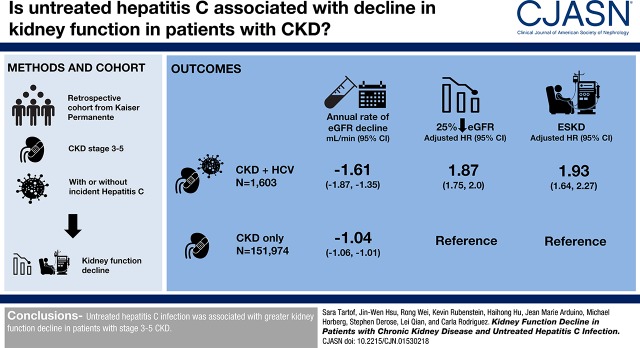
Abstract
Background and objectives
Studies evaluating the role of hepatitis C viral (HCV) infection on the progression of CKD are few and conflicting. Therefore, we evaluated the association of untreated HCV on kidney function decline in patients with stage 3–5 CKD.
Design, setting, participants, & measurements
This retrospective cohort study included members of Kaiser Permanente Southern California and Kaiser Permanente Mid-Atlantic States aged ≥18 years, with incident HCV and CKD diagnoses from January 1, 2004 to December 31, 2014. We used generalized estimating equations to compare the rate of change in eGFR between those with HCV and CKD versus CKD alone, adjusting for covariates. Cox proportional hazards models compared the risk of 25% decrease in eGFR and ESKD (defined as progression to eGFR<15 ml/min per 1.73 m2 on two or more occasions, at least 90 days apart) in those with HCV and CKD versus CKD alone, adjusting for covariates.
Results
We identified 151,974 patients with CKD only and 1603 patients with HCV and CKD who met the study criteria. The adjusted annual decline of eGFR among patients with HCV and CKD was greater by 0.58 (95% confidence interval [95% CI], 0.31 to 0.84) ml/min per 1.73 m2, compared with that in the CKD-only population (HCV and CKD, −1.61; 95% CI, −1.87 to −1.35 ml/min; CKD only, −1.04; 95% CI, −1.06 to −1.01 ml/min). Adjusted for covariates, the hazard for a 25% decline in eGFR and for ESKD were 1.87 (95% CI, 1.75 to 2.00) and 1.93 (95% CI, 1.64 to 2.27) times higher among those with HCV and CKD, respectively, compared with those with CKD only.
Conclusions
Untreated HCV infection was associated with greater kidney function decline in patients with stage 3–5 CKD.
Keywords: chronic kidney disease; clinical epidemiology; end-stage renal disease; Epidemiology and outcomes; ESRD; hepatitis; glomerular filtration rate; Proportional Hazards Models; Retrospective Studies; Renal Insufficiency, Chronic; Kidney Failure, Chronic; Hepatitis C; Disease Progression
Visual Abstract
Introduction
CKD is a worldwide health problem that affects 10%–16% of adults (1,2). Hepatitis C virus (HCV) infection is a cause of some forms of CKD and infects approximately 3% of the world population (3,4). Both diseases, particularly when left untreated, cause considerable morbidity and mortality worldwide.
The role of HCV on the development and progression of CKD is controversial. Although some studies have demonstrated an association between HCV infection and developing incident CKD or ESKD (5–11), others have not (12–17). Studies to evaluate the role of HCV on the progression of CKD are scarce, and are also conflicting. An association between HCV and the progression of CKD has been demonstrated for those with certain high-risk conditions, such as diabetic nephropathy, HIV (11,18–21), and for veterans (11). However, other evidence shows lack of association between HCV infection and CKD progression (22). Lack of consensus regarding the role of HCV on CKD progression may be because of limitations of prior work, including small sample sizes, varying definitions of CKD outcomes (12), cross-sectional study design, lack of inclusion of women, lack of HCV genotypic data, and lack of consideration of HCV treatment. Successful IFN-based treatment for HCV has been shown to reduce the risk of ESKD (23), and inclusion of this covariate is important to test associations with kidney function decline.
Therefore, we evaluated decline in kidney function in patients with stage 3–5 CKD (eGFR<60 ml/min per 1.73 m2) and untreated HCV infection. Although most studies evaluate the effect of HCV infection on the development of de novo CKD and progression in patients on dialysis and patients with kidney transplant, we provide a novel perspective in a population with stage 3–5 CKD. Restricting our HCV population to untreated patients allows a natural history perspective of the combined effect of HCV and CKD on kidney function decline. New data challenge previously established end points for CKD progression used in clinical trials, demonstrating that lesser changes in eGFR over 1, 2, and 3 years are strongly associated with subsequent ESKD and mortality (24). Knowing whether HCV infection contributes to a more rapid decline could aid the monitoring and treatment programs of patients with both CKD and HCV.
Materials and Methods
Setting
This study included patients from Kaiser Permanente Southern California (KPSC) and Kaiser Permanente Mid-Atlantic States (KPMAS; comprised of Baltimore, the District of Columbia, parts of Maryland, and northern Virginia), which collectively care for >4.7 million health plan members. At both sites, the member populations are representative of the socioeconomic and racial diversity of the area population (25). Both sites obtained data using electronic health records, which integrate medical information from all care settings. Minimal copays are a strong incentive to receive care within the system, contributing to highly complete capture of member health care utilization data.
Study Population
This retrospective cohort study included patients aged ≥18 years at the time of incident diagnoses of HCV or CKD from January 1, 2004 to December 31, 2014. An inclusion criterion was active enrollment with no membership gaps >45 days in the year before diagnosis date to allow for collection of baseline data. All patients were required to have at least one baseline eGFR measure (defined by the CKD Epidemiology Collaboration equation to estimate eGFR [26]), and one eGFR measurement during the follow-up period. The CKD Epidemiology Collaboration equation requires age, sex, and race to calculate eGFR. Patients who received HCV treatment before the index date were excluded from the study; patients who initiated dialysis or received a liver or kidney transplant before the index date were also excluded, as these procedures can render unreliable eGFR values (27,28). Further, patients with eGFR<15 ml/min per 1.73 m2 at baseline were excluded from the ESKD analyses (described below).
CKD was identified by two occasions of eGFR<60 ml/min per 1.73 m2 that are >90 days apart, with eGFR never returning to ≥60 ml/min per 1.73 m2. Chronic HCV infection was identified by at least one positive HCV RNA, HCV genotype, or positive HCV antibody test plus one or more HCV-coded visit. For CKD, the diagnosis date was attributed to the earliest date in the series of eGFR<60 ml/min per 1.73 m2. The HCV diagnosis date was attributed to the earliest date where one of the criteria above were satisfied.
Patients in the CKD-only cohort consisted of patients that were diagnosed with CKD and never received a diagnosis of HCV during the study period. Follow-up time for the CKD-only cohort started at the time of CKD diagnosis (index date), as stated above. Patients in the HCV and CKD cohort consisted of patients who were diagnosed with CKD and HCV during the study period, with either condition occurring first. For the HCV and CKD cohort, follow-up time began at the time of diagnosis of the latter of HCV or CKD (index date) (Supplemental Figure 1). When CKD was diagnosed first and HCV second, the closest eGFR value ±30 days to the time of diagnosis of HCV determined baseline eGFR, as this was the index date (Supplemental Figure 1). Patients in the HCV and CKD and CKD-only cohorts were mutually exclusive.
Outcome
Outcomes of interest included (1) rate of decline in eGFR from baseline, (2) 25% decline in eGFR (24,29,30), and (3) development of ESKD (defined as progression to eGFR<15 ml/min per 1.73 m2 on two or more occasions at least 90 days apart), dialysis, or kidney transplant approval (may or may not result in actual kidney transplant). To address fluctuations related to acute illness or various inputs (31,32), we identified the median of all serum creatinine measures taken on the same date. If there was a cluster of measurements where any two successive measurements were within 8 days of each other, we used the serum creatinine measurement taken on the median date. This median creatinine measurement was used for the eGFR calculation, such that there was no more than one eGFR measurement per week.
Follow-Up Time
Follow-up time began on the index date, defined above, and continued as long as the patient was continuously enrolled (allowing a 45-day gap for enrollment to appear in administrative databases). Follow-up time was censored at the time of initiation of HCV therapy, at the start of dialysis, actual liver or kidney transplant, death, end of the study period (December 31, 2014), or disenrollment, whichever came first. For the ESKD analyses, censoring occurred on death, disenrollment, end of the study period, actual liver transplant, or HCV treatment initiation.
Statistical Analyses
We compared the rate of change in eGFR, risk of a 25% decrease in eGFR from baseline, and risk of ESKD between patients with CKD and treatment-naïve HCV compared with those with CKD alone. To compare the estimated mean rate of change in eGFR between exposure groups, we used generalized estimating equations adjusted for potential confounding variables, including age, sex, race/ethnicity, diabetes, hypertension, acute myocardial infarction, HIV, hepatitis B virus, end-stage liver disease (ESLD), and CKD stage (see Supplemental Table 1 for detailed definitions of clinical covariates) (33). Covariates were assessed in the year before index date (baseline). Risk of a 25% decrease in eGFR and risk of ESKD were estimated using Cox proportional hazards cause-specific competing risk models adjusted for covariates as above with the exception that eGFR at baseline (i.e., closest eGFR measurement within 30 days of index date) was used in place of CKD stage (34). Death, dialysis and kidney or liver transplant were considered competing risk events for risk of a 25% change; death and liver transplant were considered competing risk events for the ESKD risk model. Log-log survival plots were used to test for assumptions of proportional hazards.
Analyses were stratified by sex, age, race, diabetes, hypertension, and CKD stage in separate models. Analyses stratifying the ESKD outcome into persistent eGFR<15 ml/min per 1.73 m2 and dialysis were also performed. Three sensitivity analyses were conducted: the first used propensity score matched models to estimate risk of 25% decline in eGFR and ESKD, the second restricted analyses to those with positive HCV RNA as sole diagnostic criteria, and the third assessed patients with and without ESLD.
All analyses were performed as complete case analyses. Analyses were conducted with SAS (version 9.3 for Windows; SAS Institute, Cary, NC). The study protocol was reviewed and approved by the KPSC Institutional Review Board, which waived requirement for informed consent.
Results
Study Cohorts
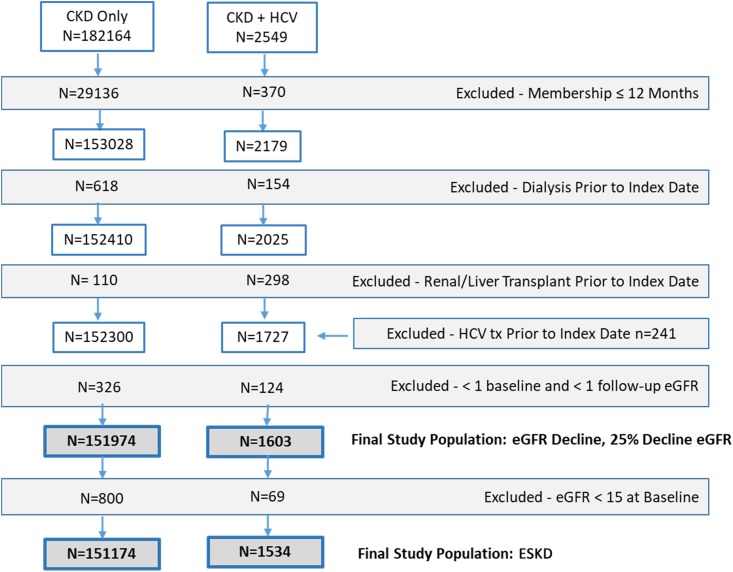
A total of 182,164 patients with stage 3–5 CKD and no HCV, and 2549 patients with HCV and CKD were identified during the study period. Of these, 30,198 (17%) and 745 (29%) had <12 months membership, dialysis, kidney or liver transplant, HCV treatment, or fewer than one baseline and one follow-up eGFR measurement documented and were excluded from the study, respectively (Figure 1). Patients were also excluded because of the requirement of complete race, age, and sex data for the eGFR equation; 3.7% of patients with at least one serum creatinine measurement were excluded because of missing race data, and 2.6% had race imputed using the algorithm reported in the Geographically Enriched Member Sociodemographics national Kaiser Permanente datamart (35). No patients had other covariate data missing. Patients excluded because of HCV treatment differed by some variables compared with included patients (Supplemental Table 2). For the ESKD analyses, an additional 800 patients with CKD only, and 69 patients with HCV and CKD were excluded because of an eGFR<15 ml/min per 1.73 m2 at baseline.
Figure 1.
Application of exclusion criteria to obtain final study population. Some patients met more than one exclusion criterion.
The final study cohort for the analyses of rate of eGFR decline and risk of 25% eGFR decline consisted of 151,974 patients with CKD only and 1603 patients with HCV and CKD. Of the HCV and CKD population, 66% were diagnosed with HCV first (median time to CKD diagnosis, 1362 days), 32% were diagnosed with CKD first (median time to HCV diagnosis, 631 days), and 2% were diagnosed on the same date. Overall, the group with HCV and CKD were younger than those with CKD only and had a higher proportion of men and black and Hispanic participants (Table 1). Patients with HCV and CKD had greater prevalence of comorbidities than those with CKD only. Most patients in both cohorts had CKD stage 3 at baseline (baseline eGFR: HCV and CKD, 44; SD, 12.86; CKD only, 50; SD, 8.21) (Table 1).
Table 1.
Clinical and sociodemographic characteristics of study cohort patients with CKD and hepatitis C infection compared with those with CKD alone
| Characteristic | Total n=153,577 | HCV and CKD n=1603 | CKD Only n=151,974 |
|---|---|---|---|
| Sex | |||
| Women | 83,050 (54%) | 691 (43%) | 82,359 (54%) |
| Men | 70,527 (46%) | 912 (57%) | 69,615 (46%) |
| Age at CKD index date | |||
| Mean (SD) | 71 (11) | 65 (11) | 71 (11) |
| Median | 72 | 64 | 72 |
| Q1, Q3 | 64.0, 80.0 | 58.0, 73.0 | 64.0, 80.0 |
| Race | |||
| Asian/native Hawaiian or other Pacific Islander | 12,400 (8%) | 78 (5%) | 12,322 (8%) |
| Black or African | 22,965 (15%) | 576 (36%) | 22,389 (15%) |
| Hispanic | 26,926 (18%) | 379 (24%) | 26,547 (18%) |
| Multiple/others | 492 (0.3%) | 5 (0.3%) | 487 (0.3%) |
| White | 90,794 (59%) | 565 (35%) | 90,229 (59%) |
| History of type 1 and type 2 diabetes mellitus | 48,735 (32%) | 675 (42%) | 48,060 (32%) |
| Hypertension | 93,885 (61%) | 1128 (70%) | 92,757 (61%) |
| HIV | 616 (0.4%) | 60 (4%) | 556 (0.4%) |
| Hepatitis B | 483 (0.3%) | 24 (2%) | 459 (0.3%) |
| End-stage liver disease | 658 (0.4%) | 141 (9%) | 517 (0.3%) |
| Acute myocardial infarction | 3854 (3%) | 38 (2%) | 3816 (3%) |
| eGFR value at baseline | |||
| Mean (SD) | 50 (8) | 44 (13) | 50 (8) |
| Median (Q1, Q3) | 52 (46, 56) | 48 (38, 54) | 52 (46, 56) |
| CKD stage at baseline | |||
| Stage 3 | 148,931 (97%) | 1384 (86%) | 147,547 (97%) |
| Stage 4 | 3777 (23%) | 150 (9%) | 3627 (2%) |
| Stage 5 | 869 (0.6%) | 69 (4%) | 800 (0.5%) |
| Number of follow-up eGFR measurements | |||
| Mean (SD) | 9.8 (10.9) | 13.6 (14.5) | 9.8 (10.8) |
| Median (Q1, Q3) | 6 (3, 13) | 8 (4, 18) | 6 (3, 13.0) |
| Total follow-up time, yr | |||
| Mean (SD) | 3.8 (2.9) | 3.1 (2.6) | 3.8 (2.9) |
| Median (Q1, Q3) | 2.9 (1.4, 5.6) | 2.4 (1.0, 4.5) | 2.9 (1.4, 5.6) |
HCV, hepatitis C virus; Q1, Quartile 1, Q3, Quartile 3.
The median number of follow-up eGFR measurements was somewhat higher in those with HCV and CKD (HCV and CKD median, 8; interquartile range, 4–18 versus CKD-only median, 6; interquartile range, 3–13). However, the median length of follow-up before censoring and outcomes was similar between the two groups (2.4 versus 2.9 years).
For the ESKD analyses, the final CKD-only cohort included 151,174 patients, and the HCV and CKD cohort included 1534 patients (Figure 1). The distribution of covariates after exclusion remained comparable with that described above (data available on request).
Comparing Rate of Change in eGFR
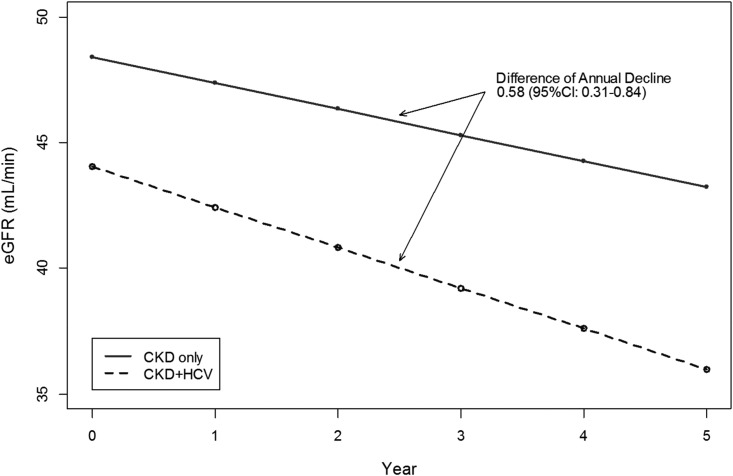
In unadjusted analyses, the annual decline in eGFR was greater by 2.39 ml/min per 1.73 m2 in patients with HCV and CKD versus those with CKD alone. After adjustment for covariates, the decline of eGFR among patients with HCV and CKD was greater by 0.58 (95% confidence interval [95% CI], 0.31 to 0.84) ml/min per 1.73 m2, compared with that in the CKD-only population (HCV and CKD, −1.61; 95% CI, −1.87 to −1.35 ml/min per 1.73 m2; CKD only, −1.04; 95% CI, −1.06 to −1.01 ml/min per 1.73 m2) (Figure 2; Supplemental Table 3).
Figure 2.
Adjusted annual decline in eGFR in patients with HCV and CKD and those with CKD alone. Adjusted for age, sex, race/ethnicity, CKD stage, diabetes, hypertension, HIV, hepatitis B infection, myocardial infarction, end-stage liver disease; and interactions between time and HCV and CKD status, diabetes, hepatitis B virus, and race.
Hazard for a 25% Decline in eGFR and ESKD
The unadjusted hazard for a 25% decline in eGFR comparing those with HCV and CKD to those with CKD alone was 2.17 (95% CI, 2.03 to 2.32) (Incidence rate [IR] in HCV and CKD, 380.5 per 100,000 person-years; IR in CKD only, 170.8 per 100,000 person-years). Adjusted for covariates, the hazard for a 25% decline in eGFR was 1.87 (95% CI, 1.75 to 2.00) times higher among those with HCV and CKD compared with those with CKD only (Table 2). Patients with ESLD, diabetes, and black and Hispanic race/ethnicity (versus white) had greater hazard for a 25% decline than patients without those characteristics. Censoring between the two comparison groups was comparable for death, but differed for dialysis and liver transplant. Overall, 2% of patients with HCV and CKD initiated HCV treatment (Table 3).
Table 2.
Adjusted cause-specific hazard ratio for 25% decrease in eGFR and ESKD in patients with CKD and hepatitis C infection compared with those with CKD alone
| Characteristics | 25% Decline in eGFR | ESKD | ||||
|---|---|---|---|---|---|---|
| No. of Patients with 25% Decrease | HRa,b | 95% CI | No. of Patients with ESKD | HRa,b | 95% CI | |
| Cohort | ||||||
| HCV and CKD | 915 | 1.87 | 1.75 to 2.00 | 162 | 1.93 | 1.64 to 2.27 |
| CKD only | 65,133 | 1.00 | Reference | 5240 | 1.00 | Reference |
| Age, yr | 66,048 | 1.00 | 1.00 to 1.00 | 5402 | 0.93 | 0.93 to 0.94 |
| Sex | ||||||
| Women | 35,529 | 1.01 | 0.99 to 1.03 | 2265 | 0.65 | 0.61 to 0.68 |
| Men | 30,519 | 1.00 | Reference | 3137 | 1.00 | Reference |
| Race | ||||||
| Asian/Pacific Islander | 5246 | 1.01 | 0.98 to 1.04 | 514 | 1.64 | 1.48 to 1.81 |
| Black | 11,123 | 1.28 | 1.25 to 1.31 | 1594 | 2.81 | 2.61 to 3.01 |
| Hispanic | 12,613 | 1.21 | 1.19 to 1.24 | 1685 | 2.31 | 2.15 to 2.48 |
| Others | 174 | 0.85 | 0.73 to 0.98 | 28 | 2.43 | 1.67 to 3.52 |
| White | 36,892 | 1.00 | Reference | 1581 | 1.00 | Reference |
| Baseline comorbidity | ||||||
| Diabetes mellitus | 25,417 | 1.71 | 1.68 to 1.74 | 3062 | 2.80 | 2.64 to 2.96 |
| Hypertension | 42,594 | 1.24 | 1.22 to 1.26 | 2791 | 0.74 | 0.70 to 0.78 |
| HIV | 250 | 1.44 | 1.26 to 1.63 | 44 | 1.24 | 0.90 to 1.71 |
| Hepatitis B | 209 | 1.16 | 1.01 to 1.33 | 27 | 1.17 | 0.78 to 1.75 |
| Myocardial infarction | 2082 | 1.48 | 1.42 to 1.55 | 161 | 1.32 | 1.13 to 1.55 |
| ESLD | 449 | 3.75 | 3.41 to 4.12 | 52 | 2.89 | 2.19 to 3.81 |
| Baseline eGFR | 66,048 | 1.02 | 1.02 to 1.02 | 5402 | 0.94 | 0.94 to 0.95 |
HR, hazard ratio; 95% CI, 95% confidence interval; HCV, hepatitis C virus; ESLD, end-stage liver disease.
Follow-up time is censored at the time of the start of dialysis, liver/kidney transplant, HCV treatment, death, disenrollment from Kaiser Permanente health plan, or the end of the study period (December 31, 2014); ESKD analyses censored on liver transplant, HCV treatment, death, disenrollment from Kaiser Permanente health plan, or the end of the study period.
Patients with baseline eGFR<15 ml/min per 1.73 m2 were excluded: CKD only, n=151,174; HCV and CKD, n=1534.
Table 3.
Censoring events in the analyses for 25% decline in eGFR and ESKD
| Status at the End of Follow-Up | Total | HCV and CKD | CKD Only |
|---|---|---|---|
| 25% decline in eGFR | n=1603 | n=15,1974 | |
| Mean follow-up time in years (SD) | 1.5 (1.6) | 2.5 (2.2) | |
| Event, 25% eGFR decrease | 66,048 (43%) | 915 (57%) | 65,133 (43%) |
| Death | 11,110 (7%) | 113 (7%) | 10,997 (7%) |
| Dialysis | 551 (0.4%) | 50 (3%) | 501 (0.3%) |
| Kidney transplant | 8 (0%) | 0 (0%) | 8 (0%) |
| Liver transplant | 38 (0%) | 14 (1%) | 24 (0%) |
| HCV treatment | 28 (0%) | 28 (2%) | 0 (0%) |
| Disenrollment | 15,445 (10%) | 138 (9%) | 15,307 (10%) |
| End of study, December 31, 2014 | 60,349 (39%) | 345 (22%) | 60,004 (40%) |
| ESKD | n=1534 | n=151,174 | |
| Mean follow-up time in years (SD) | 2.5 (2.3) | 3.7 (2.9) | |
| Event, ESKD | 5402 (4%) | 162 (11%) | 5240 (4%) |
| Death | 29,937 (20%) | 381 (25%) | 29,556 (20%) |
| Dialysis | 74 (0%) | 71 (5%) | 3 (0%) |
| Liver transplant | 71 (0%) | 22 (1%) | 49 (0%) |
| HCV treatment | 37 (0%) | 37 (2%) | 0 (0%) |
| Disenrollment | 21,666 (14%) | 232 (15%) | 21,434 (14%) |
| End of study December 31, 2014 | 95,521 (63%) | 629 (41%) | 94,892 (63%) |
HCV, hepatitis C virus.
The unadjusted hazard for ESKD comparing those with HCV and CKD with those with CKD alone was 4.72 (95% CI, 4.03 to 5.52; IR in HCV and CKD, 41.6 per 100,000 person-years; IR in CKD only, 9.4 per 100,000 person-years). The adjusted hazard for ESKD was 1.93 times higher (95% CI, 1.64 to 2.27) among those with HCV and CKD compared with CKD alone. Patients who were men and non-white, particularly black patients, had higher adjusted hazard of ESKD compared with women and white patients, respectively. Death, dialysis, and liver transplant during follow-up were more likely in the HCV and CKD group compared with the CKD group. Overall, 2% of patients with HCV and CKD initiated HCV treatment (Table 3).
Stratified Analyses
The adjusted hazard for a 25% decrease in eGFR comparing those with HCV and CKD with those with CKD alone differed by CKD stage; there were no other significant differences between subgroups. The adjusted hazard of ESKD between those with HCV and CKD versus CKD alone differed by diabetes, hypertension, and CKD status (Supplemental Table 4). The analyses stratifying the ESKD outcome into eGFR<15 ml/min per 1.73 m2 and dialysis yielded similar results to the main ESKD analyses (Supplemental Table 4).
Sensitivity Analyses
We were able to achieve balance across all covariates in the propensity score matched model. The estimate of risk for 25% decline in eGFR and ESKD were similar to the main multivariable analyses results presented in Table 2, and do not alter conclusions (Supplemental Table 5). For sensitivity analyses among only those with positive HCV RNA as diagnostic criteria (n=1372), we also found similar results to the primary analyses (Supplemental Table 5).
The rate of decline (generalized estimating equation analyses) was faster for those with ESLD compared with those without ESLD. However, the hazard of 25% decline in eGFR and ESKD was lower in those with ESLD compared with those without. The overall message of higher risk for the HCV and CKD cohort versus CKD alone remained for all analyses. The lower hazard for patients with ESLD is likely because of higher numbers of competing risks in the cohort with ESLD (data not shown).
Discussion
We found that untreated HCV has a moderate association with kidney function decline among patients with stage 3–5 CKD. Although there is general consensus that HCV contributes to the risk of some glomerular diseases, there remains controversy regarding the role of HCV infection on the progression of CKD more broadly, with particular lack of data for those with moderate to advanced CKD (9,11,12,19,22). Our results support the hypothesis that untreated HCV contributes to kidney function decline.
Several mechanisms by which HCV worsens kidney function have been postulated. Glomerular injury may result from deposition of circulating immune complexes within the subendothelium and mesangium (36), which activate the complement system, leading to mononuclear phagocyte proliferation and the release of proteases and oxidants that alter glomerular permeability (36–38). Other research suggests that HCV triggers a cascade of local or systemic immune and inflammatory responses of the kidney through inflammatory mediators (6,39,40). Epidemiologically, patients with HCV have a five-fold increased risk in the odds of membranoproliferative GN compared with control individuals without HCV (41). Among patients with GN, HCV infection increased the rate of progression of CKD, developing ESKD, and death (42).
In our study, baseline diabetes, non-white race, and ESLD were strongly associated with greater risks of all outcomes. Prior studies have shown that decompensated liver disease, hypertension, and diabetes were associated with higher risk of CKD in patients with HCV (9); however, few prior studies have demonstrated the increased risk of black and Hispanic race/ethnicity in this relationship.
This study has several strengths. We removed the potential confounding association of HCV treatment on kidney disease progression, which was documented in 10% of our original cohort before exclusions. The racial and ethnic diversity of our study population proved important to fill existing knowledge gaps, particularly for Hispanic populations. Further, more than half of our study population are women, allowing us to contribute new knowledge beyond the previous, male-dominated, large-scale studies by the Veterans Administration (11,22). Finally, to evaluate concerns of residual confounding in the main analyses, we performed stratified, propensity score-matched, and sensitivity analyses, which all supported the conclusions from the main analyses.
However, there are several potential limitations to this study. The follow-up time for the HCV and CKD population was shorter than the CKD-only population for the analyses of 25% decline and ESKD. Although this was largely driven by more frequent and faster time to the outcome events, the HCV and CKD population was also more likely to be censored before the end of the study period. If worsening CKD prompted treatment for HCV (and therefore censoring), we may underestimate decline. We may also underestimate decline due to immortality bias imposed by the 90-day requirement between first and second qualifying eGFR values to define CKD; however, the mean time between qualifying eGFR measurements was less than a year and therefore not likely to have a large effect. Finally, muscle wasting and altered creatinine metabolism can decrease creatinine generation, leading to attenuation of our findings in patients with severe HCV-associated liver disease (i.e., ESLD). In our main analyses, those with ESLD demonstrated elevated risk of kidney function decline, and more rapid decline with ESLD was seen in sensitivity analyses. Hepatorenal syndrome may play an important role in disease progression; the fact that progression may be potentially underestimated suggests a need for careful clinical observation of this high-risk group.
For HCV, delays in diagnoses and known limitations in the effectiveness of IFN-based treatments likely contributed to delays in HCV screening. As such, our results may represent the risk of kidney function decline in patients with HCV and CKD with more advanced HCV disease.
In summary, we found that untreated HCV affects kidney function decline among patients with stage 3–5 CKD over 2–3 years. As faster decline of eGFR is associated with higher risk of mortality (43), it is important to identify patients at increased risk of accelerated kidney decline. With IFN-based treatments, eradication of HCV in patients with CKD has been difficult (44–46). However, new direct-acting antiviral therapies have demonstrated excellent safety and efficacy profiles in patients with CKD (47–50). It remains to be seen whether the use of newer direct-acting HCV antivirals can mitigate the magnitude and rate of kidney function decline and progression to ESKD among patients with HCV and CKD.
Disclosures
J.M.A. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Kenilworth, NJ), and owns stock in the company. S.Y.T., M.H., K.B.R., and C.V.R. received research support from Merck Sharp & Dohme Corp. during the conduct of the study. C.V.R. owns <$5000 in Gilead stock shares.
Supplementary Material
Acknowledgments
S.Y.T., J.-W.H., R.W., K.B.R., H.H., J.M.A., M.H., S.F.D., L.Q., and C.V.R. are responsible for the work described in this paper. All authors were involved in at least one of the following: conception, design, acquisition, analysis, statistical analysis, interpretation of data, and drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Kenilworth, NJ). J.M.A., who is an employee of the company, participated in study design, analysis and interpretation of data, writing the report, and the decision to submit the report for publication.
The data presented in this manuscript were presented at Infectious Disease Week (IDWEEK), October 26–30, 2016, in New Orleans, Louisiana.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01530218/-/DCSupplemental.
References
- 1.Peralta CA, Lin F, Shlipak MG, Siscovick D, Lewis C, Jacobs DR Jr., Bibbins-Domingo K: Race differences in prevalence of chronic kidney disease among young adults using creatinine-based glomerular filtration rate-estimating equations. Nephrol Dial Transplant 25: 3934–3939, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggers PW: Has the incidence of end-stage renal disease in the USA and other countries stabilized? Curr Opin Nephrol Hypertens 20: 241–245, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Wasley A, Alter MJ: Epidemiology of hepatitis C: Geographic differences and temporal trends. Semin Liver Dis 20: 1–16, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) : KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of Hepatitis C in chronic kidney disease. Kidney Int Suppl 73: S1–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Li WC, Lee YY, Chen IC, Wang SH, Hsiao CT, Loke SS: Age and gender differences in the relationship between hepatitis C infection and all stages of Chronic kidney disease. J Viral Hepat 21: 706–715, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Fabrizi F, Verdesca S, Messa P, Martin P: Hepatitis C virus infection increases the risk of developing chronic kidney disease: A systematic review and meta-analysis. Dig Dis Sci 60: 3801–3813, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ: Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: A 6-year nationwide cohort study across Taiwan. BMC Nephrol 14: 187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann JN, Törner A, Chow WH, Ye W, Purdue MP, Duberg AS: Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: A nationwide register-based cohort study in Sweden. Eur J Cancer Prev 20: 326–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butt AA, Wang X, Fried LF: HCV infection and the incidence of CKD. Am J Kidney Dis 57: 396–402, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, Yu ML, Hwang SJ: Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One 9: e100790, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP: Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 61: 1495–1502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moe SM, Pampalone AJ, Ofner S, Rosenman M, Teal E, Hui SL: Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis 51: 885–892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asrani SK, Buchanan P, Pinsky B, Rey LR, Schnitzler M, Kanwal F: Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol 8: 79–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui JI, Vittinghoff E, Shlipak MG, O’Hare AM: Relationship between hepatitis C and chronic kidney disease: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 17: 1168–1174, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple LS, Koepsell T, Sampson J, Louie T, Dominitz JA, Young B, Kestenbaum B: Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol 2: 715–721, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka N, Ishizaka Y, Seki G, Nagai R, Yamakado M, Koike K: Association between hepatitis B/C viral infection, chronic kidney disease and insulin resistance in individuals undergoing general health screening. Hepatol Res 38: 775–783, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ: Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: A cross-sectional study. Am J Kidney Dis 56: 23–31, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Soma J, Saito T, Taguma Y, Chiba S, Sato H, Sugimura K, Ogawa S, Ito S: High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol 11: 690–699, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O’Hare AM: Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med 167: 1271–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Crook ED, Penumalee S, Gavini B, Filippova K: Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care 28: 2187–2191, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR: The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS 22: 1799–1807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogal SS, Yan P, Rimland D, Lo Re V 3rd, Al-Rowais H, Fried L, Butt AA; Electronically Retrieved Cohort of HCV Infected Veterans Study Group : Incidence and progression of chronic kidney disease after hepatitis C seroconversion: Results from ERCHIVES. Dig Dis Sci 61: 930–936, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY: Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 59: 1293–1302, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS: Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ: Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 16: 37–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos J, Martins LS: Estimating glomerular filtration rate in kidney transplantation: Still searching for the best marker. World J Nephrol 4: 345–353, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Riordan A, Wong V, McCormick PA, Hegarty JE, Watson AJ: Chronic kidney disease post-liver transplantation. Nephrol Dial Transplant 21: 2630–2636, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, Matsushita K, Hemmelgarn BR: Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant 27: 3835–3843, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J: Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617–2624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glassock RJ, Winearls C: Screening for CKD with eGFR: doubts and dangers. Clin J Am Soc Nephrol 3: 1563–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bostom AG, Kronenberg F, Ritz E: Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol 13: 2140–2144, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986 [PubMed] [Google Scholar]
- 34.Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ: When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28: 2670–2677, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Derose SF, Contreras R, Coleman KJ, Koebnick C, Jacobsen SJ: Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: The Kaiser Permanente Southern California experience. Med Care Res Rev 70: 330–345, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Daghestani L, Pomeroy C: Renal manifestations of hepatitis C infection. Am J Med 106: 347–354, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, Wilson R: Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465–470, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E, Scudeller G, Zanetti A: Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med 117: 573–577, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Fabrizi F, Dixit V, Messa P: Impact of hepatitis C on survival in dialysis patients: A link with cardiovascular mortality? J Viral Hepat 19: 601–607, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K: Hepatitis C virus infection and diabetes: Direct involvement of the virus in the development of insulin resistance. Gastroenterology 126: 840–848, 2004 [DOI] [PubMed] [Google Scholar]
- 41.El-Serag HB, Hampel H, Yeh C, Rabeneck L: Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 36: 1439–1445, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Noureddine LA, Usman SA, Yu Z, Moorthi RN, Moe SM: Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol 32: 311–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, Abbott KC, Eisen S: Rate of kidney function decline associates with mortality. J Am Soc Nephrol 21: 1961–1969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fabrizi F, Dixit V, Messa P, Martin P: Antiviral therapy (pegylated interferon and ribavirin) of hepatitis C in dialysis patients: Meta-analysis of clinical studies. J Viral Hepat 21: 681–689, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr., Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347: 975–982, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Hadziyannis SJ, Sette H Jr., Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr., Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM; PEGASYS International Study Group : Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med 140: 346–355, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, Liapakis A, Nelson DR, Fried MW, Terrault NA; HCV-TARGET : Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 36: 807–816, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr., Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W: Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): A combination phase 3 study. Lancet 386: 1537–1545, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, Bernstein DE, Cohen DE, Shulman NS, Wang D, Khatri A, Abunimeh M, Podsadecki T, Lawitz E: Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology 150: 1590–1598, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C, Colombo M, Yoshida EM, Nelson DR, Collins C, Lei Y, Kosloski M, Mensa FJ: Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med 377: 1448–1455, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.