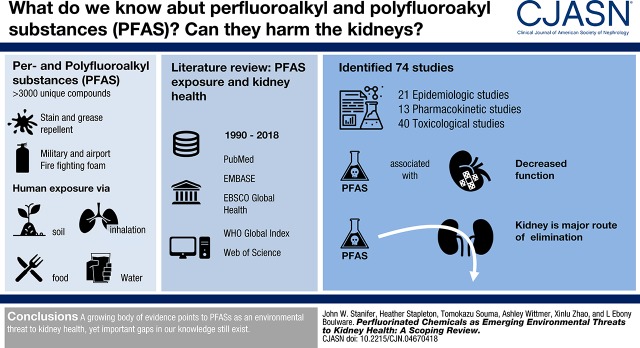
Abstract
Background and objectives
Per- and polyfluoroalkyl substances (PFASs) are a large group of manufactured nonbiodegradable compounds. Despite increasing awareness as global pollutants, the impact of PFAS exposure on human health is not well understood, and there are growing concerns for adverse effects on kidney function. Therefore, we conducted a scoping review to summarize and identify gaps in the understanding between PFAS exposure and kidney health.
Design, setting, participants, & measurements
We systematically searched PubMed, EMBASE, EBSCO Global Health, World Health Organization Global Index, and Web of Science for studies published from 1990 to 2018. We included studies on the epidemiology, pharmacokinetics, or toxicology of PFAS exposure and kidney-related health, including clinical, histologic, molecular, and metabolic outcomes related to kidney disease, or outcomes related to the pharmacokinetic role of the kidneys.
Results
We identified 74 studies, including 21 epidemiologic, 13 pharmacokinetic, and 40 toxicological studies. Three population-based epidemiologic studies demonstrated associations between PFAS exposure and lower kidney function. Along with toxicology studies (n=10) showing tubular histologic and cellular changes from PFAS exposure, pharmacokinetic studies (n=5) demonstrated the kidneys were major routes of elimination, with active proximal tubule transport. In several studies (n=17), PFAS exposure altered several pathways linked to kidney disease, including oxidative stress pathways, peroxisome proliferators-activated receptor pathways, NF-E2–related factor 2 pathways, partial epithelial mesenchymal transition, and enhanced endothelial permeability through actin filament modeling.
Conclusions
A growing body of evidence portends PFASs are emerging environmental threats to kidney health; yet several important gaps in our understanding still exist.
Keywords: Actin Cytoskeleton; Environment; Epidemiologic Studies; Epithelial-Mesenchymal Transition; Exposure; Global Health; Health Disparities; kidney; Kidney Diseases; Kidney Tubules, Proximal; oxidative stress; Permeability; Peroxisome Proliferators; Pollutants; Toxicology; NF-E2-Related Factor 2
Visual Abstract
Introduction
Per- and polyfluoroalkyl substances (PFASs) are a large group of >3000 compounds used to provide stain- and grease-repelling properties to consumer products, including textiles, papers, and food packaging (1). PFASs are also used in aqueous fire-fighting foams used for distinguishing fires near airports and military bases (1). PFASs have been detected in soil, air, and water from all regions of the world, with bioaccumulation across entire ecological food chains. As such, PFASs are now recognized as globally ubiquitous pollutants.
Humans are exposed to PFASs through ingestion of contaminated soil, food, and water, and inhalation of contaminated air (1,2). Detectable levels are found in most humans, and in the United States, nearly all adults have demonstrated some level of PFAS exposure (2). Even with efforts to reduce or eliminate production, the drinking water for >6 million United States residents still exceeds the lifetime health advisory for both perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) (3). Likewise, because of an increase in large-scale production in countries such as China, human exposure remains high worldwide (4). Furthermore, pressure to phase out some PFASs, such as PFOS and PFOA, has led to precipitous increases in the production of unstudied and unregulated novel replacement compounds such as perfluoroether carboxylic acids (e.g., GenX, Adona), chlorinated polyfluoroether sulfonates (e.g., F-53B), and fluorotelomer alcohols (e.g., Novec 1230).
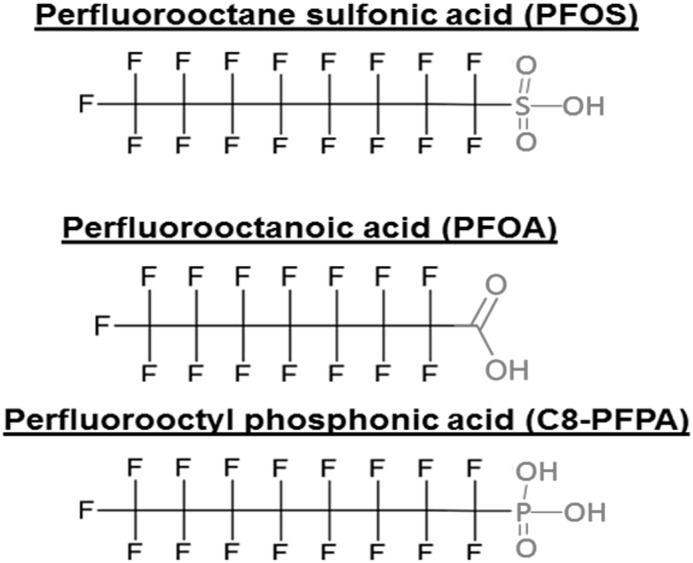
Despite widespread exposure, the impact of PFASs on human health is only recently gaining awareness. As organic isomers with charged functional groups, such as sulfonic acids, carboxylic acids, and phosphonic acids (Figure 1), PFASs are increasingly linked to carcinogenesis; disruption of endocrine, metabolic, and immunologic pathways; and reproductive and developmental toxicity (5). Most notably, the C8 Health Project—a study convened as part of a legal settlement against a Mid-Ohio Valley manufacturer to investigate the human health effects of PFAS exposure—demonstrated evidence linking PFOA exposure with testicular and genitourinary cancers, hyperlipidemia, thyroid diseases, ulcerative colitis, and gestational hypertension (6). Given their chemical properties and biologic effects, plausible concerns about PFAS exposure causing adverse kidney consequences are growing; yet, the relationship between PFAS exposure and kidney function is not well understood. Therefore, we conducted a scoping review to summarize existing knowledge and identify gaps in the epidemiologic, pharmacokinetic, and toxicological data on PFAS exposure and kidney-related health.
Figure 1.
Molecular structure for PFASs with sulfonic acid (PFOS), carboxylic acid (PFOA), and phosphonic acid (PFPA) moieties.
Materials and Methods
Search Strategy
With the assistance of a specialized medical librarian, we iteratively developed a comprehensive search strategy for the PubMed, EMBASE, EBSCO Global Health, World Health Organization (WHO) Global Index Medicus (which includes regional indices, WHO Library Information System, and Scientific Electronic Library Online), and Web of Science databases. We used Boolean logic with search terms including a combination of relevant subject headings and text words for kidney disease (e.g., kidney diseases, renal, albuminuria, etc.) and PFASs (e.g., perfluoro, polyfluoro, PFAS, etc.). We used controlled vocabularies (e.g., medical subject heading terms) to identify synonyms. We applied no language or study design restrictions, and we included both human and animal studies. We searched for studies published from January 1 1990 to February 22, 2018. We supplemented the searches by manually reviewing the reference lists from review articles. The detailed search parameters are available in the study protocol (Supplemental Appendix). The study protocol was developed in December 2017; it is not registered in the International Prospective Register of Systematic Reviews as scoping reviews are not eligible for inclusion.
Study Selection
We screened the title and abstract for all identified studies. To be included for full-text review, each study had to: (1) investigate the toxicology of PFASs in animals or humans, or (2) evaluate the epidemiology or pharmacokinetics of PFASs in humans. Review articles, editorials, case reports, and studies only reporting methodology for chemical analyses and identification were excluded. Studies were included in the final scoping review if full-text review demonstrated they investigated the pharmacokinetics, toxicology, or epidemiology of PFASs and reported a kidney-related outcome, including clinical outcomes (e.g., prevalence of kidney disease, changes in kidney function, mortality related to kidney diseases), histologic outcomes (e.g., pathologic evidence of alterations in kidney tissue), molecular outcomes (e.g., disturbances in cellular pathways of kidney cell lines or tissue), or metabolic outcomes (e.g., alterations of metabolic pathways with known links to kidney function or kidney diseases), or outcomes related to the pharmacokinetic role of the kidneys in metabolism, tissue distribution, or clearance and elimination of PFASs in humans.
Data Extraction
Two investigators independently reviewed and extracted data into standard forms to facilitate data-charting, data synthesis, and results reporting. Errors in data extraction were resolved by joint review of the original articles. In instances where insufficient data were presented in the article (e.g., abstracts), we contacted the authors for additional information. For epidemiologic studies, we extracted each study’s investigators, years of conduct, design, setting, population, study size, PFASs studied, methods for assessing PFAS exposure, kidney-related outcomes, and major findings. For pharmacokinetic studies, we extracted each study’s investigators, year of publication, PFASs studied, pharmacokinetic parameters investigated, and major findings. For toxicology studies, we extracted each study’s investigators, year of publication, design and animal model or cell line, PFASs studied, and major findings. We classified toxicology studies into mechanistic domains (clinical, histologic, cellular, or metabolic) on the basis of the major findings.
Results
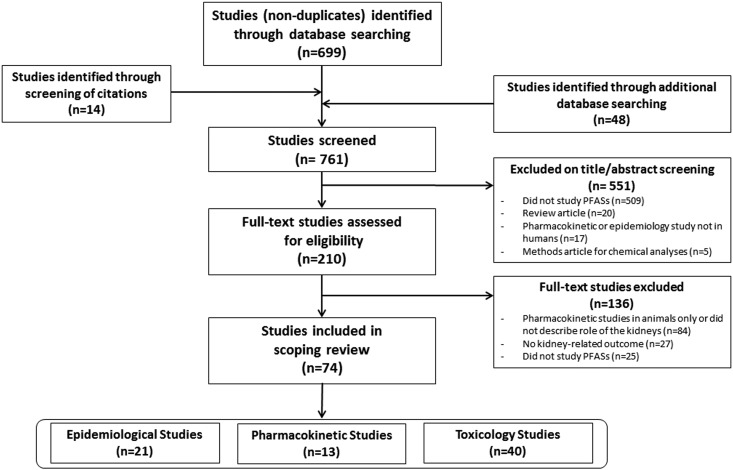
We sought to identify epidemiologic, pharmacokinetic, or toxicological studies on PFAS exposure and kidney-related health. We identified 210 studies published between 1991 and 2018 meeting inclusion criteria for full-text review (Figure 2). We excluded 136 studies that were pharmacokinetic studies conducted only in animals or not describing the pharmacokinetic role of the kidneys (n=84; 61%), did not report a kidney-related outcome (n=27; 20%), or did not investigate PFAS exposure (n=25; 18%). After full-text review, we included 74 studies, of which 21 (28%) were epidemiologic, 13 (18%) were pharmacokinetic, and 40 (54%) were toxicological studies.
Figure 2.
Flow diagram of study selection.
Human Epidemiologic Studies
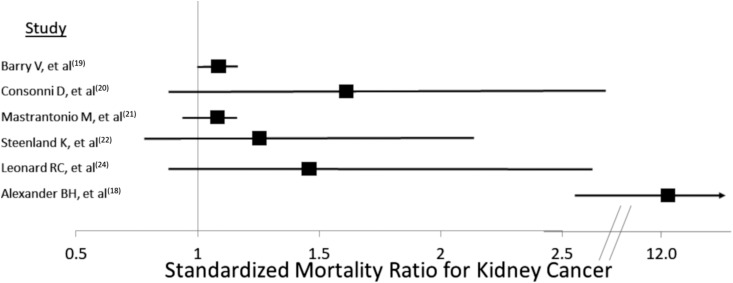
We identified 21 epidemiologic studies, all published between 2003 and 2017, investigating PFAS exposure and kidney-related health, with 11 studies directly assessing exposure through serum concentrations and ten studies indirectly estimating exposure (Table 1). All of the studies investigated PFOA and/or PFOS; a few studies additionally investigated perfluorohexane sulfonate (n=4) or perfluorononanoic acid (PFNA) (n=2). All but two studies were conducted in the United States (17,21), and all but one were cross-sectional, retrospective cohort, or ecological studies (25). In six studies, PFAS exposure was associated with increased mortality from kidney-related cancers (18–22,24); however, the strength of the association varied, with standardized mortality ratios ranging from 1.07 to 12.8 (Figure 3).
Table 1.
Human epidemiologic studies (1990–2018) investigating per- and polyfluoroalkyl substances exposure and kidney health
| Authors | Study Years | Study Design | Setting | Population | Sample Size | Exposure | Kidney Outcome | Major Findings | Summary Notes |
|---|---|---|---|---|---|---|---|---|---|
| Direct exposure assessments (n=11) | |||||||||
| Dhingra et al. (7) | 1952–2012 | Cross-sectional | Community surrounding manufacturer | Adults living in eligible area | 29,499 | PFOAa | eGFR | Association present | Negative trend in eGFR across measured serum PFOA quintiles (β=−0.64 to −1.03; P=0.01) |
| Kataria et al. (8) | 2003–2010 | Cross-sectional | NHANES | Children 12–19 yr old | 1960 | PFOS, PFOA, PFHxS, PFNA | eGFR | Association present | Increased odds (OR, 2.0; 95% CI, 1.4 to 2.9) for lower eGFR with increasing exposure levels for PFOS and PFOA |
| Shankar et al. (9) | 1999–2008 | Cross-sectional | NHANES | Adults >20 yr old | 4587 | PFOA, PFOS | eGFR, prevalent CKD | Association present | eGFR: 5.7 and 6.7 ml/min per 1.73 m2 lower with increasing exposure |
| Prevalent CKD: OR, 1.7 (95% CI, 1.0 to 2.9) and 1.8 (95% CI, 1.0 to 3.3) for PFOA and PFOS | |||||||||
| Vearrier et al. (10) | 2003–2008 | Cross-sectional | NHANES | Adults | 6305 | PFOA | Prevalent CKD, incident ESKD | Association present | Prevalent CKD: OR, 1.2 (95% CI, 1.1 to 1.3); incident ESKD: OR, 1.9 (95% CI, 1.2 to 3.0) |
| Watkins et al. (11) | 1989–2006 | Retrospective cohort | Community surrounding manufacturer | Children (1–18 yr old) living in eligible area | 9660 | PFOA, PFOS, PFHxS, PFNAa | eGFR | Association present | Negative trend in eGFR (−0.73 to −1.34 ml/min per 1.73 m2) with increasing exposure to each PFAS |
| Conway et al. (12) | 2017 | Cross-sectional | Community surrounding manufacturer | Adults living in eligible area | 53,650 | PFOA, PFOS, PFHxS, PFNA | eGFR | No observed association | No association with any PFAS |
| Emmett et al. (13) | 2003–2005 | Cross-sectional | Community surrounding manufacturer | Adults and children living in eligible areas | 371 | PFOA | Serum creatinine | No observed association | – |
| Olsen et al. (14) | 2003 | Cross-sectional | Occupational | Adult employees | 518 | PFOS | Serum creatinine | No observed association | – |
| Olsen et al. (15) | 2012 | Cross-sectional | Occupational | Male employees | 506 | PFOA, PFOA | eGFR, prevalent CKD | No observed association | No association with eGFR or prevalent CKD |
| Steenland et al. (16) | 2005–2006 | Cross-sectional | Community surrounding manufacturer | Adults living in the eligible area | 54,951 | PFOA, PFOS | Serum creatinine | No observed association | No observed association for PFOA or PFOS |
| Zhou et al. (17) | 2013 | Cross-sectional | Community surrounding manufacturer (China) | Manufacturer employees living in eligible area | 39 | PFOA, PFOS, PFHxS | Serum creatinine | No observed association | No observed association for PFOA, PFOS, or PFHxS |
| Indirect exposure assessments (n=10) | |||||||||
| Alexander et al. (18) | 1961–1997 | Retrospective cohort | Occupational | Adult employees | 2083 | PFOS | Genitourinary and kidney cancer | Association present | Genitourinary and kidney cancer: SMR, 12.8 (95% CI, 2.6 to 37.4) |
| Barry et al. (19) | 1952–2011 | Retrospective cohort | Community surrounding manufacturer | Adults living in eligible area | 32,254 | PFOA | Kidney cancer | Association present | Kidney cancer: HR, 1.1 (95% CI, 1.0 to 1.2) per each unit increase in PFOA |
| Consonni et al. (20) | 1950–2008 | Retrospective cohort | Community surrounding manufacturer | Male employees | 5879 | PFOA | Mortality from kidney cancer | Association present | Kidney cancer: SMR, 1.7 (95% CI, 0.8 to 3.1) |
| Mastrantonio et al. (21) | 1980–2013 | Retrospective cohort (ecological) | Community surrounding manufacturer | High-risk districts | 24 districts | PFOA, PFOS | Mortality from kidney cancer | Association present | Kidney cancer: SMR, 1.1 (95% CI, 0.9 to 1.2) |
| Steenland et al. (22) | 1979–2004 | Retrospective cohort | Occupational | Adult employees | 5791 | PFOAa | Mortality from kidney cancer | Association present | Kidney cancer: SMR, 1.3 (95% CI, 0.7 to 2.2) |
| Vieira et al. (23) | 1996–2005 | Retrospective cohort (ecological) | Community surrounding manufacturer | High-risk districts, counties | Six water districts, 13 counties | PFOA | Incident kidney cancer | Association present | Kidney cancer: OR, 2.0 (95% CI, 1.0 to 3.9) |
| Leonard et al. (24) | 1948–2002 | Retrospective cohort | Occupational | Adult employees | 6027 | PFAS, not specified | Mortality from kidney cancer, nephritis, or nephrosis | Association present (kidney cancer) | Kidney cancer: SMR, 1.5 (95% CI, 0.8 to 2.7) |
| No observed association (nephritis or nephrosis) | |||||||||
| Costa et al. (25) | 1978–2007 | Prospective cohort | Occupational | Male employees | 53 | PFOA | Serum creatinine | No observed association | – |
| Dhingra et al. (26) | 1952–2011 | Retrospective cohort | Community surrounding manufacturer | Adults living in eligible area | 28,240 | PFOAa | Prevalent CKD | No observed association | – |
| Raleigh et al. (27) | 1947–2002 | Retrospective cohort | Occupational | Adult employees | 9027 | Ammonium PFOA, PFOA | Mortality from kidney cancer, CKD | No observed association | No observed associations for ammonium PFOA or PFOA |
PFOA, perfluorooctanoic acid; NHANES, The National Health and Nutrition Examination Survey; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; OR, odds ratio; 95% CI, 95% confidence interval; PFAS, per- and polyfluoroalkyl substances; SMR, standardized mortality ratio; HR, hazard ratio.
Study used model-predicted cumulative serum concentrations as measure of exposure.
Figure 3.
Forest plot of studies demonstrating standardized mortality ratios associated with PFAS exposure.
We identified 14 studies investigating PFAS exposure and kidney function, of which three (21%) used indirect exposure assessments and 11 (79%) used directly measured PFAS serum concentrations, with two additionally using indirect model-based estimates. None of the studies using indirect exposure estimates demonstrated associations with CKD prevalence or kidney function, including a 30-year prospective study of only 53 adults finding no association with serum creatinine (7,11,25–27). We identified no studies investigating proteinuria outcomes.
Of the studies using direct measures of exposure, five reported significant associations between PFAS exposure and lower eGFR or greater CKD prevalence (7–11), including three population-based studies from the National Health and Nutrition Examination Survey (NHANES) (8–10). In a cross-sectional study of >4500 adults from NHANES, significant inverse associations between serum concentrations of PFOA and PFOS and eGFR were observed, with the highest quartile of exposure associated with a 5.7 and 6.7 ml/min per 1.73 m2 lower eGFR for PFOA and PFOS exposure, respectively (9). Likewise, in a cross-sectional study of 6305 adults from NHANES, serum PFOS concentrations were associated with increased odds (odds ratio, 1.15; 95% confidence interval, 1.07 to 1.25) of prevalent CKD (10). Although children have greater PFAS exposure compared with adults, we identified only two epidemiologic studies investigating kidney-related health among children (8,11). In a cross-sectional study of 1960 children from NHANES, a significant inverse association between serum concentrations of PFOA and PFOS and eGFR was observed, with the highest quartile of exposure associated with a 6.61 and 9.47 ml/min per 1.73 m2 lower eGFR for PFOA and PFOS exposure, respectively (8).
Human Pharmacokinetic Studies
We identified 13 pharmacokinetic studies, published between 2005 and 2018, investigating the role of the kidneys in metabolism, tissue distribution, or elimination of PFASs in humans (Table 2). All of the studies (n=13) investigated PFOA or PFOS; a few studies additionally investigated perfluorohexanoic acid (n=4) and perfluorobutane sulfonate (n=2). Several studies (n=5) demonstrated variation in pharmacokinetic parameters on the basis of carbon-chain length, functional group, and isomer forms (28,30,33,37,40). Three studies demonstrated that after absorption PFASs distribute widely to the serum, liver, and kidneys as well as placenta and cord serum (29,34,35), with one showing perfluorobutyrate, perfluorododecanoic acid, and perfluorodecanoic acid highly concentrated in the kidneys (35).
Table 2.
Studies (1990–2018) investigating the pharmacokinetic role of the kidneys in metabolism, tissue distribution, or clearance and elimination of per- and polyfluoroalkyl substances in humans
| Authors | Year | Exposure | Pharmacokinetic Properties | Major Findings |
|---|---|---|---|---|
| Beesoon et al. (28) | 2015 | PFOA, PFOS | Protein-binding; elimination | Key differences in protein-binding, volume of distribution, and kidney clearance related to different PFAS isomeric forms |
| Fàbrega et al. (29) | 2013 | PFOA, PFOS | Volume of distribution; tissue concentrations | Tissue concentration varied by organ (liver>plasma>kidney) |
| Model-based predictions underestimate actual kidney concentrations | ||||
| Fu et al. (30) | 2016 | PFOA, PFOS, PFHxA | Elimination | Highlighted possible nonkidney elimination pathways; |
| t1/2 (by daily clearance rates) ranged from 4.1 to 14.7 yr; | ||||
| t1/2 (by annualized decline rates) ranged from 1.7 to 3.6 yr | ||||
| Harada et al. (31) | 2005 | PFOA, PFOS | Elimination | Kidney clearance one fifth of the total clearance |
| No observed sex differences in rate of clearance | ||||
| Ingelido et al. (32) | 2018 | PFBA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUdA, PFDoA, PFBS, PFHxS, PFOS | Elimination | Elimination not mediated by OATP1A2 proximal tubule transporter |
| Olsen et al. (33) | 2007 | PFOA, PFOS, PFHxS | Elimination | t1/2 ranged from 3.8 to 8.5 yr |
| Kidney clearance effected by isomeric forms | ||||
| Pan et al. (34) | 2017 | 24 target PFASs, including Cl-PFESA | Protein-binding; volume of distribution | Placental transfer with high cord sera concentrations |
| Higher placental transfer efficiencies associated with lower eGFR | ||||
| Pérez et al. (35) | 2013 | PFOA, PFOS, PFBS, PFHxA | Volume of distribution; tissue concentrations | Tissue concentration varied by organ, with PFBS, PFDoDA, and PFDA demonstrating highest concentrations in the kidneys |
| Russell et al. (36) | 2015 | PFOA | Elimination | t1/2 was 2.4 yr, slightly longer for men compared with women |
| Elimination occurred almost exclusively by the kidneys | ||||
| Shi et al. (37) | 2016 | Cl-PFESA | Elimination | Suggest Cl-PFESA is most bio-persistent known PFAS in humans, with median t1/2 for kidney clearance of 280 yr and total body elimination of 15.3 yr |
| Worley et al. (38) | 2017 | PFOA | Metabolism; elimination | Glomerular filtration and active reabsorption and secretion by the proximal tubules via basolateral (via OAT1 and OAT3) and apical (via OAT4 and URAT1) uptake transporters |
| Yang et al. (39) | 2010 | PFOA | Elimination | Active reabsorption and secretion by the proximal tubules via apical OAT4 and URAT1; proximal tubular handling affected by extracellular pH and isomeric forms |
| Zhang et al. (40) | 2013 | PFOA, PFOS | Elimination | Key differences in kidney clearance related to different isomeric forms, including chain length, branched versus linear, and functional groups |
PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFAS, per- and polyfluoroalkyl substances; PFHxA, perfluorohexanoic acid; PFBA, perfluorobutyrate; PFHpA, perfluoroheptanoic acid; PFNA, perfluorononanoic acid; PFDA, perfluorodecanoic acid; PFUdA, perfluoroundecanoic acid; PFDoA, perfluorododecanoic acid; PFBS, perfluorobutane sulfonate; PFHxS, perfluorohexane sulfonate; OATP1A2, organic anion transporting polypeptide 1A2; Cl-PFESA, chlorinated polyfluoroalkyl ether sulfonic acid; PFDoDA, perfluorododecanoic acid; OAT1, organic anion transporter 1; OAT3, organic anion transporter 3; OAT4, organic anion transporter 4; URAT1, urate transporter 1.
Likewise, elimination varied on the basis of carbon-chain length, functional group, and isomer forms. Many studies (n=5) demonstrated the kidneys were major routes of elimination, especially for PFASs with short carbon-chain lengths (fewer than eight carbon atoms), carboxylic acid function groups, or branched isomer forms (33,36–38,40). t1/2 ranged from 1.7 to 14.7 years, with kidney elimination affected by active secretion and reabsorption in the proximal tubules (37–40). Three studies demonstrated the basolateral and apical uptake of PFASs substances into proximal tubules was mediated by transporters of the solute-carrier protein family, particularly organic anion transporter (OAT)1 and OAT3 on the basolateral side, and OAT4 and urate transporter 1 (URAT1) on the apical side (38–40). Unlike other species, although men demonstrate longer t1/2 compared with women, it remains unclear the extent to which the proximal tubule handling of PFASs is regulated through sex hormones (32,36).
Toxicology Studies
We identified 40 toxicology studies, published between 1991 and 2017, investigating PFAS exposure and kidney-related outcomes (Table 3). Among the 40 studies, 17 (40%) investigated clinical and/or histologic outcomes, 13 (33%) investigated cellular and/or histologic outcomes, and ten (25%) investigated metabolic outcomes. Most were experimental or observational animal studies, either alone (n=32) or with in vitro models (n=2). A few studies used in vitro models alone (n=5). One study conducted metabolomic profiling among humans (77). Most of the studies investigated PFOA (n=15), PFOS (n=21), or both, but several (n=14) also studied perfluorobutane sulfonate, PFNA, perfluorododecanoic acid, perfluorohepanoic acid, perfluorohexanoic acid, perfluoroundecanoic acid, and fluorotelomer precursors.
Table 3.
Studies (1990–2018) investigating the toxicology of per- and polyfluoroalkyl substances in animals or humans
| Study | Year | Study Design | Model/Cell Line | Exposure | Mechanistic Domain | Major Findings | |
|---|---|---|---|---|---|---|---|
| Chang et al. (41) | 2017 | Animal | Monkeys | PFOS | Clinical | No observed association: | Serum creatinine |
| BUN | |||||||
| Fair et al. (42) | 2013 | Animal | Dolphins | PFOS, PFOA, PFDA | Clinical | Association present: | ↑ Serum creatinine |
| ↑ BUN | |||||||
| Butenhoff et al. (43) | 2012 | Animal | Rats | PFOS | Clinical | Association present: | ↑ BUN (male and female) |
| Histologic | No observed kidney histologic changes | ||||||
| Lieder et al. (44) | 2008 | Animal | Rats | PFBS | Clinical | No observed association: | Body weight |
| Serum creatinine | |||||||
| BUN | |||||||
| Histologic | Effects observed: | Medullary and papillary tubular epithelial hyperplasia | |||||
| Interstitial infiltration with tubular basophilia and papillary edema | |||||||
| Papillary necrosis | |||||||
| Seacat et al. (45) | 2003 | Animal | Rats | PFOS | Clinical | Association present: | ↑ BUN |
| Histologic | No observed kidney histologic changes | ||||||
| Son et al. (46) | 2007 | Animal | Mouse (male) | PFOA | Clinical | No observed association: | Serum creatinine |
| BUN | |||||||
| Kidney weights | |||||||
| Histologic | No observed kidney histologic changes | ||||||
| Takahasi et al. (47) | 2014 | Animal | Rats | PFUA | Clinical | Association present: | ↑ BUN |
| Histologic | Effects observed: | Kidney tubular regeneration | |||||
| Xing et al. (48) | 2016 | Animal | Mouse (male) | PFOS | Clinical | Association present: | Acute toxicity, glomerular changes with peripheral edema |
| ↑ mortality | |||||||
| Chronic toxicity, ↓ body weight and kidney mass | |||||||
| Histologic | No observed kidney histologic changes | ||||||
| Klaunig et al. (49) | 2015 | Animal | Rats | PFHxA | Clinical | Association present: | Dose-dependent decrease in survival (females) |
| Histologic | Effects observed: | Papillary necrosis (females) | |||||
| Mild to moderate tubular atrophy | |||||||
| Serex et al. (50) | 2014 | Animal | Rats | Fluoro-telomers | Clinical | Association present: | ↑ mortality |
| Histologic | Effects observed: | Dose-dependent increase in kidney weights | |||||
| Kidney degeneration and necrosis, leading to death | |||||||
| Butenhoff et al. (51) | 2004 | Animal | Rats | aPFOA | Histologic | Effects observed: | ↑ kidney weights (parents and offspring) |
| ↓ body weights | |||||||
| Cui et al. (52) | 2009 | Animal | Rats | PFOA, PFOS | Histologic | Effects observed: | Cortical and medullary congestion with enhanced acidophilia and tumefaction of proximal tubule cells |
| Curran et al. (53) | 2008 | Animal | Rats | PFOS | Histologic | Effects observed: | ↑ kidney weights (male and female) |
| Tubular epithelial hyperplasia | |||||||
| Kim et al. (54) | 2011 | Animal | Rats | PFOS | Histologic | Effects observed: | Enhanced proximal tubular basophilia |
| Ladics et al. (55) | 2005 | Animal | Rats | Fluoro-telomers | Histologic | Effects observed: | ↑ kidney weights (males) |
| Tubular hypertrophy | |||||||
| Newsted et al. (56) | 2008 | Animal | Quail | PFBS | Histologic | No observed kidney histologic changes | |
| Rogers et al. (57) | 2013 | Animal | Rats | PFOS, PFNA | Histologic | Effects observed: | Fewer nephrons and elevated BP in offspring of maternal rats exposed during pregnancy |
| Chou et al. (58) | 2017 | Animal | Mouse | PFOS | Histologic | Effects observed: | Kidney tubular inflammation and apoptosis |
| Enhanced tubular fibrosis and cytosolic changes | |||||||
| In vitro | RTE | Cellular | Effects observed: | Epithelial mesenchymal transition induction and cell; migration via PPARγ deacetylation and Sirt1 sequestration | |||
| Wen et al. (59) | 2016 | Animal | RTE (rats) | PFOS | Histologic | Effects observed: | Loss of epithelial cells |
| Granular cytoplasmic changes in proximal tubules | |||||||
| In vitro | Cellular | Effects observed: | Dose-dependent reduction in cell proliferation | ||||
| Increased apoptosis | |||||||
| Enhanced oxidative stress (via NFAT3, PPARγ, and SIRT1) | |||||||
| Abbott et al. (60) | 2012 | Animal | Mouse | PFOA | Cellular | Effects observed: | Increased PPARα, β, γ mRNA expression in kidney tissue |
| Upregulation of Cyp4a14 gene expressing PPAR | |||||||
| Arukwe et al. (61) | 2011 | Animal | Salmon | PFOA, PFOS | Cellular | Effects observed: | PFOA: increased PPARα, γ mRNA, ACOX1, CAT expression |
| PFOS: decreased PPARα, γ mRNA expression in kidney tissue and increased expression of PPARβ, ACOX1, CAT | |||||||
| Chung (62) | 2015 | In vitro | RTE | PFOS | Cellular | Effects observed: | Enhanced expression of fibrotic and oxidative stress markers accompanied by apoptosis of RTE cells |
| Diaz et al. (63) | 1994 | Animal | Rats (male) | PFOA | Cellular | Effects observed: | Enhanced peroxisome proliferation |
| Induction of p450 in kidneys | |||||||
| Increased β oxidation of fatty acids | |||||||
| Eldasher et al. (64) | 2013 | Animal | Rats (male) | PFOA | Cellular | Effects observed: | Enhanced expression of Cyp4a14 in kidneys |
| Eroʇlu et al. (65) | 2011 | Animal | Rats | PFOS | Cellular | Effects observed: | Enhanced markers for oxidative stress (MDA, SOD, and catalase) |
| Gorrochategui et al. (66) | 2016 | In vitro | RTE (Xenopus laevis) | PFBS, PFOS, PFOA, PFNA | Cellular | Effects observed: | Reduced cellular proliferation |
| Spectral alterations of DNA/RNA structures, protein structures, and fatty acids | |||||||
| Hu et al. (67) | 2003 | In Vitro | RTE (dolphin) | PFOS, PFHA, PFBS | Cellular | Effects observed: | Carbon-chain length inhibition of intercellular communication at the gap junctions (PFOS and PFHA) |
| Qian et al. (68) | 2010 | In vitro | Microvascular endothelial cells | PFOS | Cellular | Effects observed: | Induced reactive oxygen species leading to increased vascular permeability and actin filament re-modeling, with disruption of cell junction and cell adhesions |
| Takagi et al. (69) | 1991 | Animal | Rats (male) | PFOA, PFDA, PFBA | Cellular | No observed effects: | Marker of oxidative stress and DNA damage (8-hydroxydeoxyguanosine) |
| Witzman et al. (70) | 1996 | Animal | Rats (male) | PFOA, PFDA | Cellular | Effects observed: | ↑ markers for oxidative stress, including mitochondrial markers |
| Kariuki et al. (71) | 2017 | Animal | Crustacean (Daphnia magna) | PFOS | Metabolic | Effects observed: | Disrupted several energy metabolism pathways |
| Enhanced protein degradation | |||||||
| Lankadurai et al. (72) | 2012 | Animal | Earthworm | PFOS | Metabolic | Effects observed: | Increased fatty acid oxidation |
| Disrupted glucose and energy metabolism, specifically glutamate and TCA cycle metabolites | |||||||
| Peng et al. (73) | 2013 | In vitro | Human hepatocytes | PFOA | Metabolic | Effects observed: | Disrupted carnitine metabolism |
| Disrupted cholesterol biosynthesis and lipid metabolism | |||||||
| Disrupted amino acid metabolism | |||||||
| Skov et al. (74) | 2015 | Animal | Rats (male) | PFNA | Metabolic | Effects observed: | Disrupted lipid metabolism |
| Tan et al. (75) | 2013 | Animal | Mice | PFOA | Metabolic | Effects observed: | Disrupted fatty acid metabolism |
| Wagner et al. (76) | 2017 | Animal | Crustacean (Daphnia magna) | PFOS | Metabolic | Effects observed: | Disrupted amino acid metabolism |
| Wang et al. (77) | 2017 | Human | Human | PFOA, PFOS | Metabolic | Effects observed: | Disrupted lipid and fatty acid metabolism |
| Disrupted energy metabolism, including TCA cycle and glutathione pathways | |||||||
| Disrupted xenobiotic detoxifying, anti-oxidation, and nitric oxide signal pathways | |||||||
| Yu et al. (78) | 2016 | Animal | Mouse | PFOA | Metabolic | Effects observed: | Disrupted amino acid metabolism |
| Disrupted lipid metabolism | |||||||
| Altered energy metabolism | |||||||
| Increased β oxidation of fatty acids | |||||||
| Zhang et al. (79) | 2011 | Animal | Rats (male) | PFDoA | Metabolic | Effects observed: | Disrupted kidney amino acid metabolism |
| Altered glucose and energy metabolism | |||||||
| Ding et al. (80) | 2009 | Animal | Rats (male) | PFDoA | Metabolic | Effects observed: | Disrupted lipid metabolism |
| Disrupted fatty acid metabolism | |||||||
| Disrupted amino acid metabolism | |||||||
| Clinical | Association present: | Serum creatinine | |||||
| BUN | |||||||
PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoic acid; PFDA, perfluorodecanoic acid; PFBS, perfluorobutane sulfonate; PFUA, perfluoroundecanoic acid; PFHxA, perfluorohexanoic acid; aPFOA, ammonium perfluorooctanoic acid; PFNA, perfluorononanoic acid; RTE, kidney tubular epithelial; PPAR, peroxisome proliferator receptor; Sirt1, sirtuin 1; NFAT3, nuclear factor of activated T-cells 3; Cyp4a14, cytochrome p450 4A14; ACOX1, Acyl-CoA oxidase 1; CAT, catalase; P450, cytochrome P450; MDA, malondialdehyde; SOD, superoxide dismutase; PFHA, perfluoroheptanoic acid; PFBA, perfluorobutyrate; TCA, tricarboxylic acid; PFDoA, perfluorododecanoic acid.
Clinical and Histologic Findings
Several experimental animal studies (n=8) reported short-term clinical effects related to PFAS exposure, with most studies showing small changes in BUN and/or creatinine concentrations across a wide range of exposure doses (42,43,45,47,48,59,79,80). However, the short-term clinical effects were variable, with some animal studies (n=3) showing no changes in BUN or creatinine at exposure doses as high as 600 mg/kg for PFOA (41,44,46), and others showing increased concentrations of both BUN and creatinine at exposure doses as low as 0.05–1.00 mg/kg for perfluoroundecanoic acid and perfluorododecanoic acid (47).
Histologically, experimental studies (n=12) demonstrated several changes across a range of doses related to short- and long-term PFAS exposure. The most frequently observed abnormalities were tubular epithelial hypertrophy or hyperplasia accompanied by increased kidney weights (50,51,53,55). Cytosolic changes of tubular epithelial cells, cortical and medullary congestion, with and without interstitial inflammation, focal papillary edema, fibrosis with increased collagen deposition, increased apoptotic cell death, and signs of tubular regeneration were also observed with PFAS exposure, particularly PFOS (44,52,58,59). Four studies showed high-dose exposure resulted in acute kidney toxicity, including early death from kidney failure, moderate to severe papillary necrosis, and glomerular changes with anasarca (44,48–50). One experimental study demonstrated that maternal exposure to PFOS and PFNA led to fewer nephrons and early-life hypertension among rat offspring (57), a finding consistent with human epidemiologic studies linking in utero exposure with lower birth weights (81–83). Five studies, three of which were conducted by manufacturers and included low-dose exposure, demonstrated no histologic changes in the kidneys (43,45,46,51,56).
Cellular Findings
Several studies linked PFAS exposure to increased oxidative stress in the kidneys, including enhanced expression of mitochondrial transport chain proteins (63,70,79), DNA damage (66), reduced cellular proliferation (66), and/or apoptosis (58,59,62). In six studies, a key pathway involved oxidative stress via the disruptive effects on peroxisome proliferators-activated receptors (PPAR) and their downstream functions (58–61,63,64). Two studies demonstrated that in the kidneys, exposure to PFOA dysregulated PPARα and PPARγ (60,61), key nuclear receptor hormones highly expressed in the proximal tubules and involved in adipogenesis, lipid metabolism, glucose homeostasis, and cell growth and differentiation. Three in vitro studies demonstrated kidney tubular epithelial (KTE) cells exposed to PFOS had sharp increases in apoptosis accompanied by fibrosis via a Sirt1-mediated PPARγ deacetylation (58,59,61).
Other pathogenic pathways included PFASs’ ability to induce dedifferentiation of KTE cells with partial epithelial mesenchymal transition (EMT), their role in upregulating antioxidant transcription factor NF-E2–related factor 2 (Nrf2), and their disrupting effects on epithelial cell junctions and permeability. Although debate exists as to the role of EMT in kidney fibrogenesis in vivo, two in vitro studies of KTE cells showed PFOS exposure induced EMT and cell migrations via Sirt1-mediated mechanisms, a finding consistent with prior studies linking Sirt1 to EMT programs and kidney fibrosis (58). Likewise, other studies reported significant upregulation of Nrf2 and its target gene expression in response to oxidative stress caused by PFOS exposure, with the zebrafish models demonstrating that sulforaphane, a Nrf2 inducer, attenuated the reactive oxygen species accumulation and gene expression changes (84). Although Nrf2 induction is a key defense for combating oxidative damage from chemical toxicity in the kidneys, few studies investigated the link between PFAS exposure and Nrf2 pathways. PFASs were also shown to interrupt KTE intercellular communication at gap junctions, and enhance endothelial permeability in human microvascular endothelial cells through actin filament remodeling, both of which are key features of podocyte injury (67,68).
Metabolic Findings
We identified ten studies (n=10) profiling numerous nascent metabolic changes related to PFAS exposure (71–80). Animal studies demonstrated that PFAS exposure led to derangements in lipid metabolism (73,74,78,80), glucose and mitochondrial energy metabolism (71–74,78,79), fatty acid metabolism and antioxidation (75,80), sex hormone homeostasis, and amino acid metabolism (71,72,76,78–80). In the only human study, metabolomic profiling on 181 Chinese men demonstrated lipid and amino acid metabolism, xenobiotic detoxifying, and metabolic pathways directly linked to CKD pathogenesis, including glutathione metabolism and nitric oxide generation, were disrupted by PFAS exposure (77).
Discussion
PFASs are globally pervasive environmental pollutants with widespread human exposure, and a growing body of evidence indicates PFAS exposure has adverse kidney consequences. Studies demonstrated many adverse outcomes linked to PFAS exposure, including reduced kidney function, histologic and cellular derangements in the proximal tubules, and dysregulated metabolic pathways linked to kidney disease. Nonetheless, several important gaps still exist.
We observed consistent epidemiologic associations between PFAS exposure and reduced kidney function and/or kidney cancers, including a study from the C8 Health Project with >32,000 participants (19). Despite reduced exposure to putative, traditional risk factors (e.g., cigarette smoke) in countries such as the United States, the incidence of genitourinary and/or kidney cancers continues to rise, and the potential increased risk for these cancers stemming from PFAS exposure may be of particular public health importance (85). For noncancer related kidney outcomes, a handful of studies comparing model-based PFAS exposure estimates with measured serum concentrations suggested the epidemiologic associations between PFAs exposure and reduced kidney function may be a phenomenon of reverse causation, i.e., serum concentrations of PFASs accumulate as kidney function declines (7,11,16,26). Additionally, several of the epidemiologic studies are susceptible to exposure misclassification because of indirect exposure measurements (e.g., cumulative occupational work-years), and longitudinal epidemiologic studies using direct serum PFAS measurements are needed to further characterize the epidemiologic risk of PFAS exposure.
Several toxicology studies demonstrated unequivocal histologic, cellular, and metabolic kidney-related outcomes related to PFAS exposure, including increased oxidative stress with upregulated Sirt1 and Nrf2 gene expression, enhanced apoptosis and fibrosis with tubular epithelial histologic changes, induced EMT and cell migrations, and enhanced microvascular endothelial permeability through actin filament remodeling (58,59,84). Furthermore, the relationship between kidney function and steady-state PFAS serum concentrations appears to be more complex than previous pharmacokinetic models have reported, with the limited pharmacokinetic data in humans demonstrating key differences from other species. Studies have demonstrated that humans actively transport PFASs in the proximal tubules, with greater tubular reabsorption likely responsible for the longer t1/2 in humans (33,39). Further, human proximal tubule handling of PFAS compounds differs on the basis of the carbon-chain length or functional group of the PFAS compound or the age, sex, or ethnicity of the individual, and such differences in the proximal tubular OAT-mediated transport of PFASs may be particularly salient given their putative importance in mediating other drug-induced nephrotoxicities, including aristolochic acid, cephalosporin antibiotics, and tenofovir (86). Key differences in proximal tubule transporter activity across human populations may portend different risk profiles even at similar exposure levels (87), and studies investigating the potential role of proximal tubule transporter blockade (e.g., URAT1) may facilitate a greater understanding of the risk to kidney health posed by PFAS compounds. Finally, children and adolescents may have adverse cardiovascular and kidney consequences related to increased PFAS exposure, and life-course studies will be critical to understand the long-term health impact (8,11,88,89).
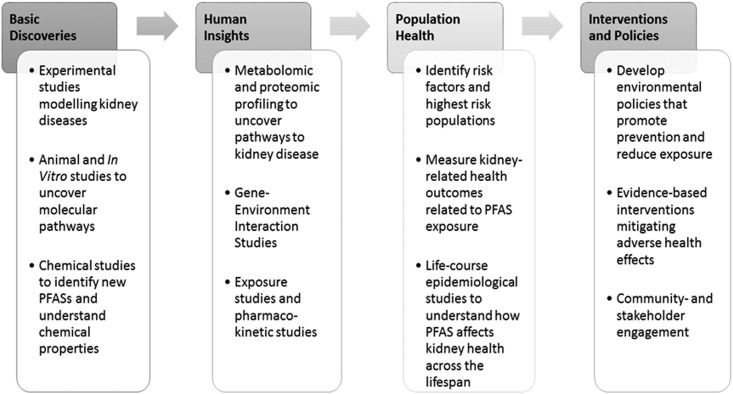
The emerging recognition of PFASs as environmental threats to human health reflects a broader understanding of the complex determinants of human health and health disparities. Environmental risk factors contribute to the development and perpetuation of health disparities around the globe, with contaminants now linked to increased burdens of chronic diseases and cancers, maternal and neonatal mortality, and developmental toxicity. In the context of kidney disease, contaminants appear to play key roles in causing CKD of unknown etiology, accelerating diabetic nephropathy, contributing to AKI, and serving as “second hits” to genetic risk factors (e.g., APOL1) (90). Nonetheless, how environmental toxins such as PFASs drive differences in kidney diseases across diverse population remains poorly understood. To understand the role environmental exposure to PFASs play in driving disparities in kidney disease, translational studies ranging from experimental models, metabolic profiling, to longitudinal life-course epidemiology will be needed (Figure 4). Furthermore, disparities in kidney disease arise from a complex interaction of factors, and studies explicating the effects of PFAS exposure with genetic, biologic, lifestyle, and other environmental risk factors (including PFAS–PFAS interactions) will be critical.
Figure 4.
Research across the translational spectrum is needed to better elucidate the potential link between PFAS exposure and adverse kidney health and eliminate potential disparities.
We note some limitations to our study. Although we included abstracts and scientific conference proceedings in our search strategy and several studies we included demonstrate negative findings, publication biases may still be present and further studies are needed. Additionally, given the paucity of data on alternative fluorinated compounds, we did not include them as a primary focus of our scoping review. However, many PFASs are being phased out of production and are being replaced by alternative PFAS compounds, which are increasingly being detected in the environment. For example, perfluoroether carboxylic acids, such as the commercial compound GenX, were very recently identified in urban municipal drinking water in North Carolina, and chlorinated polyfluorinated ether sulfonates, such as the commercial compound F-53B used in metal-plating industries, were recently detected in humans from China (34). Although these replacement compounds were manufactured as ostensibly safer alternatives to PFASs, they have chemical properties (e.g., etherification, chlorination) that prompt serious concern, and studies such as the GenX Exposure Study are only just now beginning to investigate outcomes associated with exposure to these replacement compounds. Limited data demonstrate placental transfer (34), greater binding affinities to human liver fatty acid protein, extremely long t1/2 in humans (37), and dose-dependent kidney tubular dilation and mineralization, papillary necrosis, and chronic progressive nephropathy in animal models (91). Further, many of the alternatives are themselves precursors to PFASs such as PFOA and PFOS, which through chemical breakdown or biotransformation can lead to persistent PFAS exposure despite phase-out efforts (92). Even more challenging is that hundreds of undiscovered PFAS compounds exist and their health effects are unknown, but proprietary aegis impedes development of detection methods or authentication standards to facilitate their study.
In conclusion, a growing body of evidence portends PFASs are emerging environmental threats to kidney health; yet several important gaps in our understanding still exist. Given the drastic increased production of novel replacement PFAS compounds, studies investigating the relationship between PFAS exposure and kidney disease are urgently needed.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Sarah Cantrell at the Duke University Medical Center Library and Archives for her assistance and integral role in developing the comprehensive literature search.
J.W.S. contributed to the study design, literature search, data extraction, data synthesis, and manuscript preparation. H.M.S. contributed to the literature search, data synthesis, and manuscript preparation. T.S. contributed to the data synthesis and manuscript preparation. A.W. and X.Z. contributed to the data extraction and manuscript preparation. L.E.B. contributed to the study design, data synthesis, and manuscript preparation. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04670418/-/DCSupplemental.
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP: Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag 7: 513–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL: Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect 115: 1596–1602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM: Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3: 344–350, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Environmental Protection Agency : Long-Chain Perfluorinated Chemical (PFCs): Action Plan, Washington, DC, United States Environmental Protection Agency, 2009 [Google Scholar]
- 5.Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ: Molecular mechanisms of PFOA-induced toxicity in animals and humans: Implications for health risks. Environ Int 99: 43–54, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Steenland K, Fletcher T, Savitz DA: Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 118: 1100–1108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K: A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125: 416–421, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L: Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health 14: 89, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar A, Xiao J, Ducatman A: Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 174: 893–900, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vearrier D, Jacobs D, Greenberg MI: Serum perfluorooctanoic acid concentration is associated with clinical renal disease but not clinical cardiovascular disease. Clin Toxicol (Phila) 51: 326, 2013 [Google Scholar]
- 11.Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA: Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 121: 625–630, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway BN, Innes K, Costacou T, Arthur J: Perfluoroalkyl substances and kidney function in chronic kidney disease, anemia, and diabetes. Diabetes 66: A143, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, Shaw LM: Community exposure to perfluorooctanoate: Relationships between serum levels and certain health parameters. J Occup Environ Med 48: 771–779, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen GW, Burris JM, Burlew MM, Mandel JH: Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med 45: 260–270, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Olsen G, Butenhoff J, Zobel L: PFOA, PFOS, and chronic kidney disease: Clearance comes before causation. Epidemiology 23: S610, 2012 [Google Scholar]
- 16.Steenland K, Tinker S, Shankar A, Ducatman A: Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect 118: 229–233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Shi Y, Vestergren R, Wang T, Liang Y, Cai Y: Highly elevated serum concentrations of perfluoroalkyl substances in fishery employees from Tangxun lake, china. Environ Sci Technol 48: 3864–3874, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Alexander BH, Olsen GW, Burris JM, Mandel JH, Mandel JS: Mortality of employees of a perfluorooctanesulphonyl fluoride manufacturing facility. Occup Environ Med 60: 722–729, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry V, Winquist A, Steenland K: Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121: 1313–1318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consonni D, Straif K, Symons JM, Tomenson JA, van Amelsvoort LG, Sleeuwenhoek A, Cherrie JW, Bonetti P, Colombo I, Farrar DG, Bertazzi PA: Cancer risk among tetrafluoroethylene synthesis and polymerization workers. Am J Epidemiol 178: 350–358, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A, Crosignani P: Drinking water contamination from perfluoroalkyl substances (PFAS): An ecological mortality study in the Veneto Region, Italy. Eur J Public Health 28: 180–185 2017 [DOI] [PubMed] [Google Scholar]
- 22.Steenland K, Woskie S: Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol 176: 909–917, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T: Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ Health Perspect 121: 318–323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard RC, Kreckmann KH, Sakr CJ, Symons JM: Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol 18: 15–22, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Costa G, Sartori S, Consonni D: Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med 51: 364–372, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Dhingra R, Lally C, Darrow LA, Klein M, Winquist A, Steenland K: Perfluorooctanoic acid and chronic kidney disease: Longitudinal analysis of a Mid-Ohio Valley community. Environ Res 145: 85–92, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Raleigh KK, Alexander BH, Olsen GW, Ramachandran G, Morey SZ, Church TR, Logan PW, Scott LL, Allen EM: Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup Environ Med 71: 500–506, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beesoon S, Martin JW: Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environ Sci Technol 49: 5722–5731, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Fàbrega F, Kumar V, Schuhmacher M, Domingo JL, Nadal M: PBPK modeling for PFOS and PFOA: Validation with human experimental data. Toxicol Lett 230: 244–251, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Fu J, Gao Y, Cui L, Wang T, Liang Y, Qu G, Yuan B, Wang Y, Zhang A, Jiang G: Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Sci Rep 6: 38039, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A: Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 99: 253–261, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, Zampaglioni F, Marra V, Miniero R, Valentini S, Russo F, Vazzoler M, Testai E, De Felip E: Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ Int 110: 149–159, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR: Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115: 1298–1305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Y, Zhu Y, Zheng T, Cui Q, Buka SL, Zhang B, Guo Y, Xia W, Yeung LW, Li Y, Zhou A, Qiu L, Liu H, Jiang M, Wu C, Xu S, Dai J: Novel chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: Placental transfer and relationship with serum albumin and glomerular filtration rate. Environ Sci Technol 51: 634–644, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, Farré M: Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59: 354–362, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Russell MH, Waterland RL, Wong F: Calculation of chemical elimination half-life from blood with an ongoing exposure source: The example of perfluorooctanoic acid (PFOA). Chemosphere 129: 210–216, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Vestergren R, Xu L, Zhou Z, Li C, Liang Y, Cai Y: Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs). Environ Sci Technol 50: 2396–2404, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Worley RR, Yang X, Fisher J: Physiologically based pharmacokinetic modeling of human exposure to perfluorooctanoic acid suggests historical non drinking-water exposures are important for predicting current serum concentrations. Toxicol Appl Pharmacol 330: 9–21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang CH, Glover KP, Han X: Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci 117: 294–302, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Beesoon S, Zhu L, Martin JW: Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47: 10619–10627, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Chang S, Allen BC, Andres KL, Ehresman DJ, Falvo R, Provencher A, Olsen GW, Butenhoff JL: Evaluation of serum lipid, thyroid, and hepatic clinical chemistries in association with serum perfluorooctanesulfonate (PFOS) in cynomolgus monkeys after oral dosing with potassium PFOS. Toxicol Sci 156: 387–401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fair PA, Romano T, Schaefer AM, Reif JS, Bossart GD, Houde M, Muir D, Adams J, Rice C, Hulsey TC, Peden-Adams M: Associations between perfluoroalkyl compounds and immune and clinical chemistry parameters in highly exposed bottlenose dolphins (Tursiops truncatus). Environ Toxicol Chem 32: 736–746, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Butenhoff JL, Chang SC, Olsen GW, Thomford PJ: Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology 293: 1–15, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Lieder PH, Chang SC, York RG, Butenhoff JL: Toxicological evaluation of potassium perfluorobutanesulfonate in a 90-day oral gavage study with Sprague-Dawley rats. Toxicology 255: 45–52, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, Butenhoff JL: Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183: 117–131, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Son HY, Kim SH, Shin HI, Bae HI, Yang JH: Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch Toxicol 82: 239–246, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Takahashi M, Ishida S, Hirata-Koizumi M, Ono A, Hirose A: Repeated dose and reproductive/developmental toxicity of perfluoroundecanoic acid in rats. J Toxicol Sci 39: 97–108, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Xing J, Wang G, Zhao J, Wang E, Yin B, Fang D, Zhao J, Zhang H, Chen YQ, Chen W: Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J mouse models. Environ Pollut 210: 388–396, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Klaunig JE, Shinohara M, Iwai H, Chengelis CP, Kirkpatrick JB, Wang Z, Bruner RH: Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague-Dawley rats. Toxicol Pathol 43: 209–220, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Serex T, Anand S, Munley S, Donner EM, Frame SR, Buck RC, Loveless SE: Toxicological evaluation of 6:2 fluorotelomer alcohol. Toxicology 319: 1–9, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Butenhoff JL, Kennedy GL Jr., Frame SR, O’Connor JC, York RG: The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology 196: 95–116, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB: Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 56: 338–349, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Curran I, Hierlihy SL, Liston V, Pantazopoulos P, Nunnikhoven A, Tittlemier S, Barker M, Trick K, Bondy G: Altered fatty acid homeostasis and related toxicologic sequelae in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS). J Toxicol Environ Health A 71: 1526–1541, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Kim HS, Jun Kwack S, Sik Han E, Seok Kang T, Hee Kim S, Young Han S: Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate. J Toxicol Sci 36: 201–210, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Ladics GS, Stadler JC, Makovec GT, Everds NE, Buck RC: Subchronic toxicity of a fluoroalkylethanol mixture in rats. Drug Chem Toxicol 28: 135–158, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Newsted JL, Beach SA, Gallagher SP, Giesy JP: Acute and chronic effects of perfluorobutane sulfonate (PFBS) on the mallard and northern bobwhite quail. Arch Environ Contam Toxicol 54: 535–545, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Rogers JM, Ellis-Hutchings RG, Grey BE, Zucker RM, Norwood J Jr., Grace CE, Gordon CJ, Lau C: Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol Sci 137: 436–446, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Chou HC, Wen LL, Chang CC, Lin CY, Jin L, Juan SH: From the cover: L-carnitine via PPARγ- and Sirt1-dependent mechanisms attenuates epithelial-mesenchymal transition and renal fibrosis caused by perfluorooctanesulfonate. Toxicol Sci 160: 217–229, 2017 [DOI] [PubMed] [Google Scholar]
- 59.Wen LL, Lin CY, Chou HC, Chang CC, Lo HY, Juan SH: Perfluorooctanesulfonate mediates renal tubular cell apoptosis through PPARgamma inactivation. PLoS One 11: e0155190, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abbott BD, Wood CR, Watkins AM, Tatum-Gibbs K, Das KP, Lau C: Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator-activated receptors (PPAR) and nuclear receptor-regulated genes in fetal and postnatal CD-1 mouse tissues. Reprod Toxicol 33: 491–505, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Arukwe A, Mortensen AS: Lipid peroxidation and oxidative stress responses of salmon fed a diet containing perfluorooctane sulfonic- or perfluorooctane carboxylic acids. Comp Biochem Physiol C Toxicol Pharmacol 154: 288–295, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Chung ACK: Perfluorooctane sulfonate (PFOS) promotes renal injury under diabetic condition in vitro. Hong Kong J Nephrol 17: S3–S4, 2015 [Google Scholar]
- 63.Diaz MJ, Chinje E, Kentish P, Jarnot B, George M, Gibson G: Induction of cytochrome P4504A by the peroxisome proliferator perfluoro-n-octanoic acid. Toxicology 86: 109–122, 1994 [DOI] [PubMed] [Google Scholar]
- 64.Eldasher LM, Wen X, Little MS, Bircsak KM, Yacovino LL, Aleksunes LM: Hepatic and renal Bcrp transporter expression in mice treated with perfluorooctanoic acid. Toxicology 306: 108–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eroʇlu P, Deniz M, Eke D, Çömelekoʇlu U, Çelik A, Berköz M, et al. : Projective effect of curcumin against oxidative damage in rat kidney. Turkish J Biochem 36, 317–321, 2011 [Google Scholar]
- 66.Gorrochategui E, Lacorte S, Tauler R, Martin FL: Perfluoroalkylated substance effects in xenopus laevis A6 kidney epithelial cells determined by ATR-FTIR spectroscopy and chemometric analysis. Chem Res Toxicol 29: 924–932, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu W, Jones PD, Upham BL, Trosko JE, Lau C, Giesy JP: Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 68: 429–436, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Qian Y, Ducatman A, Ward R, Leonard S, Bukowski V, Lan Guo N, Shi X, Vallyathan V, Castranova V: Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: Role in endothelial permeability. J Toxicol Environ Health A 73: 819–836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa Y: Short-term exposure to the peroxisome proliferators, perfluorooctanoic acid and perfluorodecanoic acid, causes significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett 57: 55–60, 1991 [DOI] [PubMed] [Google Scholar]
- 70.Witzmann FA, Fultz CD, Lipscomb JC: Toxicant-induced alterations in two-dimensional electrophoretic patterns of hepatic and renal stress proteins. Electrophoresis 17: 198–202, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Kariuki MN, Nagato EG, Lankadurai BP, Simpson AJ, Simpson MJ: Analysis of sub-lethal toxicity of perfluorooctane sulfonate (PFOS) to daphnia magna Using 1H nuclear magnetic resonance-based metabolomics. Metabolites 7: E15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lankadurai BP, Simpson AJ, Simpson MJ: H-1 NMR metabolomics of Eisenia fetida responses after sub-lethal exposure to perfluorooctanoic acid and perfluorooctane sulfonate. Environ Chem 9: 502–511, 2012 [Google Scholar]
- 73.Peng S, Yan L, Zhang J, Wang Z, Tian M, Shen H: An integrated metabonomics and transcriptomics approach to understanding metabolic pathway disturbance induced by perfluorooctanoic acid. J Pharm Biomed Anal 86: 56–64, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Skov K, Kongsbak K, Hadrup N, Frandsen HL, Svingen T, Smedsgaard J, et al. : Exposure to perfluorononanoic acid combined with a low-dose mixture of 14 human-relevant compounds disturbs energy/lipid homeostasis in rats. Metabolomics 11: 1451–1464, 2015 [Google Scholar]
- 75.Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, Sun X, Jia W, Zhou Z: High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 8: e61409, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner ND, Simpson AJ, Simpson MJ: Metabolomic responses to sublethal contaminant exposure in neonate and adult Daphnia magna. Environ Toxicol Chem 36: 938–946, 2017 [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Liu L, Zhang W, Zhang J, Du X, Huang Q, Tian M, Shen H: Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative /nitrosative stress in humans. Environ Pollut 229: 168–176, 2017 [DOI] [PubMed] [Google Scholar]
- 78.Yu N, Wei S, Li M, Yang J, Li K, Jin L, Xie Y, Giesy JP, Zhang X, Yu H: Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci Rep 6: 23963, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H, Ding L, Fang X, Shi Z, Zhang Y, Chen H, Yan X, Dai J: Biological responses to perfluorododecanoic acid exposure in rat kidneys as determined by integrated proteomic and metabonomic studies. PLoS One 6: e20862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding L, Hao F, Shi Z, Wang Y, Zhang H, Tang H, Dai J: Systems biological responses to chronic perfluorododecanoic acid exposure by integrated metabonomic and transcriptomic studies. J Proteome Res 8: 2882–2891, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Kishi R, Nakajima T, Goudarzi H, Kobayashi S, Sasaki S, Okada E, Miyashita C, Itoh S, Araki A, Ikeno T, Iwasaki Y, Nakazawa H: The association of prenatal exposure to perfluorinated chemicals with maternal essential and longchain polyunsaturated fatty acids during pregnancy and the birth weight of their offspring: The hokkaido study. Environ Health Perspect 123: 1038–1045, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, Chen PC, Hsieh WS: Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One 7: e42474, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Cupul-Uicab LA, Brantsaeter AL, Longnecker MP: Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol 175: 1209–1216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi X, Zhou B: The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci 115: 391–400, 2010 [DOI] [PubMed] [Google Scholar]
- 85.King SC, Pollack LA, Li J, King JB, Master VA: Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol 191: 1665–1670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagos Y, Wolff NA: Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins (Basel) 2: 2055–2082, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W: The organic anion transporter (OAT) family: A systems biology perspective. Physiol Rev 95: 83–123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin CY, Lin LY, Wen TW, Lien GW, Chien KL, Hsu SH, Liao CC, Sung FC, Chen PC, Su TC: Association between levels of serum perfluorooctane sulfate and carotid artery intima-media thickness in adolescents and young adults. Int J Cardiol 168: 3309–3316, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Qin XD, Qian Z, Vaughn MG, Huang J, Ward P, Zeng XW, Zhou Y, Zhu Y, Yuan P, Li M, Bai Z, Paul G, Hao YT, Chen W, Chen PC, Dong GH, Lee YL: Positive associations of serum perfluoroalkyl substances with uric acid and hyperuricemia in children from Taiwan. Environ Pollut 212: 519–524, 2016 [DOI] [PubMed] [Google Scholar]
- 90.Stanifer JW, Muiru A, Jafar TH, Patel UD: Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant 31: 868–874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caverly Rae JM, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL: Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague-Dawley rats. Toxicol Rep 2: 939–949, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Cousins IT, Scheringer M, Hungerbühler K: Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60: 242–248, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.