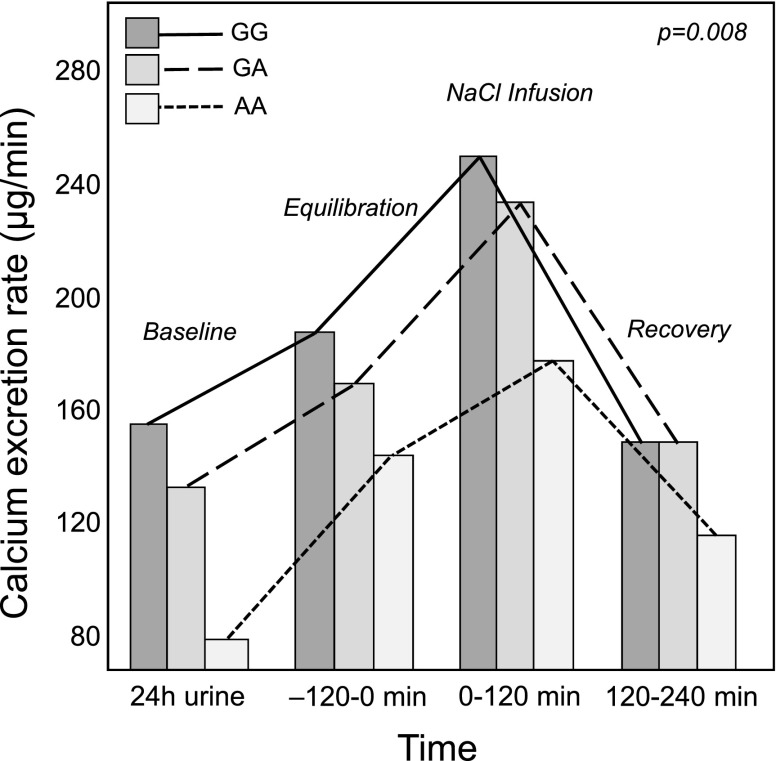
Patients undergoing the NaCl infusion test showed a significant increase of calcium excretion during the test (Table 1). Considering the SNP rs219755, calcium excretion during saline infusion was significantly lower in patients with AA than patients with GA and patients with GG (Table 2). These differences were confirmed using ANOVA for repeated measures (Figure 2); these findings remained significant after correction for age, sex, body mass index, history of kidney stones, level of BP, and sodium excretion during saline infusion. Similar results were observed for four of six CLDN14 SNPs identified in the previous step of our analysis: rs219739, rs2835364, 21:37835184, and rs219780 (Table 4).
Figure 2.
Calcium excretion during the sodium chloride (NaCl) infusion test in 370 patients according to claudin-14 rs219755 (A>G) genotype. Patients carrying the A allele had lower calcium excretion in response to NaCl infusion compared with patients with the GG genotype. Analysis was conducted with ANOVA for repeated measures (P<0.01). The analysis was also significant when adjusted for age, sex, body mass index, and urinary sodium excretion at the end of infusion (P<0.01). Pairwise comparisons: P=0.003, AA versus GA; P<0.001, AA versus GG. Calcium excretion was expressed as micrograms per minute in 24-hour urine and three urine samples collected during the NaCl infusion test (equilibration, NaCl infusion, and recovery). Sample size: AA, n=20; GA, n=148; GG, n=202.
Calcium Excretion and SNPs in CLDN16 and CLDN19
No significant association was found between 24-hour calcium excretion and 97 CLDN16 gene SNPs (significance threshold defined by the multiple comparison test: P<0.01) and 37 CLDN19 gene SNPs (significance threshold defined by the multiple comparison test: P<0.01). Moreover, no association was found with calcium excretion measured before, during, and after NaCl infusion.
Bioinformatic Analyses for CLDN14
Tag SNPs and one candidate (rs219780) span for 23 kb in the 3′ region of the CLDN14 gene. On the basis of recent GENCODE and Ensembl data, all tag SNPs map on intron regions of a nonprotein coding gene GENCODE AP000695.2 (ENST00000428667.1) and partially on GENCODE AP000695.1 (ENST00000429588.1), both antisense transcripts (Supplemental Figure 2). They are expressed in liver and kidney cortex as CLDN14 but also in skeletal muscle, subcutaneous adipose tissue, breast tissue, adrenal gland, and different arteries. No overlap with other regulatory elements (open chromatin, transcription factor binding site, promoter flank, and motif feature) appeared for each SNP site by Ensembl 91 annotation.
In silico analysis with ESEfinder found that the candidate synonymous SNP rs219780 causes a gain of SRSF1 (IgM-BRCA1), SRSF2, and SRSF5 binding sites in the context sequence of A minor variant, thus potentially influencing splicing (Supplemental Figure 3A). Moreover, the same A allele variant promotes a gain of a new branch site for splicing event (Supplemental Figure 3B).
Discussion
This study evaluated the association between urinary calcium excretion and SNPs in CLDN14, CLDN16, and CLDN19 genes coding for CLDN14, CLDN16, and CLDN19 that regulate permeability of tight junction pores involved in paracellular calcium and magnesium reabsorption in the thick ascending limb of Henle loop (5,6). The 3′ region of CLDN14 was identified as a locus potentially influencing tubular calcium reabsorption that is associated with calcium excretion in 24-hour urine and after NaCl infusion (2,30). Minor alleles of SNPs in this CLDN14 region were associated with lower calcium excretion and could be protective against hypercalciuria. This association was independent of sodium excretion and GFR, because confirmed after adjustment for these two variables. Conversely, no CLDN16 or CLDN19 SNPs were associated with calcium excretion.
CLDN14, CLDN16, and CLDN19 are expressed in the same tight junction pores (2,6–8), but the activity of CLDN14 seems to be the most relevant and dynamic, because it acts as a modulator of tight junction permeability without being a stable component of their pores (9–11). Genetic variants may blunt CLDN14 activity and lead to a more efficient paracellular bivalent cation reabsorption in the ascending limb. The effect size in homozygous and heterozygous patients indicated that minor alleles of CLDN14 SNPs did not have dominant activity on calcium reabsorption. Contrary to calcium excretion, values of serum calcium did not change according to the CLDN14 genotype during the saline test. This agrees with the normal serum calcium usually detected in patients with idiopathic hypercalciuria (31) and might be explained by the involvement of bone cells in serum calcium control (32,33): bone lining cells express CLDN14 and mediate calcium flux from bone tissue to blood (34). CLDN14 genotype could modify this flux, which is not related to bone remodeling and may be influenced by PTH (32,33). A previous study observed that carriers of the minor allele at rs219780 showed lower serum PTH, lower calcium excretion, and higher bone mineral density compared with homozygotes for the more common allele (14).
The larger proportion of CLDN14 SNPs associated with calcium excretion is located in the 3′ region of the gene, close to the unique coding exon, and it may have a regulatory function on CLDN14 expression. On the basis of recent bioinformatics data, associated tag SNPs map on intron regions of two antisense transcripts (nonprotein coding genes) with unknown function. Despite the concordance between them and CLDN14 expression in tissues, such as liver and kidney cortex, no information on a possible relationship among them is currently available. Moreover, no overlap with other regulatory elements was found in this gene region. Only one SNP (rs219780) among those associated with calcium excretion was exonic but synonymous. Although its minor allele does not affect amino acid sequence, it may represent a potential exonic splicing enhancer element that affects splicing events and tubular CLDN14 expression. In silico analysis found that the A minor variant at rs219780 introduces new binding sites for SRSF1 (IgM-BRCA1), SRSF2, and SRSF5 and also promotes a new branch site for splicing event. However, in vivo experiments are required to validate this in silico hypothesis. A previous in silico study hypothesized that rs78250838 might introduce a new transcription factor binding site within CLDN14, but this SNP was not associated with calcium excretion in our patients and was not in linkage with rs219780 and rs219755 (35).
CLDN14 expression may be stimulated by serum calcium and dietary calcium intake, which inhibit the production of two miRs, miR-9 and miR-374, that are suppressive of CLDN14 transcription through calcium-sensing receptor activity (8). SNPs associated with calcium excretion do not seem to affect binding sites for these miRs in the 3′ untranslated region of the CLDN14 gene (9), but they could have a role in the genetic background underlying kidney response to environmental stimuli and PTH (13).
These findings suggest that minor alleles of CLDN14 SNPs might play a protective role against hypercalciuria and kidney stones as described in populations of different origin (4,14–16,30). We confirmed the association of CLDN14 3′ region SNPs with 24-hour calcium excretion, but different SNPs were implicated with respect to previous studies (4,14–16). Conversely, we did not find association with kidney stone disease in our population. It should be emphasized that the presence of stones was “self-reported” by our patients, without any additional imaging tests aimed at finding silent stones. Moreover, the population under study was relatively young, and it was gathered to investigate essential hypertension and not kidney stones. These characteristics may explain our negative results about association with kidney stones, but they allowed us to explore the association of CLDN14, CLDN16, and CLDN19 SNPs with BP measured before and during the NaCl infusion. However, we did not find any associations of these SNPs with hypertension or BP.
In conclusion, our results suggest that CLDN14 SNPs are associated with urinary calcium excretion in 24-hour urine and in response to NaCl load. Variant alleles of CLDN14 SNPs may decrease expression of CLDN14 in the ascending limb of Henle loop and its inhibitory effect on paracellular calcium reabsorption. They may protect against hypercalciuria and potentially, disorders associated with hypercalciuria, like kidney stones and osteoporosis.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Inching toward a Greater Understanding of Genetic Hypercalciuria: The Role of Claudins,” on pages 1460–1462.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01770218/-/DCSupplemental.
References
- 1.Yu AS: Claudins and the kidney. J Am Soc Nephrol 26: 11–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olinger E, Houillier P, Devuyst O: Claudins: A tale of interactions in the thick ascending limb. Kidney Int 93: 535–537, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Günzel D, Yu ASL: Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corre T, Olinger E, Harris SE, Traglia M, Ulivi S, Lenarduzzi S, Belge H, Youhanna S, Tokonami N, Bonny O, Houillier P, Polasek O, Deary IJ, Starr JM, Toniolo D, Gasparini P, Vollenweider P, Hayward C, Bochud M, Devuyst O: Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine. Pflugers Arch 469: 91–103, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Vezzoli G, Terranegra A, Soldati L: Calcium-sensing receptor gene polymorphisms in patients with calcium nephrolithiasis. Curr Opin Nephrol Hypertens 21: 355–361, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M, Günzel D: Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci U S A 114: E219–E227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong Y, Hou J: Claudins in barrier and transport function-the kidney. Pflugers Arch 469: 105–113, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toka HR: New functional aspects of the extracellular calcium-sensing receptor. Curr Opin Nephrol Hypertens 23: 352–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J: Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, Hou J: Claudin-14 underlies Ca++-sensing receptor-mediated Ca++ metabolism via NFAT-microRNA-based mechanisms. J Am Soc Nephrol 25: 745–760, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toka HR, Al-Romaih K, Koshy JM, DiBartolo S 3rd, Kos CH, Quinn SJ, Curhan GC, Mount DB, Brown EM, Pollak MR: Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 23: 1879–1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikari A, Okude C, Sawada H, Sasaki Y, Yamazaki Y, Sugatani J, Degawa M, Miwa M: Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim Biophys Acta 1778: 283–290, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Courbebaisse M, Ide N, Fan Y, Hanai JI, Kaludjerovic J, Densmore MJ, Yuan Q, Toka HR, Pollak MR, Hou J, Lanske B: Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin14. Proc Natl Acad Sci U S A 114: E3344–E3353, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d’Ancona FCH, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K: Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41: 926–930, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Oddsson A, Sulem P, Helgason H, Edvardsson VO, Thorleifsson G, Sveinbjörnsson G, Haraldsdottir E, Eyjolfsson GI, Sigurdardottir O, Olafsson I, Masson G, Holm H, Gudbjartsson DF, Thorsteinsdottir U, Indridason OS, Palsson R, Stefansson K: Common and rare variants associated with kidney stones and biochemical traits. Nat Commun 6: 7975, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guha M, Bankura B, Ghosh S, Pattanayak AK, Ghosh S, Pal DK, Puri A, Kundu AK, Das M: Polymorphisms in CaSR and CLDN14 genes associated with increased risk of kidney stone disease in patients from the eastern part of India. PLoS One 10: e0130790, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology : 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 23: 3–16, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Yamakawa H, Suzuki H, Nakamura M, Ohno Y, Saruta T: Disturbed calcium metabolism in offspring of hypertensive parents. Hypertension 19: 528–534, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Cappuccio FP, Elliott P, Allender PS, Pryer J, Follman DA, Cutler JA: Epidemiologic association between dietary calcium intake and blood pressure: A meta-analysis of published data. Am J Epidemiol 142: 935–945, 1995 [DOI] [PubMed] [Google Scholar]
- 20.McCarron DA, Pingree PA, Rubin RJ, Gaucher SM, Molitch M, Krutzik S: Enhanced parathyroid function in essential hypertension: A homeostatic response to a urinary calcium leak. Hypertension 2: 162–168, 1980 [DOI] [PubMed] [Google Scholar]
- 21.Quereda C, Orte L, Sabater J, Navarro-Antolin J, Villafruela JJ, Ortuño J: Urinary calcium excretion in treated and untreated essential hypertension. J Am Soc Nephrol 7: 1058–1065, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T: Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, Arima H, Hoggart C, Tichet J, Nikitin YP, Conti C, Seidlerova J, Tikhonoff V, Stolarz-Skrzypek K, Johnson T, Devos N, Zagato L, Guarrera S, Zaninello R, Calabria A, Stancanelli B, Troffa C, Thijs L, Rizzi F, Simonova G, Lupoli S, Argiolas G, Braga D, D’Alessio MC, Ortu MF, Ricceri F, Mercurio M, Descombes P, Marconi M, Chalmers J, Harrap S, Filipovsky J, Bochud M, Iacoviello L, Ellis J, Stanton AV, Laan M, Padmanabhan S, Dominiczak AF, Samani NJ, Melander O, Jeunemaitre X, Manunta P, Shabo A, Vineis P, Cappuccio FP, Caulfield MJ, Matullo G, Rivolta C, Munroe PB, Barlassina C, Staessen JA, Beckmann JS, Cusi D: Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 59: 248–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manunta P, Cusi D, Barlassina C, Righetti M, Lanzani C, D’Amico M, Buzzi L, Citterio L, Stella P, Rivera R, Bianchi G: Alpha-adducin polymorphisms and renal sodium handling in essential hypertensive patients. Kidney Int 53: 1471–1478, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR: ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res 31: 3568–3571, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard JK, Przeworski M: Linkage disequilibrium in humans: Models and data. Am J Hum Genet 69: 1–14, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Yekutieli D: The control of the false discovery rate in multiple testing under dependency. Ann Stat 29: 1165–1188, 2001 [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC: PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vezzoli G, Terranegra A, Aloia A, Arcidiacono T, Milanesi L, Mosca E, Mingione A, Spotti D, Cusi D, Hou J, Hendy GN, Soldati L, Paloschi V, Dogliotti E, Brasacchio C, Dell’Antonio G, Montorsi F, Bertini R, Bellinzoni P, Guazzoni G, Borghi L, Guerra A, Allegri F, Ticinesi A, Meschi T, Nouvenne A, Lupo A, Fabris A, Gambaro G, Strazzullo P, Rendina D, De Filippo G, Brandi ML, Croppi E, Cianferotti L, Trinchieri A, Caudarella R, Cupisti A, Anglani F, Del Prete D; GENIAL network (Genetics and Environment in Nephrolithiasis Italian Alliance) : Decreased transcriptional activity of calcium-sensing receptor gene promoter 1 is associated with calcium nephrolithiasis. J Clin Endocrinol Metab 98: 3839–3847, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vezzoli G, Soldati L, Gambaro G: Hypercalciuria revisited: One or many conditions? Pediatr Nephrol 23: 503–506, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Marenzana M, Shipley AM, Squitiero P, Kunkel JG, Rubinacci A: Bone as an ion exchange organ: Evidence for instantaneous cell-dependent calcium efflux from bone not due to resorption. Bone 37: 545–554, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM: Large calcium fluxes that are not related to remodeling exist. Bone 33: 269, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Wongdee K, Riengrojpitak S, Krishnamra N, Charoenphandhu N: Claudin expression in the bone-lining cells of female rats exposed to long-standing acidemia. Exp Mol Pathol 88: 305–310, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Ure ME, Heydari E, Pan W, Ramesh A, Rehman S, Morgan C, Pinsk M, Erickson R, Herrmann JM, Dimke H, Cordat E, Lemaire M, Walter M, Alexander RT: A variant in a cis-regulatory element enhances claudin-14 expression and is associated with pediatric-onset hypercalciuria and kidney stones. Hum Mutat 38: 649–657, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.