Abstract
Patients with CKD exhibit a disproportionate burden of cardiovascular mortality, which likely stems from the presence of unique, nontraditional risk factors that accompany deteriorating kidney function. Mounting evidence suggests that alterations to the intestinal microbiome in CKD may serve as one such risk factor. The human intestinal tract is home to >100 trillion micro-organisms made up of a collection of commensal, symbiotic, and pathogenic species. These species along with their local environment constitute the intestinal microbiome. Patients with CKD show intestinal dysbiosis, an alteration of the gut micro-organism composition and function. Recent evidence links byproducts of intestinal dysbiosis to vascular calcification, atherosclerosis formation, and adverse cardiovascular outcomes in CKD. CKD-associated intestinal dysbiosis may also be accompanied by defects in intestinal barrier function, which could further enhance the negative effects of pathogenic intestinal bacteria in the human host. Thus, intestinal dysbiosis, defective intestinal barrier function, and a reduced capacity for clearance by the kidney of absorbed bacterial byproducts may all potentiate the development of cardiovascular disease in CKD. This narrative review focuses on microbiome-mediated mechanisms associated with CKD that may promote atherosclerosis formation and cardiovascular disease. It includes (1) new data supporting the hypothesis that intestinal barrier dysfunction leads to bacterial translocation and endotoxemia that potentiate systemic inflammation, (2) information on the accumulation of dietary-derived bacterial byproducts that stimulate pathways promoting atheromatous changes in arteries and cardiovascular disease, and (3) potential interventions. Despite great scientific interest in and a rapidly growing body of literature on the relationship between the microbiome and cardiovascular disease in CKD, many important questions remain unanswered.
Keywords: arteries, Atherosclerosis, Bacteria, Bacterial Translocation, Cardiovascular Diseases, Dysbiosis, Endotoxemia, Gastrointestinal Microbiome, Humans, Intestines, Microbiota, Renal Insufficiency, Chronic, risk factors, vascular calcification
Introduction
CKD affects 14% of adults in the United States (1). Patients with CKD are at a far greater risk of developing cardiovascular disease compared with the general population (2), and it is evident that this cardiovascular disease burden is not entirely driven by a higher prevalence of traditional risk factors. Accumulating evidence supports metabolites produced by intestinal microbes as nontraditional risk factors for cardiovascular disease in CKD. Thus, scientific interest is rapidly evolving to better understand the contribution of the intestinal microbiome to cardiovascular disease in patients with CKD. This review focuses on new evidence linking gut-derived microbial byproducts to atherosclerosis and cardiovascular outcomes in CKD.
Microbiome Alterations in CKD
The human intestinal tract is home to >100 trillion micro-organisms, which consist of a collection of commensal, symbiotic, and pathogenic species contributing to a local ecologic community termed the microbiome. The microbiome aids in food digestion, vitamin biosynthesis, bile acid biotransformation, innate immunity, and intestinal barrier maintenance. In humans, most intestinal bacteria belong to five phyla; however, substantial species-level variability exists and is determined by genetic and environmental factors (3). Recent studies show dysbiosis, which is characterized by shifts in the relative abundance of major bacterial populations, in fecal samples from patients with CKD (4) and patients with ESKD versus healthy controls (5–7). The factors contributing to dysbiosis in kidney disease remain largely undefined; however, dietary restriction (3), use of phosphate binders (8) and antibiotics (3,9), and even CKD (10) itself may play a role.
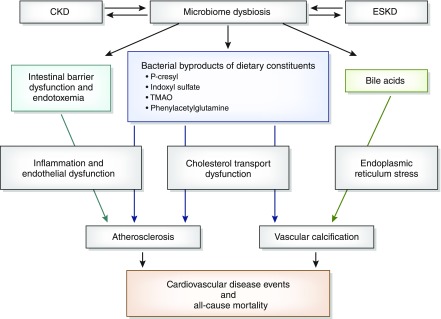
Intestinal dysbiosis that is observed in CKD and ESKD may increase the production of bacterial byproducts that are rapidly absorbed from the intestinal lumen. When increased absorption is coupled with decreased clearance by the kidneys, levels of gut-derived toxins rise in circulation and may promote cardiovascular disease. Additionally, animal studies suggest the presence of defects in intestinal barrier function in CKD (3). The endotoxemia and systemic inflammation, which could result from intestinal barrier defects, are also established risk factors for cardiovascular disease (11). A comprehensive review of the intestinal microbiome and CKD was recently published by Ramezani and Raj (3). In this review, we focus on new evidence that supports the mechanisms by which intestinal dysbiosis associated with kidney disease potentially contributes to cardiovascular disease (Figure 1, Table 1) and discuss potential interventions (Table 2).
Figure 1.
Mechanisms by which microbiome dysbiosis in kidney disease may lead to cardiovascular disease. TMAO, trimethylamine-N-oxide.
Table 1.
Microbiome-related mediators of cardiovascular disease and poor cardiovascular outcomes in CKD
| Cardiovascular Risk Factor and Potential Mediators | Mechanism of Cardiovascular Disease Promotion | Relationship to Cardiovascular Outcomes in Kidney Disease |
|---|---|---|
| Intestinal barrier disruption, endotoxemia, and inflammation | ||
| Bacterial endotoxins | ||
| Bloodstream entry facilitated by intestinal barrier dysfunction (3,10) causing systemic inflammation (10) | Innate immunity activation causing inflammation (11) | Higher endotoxin levels among patients with CKD and patients with ESKD compared with healthy controls (3) |
| Intestinal dysbiosis favoring pathologic bacterial populations (5) | Vascular endothelial dysfunction (3,11) | Endotoxin levels are higher among patients with ESKD and cardiovascular disease compared with those without cardiovascular disease (3) |
| Endotoxin levels and inflammation positively correlate with carotid intimal medial thickness in patients with ESKD (3) | ||
| Higher endotoxin levels are significantly associated with all-cause mortality in patients with ESKD (3) | ||
| Bacterial byproducts of dietary constituents | ||
| Indoxyl sulfate | ||
| Bacterial byproduct of tryptophan metabolism (4) | Induces inflammation and oxidative stress causing vascular endothelial cell injury by (13) leukocyte activation and adhesion, increasing proinflammatory cytokines and reactive oxygen species, and reducing NO production | Positively correlates with measures of vascular dysfunction (14,15) and aortic calcification (15) |
| Decreases macrophage cholesterol efflux | Independently associated with cardiovascular disease (15–17) and atherosclerosis (18) | |
| p-Cresyl | ||
| Derived from bacterial fermentation of tyrosine and pheylalanine (4) | Induces inflammation and oxidative stress causing vascular endothelial cell injury by (13) leukocyte activation and adhesion, increasing proinflammatory cytokines and reaction oxygen species, and reducing NO production | Positively correlates with measures of vascular dysfunction (14,15) and aortic calcification (15) |
| Independently associated with cardiovascular disease (15–17)and atherosclerosis (18) | ||
| TMAO | ||
| Bacterial conversion of l-carnitine and choline to TMA precursor (19–21) | Increases macrophage cholesterol scavenger receptors (19) | Independently associated with all-cause mortality (23,26,27,29) |
| Shift in dominant intestinal bacteria composition in CKD leads to altered gene expression favoring TMA production (4) | Reduces reverse cholesterol transport (20) | Independently associated with cardiovascular events and mortality (27) |
| TMA conversion to TMAO by hepatic Flavin mono-oxygenase (22) | Independently associated with greater atherosclerotic burden (23) | |
| Independently associated with cardiovascular ischemic events (25) | ||
| Phenylacetylglutamine | Mechanism not determined | Independently associated with cardiovascular disease events in CKD (31) and ESKD (32) |
| Bacterial conversion of phenylalanine to phenylacetic acid, which is then conjugated with glutamine | ||
| Bile acids | ||
| DCA | Induces vascular calcification through endoplasmic reticulum stress mechanism (37) | Independently associated with greater coronary artery calcification (39) |
| Intestinal bacteria biotransformation of primary bile acid (cholic acid) to secondary bile acid DCA (33) |
NO, nitric oxide; TMAO, trimethylamine-N-oxide; TMA, trimethylamine; DCA, deoxycholic acid.
Table 2.
Therapies that modify the human intestinal microbiome and bacterial byproduct metabolism in kidney disease
| Study Design | Intervention | Subjects | Primary Outcome | Secondary Outcomes | Ref. |
|---|---|---|---|---|---|
| Adsorbent | |||||
| RCT, 1:1 active versus placebo, 189-wk EPPIC1, 170-wk EPPIC2 | AST-120 | CKD 4, n=2035 | No change in time to kidney disease progressiona | No change in time to kidney disease progression,a including death | 40 |
| Synbiotic | |||||
| RCT, 2:1 active versus placebo, 30 d | Probinul Neutra | After kidney transplant, n=36 | ↓ p-Cresol | No change in kidney function, glycemia, lipids, albumin | 41 |
| RCT, active versus placebo, 6-wk crossover, 4-wk washout | Synbiotic | CKD 3–4, n=37 | No change in indoxyl sulfate | ↓ p-Cresol; significant change in microbiome; ↑ albuminuria; no change in eGFR, urinary kidney injury molecule-1, serum inflammatory, or oxidative stress biomarkers | 42 |
| RCT, 1:1 active versus placebo, 30 d | Probinul Neutra | CKD 3–4, n=30 | ↓ p-Cresol | No change in gastrointestinal symptoms | 43 |
| RCT, 1:1 active versus placebo, 2 mo | Synbiotic | ESKD, n=18 | ↑ Stool bifidobacterial counts | ↓ Gastrointestinal symptoms | 44 |
| RCT, 1:1 active versus placebo, 2 mo | Synbiotic | ESKD, n=44 | ↓ Gastrointestinal symptoms | ↓ Markers of malnutrition and serum CRP and TNF-α levels (NS) | 45 |
| Prebiotic | |||||
| RCT, active versus placebo, 6-wk crossover, 4-wk washout | Arabinoxylan oligosaccharides | CKD 3–4, n=39 | ↓ TMAO; no change in urea, p-cresyl sulfate, p-cresyl glucuronide, indoxyl sulfate, phenylacetylglutamine | No change in urinary excretion of urea, p-cresyl sulfate, p-cresyl glucuronide, indoxyl sulfate, phenylacetylglutamine, TMAO; no change in insulin resistance | 46 |
| Sevelamer | |||||
| RCT, 1:1 active versus placebo, 3 mo | Sevelamer | CKD 3–5 (predialysis), n=69 | ↓ p-Cresol | ↓ LDL and serum phosphorus | 8 |
| Dietary fiber | |||||
| Single blinding, 12 wk | Dietary fiber | CKD 3, n=13 | ↓ p-Cresol | ↑ Stool frequency; no change in quality of life | 47 |
| RCT, 1:1 active versus placebo, 6 wk | Dietary fiber | ESKD, n=40 | ↓ Indoxyl sulfate; ↓ p-cresol (NS) | No change in predialysis weight, BUN, serum albumin, prealbumin, CRP, phosphorus, or KDQOL-36 | 48 |
| Antibiotics | |||||
| Observational, 28 d | Single 250-mg oral dose of vancomycin | ESKD, n=10 | ↓ Indoxyl sulfate; ↓ p-cresyl sulfate | Decrease in gut microbiome diversity | 9 |
RCT, randomized, controlled trial; EPPIC1, Evaluating Prevention of Progression of CKD 1; EPPIC2, Evaluating Prevention of Progression of CKD 2; CRP, C-reactive protein; NS, nonsignificant; TMAO, trimethylamine-N-oxide; KDQOL-36, Kidney Disease Quality of Life 36 instrument.
Composite of dialysis initiation, kidney transplant, and serum creatinine doubling.
Dysbiosis, Intestinal Barrier Disruption, Endotoxemia, and Inflammation
Systemic inflammation is a consequence of reduced kidney function and a nontraditional risk factor for cardiovascular disease (11). Although the mechanisms triggering innate immunity and associated inflammation in CKD are debated, one hypothesis is that low levels of bacterial endotoxin are introduced into the bloodstream of patients with CKD and stimulate these pathways. Endotoxemia is observed to be more prevalent in patients with CKD and patients with ESKD versus controls (3), and it is associated with cardiovascular disease in CKD (3) and non-CKD populations (11). In addition to being proinflammatory stimuli, endotoxins directly cause endothelial dysfunction, one of the earliest findings in atherosclerosis (11).
The mechanisms whereby bacterial endotoxin is introduced into the circulation in patients with CKD remain unclear. Among patients undergoing dialysis, endotoxemia may result from the dialysis procedure. However, endotoxemia is noted in earlier stages of CKD (3), suggesting the contribution of factors unrelated to dialysis. As reviewed previously, disruption of the normal intestinal barrier may facilitate passage of bacterial endotoxins into the circulation. Translational studies support this hypothesis by showing significant reductions in intestinal epithelial cell tight junction proteins (claudin-1, occludin, and zona occludins 1) in rat models of CKD (3). Likewise, incubating human enterocytes in uremic plasma increases epithelial permeability and reduces tight junction protein expression (3).
New data show a relationship between CKD-related dysbiosis and systemic inflammation in both animals and humans (7,10). Furthermore, animal data suggest that dysbiosis itself alters intestinal barrier function, leading to bacterial translocation and systemic inflammation. More specifically, in a CKD mouse model showing systemic inflammation, endotoxemia, and intestinal dysbiosis, elimination of facultative anaerobic microbiota prevented bacterial translocation and reduced endotoxemia and systemic markers of inflammation (10). However, although dysbiosis in a small ESKD cohort was associated with systemic inflammation, there was no evidence of intestinal inflammation or bacterial translocation (7). If intestinal barrier function is compromised and leads to systemic inflammation, the origin of these changes in humans with CKD remains uncertain.
Bacterial Byproducts of Dietary Constituents
Indoxyl Sulfate and p-Cresyl
Indoxyl sulfate and p-cresyl are two well studied uremic toxins that are byproducts of bacterial metabolism of dietary constituents. Indoxyl sulfate and p-cresyl are excreted by the kidney, and serum concentrations progressively increase as GFR declines. These metabolites are highly protein bound; thus, they are poorly removed by dialysis. Intestinal bacteria tryptophanase enzymes convert dietary tryptophan to indole, which is absorbed and metabolized to indoxyl sulfate in the liver. p-Cresyl is derived from p-cresol, a product of bacterial fermentation of tyrosine and phenylalanine in the colon (3). Although elevated serum concentrations of indoxyl sulfate and p-cresyl in CKD may result from decreased excretion by the kidney, new data suggest that changes in the microbiome favor increased production of these compounds. A potential mechanism for increased formation of these uremic toxins in kidney disease could be epigenetic changes induced by gut dysbiosis, which could alter amino acid metabolism to favor indoxyl sulfate and p-cresyl generation. In support of this theory, new data from a CKD rat model with disordered amino acid metabolism and elevated indoxyl sulfate and p-cresyl showed that shifts in the abundance of specific intestinal bacteria were associated with activation of genes related to amino acid metabolism (12). Another recent investigation observed a greater relative abundance of intestinal bacteria containing enzymes that facilitate the generation of indoxyl sulfate and p-cresyl among patients with ESKD (5).
Both indoxyl sulfate and p-cresyl are purported vascular toxins. Cell culture and animal studies show that these metabolites cause vascular endothelial cell injury by increasing leukocyte activation and adhesion and contributing to local inflammation and oxidative stress (13). Indoxyl sulfate also seems to decrease macrophage cholesterol efflux (13), an important pathway for preventing foam cell formation. Human data support these mechanistic findings. Higher concentrations of circulating indoxyl sulfate and p-cresyl correlate with measures of vascular dysfunction (14,15) and aortic calcification (15), and they are independently associated with cardiovascular disease among individuals with kidney disease (14–17). However, not all studies agree with these findings. In a large cohort of 1273 patients on dialysis, indoxyl sulfate and p-cresol were not associated with cardiovascular events (18). Only in a subcohort of patients on dialysis with low serum albumin was p-cresyl significantly associated with cardiovascular events (18), suggesting that other factors may modify this relationship.
Trimethylamine-N-Oxide
Trimethylamine-N-oxide (TMAO) is a byproduct of bacterial metabolism of dietary choline and l-carnitine, and recent studies suggest that it directly promotes atherosclerosis (19–21). Intestinal bacteria metabolize dietary choline and l-carnitine to trimethylamine (19–21), which undergoes rapid intestinal absorption and subsequent oxidation in the liver by flavin mono-oxygenase enzymes to TMAO (22). Serum concentrations of TMAO increase with advancing CKD and are at least 30-fold higher in patients with ESKD (23). Data are conflicting as to whether these marked elevations result solely from decreased clearance by the kidneys or are due to increased production of this metabolite. Animal studies suggest that differences in microbiota diversity (24) may explain the significant interpatient variability of blood TMAO levels among patients with similar GFR (25). Recent human and animal data suggest that dysbiosis is directly responsible for higher TMAO levels observed in CKD (4). In these studies, patients with CKD showed a shift in the relative abundance of dominant intestinal bacteria compared with healthy controls; this shift in gut bacterial composition was accompanied by altered gene expression favoring trimethylamine production in patients with CKD. Moreover, when the gut flora from patients with CKD was used to repopulate the intestines of antibiotic-treated mice, serum TMAO levels increased (4).
In mice, TMAO directly induces atherosclerosis by increasing macrophage cholesterol scavenger receptors (19) and reducing reverse cholesterol transport (20), resulting in greater foam cell formation, a characteristic of early atherosclerosis. TMAO is independently associated with both prevalent and incident cardiovascular disease risk, including atherosclerosis, among non-CKD populations (19–21). Furthermore, higher circulating concentrations of TMAO are associated with all-cause mortality (23,26,27) and greater burden of coronary atherosclerosis (23) and ischemic cardiovascular events (25) among patients with CKD. However, among patients with ESKD, data are conflicting (28,29). Thus, if TMAO is a cardiovascular toxin, it seems that serum levels may better predict cardiovascular events among patients with preserved kidney function or moderate CKD (21,23,25).
Phenylacetylglutamine
Phenylacetylglutamine is a newly identified bacterial metabolite of amino acid fermentation. It is derived from the bacterial conversion of phenylalanine to phenylacetic acid, which is then conjugated with glutamine. Phenylacetylglutamine is absorbed into the circulation and efficiently cleared by the kidneys. Serum levels of this compound are elevated in advanced CKD (30). Although decrements in glomerular filtration may largely explain elevated serum concentrations of phenylacetylglutamine in CKD (30), a study evaluating 24-hour urinary excretion of phenylacetylglutamine among patients with predialysis CKD and patients with new kidney transplants suggests that microbiome changes may enhance the production and intestinal absorption of phenylacetylglutamine (31). Moderately sized observational studies suggest an independent relationship between higher serum phenylacetylglutamine levels and adverse outcomes among individuals with kidney disease. Among 488 patients with predialysis CKD, serum phenylacetylglutamine was independently associated with greater mortality and cardiovascular disease (31). Likewise, among 394 patients on hemodialysis, serum phenylacetylglutamine was also independently associated with cardiovascular disease events (32). However, in a larger cohort (n=1273) of patients on dialysis, there was no association with cardiovascular events (18). Because phenylacetylglutamine is a bacterial byproduct of amino acid metabolism and because there is some evidence that it may be influenced by the microbiome composition (32), it is a candidate for further investigation into the intersection of dysbiosis and cardiovascular disease in kidney disease.
Bile Acid Metabolism
Intestinal bacteria induce biotransformation of primary bile acids to secondary bile acids, including deoxycholic acid (DCA) (33). In CKD, although all circulating bile acids are elevated (34,35), the proportion of circulating DCA is increased compared with others (34). Thus, CKD-associated dysbiosis may be responsible for elevated circulating DCA levels. Bile acids, especially cheno-DCA, stimulate the farnesoid X nuclear receptor (FXR) to regulate lipid and glucose metabolism. The FXR may influence cardiovascular disease not only because dysregulation of lipid and glucose metabolism promotes endothelial dysfunction and atherosclerosis but also because FXR has been identified in atherosclerotic lesions and endothelial cells (36). Moreover, DCA is directly toxic to cultured vascular smooth muscle cells (35); thus, it may also have direct vascular toxicity. FXR activation decreases DCA levels and attenuates vascular toxicity by reducing vascular calcification (35,37) and atherosclerotic plaque formation in animal models (38). It is plausible that elevated circulating DCA levels along with disproportionately lower levels of other bile acids that have a higher affinity for FXR promote vascular calcification and atherosclerosis in CKD. We recently described an independent relationship between elevated circulating DCA levels and coronary artery calcification among patients with moderate CKD (39). Among 112 patients with a mean eGFR of 31.5±8.7 ml/min per 1.73 m2, those with a DCA level greater than the median of 58 ng/ml had significantly greater coronary artery calcification scores compared with those whose DCA level was less than or equal to the median. Likewise, when modeled as a continuous variable, higher DCA levels were associated with greater coronary artery calcification scores. These relationships were unchanged and remained significant after adjustment for demographics, comorbidities, and markers of mineral metabolism, including calcium, phosphorus, parathyroid hormone, and fibroblast growth factor 23.
Potential Interventions
Modifying the intestinal microbiome may be a candidate therapy to mitigate cardiovascular disease among individuals with kidney disease. Therapies are aimed at changing the relative abundance of intestinal microbial species to improve inflammation and lower serum concentrations of bacteria-derived toxins, which are associated with cardiovascular disease. In recent small interventional trials, synbiotics (pre- and probiotics), antibiotics, sevelamer, and a high-fiber diet showed the ability to reduce serum indoxyl sulfate and p-cresol as well as change the composition of some intestinal microbial species among patients with CKD and patients with ESKD (Table 1). In animal models and small human trials, the oral adsorbent, AST-120, partially restored intestinal epithelial tight junction proteins, reduced inflammation and serum levels of indoxyl sulfate, and slowed kidney disease progression (3). However, a large randomized, placebo-controlled trial among 2035 subjects with CKD stage 4 showed no significant benefit in kidney disease progression or death of AST-120 (40). These conflicting results expose a potential gap in the understanding of the microbiome and its relationship to clinical outcomes, such as mortality and cardiovascular disease, in kidney disease. Interventions that shift the composition of the microbiome and even improve circulating concentrations of bacterial byproducts with purported organ toxicity do not necessarily translate to improved clinical outcomes. This conundrum illustrates the complexity of the microbiome and our ability to alter it to improve cardiovascular outcomes in kidney disease. More large interventional trials are needed to determine whether other therapies that modify the intestinal microbiome improve hard clinical end points in kidney disease.
Conclusions
Mounting evidence suggests that CKD is accompanied by shifts in the relative abundance of gut bacterial populations and defects in intestinal barrier function leading to systemic inflammation. The retention of metabolites derived from intestinal bacteria is a common observation in patients with CKD, possibly resulting from reduced clearance by the kidneys of these substances and alterations in their production and intestinal absorption. Translational research studies show that many bacterial byproducts directly stimulate pathways that promote deleterious changes in arteries. Numerous epidemiologic studies show a relationship between gut-derived vascular toxins and cardiovascular events in patients with CKD. Although various interventions may alter the composition of intestinal microbiota in CKD and ESKD, clinical benefit is not yet confirmed. As a result, the intestinal microbiome is emerging as a key target for research focused on understanding the contribution of nontraditional risk factors to cardiovascular morbidity in CKD. As an important first step to realizing the gut microbiome as a therapeutic target, efforts to better define microbiome changes in CKD are currently underway (NCT02572882 and NCT03265639).
Disclosures
A.J. and J.S. have no disclosures. T.I. received consulting honorarium from Bayer and receives investigational drugs for ongoing study from Shire and Endurance Products Co.
Acknowledgments
The work reported in this study was supported by Veterans Administration grant CDA 5IK2CX001030-03 (to A.J.) and National Institutes of Health grant R01DK110087 (to T.I.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.United States Renal Data System: United States Renal Data System Annual Data Report 2015. CKD in the General Population. Available at: https://www.usrds.org/2015/view/v1_01.aspx. Accessed September 25, 2017
- 2.Kuznik A, Mardekian J, Tarasenko L: Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: An analysis of national health and nutritional examination survey data, 2001-2010. BMC Nephrol 14: 132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramezani A, Raj DS: The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, Nie J, Zhou HW, Yin J: Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep 7: 1445, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND: Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang S, Xie S, Lv D, Wang P, He H, Zhang T, Zhou Y, Lin Q, Zhou H, Jiang J, Nie J, Hou F, Chen Y: Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep 7: 2870, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadlbauer V, Horvath A, Ribitsch W, Schmerböck B, Schilcher G, Lemesch S, Stiegler P, Rosenkranz AR, Fickert P, Leber B: Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci Rep 7: 15601, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riccio E, Sabbatini M, Bruzzese D, Grumetto L, Marchetiello C, Amicone M, Andreucci M, Guida B, Passaretti D, Russo G, Pisani A: Plasma p-cresol lowering effect of sevelamer in non-dialysis CKD patients: Evidence from a randomized controlled trial [published online ahead of print November 20, 2017]. Clin Exp Nephrol doi:10.1007/s10157-017-1504-8 [DOI] [PubMed] [Google Scholar]
- 9.Nazzal L, Roberts J, Singh P, Jhawar S, Matalon A, Gao Z, Holzman R, Liebes L, Blaser MJ, Lowenstein J: Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol Dial Transplant 32: 1809–1817, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Andersen K, Kesper MS, Marschner JA, Konrad L, Ryu M, Kumar Vr S, Kulkarni OP, Mulay SR, Romoli S, Demleitner J, Schiller P, Dietrich A, Müller S, Gross O, Ruscheweyh HJ, Huson DH, Stecher B, Anders HJ: Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol 28: 76–83, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Li J, Yu J, Wang Y, Lu J, Shang EX, Zhu Z, Guo J, Duan J: Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. J Pharm Biomed Anal 149: 425–435, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Sirich TL, Meyer TW, Gondouin B, Brunet P, Niwa T: Protein-bound molecules: A large family with a bad character. Semin Nephrol 34: 106–117, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Rossi M, Campbell K, Johnson D, Stanton T, Pascoe E, Hawley C, Dimeski G, McWhinney B, Ungerer J, Isbel N: Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: An observational study. Nutr Metab Cardiovasc Dis 24: 1035–1042, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox): Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P: p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5: 1182–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CP, Lu LF, Yu TH, Hung WC, Chiu CA, Chung FM, Yeh LR, Chen HJ, Lee YJ, Houng JY: Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis severity in stable angina patients with early stage of renal failure. Atherosclerosis 211: 579–583, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Shafi T, Sirich TL, Meyer TW, Hostetter TH, Plummer NS, Hwang S, Melamed ML, Banerjee T, Coresh J, Powe NR: Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int 92: 1484–1492, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL: Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19: 576–585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL: Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368: 1575–1584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, Philpot RM, Rettie AE: Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3. Biochem Pharmacol 56: 1005–1012, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS: Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 27: 305–313, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano KA, Vivas EI, Amador-Noguez D, Rey FE: Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 6: e02481, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto C, Levin A; CanPREDDICT Investigators: Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int 89: 1144–1152, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, Stenvinkel P, Bergman P: Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 11: e0141738, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL: Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116: 448–455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O: Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr 25: 351–356, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafi T, Powe NR, Meyer TW, Hwang S, Hai X, Melamed ML, Banerjee T, Coresh J, Hostetter TH: Trimethylamine o-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol 28: 321–331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poesen R, Claes K, Evenepoel P, de Loor H, Augustijns P, Kuypers D, Meijers B: Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol 27: 3479–3487, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poesen R, Evenepoel P, de Loor H, Bammens B, Claes K, Sprangers B, Naesens M, Kuypers D, Augustijns P, Meijers B: The influence of renal transplantation on retained microbial-human co-metabolites. Nephrol Dial Transplant 31: 1721–1729, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J, Powe NR: Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: Results from the retained organic solutes and clinical outcomes (ROSCO) investigators. PLoS One 10: e0126048, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridlon JM, Kang DJ, Hylemon PB: Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Jimenez F, Monte MJ, El-Mir MY, Pascual MJ, Marin JJ: Chronic renal failure-induced changes in serum and urine bile acid profiles. Dig Dis Sci 47: 2398–2406, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki M, Miyazaki-Anzai S, Masuda M, Kremoser C: Deoxycholic acid contributes to chronic kidney disease-dependent vascular calcification. Circulation 128: A14937, 2013 [Google Scholar]
- 36.Cariou B, Staels B: FXR: A promising target for the metabolic syndrome? Trends Pharmacol Sci 28: 236–243, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki-Anzai S, Levi M, Kratzer A, Ting TC, Lewis LB, Miyazaki M: Farnesoid X receptor activation prevents the development of vascular calcification in ApoE-/- mice with chronic kidney disease. Circ Res 106: 1807–1817, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki-Anzai S, Masuda M, Levi M, Keenan AL, Miyazaki M: Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS One 9: e108270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jovanovich A, Isakova T, Block G, Stubbs J, Smits G, Chonchol M, Miyazaki M: Deoxycholic acid, a metabolite of circulating bile acids, and coronary artery vascular calcification in CKD. Am J Kidney Dis 71: 27–34, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M: Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 26: 1732–1746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guida B, Cataldi M, Memoli A, Trio R, di Maro M, Grumetto L, Capuano I, Federico S, Pisani A, Sabbatini M: Effect of a short-course treatment with synbiotics on plasma p-cresol concentration in kidney transplant recipients. J Am Coll Nutr 36: 586–591, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto CC, McWhinney BC, Ungerer JP, Campbell KL: Synbiotics easing renal failure by improving gut microbiology (SYNERGY): A randomized trial. Clin J Am Soc Nephrol 11: 223–231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, Barbato F, Cataldi M: Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr Metab Cardiovasc Dis 24: 1043–1049, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, García-García G, Parra-Rojas I, Castro-Alarcón N: Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr 24: 330–335, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Viramontes-Hörner D, Márquez-Sandoval F, Martín-del-Campo F, Vizmanos-Lamotte B, Sandoval-Rodríguez A, Armendáriz-Borunda J, García-Bejarano H, Renoirte-López K, García-García G: Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J Ren Nutr 25: 284–291, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Poesen R, Evenepoel P, de Loor H, Delcour JA, Courtin CM, Kuypers D, Augustijns P, Verbeke K, Meijers B: The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: A randomized controlled trial. PLoS One 11: e0153893, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmean YA, Segal MS, Palii SP, Dahl WJ: Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J Ren Nutr 25: 316–320, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW: Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9: 1603–1610, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]