Abstract
Background and objectives
An association between proton pump inhibitor (PPI) use and hip fracture risk has been described in the general population, where the primary causative hypothesis focuses on impaired gastrointestinal calcium absorption. The impact of acid suppressor use on hip fracture risk in a high-risk subset, patients with ESKD requiring hemodialysis, is unknown and could help further distinguish the reason for higher susceptibility among PPI users.
Design, setting, participants, & measurements
Using the US Renal Data System, we identified all hip fracture events recorded between 2009 and 2014 among patients dependent on hemodialysis. Eligible cases were matched on index date with ten controls. We identified PPI and histamine-2 receptor antagonist use from Medicare Part D claims covering 3 years before the index date and stratified according to proportion of days covered by filled prescriptions. Using logistic regression with multiple imputation for missing data, we estimated unadjusted and multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs).
Results
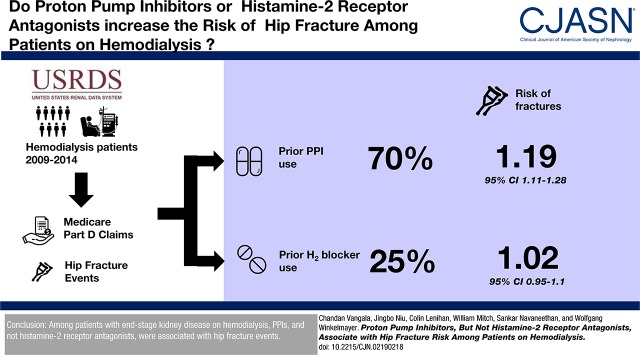
We studied 4551 cases and 45,510 controls. Patients were older, more likely to be female and white, and had shorter dialysis vintage; fewer were obese. A larger proportion of patients had any prior PPI (70% versus 63%) or histamine-2 receptor antagonist (25% versus 23%) use. Use of PPI was associated with higher risk of hip fracture (adjusted OR, 1.19; 95% CI, 1.11 to 1.28). This association remained within subgroups of low, moderate, and high PPI use, yielding adjusted ORs of 1.16 (95% CI, 1.06 to 1.27), 1.21 (95% CI, 1.11 to 1.31), and 1.19 (95% CI, 1.08 to 1.31), respectively.
Conclusions
Among patients with ESKD on hemodialysis, PPIs and not histamine-2 receptor antagonists were associated with hip fracture events.
Keywords: end-stage renal disease; Epidemiology and outcomes; drug safety; case-control; USRDS; Odds Ratio; Proton Pump Inhibitors; renal dialysis; Logistic Models; Histamine; Medicare Part D; Histamine H2 Antagonists; Kidney Failure, Chronic; Hip Fractures; obesity
Visual Abstract
Introduction
Hip fractures are devastating events with significant downstream morbidity, mortality, and excess health care cost. Among patients with ESKD requiring hemodialysis, hip fracture risk is estimated to be four times higher than in the general population (1,2). Patients dependent on hemodialysis who sustain a hip fracture face a 30-day mortality of 16% (3), a 1-year mortality twice that of the general population, and a median length of stay 3 days longer (4). Distorted bone and mineral metabolism, dysautonomia, cachexia, inflammation, and additional medication burden may all be substantial contributors to increased risk. However, little is known about the effect of commonly prescribed medications on hip fracture risk in this unique population.
Proton pump inhibitor (PPI) use is associated with hip fracture risk in the general population (5,6). The hypothesized mechanisms underlying this association focus on reduced calcium absorption (7,8), but also include gastrin-mediated parathyroid gland stimulation (9), vitamin B12 deficiency (10), and impaired function of osteoblasts and osteoclasts (11). Patients with ESKD are a subgroup in whom calcinuria does not balance out gastrointestinal absorption of calcium. Therefore, total body calcium storage relies largely on external influences such as nutrition, medications, and dialysate concentration. Accordingly, fracture risk hinges less on calcium supply as absorption is likely mitigated by limited or absent urine output. Theoretically, histamine-2 receptor antagonists, which also reduce gastric acid production, would yield a similar association with hip fracture events if the mechanism of proposed risk involved acid suppression alone. Yet, studies in the general population conflict on whether a demonstrable association exists between histamine-2 receptor antagonists and fracture events (12,13).
Thus, with bone pathology distinctive from more frequently seen osteoporosis (14) and less concern for deficient total body stores of calcium, the impact of commonly prescribed acid suppressants on hip fracture risk in the high-risk subset with ESKD remains uncertain. We designed this study to determine whether PPI and histamine-2 receptor antagonist use associated with hip fracture events in patients with ESKD requiring hemodialysis in the United States.
Materials and Methods
Study Design and Source Population
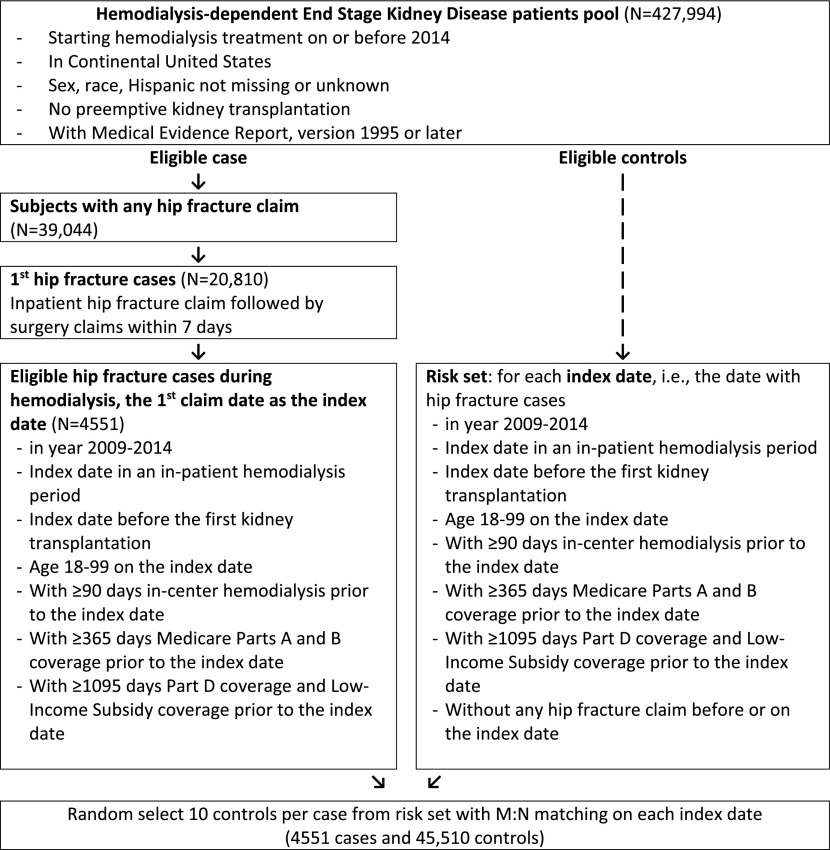
Using the US Renal Data System (USRDS), the national registry of persons with ESKD (15), we conducted a retrospective, case-control study. This study of all Medicare (Parts A and B)-insured patients on hemodialysis was essentially nested within the person-time between 2006 and 2014. The study population required that patients had comprehensive prescription drug coverage through Medicare Part D and had received a Low-Income Subsidy, which greatly limits or eliminates copayments for medications. The patient pool was further restricted to exclude those with preemptive kidney transplantation, missing treatment start date, missing demographic data, or missing Medical Evidence Reports from 1995 or later. The hip fracture and matching control index date must have occurred before a first kidney transplantation. Before the index date, all participants were required to have ≥365 days of Medicare Parts A and B coverage, ≥1095 days of both Part D and Low-Income Subsidy coverage, and at least 90 days of hemodialysis therapy.
We identified potential cases from inpatient claims containing the International Classification of Diseases, Ninth Edition diagnosis codes 820.xx or 821.xx. The date of hip fracture diagnosis was defined as the index date. To further ensure a specific hip fracture identification algorithm, we required cases to have an International Classification of Diseases, Ninth Edition surgical procedure code among 78.55, 79.15, 81.52, 79.05, 79.25, or 81.40 within 7 days of diagnosis (3). On each index date, ten controls per case were randomly selected from the risk set with M:N matching. For n cases on each index date, 10×n number of controls were selected without specifically linking to an individual case. This pooled strategy is an efficient way to organize sets and is useful in case-control studies with random selection of participants (16,17). Controls could subsequently become cases.
Exposure of Interest
The exposures of interest were PPI and histamine-2 receptor antagonist use as recorded over the 3 years preceding the index date from Part D prescription claims representing filled prescriptions. We categorized any use as the presence of at least one prescription claim in the 3 years before the index date. Among these users, those labeled “low use” received pharmacy-dispensed pills covering <20% of the 1095 days before the index date. We labeled PPI users “moderate use” if they received pills covering ≥20% but <80% of the 1095 days before the index date. “High use” was reserved for users with ≥80% of the 1095 days before the index date. Lastly, we also recorded PPI use as a continuous variable capturing the total number of exposure months.
Covariates
Patient characteristics were drawn from Medical Evidence Reports and Medicare billing claims (hospitalization data files and physician supplier files) compiled by the USRDS. Age, sex, race (white, black, or other), Hispanic ethnicity, and body mass index (BMI) have well known associations with hip fracture, and these characteristics were abstracted from the Medical Evidence Report along with the duration of dialysis before the index date (vintage) and the reported primary cause of ESKD. With a required ≥1 year of Part A and B coverage, we aimed to incorporate specific comorbid conditions that could potentially affect hip fracture risk. Consequently, we included hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, peripheral vascular disease, arrhythmia, rheumatologic disorder, osteoporosis, depression, and tobacco use as potential confounders (see Supplemental Table 1 for specifications) (18–24). As there is precedent for geographic variation of hip fracture incidence (25,26), census division was also included in multivariable analysis. Prior bisphosphonate and steroid use was defined from Part D claims as any pharmacy-dispensed pills in the 3 years before the index date.
Statistical Analyses
Unadjusted and multivariable-adjusted, conditional logistic regression models were fit to estimate (independent) associations between hip fracture case (versus control) status and prior PPI and histamine-2 receptor antagonist use. We expressed estimates of association as odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs), comparing nonuse with any use, as well as with low use, moderate use, and high use. We incorporated baseline characteristics and comorbidities outlined in Table 1 in the multivariable analysis. We further examined for potential interactions of the main associations with several characteristics and presented findings from subgroups including age, sex, race, ethnicity, and BMI. Lastly, we performed a separate analysis in which we excluded patients that had exposure to both PPI and histamine-2 receptor antagonist use.
Table 1.
Characteristics of hip fracture cases and controls
| Variable | Cases, n=4551 | Controls, n=45,510 |
|---|---|---|
| Mean±SD or n (%) | Mean±SD or n (%) | |
| Age, yr | 71±12 | 61±14 |
| Median [IQR] | 72 [63–79] | 62 [52–72] |
| Sex | ||
| Women | 2666 (59) | 23,599 (52) |
| Men | 1885 (41) | 21,911 (48) |
| Ethnicity/race | ||
| Non-Hispanic black | 1328 (29) | 22,404 (49) |
| Non-Hispanic white | 1812 (40) | 11,383 (25) |
| Non-Hispanic other | 376 (8) | 2840 (6) |
| Hispanic black | 16 (0.4) | 285 (0.6) |
| Hispanic white | 1001 (22) | 8432 (19) |
| Hispanic other | 18 (0.4) | 166 (0.4) |
| BMI, kg/m2; missing in 959 | 28±8 | 30±9 |
| Median [IQR] | 26.7 [23.1–31.9] | 28.4 [24.0–34.8] |
| Underweight | 194 (4) | 1550 (3) |
| Normal | 1528 (34) | 12,233 (27) |
| Overweight | 1285 (28) | 11,797 (26) |
| Obese | 1137 (25) | 13,214 (29) |
| Severely obese | 338 (7) | 5826 (13) |
| Dialysis vintage, yr | 5.1±3.4 | 6.0±3.7 |
| Median [IQR] | 4.4 [2.5–7.0] | 5.2 [3.4–7.9] |
| Census division | ||
| New England | 138 (3) | 1288 (3) |
| Middle Atlantic | 479 (11) | 5371 (12) |
| East North Central | 578 (13) | 6117 (13) |
| West North Central | 239 (5) | 1937 (4) |
| South Atlantic | 958 (21) | 10,912 (24) |
| East South Central | 350 (8) | 3810 (8) |
| West South Central | 765 (17) | 7609 (17) |
| Mountain | 250 (6) | 1634 (4) |
| Pacific | 794 (18) | 6732 (15) |
| Comorbidities | ||
| Hypertension | 4477 (98) | 44,381 (98) |
| Diabetes mellitus | 3518 (77) | 30,334 (67) |
| Coronary artery disease | 2712 (60) | 20,975 (46) |
| Peripheral vascular disease | 1920 (42) | 14,315 (31) |
| Cerebrovascular disease | 1246 (27) | 8964 (20) |
| Heart failure | 2974 (65) | 24,579 (54) |
| Arrhythmia | 1437 (32) | 10,124 (22) |
| Osteoporosis | 30 (0.7) | 136 (0.3) |
| Rheumatological disorder | 176 (4) | 1843 (4) |
| Tobacco use | 697 (15) | 7584 (17) |
| Depression | 1149 (25) | 8109 (18) |
| History of steroid use | 1379 (30) | 13,185 (29) |
| History of bisphosphonate use | 193 (4) | 825 (2) |
| Primary cause of ESKD; missing in 4 | ||
| Diabetes mellitus | 2569 (57) | 21,613 (48) |
| GN/vasculitis | 248 (6) | 4697 (10) |
| Interstitial/pyelonephritis | 125 (3) | 1226 (3) |
| Hypertensive/large vessel disease | 1230 (27) | 13,643 (30) |
| Cystic/hereditary/congenital | 77 (2) | 1168 (3) |
| Neoplasm/tumor | 42 (0.9) | 316 (0.7) |
| Miscellaneous | 260 (6) | 2843 (6) |
IQR, interquartile range; BMI, body mass index.
Given the required Medical Evidence Report, we expected limited missing data and assumed these data to be missing at random. Multiple imputation using chained equations were used to create and analyze five imputed datasets (27). Incomplete variables were imputed under fully conditional specification and the imputation model included all of the variables in the analysis plus a fixed effect for pair identification to account for the matching of controls to cases (28,29). Model parameters were estimated applying the analysis model to each imputed data set separately. These estimates and their SEMs were then combined using Rubin rules. Analyses were conducted using SAS software (version 9.3; SAS Institute Inc., Cary, NC) and StataMP (version 14; StataCorp, College Station, TX).
Results
Patient Selection and Baseline Characteristics
We identified 4551 hip fracture cases between 2009 and 2014 who satisfied all inclusion criteria. We randomly selected exactly ten controls per case with M:N matching on index date, resulting in 45,510 controls (Figure 1). Cases were older than controls with a mean difference in age of 9.3 years (Table 1). The proportion of non-Hispanic white cases among cases was greater than in controls (40% versus 25%) whereas the proportion of non-Hispanic black cases was lower (29% versus 49%). The control patients had higher BMI (with a mean difference of 1.9 kg/m2) and a greater proportion were obese or severely obese. Cases had a shorter duration since initiating hemodialysis treatment compared with cases (mean difference of 0.8 years) and had higher proportions of almost all of the measured comorbid conditions.
Figure 1.
Eligible cases and the risk set from which controls were drawn had equivalent selection criteria. Subjects required 1 year of Parts A and B coverage in order to capture comorbid conditions that could influence hip fracture risk. Requiring 3 years of Part D and Low-Income subsidy coverage granted access to degree of medication use.
Cases had higher proportions of any recorded PPI use before their hip fracture event (70% versus 63%) and also had greater proportions that fell into categories of moderate or high use compared with controls. Almost 15% of the overall population had ≥80% of the proportion of days covered with PPI pills received. By contrast, fewer patients filled prescriptions for histamine-2 receptor antagonists in the previous 3 years, 24% overall, with no significant differences between cases and controls in any use or any of the categories of proportion of days covered. Of the entire sample of cases and controls, 15.9% of the patients had exposure to both PPI use and histamine-2 receptor antagonist use.
Two variables from the Medical Evidence Report, BMI and cause of ESKD, contained missing observations. We found 959 missing BMI values and four missing labels for cause of ESKD, comprising nearly 2% and <0.01% of the total expected observations, respectively.
Associations between PPI Use and Fracture
The unadjusted OR of hip fracture status with any versus no PPI use was 1.39 (95% CI, 1.30 to 1.49; Table 2). The unadjusted OR for less (<20% of proportion of days covered), moderate (≥20% and <80% of proportion of days covered), and high (≥80% of proportion of days covered) PPI use were 1.21 (95% CI, 1.11 to 1.32), 1.44 (95% CI, 1.33 to 1.55), and 1.55 (95% CI, 1.42 to 1.70), respectively. Multivariable adjustment for demographics, BMI, dialysis vintage, comorbid conditions, cause of ESKD, and Census region attenuated these findings. The multivariable-adjusted ORs of hip fracture status with any versus no PPI use was 1.19 (95% CI, 1.11 to 1.28), whereas the adjusted odds ratios for low, moderate, and high PPI use were 1.16 (95% CI, 1.06 to 1.27), 1.21 (95% CI, 1.11 to 1.31), and 1.19 (95% CI, 1.08 to 1.31), respectively. The unadjusted association of hip fracture events with the continuous variable, years of PPI exposure, resulted in an OR of 1.16 (95% CI, 1.13 to 1.19). After multivariable adjustment, the strength of association was attenuated to an OR of 1.06 (95% CI, 1.03 to 1.09) for each additional year of PPI exposure. No significant interaction with other variables was detected, and consistency was maintained in examining the association among subgroups (Supplemental Figure 1). The association also remained unchanged after excluding patients with any exposure to bisphosphonates during the 3-year window.
Table 2.
Proton pump inhibitor and histamine-2 receptor antagonist use in hip fracture cases and controls and measures of association
| Acid Suppressant | Proportion of Days Covered | Cases (n=4551), N (%) | Controls (n=45,510), N (%) | Unadjusted Odds Ratio | P Value | Adjusted Odds Ratio | P Value |
|---|---|---|---|---|---|---|---|
| Proton pump inhibitor | No use | 1347 (30) | 16,773 (37) | 1.00 (referent) | – | 1.00 (referent) | – |
| Any use | 3204 (70) | 28,737 (63) | 1.39 (1.30 to 1.49) | <0.001 | 1.19 (1.11 to 1.28) | <0.001 | |
| <20% PDC | 906 (19) | 9325 (21) | 1.21 (1.11 to 1.32) | <0.001 | 1.16 (1.06 to 1.27) | <0.001 | |
| ≥20% and <80% PDC | 1483 (33) | 12,864 (28) | 1.44 (1.33 to 1.55) | <0.001 | 1.21 (1.11 to 1.31) | <0.001 | |
| ≥80% PDC | 815 (18) | 6548 (14) | 1.55 (1.42 to 1.70) | <0.001 | 1.19 (1.08 to 1.31) | <0.001 | |
| H2-receptor antagonist | No use | 3429 (75) | 35,011 (77) | 1.00 (referent) | – | 1.00 (referent) | – |
| Any use | 1122 (25) | 10,499 (23) | 1.09 (1.02 to 1.17) | 0.02 | 1.02 (0.95 to 1.10) | 0.54 | |
| <20% PDC | 568 (13) | 5755 (13) | 1.01 (0.92 to 1.11) | 0.87 | 0.99 (0.90 to 1.09) | 0.09 | |
| ≥20% and <80% PDC | 431 (10) | 3378 (8) | 1.23 (1.11 to 1.37) | <0.001 | 1.08 (0.97 to 1.21) | 0.20 | |
| ≥80% PDC | 123 (3) | 1166 (3) | 1.08 (0.89 to 1.30) | 0.44 | 0.96 (0.79 to 1.17) | 0.16 |
PDC, proportion of days covered (sum of the number of days of drug supplied in prescriptions divided by the number of days of the interval; here: 1095 days).
By contrast, the unadjusted conditional OR of hip fracture status with any versus no histamine-2 receptor antagonist use was 1.09 (95% CI, 1.02 to 1.17; Table 2). The unadjusted ORs for less, moderate, and high histamine-2 receptor antagonist use were 1.01 (95% CI, 0.92 to 1.11), 1.23 (95% CI, 1.11 to 1.37), and 1.08 (95% CI, 0.89 to 1.30), respectively. However, multivariable-adjustment rendered all of these associations null.
After excluding patients with exposure to both PPIs and histamine-2 receptor antagonists, the associations were unchanged among the remaining 84.1% of the sample. The multivariable-adjusted OR of hip fracture status with any versus no PPI use was 1.18 (95% CI, 1.10 to 1.27) whereas with any versus no histamine-2 receptor antagonist use, it was 0.89 (95% CI, 0.79 to 1.02).
Discussion
Using a large nationwide registry of United States patients with ESKD requiring hemodialysis, we found that almost three quarters of patients who had a hip fracture had used a PPI in the 3 years preceding their event, with close to 15% having filled PPI prescriptions representing supplies in excess of 80% of the days during that time interval. When compared with controls frequency matched on the index date, we found a significant association between case status and prior PPI use. The association remained significant after multivariable adjustment. Conversely, no associations were identified with histamine-2 receptor antagonist use; however, only 3% of patients dependent on hemodialysis received sufficient medications to be labeled as high users.
This investigation confirms needed vigilance for unnecessary long-term PPI use. With 42% of Part D-enrolled patients on dialysis having received a PPI in 2014, PPIs were the sixth highest class of drugs received (15). Only phosphate-binding agents, opiate agonists, and various cardiovascular agents were received in higher quantity than PPIs. An examination of the Danish national database revealed a four-fold increase in PPI use from 2002 to 2014 and also noted that the proportion maintaining treatment beyond 2 years increased with each passing year (30). By examining acid suppressor use in the 3 years before the index date, we assessed both cumulative exposure and the proportion of long-term users. Clinical guidelines do not outline a definition of long-term use, but the US Food and Drug Administration has requested that manufacturers supply 3-year data if available (31).
Several observational studies have identified an association between PPI use and hip fracture events in the general population (5,6,12). In the first large-scale study, Yang et al. (6) analyzed 13,556 hip fracture cases and 135,386 controls matched on age, sex, calendar period, and index date and reported an adjusted OR of 1.44, (95% CI, 1.30 to 1.59) with more than 1 year of PPI therapy. More recently, Zhou et al. (5) performed a meta-analysis involving 18 observational studies, where the pooled analysis demonstrated that PPI use moderately increased the risk (relative risk, 1.26; 95% CI, 1.16 to 1.36) of hip fracture.
Mechanisms of increased risk are still undetermined, but the lack of an association between PPI use and bone mineral density (32,33) suggests that PPI use may be more influential on bone quality. Costa-Rodrigues et al. (11) conducted experiments where three different types of PPI-containing media exhibited dose-dependent inhibitory effects on osteoblast and osteoclast activity. In vitro studies suggest that PPIs may inhibit osteoclast function through irreversible binding to their vacuolar type of H+-ATPase. Further supporting this mechanism, the development of an H+K+-ATPase beta subunit knockout mouse resulted in decreases in bone mineral content, mechanical strength, and cortical thickness (34). Mizunashi et al. conducted a study of patients with history of gastric ulcer maintained on histamine-2 receptor antagonists. Changing therapy to omeprazole reduced urinary hydroxyproline whereas control patients without therapy noted no changes in bone parameters (35). Jo et al. (36) randomized 39 patients to revaprazan, a reversible inhibitor of H+K+-ATPase, and pantoprazole and noted distinction in the levels of another marker of bone turnover, urine deoxypyridinoline. Pathologic changes in bone resorption may explain the persistent fracture susceptibility despite the lack of BMD findings or diagnosis of osteoporosis.
Discourse has mainly focused on the potential for PPI-induced reductions in calcium absorption. In a small study of eight patients using gut lavage, Bo-Linn et al. (7) reported no significant difference in net calcium absorption with cimetidine-induced changes in gastric pH from 3.0 to 7.4. Another similar study of omeprazole-induced increases in gastric pH did not result in significant changes in mineral (calcium, magnesium, phosphorus, or zinc) absorption (8). Opposingly, O’Connell et al. (37) noted that after 2 weeks of omeprazole therapy, 18 patients demonstrated an absolute difference in fractional calcium absorption of −5.5%. Lastly, a study of 16 patients with ESKD dependent on hemodialysis, receiving therapy with calcium carbonate revealed a slight but significant decrease in corrected serum calcium level (2.41±0.18 to 2.36±0.16 mmol) after 1 month of omeprazole therapy (38). Differing methods for detecting net calcium absorption likely contribute to conflicting results. Importantly, patients with ESKD are more often than not believed to be in net positive calcium balance with excess calcium administration, resulting in extraosseous deposition (39).
Hypomagnesemia can provoke parathyroid hormone secretion, increasing osteoclast activity and resultant bone resorption. Sakaguchi et al. (40) recently conducted an analysis of data extracted from the Japanese Society for Dialysis Therapy Renal Data Registry that demonstrated clinical evidence for the influence of magnesium levels on parathyroid hormone secretion and fracture risk. Several studies report an association between PPI use and hypomagnesemia in the general population (41,42), and reduced magnesium levels have even been reported in multiple studies of patients dependent on hemodialysis (43–46). Importantly, Kieboom et al. noted similar associations with hypomagnesemia among both histamine-2 receptor antagonist users (adjusted OR, 2.00; 95% CI, 1.08 to 3.72) and PPI users (adjusted OR, 2.00; 95% CI, 1.36 to 2.93) (42). However, unless the requirement of hemodialysis differentially affects the impact of PPIs and histamine-2 receptor antagonists on magnesium absorption, the lack of association with hip fracture events in our examination of histamine-2 receptor antagonist use argues against a cation-related pathology.
Previous studies have also attributed increased hip fracture risk to reduced absorption of pH-mediated nutrients other than mineral cations. Vitamin B12 deficiency is found in greater proportions among patients who are prescribed either group of acid suppressors (10). This deficiency could affect fall risk through neurologic disturbance or disrupt bone remodeling by weakening collagen crosslinks via hyperhomocysteinemia. Use of omeprazole in chickens has led to parathyroid hypertrophy and hyperplasia (9). However, much like B12 deficiency, gastrin-mediated increases in parathyroid hormone secretion would be equally as likely among histamine-2 receptor antagonist use. Also, this finding has not been replicated in human studies (36). Furthermore, a meta-analysis by Clark et al. (47) concluded that ranitidine, a histamine-2 receptor antagonist, was more effective than PPIs at increasing gastric pH. Thus, the lack of significant association among histamine-2 receptor antagonist users suggest that secondary hypergastrinemia and the potential effect of gastrointestinal pH on absorption of cations and nutrients are unlikely culprits for conferring increased risk.
PPI use has no known effect on the bioavailability of commonly used medications to address mineral bone disorder associated with CKD, namely phosphate binders, vitamin D analogs, and calcimimetics. Phosphate binders, specifically sevelamer (48) and calcium carbonate (49), have been explicitly studied and their effectiveness in lowering serum phosphorus levels remains unchanged with PPI use. A study of 26 healthy participants receiving paricalcitol yielded no changes in peak serum concentration or area under the plasma drug concentration-time curve after single doses of omeprazole (50).
Current treatments of mineral bone disease associated with CKD focus on following biomarkers and preventing distortions in bone turnover. Unfortunately, no single element of this medical therapy has demonstrated a reduction in fracture outcomes. Additionally, bisphosphonates are not indicated in patients with ESKD, limiting fracture prevention strategies even further.
A trend among varying degrees of adherence was found in unadjusted analyses, but less obvious in adjusted regression models. This could potentially result from an inability to sufficiently differentiate moderate and high use. We could also be capturing an association with a surrogate of the underlying pathology, gastric acidity. However, the lack of significant findings with regards to histamine-2 receptor antagonist use make this possibility less likely. The estimated measure of association between hip fractures and each additional month of PPI use as a continuous variable appears slight. Yet, when considering a cumulative year of use, this estimate is consistent with the magnitude of difference between the lowest and highest categories of proportion of days covered. Given the abundance of observational studies reporting this association in the general population, we suspect that the uncovered association is not only accurate, but in fact, this association in the hemodialysis-dependent subset helps shed light on the underlying manner in which PPI use elevates hip fracture risk.
A potential limitation of this study is the inability to incorporate representative markers of the mineral bone disease axis. Secondary and tertiary hyperparathyroidism, along with the opposite scenario of adynamic bone disease, are highly influential on bone turnover and resultant quality, but parathyroid hormone levels were unavailable in our database. Both PPI and histamine-2 receptor antagonist use have been available over the counter during the time period studied. If the relatively reduced prevalence of histamine-2 receptor antagonist use does reflect uncaptured over-the-counter use, this could affect our power to detect sizable differences between cases and controls. Absent a financial incentive, however, restriction to recipients of Low-Income Subsidy with mostly first dollar coverage of prescription drugs limits this concern for uncaptured over the counter use. Significant overlap between PPI and histamine-2 receptor antagonist use may skew understandings of these exposure; however, the results remained consistent when the analysis was restricted to only PPI use and only histamine-2 receptor use. Although the required Medicare coverage does assist with capturing influential aspects of fracture risk, the requirement may also lend to potential selection bias and concerns regarding survivorship. Additionally, as in all observational studies, residual confounding by unmeasured or imprecisely measured confounders may exist. Considering that further subspecification of the exposure would limit the power of this study, we did not differentiate among specific types of drugs within their respective classes and their available dosages. Lastly, we were unable to capture the specific indication for which acid suppressor use was prescribed.
Prevalent long-term PPI use in patients dependent on hemodialysis warrants heightened vigilance over the continued necessity of these medications. In a broadly representative study of patients dependent on hemodialysis in the United States, we identified an independent association of hip fracture events with prior PPI use. The lack of an association with prior histamine-2 receptor antagonist use and the study patients’ hemodialysis-dependent status may suggest a more direct influence of PPIs on bone quality, as opposed to an effect on cation and nutrient stores. Thus, we recommend interval assessment of continued PPI use in patients dependent on hemodialysis who already experience substantial medication burden.
Disclosures
W.C.W. reports having served as an advisor or consultant, unrelated to the topic of this manuscript, to Akebia, Amgen, Astra-Zeneca, Bayer, Daichii-Sankyo, Relypsa, Vifor-Fresenius Medical Care Renal Pharma, and ZS Pharma. S.D.N. serves on the independent event adjudication committee for clinical trials sponsored by Bayer and Boehringer Ingelheim (unrelated to the topic of this manuscript). All other authors have no financial disclosures to report.
Supplementary Material
Acknowledgments
Data were provided through a data use agreement between the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) and W.C.W. An NIDDK officer reviewed this manuscript for privacy and approved of its publication. An Institutional Review Board at Baylor College of Medicine approved this study (H-36408).
C.V. participated in study design, performed statistical analysis, drafted manuscript, performed bibliographic search, and interpreted results of analysis. J.N. participated in study design and statistical analyses. C.R.L. substantially influenced conception and study design and provided significant contributions to interpretation of results. S.D.N. and W.E.M. were involved in interpretation of results and made important contributions to revising the manuscript for important intellectual content. W.C.W. was instrumental to acquisition of data, conception, drafting study design, and interpreting analysis. Each author contributed important intellectual content to manuscript drafting and revision. All authors approved the final manuscript.
C.V. was supported by a gift from Dr. and Mrs. Harold Selzman. C.R.L. receives grant support through a Mentored Clinical and Population Research Award from the American Heart Association (Western State Affiliate) and a Norman S. Coplon Extramural Grant for Clinical Applied Research from Satellite Healthcare. W.C.W. receives support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Proton Pump Inhibitors in Kidney Disease,” on pages 1458–1459.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02190218/-/DCSupplemental.
References
- 1.Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St Peter WL: Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis 62: 747–754, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Mittalhenkle A, Gillen DL, Stehman-Breen CO: Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 44: 672–679, 2004 [PubMed] [Google Scholar]
- 3.Nair SS, Mitani AA, Goldstein BA, Chertow GM, Lowenberg DW, Winkelmayer WC: Temporal trends in the incidence, treatment, and outcomes of hip fracture in older patients initiating dialysis in the United States. Clin J Am Soc Nephrol 8: 1336–1342, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA: Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol 24: 1461–1469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou B, Huang Y, Li H, Sun W, Liu J: Proton-pump inhibitors and risk of fractures: An update meta-analysis. Osteoporos Int 27: 339–347, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Yang YX, Lewis JD, Epstein S, Metz DC: Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 296: 2947–2953, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bo-Linn GW, Davis GR, Buddrus DJ, Morawski SG, Santa Ana C, Fordtran JS: An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest 73: 640–647, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR: Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr 14: 364–368, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Gagnemo-Persson R, Håkanson R, Sundler F, Persson P: Growth of the parathyroid glands in omeprazole-treated chickens. Scand J Gastroenterol 29: 493–497, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Lam JR, Schneider JL, Zhao W, Corley DA: Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 310: 2435–2442, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Costa-Rodrigues J, Reis S, Teixeira S, Lopes S, Fernandes MH: Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J 280: 5052–5064, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Corley DA, Kubo A, Zhao W, Quesenberry C: Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 139: 93–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok CS, Yeong JK, Loke YK: Meta-analysis: Risk of fractures with acid-suppressing medication. Bone 48: 768–776, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group : KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 7: 1–59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA: US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67[Suppl 1]: S1–S305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha-Chaudhuri P, Umbach DM, Weinberg CR: Pooled exposure assessment for matched case-control studies. Epidemiology 22: 704–712, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg CR, Umbach DM: Using pooled exposure assessment to improve efficiency in case-control studies. Biometrics 55: 718–726, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaëlsson K: Cardiovascular diseases and risk of hip fracture. JAMA 302: 1666–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Fan Y, Wei F, Lang Y, Liu Y: Diabetes mellitus and risk of hip fractures: A meta-analysis. Osteoporos Int 27: 219–228, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE; Osteoporotic Fractures in Men (MrOS) Study Group : Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation 119: 2305–2312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law MR, Hackshaw AK: A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: Recognition of a major effect. BMJ 315: 841–846, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy R, Cooper MS: Bone loss in inflammatory disorders. J Endocrinol 201: 309–320, 2009 [DOI] [PubMed] [Google Scholar]
- 23.van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C: Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54: 3104–3112, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Cook WL, Tomlinson G, Donaldson M, Markowitz SN, Naglie G, Sobolev B, Jassal SV: Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 1: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bacon WE, Smith GS, Baker SP: Geographic variation in the occurrence of hip fractures among the elderly white US population. Am J Public Health 79: 1556–1558, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee G, Zullo AR, Berry SD, Lee Y, McConeghy K, Kiel DP, Mor V: Geographic variation in hip fracture among United States longstay nursing home residents. J Am Med Dir Assoc 17: 865.e1–865.e3, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB: Multiple Imputations for Nonresponse in Surveys, New York, John Wiley & Sons, 2004 [Google Scholar]
- 28.Montez-Rath ME, Winkelmayer WC, Desai M: Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 9: 1328–1335, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Buuren S: Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16: 219–242, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pottegård A, Broe A, Hallas J, de Muckadell OB, Lassen AT, Lødrup AB: Use of proton-pump inhibitors among adults: A Danish nationwide drug utilization study. Therap Adv Gastroenterol 9: 671–678, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz DC: Long-term use of proton-pump inhibitor therapy. Gastroenterol Hepatol (N Y) 4: 322–325, 2008 [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon DH, Diem SJ, Ruppert K, Lian YJ, Liu CC, Wohlfart A, Greendale GA, Finkelstein JS: Bone mineral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: A SWAN cohort study. J Bone Miner Res 30: 232–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Targownik LE, Lix LM, Leung S, Leslie WD: Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 138: 896–904, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Fossmark R, Stunes AK, Petzold C, Waldum HL, Rubert M, Lian AM, Reseland JE, Syversen U: Decreased bone mineral density and reduced bone quality in H(+) /K(+) ATPase beta-subunit deficient mice. J Cell Biochem 113: 141–147, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Mizunashi K, Furukawa Y, Katano K, Abe K: Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int 53: 21–25, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Jo Y, Park E, Ahn SB, Jo YK, Son B, Kim SH, Park YS, Kim HJ: A proton pump inhibitor’s effect on bone metabolism mediated by osteoclast action in old age: A prospective randomized study. Gut Liver 9: 607–614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ: Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am J Med 118: 778–781, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Hardy P, Sechet A, Hottelart C, Oprisiu R, Abighanem O, Said S, Rasombololona M, Brazier M, Moriniere P, Achard JM, Pruna A, Fournier A: Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artif Organs 22: 569–573, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM: Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Sakaguchi Y, Hamano T, Wada A, Hoshino J, Masakane I: Magnesium and risk of hip fracture among patients undergoing hemodialysis. J Am Soc Nephrol 29: 991–999, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, Howell MD, Celi LA, Mukamal KJ: Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 83: 692–699, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, Franco OH, Kuipers EJ, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Proton pump inhibitors and hypomagnesemia in the general population: A population-based cohort study. Am J Kidney Dis 66: 775–782, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Ago R, Shindo T, Banshodani M, Shintaku S, Moriishi M, Masaki T, Kawanishi H: Hypomagnesemia as a predictor of mortality in hemodialysis patients and the role of proton pump inhibitors: A cross-sectional, 1-year, retrospective cohort study. Hemodial Int 20: 580–588, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Nakashima A, Ohkido I, Yokoyama K, Mafune A, Urashima M, Yokoo T: Proton pump inhibitor use and magnesium concentrations in hemodialysis patients: A cross-sectional study. PLoS One 10: e0143656, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alhosaini M, Walter JS, Singh S, Dieter RS, Hsieh A, Leehey DJ: Hypomagnesemia in hemodialysis patients: Role of proton pump inhibitors. Am J Nephrol 39: 204–209, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Misra PS, Alam A, Lipman ML, Nessim SJ: The relationship between proton pump inhibitor use and serum magnesium concentration among hemodialysis patients: A cross-sectional study. BMC Nephrol 16: 136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark K, Lam LT, Gibson S, Currow D: The effect of ranitidine versus proton pump inhibitors on gastric secretions: A meta-analysis of randomised control trials. Anaesthesia 64: 652–657, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Lai B, Cervelli MJ: Effect of gastric acid suppression with pantoprazole on the efficacy of sevelamer hydrochloride as a phosphate binder in haemodialysis patients: A pilot study. Nephrology (Carlton) 17: 402–406, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Cervelli MJ, Shaman A, Meade A, Carroll R, McDonald SP: Effect of gastric acid suppression with pantoprazole on the efficacy of calcium carbonate as a phosphate binder in haemodialysis patients. Nephrology (Carlton) 17: 458–465, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Palaparthy R, Pradhan RS, Chan J, Rieser M, Chira T, Galitz L, Awni W, Williams LA: Effect of omeprazole on the pharmacokinetics of paricalcitol in healthy subjects. Biopharm Drug Dispos 28: 65–71, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.