Abstract
Background and objectives
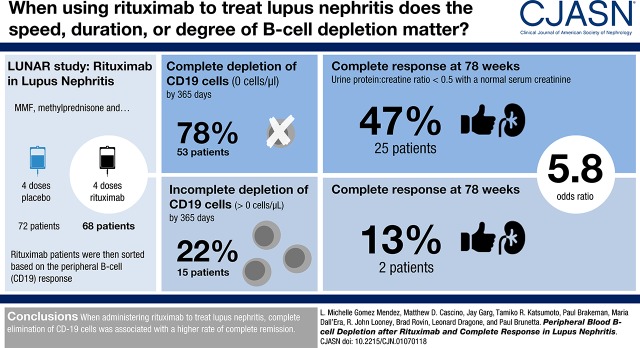
Incomplete peripheral blood B cell depletion after rituximab in lupus nephritis might correlate with inability to reduce tubulointerstitial lymphoid aggregates in the kidney, which together could be responsible for inadequate response to treatment. We utilized data from the Lupus Nephritis Assessment with Rituximab (LUNAR) study to characterize the variability of peripheral blood B cell depletion after rituximab and assess its association with complete response in patients with lupus nephritis.
Design, setting, participants, & measurements
We analyzed 68 participants treated with rituximab. Peripheral blood B cell depletion was defined as 0 cells/µl, termed “complete peripheral depletion,” assessed over 78 weeks. Logistic regression was used to estimate the association between characteristics of complete peripheral depletion and complete response (defined as urine protein-to-creatinine ratio <0.5 mg/mg, and normal serum creatinine or an increase in creatinine <15%, if normal at baseline), assessed at week 78.
Results
A total of 53 (78%) participants achieved complete peripheral depletion (0 cells/µl) in a median time of 182 days (interquartile range, 80–339).The median duration of complete peripheral depletion was 71 days (interquartile range, 14–158). Twenty-five (47%) participants with complete peripheral depletion achieved complete response, compared with two (13%) without. Complete peripheral depletion was associated with complete response (unadjusted odds ratio [OR], 5.8; 95% confidence interval [95% CI], 1.2 to 28; P=0.03). Longer time to achieving complete peripheral depletion was associated with a lower likelihood of complete response (unadjusted OR, 0.89; 95% CI, 0.81 to 0.98; P=0.02). Complete peripheral depletion lasting >71 days (the median) was associated with complete response (unadjusted OR, 4.1; 95% CI, 1.5 to 11; P=0.008).
Conclusions
There was substantial variability in peripheral blood B cell depletion in patients with lupus nephritis treated with rituximab from the LUNAR trial. Achievement of complete peripheral depletion, as well as the rapidity and duration of complete peripheral depletion, were associated with complete response at week 78.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_09_06_CJASNPodcast_18_10_.mp3
Keywords: lupus nephritis, rituximab, b cell depletion, LUNAR, renal response, systemic lupus erythematosus
Visual Abstract
Introduction
In lupus nephritis, immune complexes accumulate in the glomeruli, activate complement, and initiate an inflammatory response (1,2). Attracted by chemokines, B cells migrate from the circulation into the kidney (3) and aggregate in the tubulointerstitium (4,5). These lymphoid aggregates foster a high B cell activating factor (BAFF) environment in which B cells undergo clonal expansion and somatic hypermutation in response to local antigens (4–6). This process perpetuates a cycle of interstitial inflammation and damage, the extent of which is associated with progression to kidney failure (7). B cell–directed therapies have the potential to limit tissue damage by reducing both immune complexes in serum and lymphoid aggregates in the kidney and have therefore been attractive for use in lupus nephritis (8,9).
Rituximab is a chimeric anti-CD20 mAb that depletes CD20+ B cells. The Lupus Nephritis Assessment with Rituximab (LUNAR) study, the largest randomized trial of rituximab for the treatment of lupus nephritis to date, failed to demonstrate increased efficacy of rituximab despite achieving B cell depletion to the target of <20 cells/µl in peripheral blood (10). Further characterization of the effects of rituximab in mouse models of lupus nephritis and patients with SLE has revealed that rituximab does not consistently result in complete or prolonged depletion of peripheral blood B cells and that lymphoid tissue B cell depletion may be incomplete as well (11–21). Incomplete peripheral blood B cell depletion has been associated with a lack of response to rituximab in patients with SLE (20) and it is hypothesized that a lack of complete peripheral depletion in peripheral blood and/or the kidney tubulointerstitium may attenuate the efficacy of rituximab in lupus nephritis (22).
The LUNAR trial offers a unique opportunity to evaluate the dynamics of B cell depletion. We hypothesized that the participants of the LUNAR trial would demonstrate variability in the degree and duration of peripheral blood B cell depletion after administration of rituximab, and that these measures of peripheral blood B cell depletion would be associated with an increased probability of achieving complete response.
Materials and Methods
Participants and Setting
The LUNAR trial (Clinicaltrials.gov identifier NCT00282347, registration date January 24, 2006) (10) enrolled 144 participants with lupus nephritis from January 2006 to January 2009, from 52 centers in the United States and Latin America. Participants were required to be 16–75 years of age, have a diagnosis of SLE on the basis of the American College of Rheumatology criteria, a history of antinuclear antibody positivity, and lupus nephritis class III or class IV (alone or in combination with class V) confirmed by kidney biopsy and urine protein-to-creatinine ratio >1.0 mg/mg. Patients with >50% glomerular sclerosis on biopsy or an eGFR of <25 ml/min were ineligible for participation. Participants were randomized to receive rituximab (n=72) or placebo (n=72). Only participants who received rituximab were evaluated, including one participant from the placebo arm that was inadvertently treated with rituximab. Five participants without complete CD19 measurements for at least 300 days were excluded, allowing a total of 68 participants for analysis. The LUNAR study protocol was approved by institutional review boards and ethics committees and adhered to the Declaration of Helsinki. The participants provided written informed consent.
Treatment Protocol
Participants received 1 g of rituximab on days 1, 15, 168, and 182. Mycophenolate mofetil was maintained at 3 g/d. We administered 1 g of methylprednisolone on days 1 and 3. Participants received 100 mg of methylprednisolone prerituximab on days 15, 168, and 182. Oral prednisone was started at 0.75 mg/kg per day, then tapered to 10 mg/d by week 16, similar to the Aspreva Lupus Management Study trial (23). Participants were followed for a total of 78 weeks.
B Cell Measurements
Routine four-color flow cytometry analyses were performed approximately every 2 weeks during the first month of the trial, every 4 weeks until week 52, and every 12 weeks until week 78. Blood samples were incubated with four distinct fluorescence-conjugated antibodies using Becton Dickinson MultiTEST reagents in TruCount Absolute Count tubes and analyzed by FACSCalibur, according to manufacturer’s protocols (Becton Dickinson). Data analysis was completed with FACSCalibur and Becton Dickinson MultiSET software.
Clinical Definitions and Laboratory Measurements
Three definitions of peripheral blood B cell depletion were utilized: the LUNAR study protocol definition (CD19 count <20 cells/µl), the cutoff from the earliest phase I trials of rituximab in SLE (CD19 count <5 cells/µl) (16,17), and a more stringent, exploratory definition (CD19 count 0 cells/µl) hereafter termed “complete peripheral depletion.” eGFR was calculated using the Modification of Diet in Renal Disease Study equation. Nephrotic syndrome was defined as baseline albumin <3 g/dl and baseline urine protein-to-creatinine ratio ≥3.5 mg/mg. Peak rituximab levels were measured 30 minutes after administration of rituximab. For these analyses, peak rituximab level was defined as the overall highest level of rituximab achieved out of the four infusions administered.
Study End Points
Complete response for these analyses was defined as urine protein-to-creatinine ratio <0.5 mg/mg from a 24-hour urine collection, in conjunction with normal serum creatinine or, if normal at baseline, <15% increase from baseline. The LUNAR trial’s composite primary end point included the former criteria in addition to an inactive urinary sediment (fewer than five red blood cells per high-power field). For these analyses, urinary sediment was not included in the definition of complete response because of missing measurements and recent data demonstrating that sediment is not a useful predictor of long-term kidney outcomes (24,25). Inclusion of urinary sediment did not qualitatively affect the results presented here (see Supplemental Table 1). Complete response was assessed at week 52 and at week 78. An additional end point assessed was time to first complete response.
Statistical Analyses
Baseline differences in populations were compared using t test, Fisher exact test, or Mann–Whitney U test as appropriate. Kaplan–Meier survival analyses were used for time-to-event analyses. Logistic regression was used to estimate associations between measures of complete peripheral depletion and complete response. To identify variables most closely associated with achievement of complete peripheral depletion, variables were evaluated individually in a univariable logistic regression model with complete peripheral depletion as the outcome. Only the variables which were significantly associated with the outcome (P<0.05) were included in a multivariable logistic regression model. The variables evaluated included baseline characteristics that were significantly different among those who achieved complete peripheral depletion versus those who did not, baseline monocyte levels which are important in B cell depletion (26,27), duration of lupus nephritis, race, and peak rituximab levels. Missing week 78 values of urine protein-to-creatinine ratio and/or creatinine for seven participants were imputed with values carried forward from up to 60 days prior. Analyses were performed using STATA 14.2 software (Stata Corporation, College Station, TX). A two-sided P value of <0.05 was considered statistically significant.
Results
Achievement of Complete Peripheral Depletion
All 68 participants achieved CD19 <20 cells/µl within 86 days and <5 cells/µl within 182 days (Supplemental Figure 1, A and B). Fifty-three (78%) participants achieved complete peripheral depletion (0 cells/µl) within 365 days (Table 1). No participant achieved complete peripheral depletion for the first time after 365 days. The breakdown of the first time participants achieved complete peripheral depletion is as follows: eight (12%) achieved it directly after the first infusion of rituximab, 24 (35%) after the second, five (7%) after the third, 16 (24%) after the fourth, and 15 (22%) never achieved it. Participants who achieved complete peripheral depletion had significantly higher mean eGFR (79 versus 58 ml/min; P=0.02), median C3 (69 versus 59 mg/dl; P=0.03), and mean serum albumin (2.8 versus 2.1 g/dl; P<0.001), were more likely to be anti–Smith-positive (32% versus 7%; P=0.05) at baseline, and achieved higher peak rituximab levels (456 versus 386 µg/ml; P=0.05). Participants who achieved complete peripheral depletion also had a lower urine protein-to-creatinine ratio (3.3 versus 5.2 mg/mg; P=0.008) and were less likely to have nephrotic syndrome (28% versus 73%; P=0.002) at baseline (Table 2).
Table 1.
Number of patients from the LUNAR trial treated with rituximab that achieved the three different definitions of B cell depletion at week 52 and 78 and maximum time to depletion in days
| Definition of B Cell Depletion | Patients Who Achieved Depletion by Week 52, n (% of Total) | Patients Who Achieved Depletion by Week 78, n (% of Total) | Maximum Time to Achievement of Depletion in Days |
|---|---|---|---|
| CD19<20 cells/μl | 68 (100) | 68 (100) | 86 |
| CD19<5 cells/μl | 68 (100) | 68 (100) | 182 |
| CD19=0 cells/μl | 53 (78) | 53 (78) | 365 |
LUNAR, the Lupus Nephritis Assessment with Rituximab study.
Table 2.
Comparison of baseline characteristics of patients from the LUNAR trial treated with rituximab who achieved complete peripheral depletion versus those who never achieved complete peripheral depletion
| Characteristics | Complete Peripheral Depletion, n=53 | Incomplete Peripheral Depletion, n=15 |
|---|---|---|
| Age, yr, mean±SD | 32±8 | 31±10 |
| Women | 48 (90%) | 11 (73%) |
| Duration of lupus nephritis, mo, median (range) | 10.5 (0.4–210) | 17.8 (0.4–130) |
| Race | ||
| White | 15 (28%) | 4 (26%) |
| Hispanic | 21 (39%) | 6 (41%) |
| Black | 15 (28%) | 4 (26%) |
| Asian | 2 (4%) | 1 (7%) |
| Biopsy class | ||
| Class III only | 6 (11%) | 2 (13%) |
| Class III and V | 11 (21%) | 5 (33%) |
| Class IV only | 28 (53%) | 7 (47%) |
| Class IV and V | 8 (15%) | 1 (7%) |
| Baseline CD19, cells/μl, median (range) | 166 (11–1477) | 106 (20–1839) |
| Creatinine, mg/dl, mean±SD | 0.95±0.4 | 1.17±0.5 |
| eGFR, ml/min, mean±SD | 79±33 | 58±24 |
| Urine protein/creatinine ratio, mg/mg, mean±SD | 3.3±2.3 | 5.2±2.45 |
| Albumin, g/dL, mean±SD | 2.8±0.67 | 2.1±0.87 |
| Nephrotic syndrome present | 15 (28%) | 11 (73%) |
| IgG, g/L, median (range) | 9.9 (3.4–22) | 7.7 (1.2–20) |
| C3, mg/dl, median (range) | 69 (23–151) | 59 (32–130) |
| C4, mg/dl, median (range) | 13 (5–42) | 9.5 (5–24) |
| Anti-dsDNA positivity | 42 (80%) | 13 (87%) |
| Anti-Smith positivity | 17 (32%) | 1 (7%) |
| BAFF levels, ng/ml, median (range) | 3.1 (0.1–19) | 3.5 (1.4–24) |
| Peak rituximab levels, μg/ml, median (range) | 456 (290–1070) | 386 (293–565) |
LUNAR, the Lupus Nephritis Assessment with Rituximab study; dsDNA, double-stranded DNA; BAFF, B cell activating factor.
Complete Peripheral Depletion and Achievement of Complete Response
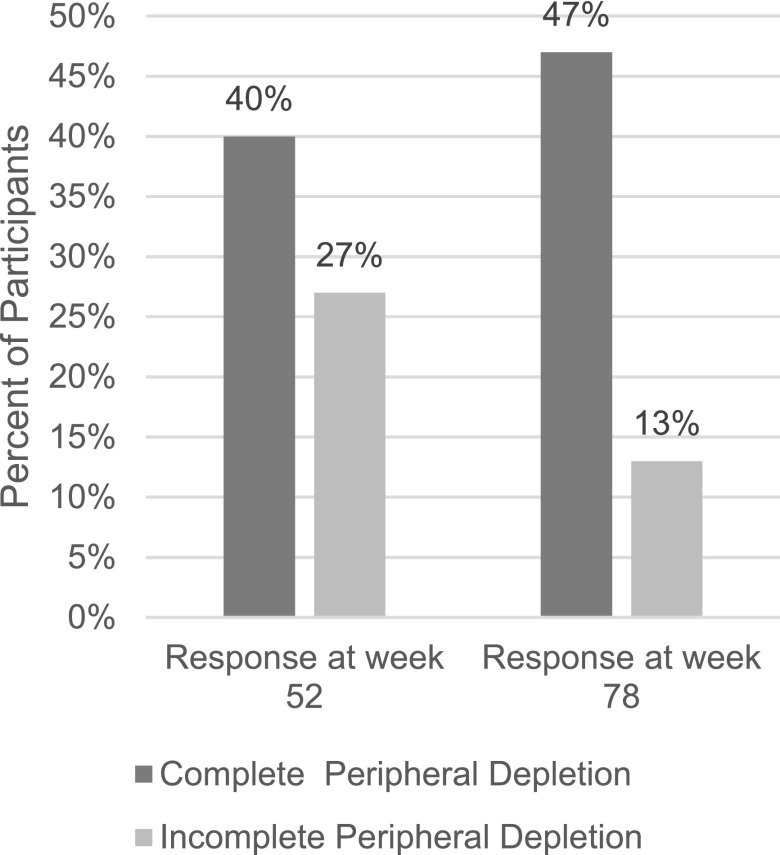
Sixty-eight participants had complete data for both week 52 and week 78 assessments. Achievement of CD19 <20 cells/µl or <5 cells/µl did not distinguish participants who achieved complete response from those who did not (Supplemental Table 2). Twenty-one (40%) participants who achieved complete peripheral depletion had complete response at week 52 and 25 (47%) at week 78 (Figure 1). In logistic regression, achievement of complete peripheral depletion was associated with increased odds of complete response at week 78 (unadjusted odds ratio [OR], 5.8; 95% confidence interval (95% CI), 1.2 to 28; P=0.03). The median time participants remained at complete peripheral depletion was 64 days (interquartile range [IQR], 12–143) through week 52 and 71 days (IQR, 14–158) through week 78. Thirty-four participants (50%) had complete peripheral depletion lasting greater than the median time (71 days), which was associated with a greater likelihood of complete response at week 78 (unadjusted OR, 4.1; 95% CI, 1.5 to 11; P=0.008) compared with the 34 (50%) who had complete peripheral depletion lasting fewer days than the median (Table 3). Neither duration nor achievement of complete peripheral depletion were significantly associated with complete response at week 52 (Supplemental Table 3).
Figure 1.
A larger percentage of participants from the LUNAR trial who achieved complete peripheral depletion (n = 53) achieved complete response at week 52 and at week 78, compared to participants who did not achieve peripheral depletion (n = 15).
Table 3.
Univariable logistic regressions assessing whether characteristics of complete peripheral depletion associate with complete response at week 78
| Characteristics | Complete Response, N (%) | Unadjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| Complete peripheral depletion | 5.8 (1.2 to 28) | 0.03 | |
| Achieved (n=53) | 25 (47) | ||
| Never achieved (n=15) | 2 (13) | ||
| Duration of complete peripheral depletion | 4.1 (1.5 to 11) | 0.008 | |
| ≥71 d (n=34) | 19 (70) | ||
| <71 d (n=34) | 8 (30) | ||
| Each incremental delay of 30 d to complete peripheral depletion | 53 (78) | 0.89 (0.81 to 0.98) | 0.02 |
95% CI, 95% confidence interval.
Time to Complete Peripheral Depletion and Achievement of Complete Response
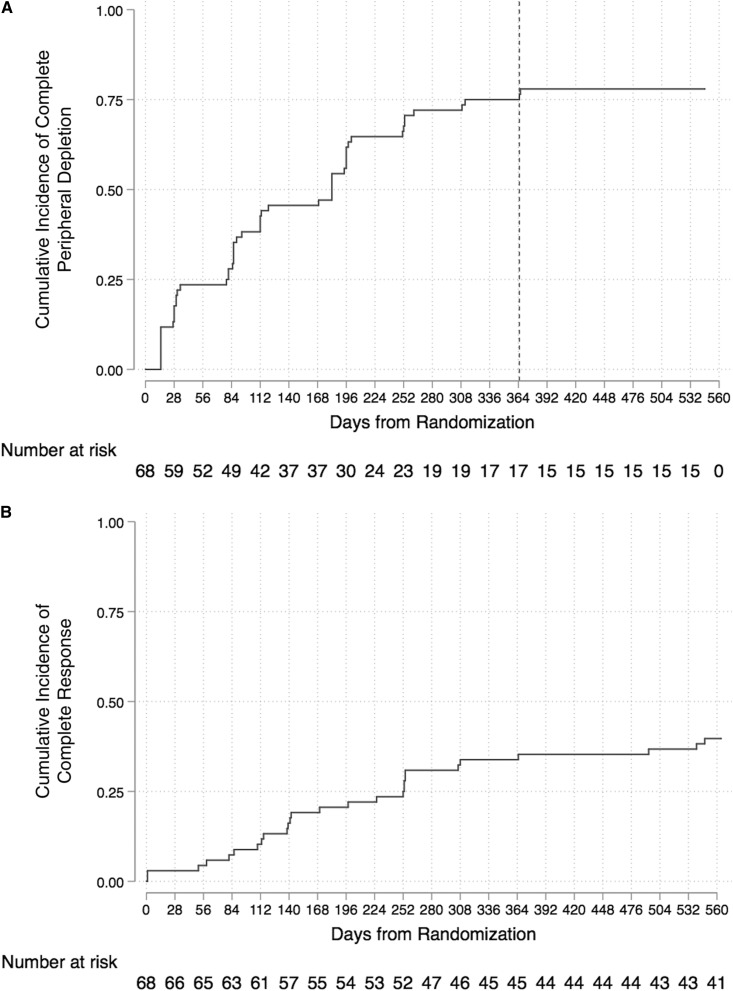
Among all participants (n=68), complete peripheral depletion was achieved in a median time of 182 days (IQR, 80–339) (Figure 2A). Among those who achieved complete response (n=27), median time to response was 170 days (IQR, 109–254) (Figure 2B). In logistic regression, each incremental delay of 30 days to achievement of complete peripheral depletion was associated with lower likelihood of complete response at week 78 (unadjusted OR, 0.89; 95% CI, 0.81 to 0.98; P=0.02) (Table 3). Delay in depletion was not significantly associated with complete response at week 52 (Supplemental Table 3).
Figure 2.
Kaplan Meier graphs demonstrating wide variability in time to complete peripheral depletion and in time to complete response in participants treated with rituximab from the LUNAR trial. (A) Time to complete peripheral depletion, in days. Line denotes day 365, which is the last day that participants ever achieved CD19 of 0 cells/µl. (B) Cumulative incidence of complete response up to week 78, in days.
Variables that Associate with Complete Peripheral Depletion
The following baseline variables were analyzed individually in univariable logistic regression with complete peripheral depletion as the outcome: age, sex, eGFR, C3, C4, urine protein-to-creatinine ratio, albumin, monocytes, lymphocytes, duration of lupus nephritis, race, anti-Smith positivity, and nephrotic syndrome. An additional variable assessed was peak rituximab levels. In multivariable logistic regression, baseline nephrotic syndrome status, baseline anti-Smith antibody positivity, and peak rituximab levels were found to be significantly associated with achievement of complete peripheral depletion (Table 4).
Table 4.
Multivariable logistic regression model including the three variables that significantly associated with complete peripheral depletion by week 78 when evaluated in a univariable model
| Variables | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Baseline nephrotic syndrome | 0.12 (0.03 to 0.50) | 0.004 |
| Baseline anti-Smith positivity | 13.8 (1.4 to 136) | 0.02 |
| Each 100 µg/ml increment above 440 µg/ml in peak rituximab levels | 2.3 (0.98 to 5.2) | 0.05 |
95% CI, 95% confidence interval.
Discussion
We hypothesized that after administration of rituximab, participants of the LUNAR trial would demonstrate variability in peripheral blood B cell depletion and that greater degree and duration of depletion would be associated with complete response. Achievement of complete peripheral depletion, more prolonged duration of complete peripheral depletion, and shorter time to achievement of complete peripheral depletion were all found to be associated with achievement of complete response at week 78 but not week 52. All participants met the original LUNAR study protocol definition for peripheral blood B cell depletion of <20 cells/µl, as well as the definition used in the earliest trials of rituximab in SLE of <5 cells/µl (16,17), therefore these definitions of B cell depletion were not useful in differentiating those who achieved complete response from those who did not.
Although our results largely support our hypothesis when week 78 response is considered, we did not find associations between measures of complete peripheral depletion and week 52 complete response. It may be that more than 52 weeks are required for B cell depletion to achieve complete efficacy in lupus nephritis. Among patients with complete peripheral depletion, rates of complete response increased from week 52 to week 78, whereas among patients with incomplete peripheral depletion, rates of complete response decreased from week 52 to week 78. This could be a manifestation of delayed healing in the kidney from the original immunologic insult. Alternatively, murine models have demonstrated that B cells in lymphoid structures and the kidney tubulointerstitium are resistant to depletion with rituximab (6,11–13). Depletion of peripheral blood B cells is thought to decrease B cell burden in target tissues by preventing their migration into lymphoid structures (8). This process may require a relatively long time to lower B cell and plasma cell numbers in situ in the kidney to the degree necessary to see an effect on complete response. In those with incomplete peripheral depletion, B cells might more rapidly reseed these lymphoid structures, accounting for the decrease in complete response observed at week 78.
Despite the apparent benefit of achieving complete peripheral depletion, less than half of participants with complete peripheral depletion achieved complete response. Autoreactive pathogenic B cells may persist in protected microenvironments such as lymphoid structures, the kidney tubulointerstitium, and the bone marrow even with adequate peripheral B cell depletion (6,11–13). These cells cannot be routinely measured and may continue autoimmune activity that results in ongoing kidney injury (4,5). There may also be unintended effects of rituximab, such as large increases in BAFF, which could promote autoreactive B cell survival and limit rituximab’s efficacy (28). This hypothesis is currently being tested by Synergetic B-cell Immodulation in SLE (Clinicaltrials.gov identifier NCT02284984), a proof-of-concept study of rituximab followed by belimumab, which is a BAFF inhibitor. The LUNAR study did not require repeat biopsies to be performed and therefore whether continued inflammation was the cause of continued proteinuria cannot be ascertained.
Therapeutic agents that enhance B cell depletion in SLE may result in a more homogenous B cell depletion profile across participants and increased efficacy in lupus nephritis. Obinutuzumab is a recently developed type II mAb against CD20 that has a nonapoptotic B cell–killing mechanism and improved antibody-dependent cellular cytotoxicity in comparison with rituximab (29–32). Obinutuzumab has been shown to induce greater B cell depletion in peripheral blood and lymph tissue than rituximab in chronic lymphocytic leukemia (32). Because of its increased depletion in lymph nodes, it is possible obinutuzumab could also achieve increased B cell depletion in situ in the kidney. The Study to Evaluate the Safety and Efficacy of Obinutuzumab Compared With Placebo in Participants With Lupus Nephritis is an ongoing phase II trial in participants with proliferative lupus nephritis randomized to either obinutuzumab or placebo plus background therapy of mycophenolate and steroids (Clinicaltrials.gov identifier NCT01905943) that aims to test the hypothesis that greater B cell depletion can lead to increased rates of response in patients with lupus nephritis.
Our multivariable logistic regression model suggests that lack of nephrotic syndrome at presentation and higher peak rituximab levels are associated with complete peripheral depletion. Patients with SLE achieve significantly lower levels of rituximab compared with patients with rheumatoid arthritis. This remains true even in SLE participants with no proteinuria, and could be due to increased internalization of rituximab and increased Ig catabolism (33). Furthermore, the t1/2 of rituximab in patients with nephrotic syndrome due to membranous nephropathy is significantly decreased compared with rheumatoid arthritis (34,35). Patients with nephrotic syndrome treated with rituximab have been found to have elevated rituximab urine levels, regardless of the original diagnosis that led to nephrotic syndrome (36,37). In membranous nephropathy, a rituximab regimen with 4 weekly 375 mg/m2 doses achieved higher rituximab levels for a longer period of time and resulted in improved B cell depletion compared with two doses of 1 g given 15 days apart (34,35). These findings suggest that patients with lupus nephritis, especially those with concomitant nephrotic syndrome, may require a more prolonged exposure to rituximab to achieve more profound peripheral blood B cell depletion, which could lead to greater efficacy of rituximab. A more in-depth pharmacokinetic study is necessary to understand the variability in peak levels observed in the LUNAR trial and explore whether they are related to nephrotic syndrome. Baseline anti-Smith positivity was also associated with complete peripheral depletion. Anti-Smith is the most specific antibody in SLE and has been associated with more severe features and poor outcomes (38–40). It is unclear why this association was found, but if it were to be reproduced in subsequent studies then further investigation into the relationship would be warranted.
Limitations of this study include that it is a post hoc analysis of a randomized, controlled trial with no statistical corrections to multiple testing. The flow cytometry used for this trial has not been internally validated for a threshold lower than <5 cells/µl and may lack precision below this level. The duration of follow-up was limited to week 78. Importantly, rituximab is not approved for use in lupus nephritis.
In summary, our study supports the hypothesis that there is variability in peripheral blood B cell depletion after administration of rituximab in lupus nephritis and that greater degree and duration, as well as shorter time to complete peripheral depletion are associated with complete response at week 78. In conclusion, rituximab in combination with mycophenolate did not achieve a uniform degree of peripheral blood B cell depletion using the most stringent definition of depletion as measured by conventional flow cytometry, and this may have contributed to the observed lack of efficacy in the LUNAR trial. We hypothesize that enhanced peripheral blood B cell depletion may improve outcomes with B cell–depleting agents in lupus nephritis. This could be achieved by combining rituximab with other agents such as belimumab, developing a deeper understanding of the pharmacokinetics of rituximab that could lead to alternative dosing regimens, or with the use of newer therapies such as obinutuzumab.
Disclosures
L.M.G.M. has received research support from Roche/Genentech. M.D.C., J.G., T.R.K., and P. Brunetta are employees of Roche/Genentech. L.D. is an employee of Merck and a stockholder of Roche/Genentech. P. Brakeman, M.D.E., R.J.L., and B.R. have no disclosures.
Supplementary Material
Acknowledgments
The LUNAR trial was supported by Genentech and Biogen Idec.
A version of this article was presented as a poster at the Annual European Congress of Rheumatology in Madrid, Spain, June 14–17, 2017.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01070118/-/DCSupplemental.
References
- 1.Rahman A, Isenberg DA: Systemic lupus erythematosus. N Engl J Med 358: 929–939, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE: New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 12: 716–730, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA: Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur J Immunol 31: 2726–2732, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR: In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MR, Trotter K, Chang A: The pathogenesis and therapeutic implications of tubulointerstitial inflammation in human lupus nephritis. Semin Nephrol 35: 455–464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC: Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174: 817–826, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR: Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 63: 865–874, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman GJ, Boyle DL: Understanding the mechanistic basis in rheumatoid arthritis for clinical response to anti-CD20 therapy: The B-cell roadblock hypothesis. Immunol Rev 223: 175–185, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Martin F, Chan AC: B cell immunobiology in disease: Evolving concepts from the clinic. Annu Rev Immunol 24: 467–496, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G; LUNAR Investigator Group : Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64: 1215–1226, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ: Depletion of B cells in murine lupus: Efficacy and resistance. J Immunol 179: 3351–3361, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Ahuja A, Teichmann LL, Wang H, Dunn R, Kehry MR, Shlomchik MJ: An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in Lupus. J Immunol 187: 3888–3894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH: Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum 62: 2443–2457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, Pullman-Mooar S, Barnack F, Striebich C, Looney RJ, Prak ET, Kimberly R, Zhang Y, Eisenberg R: Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis 67: 1724–1731, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Dias SS, Rodriguez-Garcia V, Nguyen H, Pericleous C, Isenberg D: Longer duration of B cell depletion is associated with better outcome. Rheumatology (Oxford) 54: 1876–1881, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA: An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 46: 2673–2677, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I: B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50: 2580–2589, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG: Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 62: 222–233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy V, Cambridge G, Isenberg DA, Glennie MJ, Cragg MS, Leandro M: Internalization of rituximab and the efficiency of B Cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol 67: 2046–2055, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, Ponchel F, Rawstron AC, Emery P: B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 63: 3038–3047, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Gunnarsson I, Sundelin B, Jónsdóttir T, Jacobson SH, Henriksson EW, van Vollenhoven RF: Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum 56: 1263–1272, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Reddy V, Dahal LN, Cragg MS, Leandro M: Optimising B-cell depletion in autoimmune disease: Is obinutuzumab the answer? Drug Discov Today 21: 1330–1338, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Sinclair A, Appel G, Dooley MA, Ginzler E, Isenberg D, Jayne D, Wofsy D, Solomons N: Mycophenolate mofetil as induction and maintenance therapy for lupus nephritis: Rationale and protocol for the randomized, controlled Aspreva Lupus Management Study (ALMS). Lupus 16: 972–980, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Dall’Era M, Cisternas MG, Smilek DE, Straub L, Houssiau FA, Cervera R, Rovin BH, Mackay M: Predictors of long-term renal outcome in lupus nephritis trials: Lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol 67: 1305–1313, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Tamirou F, Lauwerys BR, Dall'Era M, Mackay M, Rovin B, Cervera R, Houssiau FA, MAINTAIN Nephritis Trial Investigators : A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: Data from the MAINTAIN nephritis trial. Lupus Sci Med 2: e000123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F: Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity 35: 932–944, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF: The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 199: 1659–1669, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R: Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20: 785–798, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Honeychurch J, Alduaij W, Azizyan M, Cheadle EJ, Pelicano H, Ivanov A, Huang P, Cragg MS, Illidge TM: Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood 119: 3523–3533, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, Ferrara C, Sondermann P, Jäger C, Strein P, Fertig G, Friess T, Schüll C, Bauer S, Dal Porto J, Del Nagro C, Dabbagh K, Dyer MJ, Poppema S, Klein C, Umaña P: Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, Leandro MJ: Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford) 56: 1227–1237, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Döhner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M: Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370: 1101–1110, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Reddy V, Croca S, Gerona D, De La Torre I, Isenberg D, McDonald V, Leandro M, Cambridge G: Serum rituximab levels and efficiency of B cell depletion: Differences between patients with rheumatoid arthritis and systemic lupus erythematosus. Rheumatology (Oxford) 52: 951–952, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC: Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs R, Langer-Jacobus T, Duong M, Stahl K, Haller H, Schmidt RE, Schiffer M: Detection and quantification of rituximab in the human urine. J Immunol Methods 451: 118–121, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Stahl K, Duong M, Schwarz A, Wagner AD, Haller H, Schiffer M, Jacobs R: Kinetics of rituximab excretion into urine and peritoneal fluid in two patients with nephrotic syndrome. Case Rep Nephrol 2017: 1372859, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn SS, Yoo BW, Song JJ, Park YB, Lee SK, Lee SW: Anti-Sm is associated with the early poor outcome of lupus nephritis. Int J Rheum Dis 19: 897–902, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Arroyo-Ávila M, Santiago-Casas Y, McGwin G Jr., Cantor RS, Petri M, Ramsey-Goldman R, Reveille JD, Kimberly RP, Alarcón GS, Vilá LM, Brown EE: Clinical associations of anti-Smith antibodies in PROFILE: A multi-ethnic lupus cohort. Clin Rheumatol 34: 1217–1223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr., Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr., Magder LS: Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64: 2677–2686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.