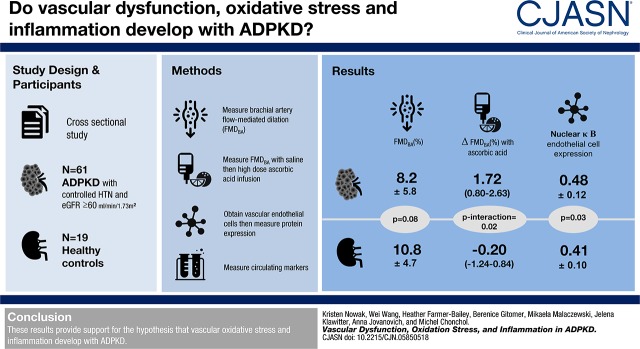
Abstract
Background and objectives
Both increased arterial stiffness and vascular endothelial dysfunction are evident in patients with autosomal dominant polycystic kidney disease, even early in the course of the disease when kidney function in preserved. Vascular dysfunction in autosomal dominant polycystic kidney disease is thought to be related to vascular oxidative stress and inflammation, but direct evidence is lacking.
Design, setting, participants, & measurements
We assessed carotid-femoral pulse-wave velocity (arterial stiffness) and brachial artery flow-mediated dilation (vascular endothelial function) in participants with early-stage autosomal dominant polycystic kidney disease (eGFR≥60 ml/min per 1.73 m2) and a history of controlled hypertension and in healthy controls. Brachial artery flow-mediated dilation was also assessed after infusion of ascorbic acid to inhibit vascular oxidative stress compared with saline. Vascular endothelial cells were collected from a peripheral vein to measure expression of proteins, and circulating markers were also assessed by ELISA or liquid chromatography-tandem mass spectrometry.
Results
In total, 61 participants with autosomal dominant polycystic kidney disease (34±9 years old [mean±SD]) and 19 healthy controls (30±5 years old) were studied. Carotid-femoral pulse-wave velocity was higher in participants with autosomal dominant polycystic kidney disease compared with healthy controls (650±131 versus 562±81 cm/s; P=0.007). Brachial artery flow-mediated dilation was 8.2%±5.8% in participants with autosomal dominant polycystic kidney disease and 10.8%±4.7% in controls (P=0.08). Among participants with autosomal dominant polycystic kidney disease, flow-mediated dilation increased from 7.7%±4.5% to 9.4%±5.2% with ascorbic acid, a difference of 1.72 (95% confidence interval, 0.80 to 2.63), whereas in control participants, flow-mediated dilation decreased nonsignificantly from 10.8%±4.7% to 10.6%±5.4%, a difference of −0.20 (95% confidence interval, −1.24 to 0.84; P interaction =0.02). Endothelial cell protein expression of NF-κB was greater in participants with autosomal dominant polycystic kidney disease (0.48±0.12 versus 0.41±0.10 [intensity versus human umbilical vein endothelial cell control]; P=0.03). However, circulating oxidative stress markers and bioactive lipid mediators did not significantly differ according to the autosomal dominant polycystic kidney disease diagnosis.
Conclusions
These results provide support for the hypothesis that vascular oxidative stress and inflammation develop with autosomal dominant polycystic kidney disease.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_09_18_CJASNPodcast_18_10_.mp3
Keywords: ADPKD; endothelium; polycystic kidney disease; pulse wave velocity; vascular; inflammation; Pulse Wave Analysis; Brachial Artery; Vascular Stiffness; Polycystic Kidney, Autosomal Dominant; Ascorbic Acid; Complement Factor B; glomerular filtration rate; Confidence Intervals; Dilatation; Tandem Mass Spectrometry; endothelial cells; Dilatation, Pathologic; oxidative stress; Inflammation; Enzyme-Linked Immunosorbent Assay; Chromatography, Liquid; hypertension; lipids
Visual Abstract
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening genetic disease, affecting approximately 1:500–1:1000 live births (1). Although the hallmark of ADPKD is the development and continued growth of multiple kidney cysts that result in ultimate loss of kidney function (2), the leading causes of death among affected patients are cardiovascular complications and disorders (3,4). The proteins encoded by the PKD1 and PKD2 genes, polycystin-1 and polycystin-2, are expressed in vascular endothelial cells and smooth cells of all major vessels, resulting in systemic manifestations of the disease (5).
Vascular endothelial dysfunction, most commonly assessed as impaired endothelium-dependent dilation, and stiffening of the large elastic arteries, typically assessed as carotid-femoral pulse-wave velocity (PWV), are two of the greatest contributors to dysfunction and disorders of arteries (6). Importantly, both measures are independently associated with future cardiovascular events and mortality (7–10). Both vascular endothelial dysfunction and increased arterial stiffness are evident in patients with ADPKD, even early in the course of the disease when kidney function is preserved (11,12).
Oxidative stress and inflammation are also evident in patients with ADPKD (13–15) and may contribute to the development of vascular dysfunction; however, the mechanisms underlying vascular dysfunction in ADPKD are incompletely understood. Whether oxidative stress and inflammation are increased locally at the vascular endothelial cell layer in humans and whether vascular oxidative stress contributes to endothelial dysfunction in patients with ADPKD are currently unknown.
The goal of this study was to compare vascular function and indicators of vascular oxidative stress and inflammation in adults with early-stage ADPKD with age- and sex-matched healthy controls. We used novel methodology to assess mechanisms contributing to vascular dysfunction in humans. An acute supraphysiologic infusion of ascorbic acid known to scavenge superoxide was used to assess the contribution of oxidative stress to vascular endothelial dysfunction. Additionally, vascular endothelial cells were collected from participants to measure expression of proteins indicating vascular oxidative stress and inflammation. We hypothesized that vascular dysfunction in participants with early-stage ADPKD would be characterized by increased vascular oxidative stress and inflammation, including alterations in circulating bioactive lipid mediators.
Materials and Methods
Study Design and Participants
This was a cross-sectional study comparing mechanisms of vascular dysfunction in adults with early-stage ADPKD and age-matched healthy controls. Patients with ADPKD participated in a randomized, placebo-controlled trial of spironolactone administration (NCT01853553), and data presented were collected at their baseline visit, with enrollment between July 2014 and June 2016. Healthy controls were prospectively recruited through university and community advertisement for comparison with participants with ADPKD, with enrollment between October 2015 and May 2017. The study was conducted at the University of Colorado Anschutz Medical Campus Division of Renal Diseases and Hypertension Clinical Vascular Physiology Laboratory. Analysts were blinded to group (ADPKD or healthy control) in the assessment of vascular measurements and circulating and cellular markers.
All participants with ADPKD randomized in the clinical trial were included in this analysis. Inclusion criteria for the trial were age between 20 and 55 years of age (women were required to be premenopausal), diagnosis of ADPKD on the basis of the Ravine criteria (in participants ≥30 years old) (16), and the PKD1 genotype (or presumed PKD1 genotype on the basis of family history). Additional inclusion criteria were total kidney volume between 500 and 2500 ml, eGFR by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (17) ≥60 ml/min per 1.73 m2, and a history of hypertension treated with a stable maximal tolerable dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) and currently controlled. All participants with ADPKD were required to have controlled BP (to <160/90 mm Hg), and they were on a stable antihypertensive regimen at the time of enrollment in the study.
Healthy control participants were 23–38 years of age (recruited after partial completion of ADPKD enrollment to best match the mean age of participants with ADPKD; women were required to be premenopausal). Inclusion criteria were healthy (free from kidney disease, cardiovascular disease, or other chronic disease as assessed by self-report, physical examination [including resting 12-lead electrocardiogram], and screening laboratory values), free from hypertension on the basis of guidelines at this time (systolic BP <140/90 mm Hg and no antihypertensive agents), and an eGFR by the CKD-EPI equation (17) ≥60 ml/min per 1.73 m2. Control participants were recruited to be nonhypertensive, because we were interested in vascular changes in ADPKD, which is complicated by hypertension in the majority of individuals (18), compared with healthy controls free from hypertension.
Additional inclusion/exclusion criteria as well as detailed procedures are described in Supplemental Material.
Procedures
Vascular Measurements.
All measurements were made following standard recommendations, including an overnight fast (19). Brachial artery flow-mediated dilation (FMDBA) was determined using duplex ultrasonography (Xario 200; Toshiba, Tustin, CA) with ECG-gated end diastolic ultrasound images analyzed by a single blinded analyst using a commercially available software package (Vascular Analysis Tools 5.8.1; Medical Imaging Applications, Coralville, IA) as described in detail previously (20,21). Endothelium-independent dilation (brachial artery dilation to 0.4 mg of sublingual nitroglycerin) was assessed as a standard index of smooth muscle cell sensitivity to exogenous nitric oxide (NO) (21,22). Nitroglycerin was administered to 12 control participants and 37 participants with ADPKD (missing due to low HR and/or low systolic BP [n=7 control; n=16 ADPKD], intravenous failure [n=6 ADPKD], drug contraindication [n=1 ADPKD], or other [n=1 ADPKD not administered due to prior apparent vasovagal response]).
Carotid-femoral PWV was measured as described in detail previously (20,21). Briefly, a transcutaneous custom tonometer (Noninvasive Hemodynamics Workstation; Cardiovascular Engineering Inc., Norwood, MA) was positioned at the carotid, brachial, radial, and femoral arteries to noninvasively assess carotid-femoral PWV and carotid-radial PWV (an index of peripheral stiffness).
Additionally, as secondary indices of arterial stiffness, the tonometry assessment in conjunction with ultrasound imaging of the carotid artery also provided blinded assessment of carotid artery compliance and carotid artery β-stiffness index as described previously (20,21). Carotid intimal medial thickness and carotid systolic BP were also assessed (20,21).
The influence of oxidative stress on FMDBA was assessed by infusing a supraphysiologic dose of ascorbic acid that produces plasma concentrations known to inhibit superoxide production in vitro (23) or isovolumic saline and measuring FMDBA during the “drip infusion” when peak plasma concentrations occur as described previously (21,24). A priming bolus of 0.075 g of ascorbic acid per 1 kg of fat-free mass (maximal dosage set at 5.0 g) dissolved in 150 ml of saline was infused intravenously at 5 ml/min for 20 minutes followed immediately by a “drip infusion” of 0.5 ml/min over the period when FMDBA was measured. Fat-free mass was estimated using a previously validated equation considering age, sex, and body mass index (25). Infusions were performed in all 19 controls and 52 participants with ADPKD (missing in n=7 due to intravenous failure; n=1 due to prior vasovagal response; n=1 due to intravenous pump malfunction). Characteristics of participants with ADPKD included in the infusions did not significantly differ from those excluded for reasons described above (Supplemental Table 1). Circulating ascorbic acid levels were measured before and after the ascorbic acid infusion to show effective elevation of plasma levels in a small subgroup of participants with ADPKD (n=5) and control participants(n=5) by ARUP Laboratories using quantitative high-performance liquid chromatography.
Cellular Markers of Oxidative Stress and Inflammation.
Vascular endothelial cells were obtained immediately before other vascular measurements from the intima of an antecubital vein (n=9–17 control participants per protein analyzed and n=32–43 participants with ADPKD per protein analyzed; not available in all participants and for all proteins due to intravenous failure or low cell yield). Cells were recovered and fixed, and slides were prepared and frozen for later staining. Positive identification of endothelial cells, assessment of nuclear integrity, and quantification of protein expression of NAD(P)H oxidase (p47phox, 1:1000; Millipore, Billerica, MA), IL-6 (1:50; Santa Cruz, Dallas, TX); NF-κB (1:300; Santa Cruz), and phosphorylated endothelial nitric oxide synthase (PeNOS; 1:100; Cell Signaling, Danvers, MA) were determined by immunofluorescence staining (Nikon Eclipse Ti, Melville, NY) by a blinded analyst as described previously (21,26,27). These markers were chosen as markers of oxidative stress, inflammation, and vascular endothelial NO production.
Circulating Markers of Oxidative Stress, Inflammation, and Bioactive Lipid Mediators.
Serum high-sensitivity C-reactive protein (hsCRP) and IL-6 levels were measured by ELISA (MSD, Rockville, MD). Targeted liquid chromatography-tandem mass spectrometry analysis of markers of oxidative stress (prostaglandins [PGs], including marker 8-isoprostane as well as PGF2α, PGD2, and PGE2) and bioactive lipid mediators (hydroxyoctadecadienoic acids [HODEs; 9-HODE and 13-HODE], hydroxyeicosatetraenoic acids [HETEs; 5-HETE, 8-HETE, 9-HETE, 11-HETE, and 12-HETE], epoxyeicosatrienoic acids [EETs; 8,9-EET, 11,12-EET, and 14–15-EET), and hydroxyeicosapentanoic acids [HEPEs; 5-HEPE and 12-HEPE]) was also performed on serum samples using validated assays as described in detail previously (15,28,29).
Statistical Analyses
Normality was evaluated using the Shapiro–Wilk test of normality. Differences in baseline variables between groups were assessed using independent sample t tests, chi-squared tests, or Fisher exact tests. Differences in vascular parameters and circulating markers between groups were assessed using an independent samples t test, and changes in FMDBA in response to ascorbic acid infusion were assessed using a 2×2 ANOVA. The influence of mean arterial pressure on group differences in carotid-femoral PWV, as recently recommended (30), was analyzed by analysis of covariance. Non-normally distributed variables were log transformed before analysis. All data are reported as means±SD or medians (interquartile ranges), with figures presented as means±SEM. Missing data for any variables are described above, and analysis was completed only on individuals with complete data for the outcome of interest. Analyses were performed using SPSS 24, and statistical significance was set at P<0.05. Because the data were considered mechanistic and hypothesis generating, adjustment was not made for multiple comparisons.
A sample size of 19 control subjects was calculated on the basis of 95% power at an α-level of 0.05 (two sided) to detect a group difference of 1.9 in the change in FMDBA with ascorbic acid infusion on the basis of previously published data assessing the effect of ascorbic acid on FMDBA in healthy middle-aged and older adults compared with young healthy controls (mean±SD change in percentage FMDBA for each group: young healthy controls: 0.2±2.0; older adults: 2.1±0.9) (24) and assuming a similar effect size in ADPKD. Although only 19 participants with ADPKD were required to provide 95% power, the ADPKD group included all participants from the clinical trial. These sample sizes (n=19 controls and n=61 participants with ADPKD) also provided 81% power to detect a group difference of at least 3.3±4.4 in percentage FMDBA (11), 90% power to detect a group difference of 66±77 cm/s in carotid-femoral PWV (20) on the basis of previous publications in ADPKD, and 98% power to detect a group difference of 0.20±0.19 in endothelial cell protein expression of NF-κB on the basis of data in healthy middle-aged and older adults compared with young healthy controls (26).
Study Approval
All procedures were approved by the institutional review board of the University of Colorado Anschutz Medical Campus and adhere to the Declaration of Helsinki. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Results
Demographic and Clinical Characteristics
Sixty-six participants with ADPKD were assessed for eligibility in the clinical trial, and five were excluded from enrollment (one did not meet inclusion/exclusion criteria and four declined to participate); therefore, the total cohort was 61. Twenty-four control participants were assessed for eligibility and five were excluded from enrollment (three did not meet inclusion/exclusion criteria and two declined to participate) for a total cohort of 19. Participants with ADPKD were slightly older and had higher BP than healthy controls (Table 1). All participants with ADPKD had a history of hypertension treated with an ACEi/ARB, and BP was well controlled. Participants with ADPKD were also more likely to use a diuretic or statin (no control participants used these medications). No control participants were hypertensive due to inclusion criteria for enrollment. Participants with ADPKD and controls did not differ in use of other medications, sex, race/ethnicity, body mass index, or cholesterol levels.
Table 1.
Demographics and clinical characteristics of participants with autosomal dominant polycystic kidney disease and control participants
| Variable | ADPKD, n=61 | Control, n=19 |
|---|---|---|
| Age, yra | 34±9 | 30±5 |
| Sex, % men | 46 | 58 |
| Race/ethnicity, % non-Hispanic white | 82 | 74 |
| BMI, kg/m2 | 27.0±4.8 | 25.9±5.1 |
| Systolic BP, mm Hga | 120±12 | 114±12 |
| Diastolic BP, mm Hga | 78±10 | 69±6 |
| CKD-EPI eGFR, ml/min per 1.73 m2 | 94±21 | 104±16 |
| LDL cholesterol, mg/dl | 96±28 | 84±17 |
| HDL cholesterol, mg/dl | 50±13 | 57±15 |
| Total cholesterol, mg/dl | 165±33 | 155±20 |
| Hypertension, %a | 100 | 0 |
| ACEi/ARB, %a | 100 | 0 |
| Diuretic, %a | 21 | 0 |
| Calcium channel blockers, % | 7 | 0 |
| Statin, %a | 21 | 0 |
| Antidepressant or antianxiety medication, % | 15 | 11 |
| Thyroid medication, % | 7 | 5 |
Data are mean±SD or n (%). ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; CKD-EPI; Chronic Kidney Disease Epidemiology Collaboration equation; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P<0.05 by chi-squared test or Fisher exact test for categorical data and independent sample t test for continuous variables.
Vascular Parameters
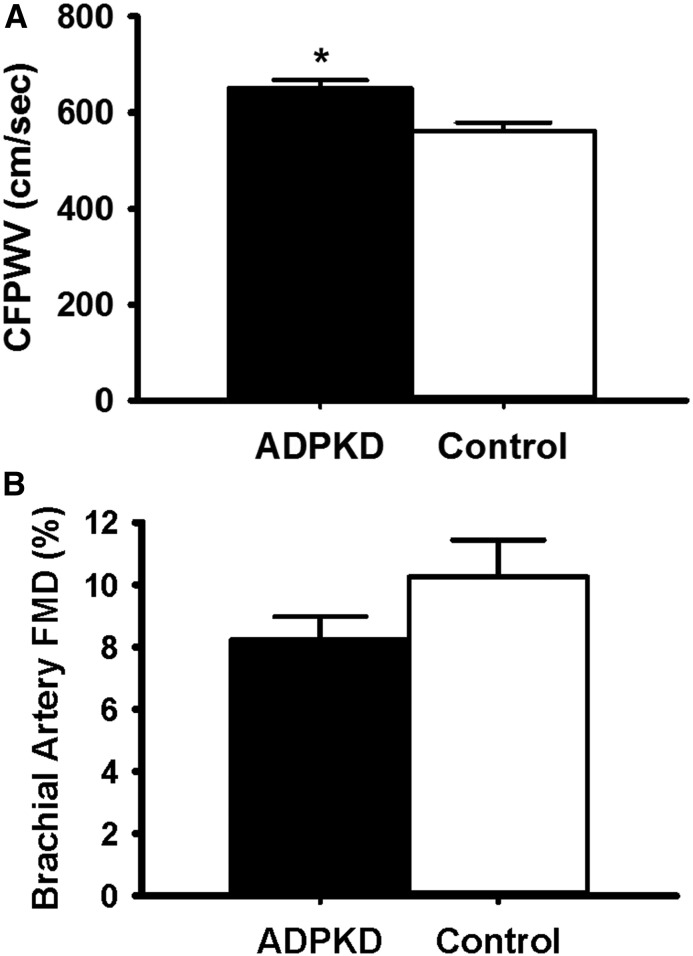
Participants with ADPKD had a 15% higher carotid-femoral PWV (ADPKD: 650±131 cm/s; control: 562±81 cm/s; P=0.007), indicating greater aortic stiffness, without a statistically significant difference in FMDBA (ADPKD: 8.2%±5.8%; control: 10.8%±4.7%; P=0.08) compared with healthy controls (Figure 1). The group difference in carotid-femoral PWV was no longer significant after adjusting for mean arterial pressure (P=0.66). Groups did not differ in baseline diameter or peak shear rate (potential covariates) (Table 2); thus, statistical correction for these variables was not performed. There were no group differences in endothelium-independent dilation to sublingual nitroglycerin. Participants with ADPKD had greater carotid-radial PWV (an index of peripheral stiffness) and carotid systolic BP, with no difference in carotid artery compliance, carotid artery β-stiffness index, or carotid intimal medial thickness.
Figure 1.
Participants with autosomal dominant polycystic kidney disease (ADPKD) demonstrated higher carotid-femoral pulse-wave velocity (CFPWV) compared with healthy controls (P<0.01), without a statistically significant difference in brachial artery flow-mediated dilation (FMD) (P=0.08). (A) CFPWV and (B) FMD in participants with ADPKD and healthy controls are shown in black and white bars, respectively. Values are mean±SEM. *P=0.007.
Table 2.
Vascular parameters in participants with autosomal dominant polycystic kidney disease and control participants
| Variable | ADPKD, n=61 | Control, n=19 | P Value |
|---|---|---|---|
| FMDBA, mm | 0.26±0.15 | 0.32±0.13 | 0.15 |
| Baseline FMD diameter, mm | 3.5±0.7 | 3.2±0.5 | 0.13 |
| Shear rate, s−1 | 1075±459 | 1134±306 | 0.20 |
| Brachial artery dilation to nitroglycerin, % | 28.3±7.3 | 30.1±7.3 | 0.46 |
| Baseline nitroglycerin diameter, mm | 3.5±0.7 | 3.3±0.47 | 0.29 |
| Carotid-radial PWV, cm/s | 921±157 | 813±136 | <0.008 |
| Carotid artery compliance, (mm/mm Hg) ×10−1 | 0.12±0.04 | 0.12±0.04 | 0.50 |
| Carotid β-stiffness index, AU | 6.0±2.0 | 5.4±1.3 | 0.20 |
| Carotid IMT, mm | 0.48±0.08 | 0.45±0.05 | 0.23 |
| Carotid systolic BP, mm Hg | 119±15 | 106±13 | 0.001 |
| Mean arterial BP, mm Hg | 91±9 | 80±6 | <0.001 |
Data are mean±SD. In total, n=12 control participants and n=37 participants with ADPKD were administered nitroglycerin, and n=60 participants with ADPKD and all (n=19) control participants had measurements of carotid artery compliance, β-stiffness index, and carotid IMT. ADPKD, autosomal dominant polycystic kidney disease; FMDBA, brachial artery flow-mediated dilation; FMD, flow-mediated dilation; PWV, pulse-wave velocity; IMT, intimal medial thickness.
Acute Inhibition of Vascular Oxidative Stress
The acute infusion of ascorbic acid known to inhibit superoxide production significantly raised plasma ascorbic acid levels in both the control (before: 86±40 μmol/L; after: 1163±197 μmol/L; P<0.001) and ADPKD groups (before: 34±5 μmol/L; after: 1094±330 μmol/L; P=0.002). However, the infusion (compared with isovolumetric saline) only improved FMDBA in participants with ADPKD (difference of 1.72; 95% confidence interval, 0.80 to 2.63), indicating the presence of vascular oxidative stress, and there was no effect in healthy controls (difference of −0.20; 95% confidence interval, −1.24 to 0.84; group × condition interaction P=0.02) (Table 3).
Table 3.
Change in brachial artery flow-mediated dilation with ascorbic acid infusion in participants with autosomal dominant polycystic kidney disease and control participants
| Variable | FMDBA with Saline Infusion | FMDBA with Ascorbic Acid Infusion | Change in FMDBA |
|---|---|---|---|
| ADPKD, n=52 | 7.7±4.5 | 9.4±5.2 | 1.72 (0.80 to 2.63) |
| Control, n=19 | 10.8±4.7 | 10.6±5.4 | −0.20 (−1.24 to 0.84) |
Data are mean±SD or mean (95% confidence interval). P interaction (group × condition) =0.02. FMDBA, brachial artery flow-mediated dilation; ADPKD, autosomal dominant polycystic kidney disease.
Cellular and Circulating Markers of Oxidative Stress, Inflammation, and Bioactive Lipid Mediators
Cellular Markers.
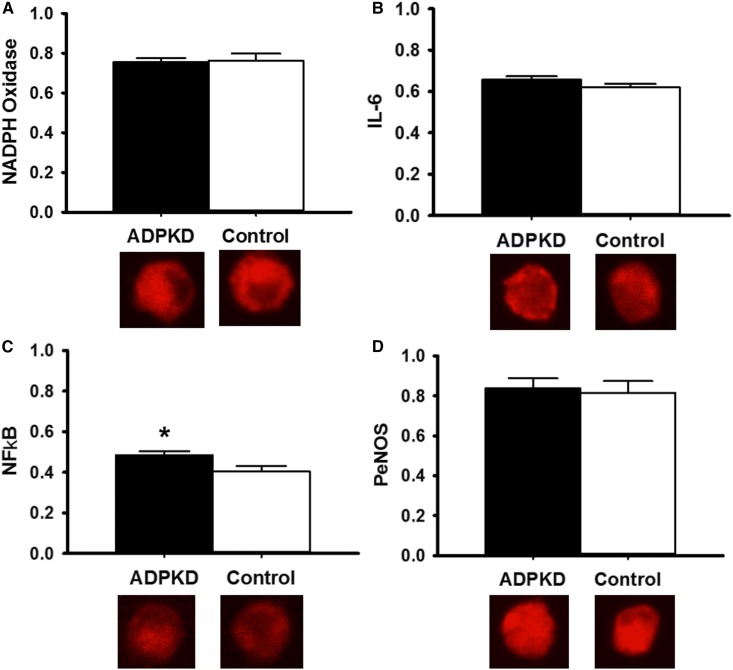
Vascular endothelial cell protein expressions of NADPH oxidase (Figure 2A), IL-6 (Figure 2B), NF-κB (Figure 2C), and PeNOS (Figure 2D) are shown in Figure 2. Participants with ADPKD had higher expression of the proinflammatory transcription factor NF-κB compared with controls (0.48±0.12 versus 0.41±0.10 [intensity versus human umbilical vein endothelial cell control]; P=0.03). There were no differences in expression of other vascular endothelial cell proteins between the two groups. Participants with NF-κB expression below the median had a higher FMDBA (10.6%±6.91%) compared with those with NF-κB expression above the median (6.7%±4.4%; P=0.01).
Figure 2.
Vascular endothelial cell protein expression of the pro-inflammatory transcription factor NF-κB is increased in participants with autosomal dominant polycystic kidney disease (ADPKD) compared with healthy controls. Protein expression of (A) NADPH oxidase (ADPKD: 0.75±0.14; control: 0.76±0.15; P=0.86), (B) IL-6 (ADPKD: 0.66±0.11; control: 0.62±0.11; P=0.26), (C) NF-κB (ADPKD: 0.48±0.12; control: 0.41±0.10; P=0.03), and (D) phosphorylated endothelial nitric oxide synthase (PeNOS; ADPKD: 0.84±0.28; control: 0.81±0.18; P=0.82) in vascular endothelial cells collected from a peripheral vein of participants with ADPKD (black bars) compared with healthy controls (white bars) Expression (by quantitative immunofluorescence) is relative to human umbilical vein endothelial cell control, with representative images shown below. Values are mean±SEM. *P<0.05.
Circulating Markers.
Participants with early-stage ADPKD did not differ from controls in markers of inflammation (hsCRP and IL-6 levels) (Table 4). Participants with ADPKD also did not differ from healthy controls in the majority of the circulating markers of oxidative stress and bioactive lipid mediators (Table 4). Participants with ADPKD had lower circulating levels of 13-HODE compared with healthy controls.
Table 4.
Circulating markers of oxidative stress, inflammation, and lipid mediators in participants with autosomal dominant polycystic kidney disease and control participants
| Variable | ADPKD, n=61 | Control, n=19 | P Value |
|---|---|---|---|
| hsCRP, mg/L | 1.09 (0.38–3.39) | 1.36 (0.46–2.55) | 0.77 |
| IL-6, pg/ml | 0.56 (0.37–0.92) | 0.48 (0.33–0.67) | 0.29 |
| 8-Isoprostane, pg/ml | 4.9 (3.8–6.2) | 4.6 (3.7–6.0) | 0.51 |
| PGF2α. pg/ml | 27.4 (13.0–67.1) | 29.2 (20.3–46.8) | 0.60 |
| PGD2, pg/ml | 83.9 (49.4–128.6) | 78.8 (60.9–108.6) | 0.41 |
| PGE2, pg/ml | 150.2 (52.4–389.1) | 182.9 (139.6–210.2) | 0.36 |
| 9-HODE, ng/ml | 12.7 (7.2–20.4) | 11.9 (10.2–18.1) | 0.55 |
| 13-HODE, ng/ml | 10.5 (6.8–17.2) | 17.7 (10.9–27.0) | 0.04 |
| 5-HETE, ng/ml | 2.1 (0.80–3.0) | 1.9 (1.2–3.2) | 0.32 |
| 8-HETE, ng/ml | 2.0 (1.2–2.9) | 0.71 (0.53–0.89) | 0.57 |
| 9-HETE, ng/ml | 2.0 (1.0–5.1) | 1.6 (1.2–2.5) | 0.20 |
| 11-HETE, ng/ml | 1.1 (0.61–2.8) | 1.5 (1.0–2.1) | 0.58 |
| 12-HETE, ng/ml | 43.6 (22.5–110.1) | 46.2 (22.4–92.7) | 0.87 |
| 11,12-EET, ng/ml | 1.3 (0.47–3.4) | 1.6 (1.1–2.1) | 0.58 |
| 14,15-EET, ng/ml | 1.0 (0.67–2.0) | 1.4 (0.9–1.7) | 0.48 |
| 5-HEPE, ng/ml | 0.71 (0.46–0.95) | 0.61 (0.37–0.72) | 0.19 |
| 12-HEPE, ng/ml | 28.4 (11.4–71.2) | 36.4 (27.2–68.8) | 0.20 |
Data are median (interquartile range). P values are independent t test comparisons between groups using log-transformed variables. ADPKD, autosomal dominant polycystic kidney disease; hsCRP, high-sensitivity C-reactive protein; PG, prostaglandin; HODE, hydroxyoctadecadienoic acid; HETE, hydroxyeicosatetraenoic acid; EET epoxyeicosatrienoic acid, HEPE, hydroxyeicosapentanoic acid.
Discussion
In this cross-sectional, translational study comparing adults with early-stage ADPKD with healthy controls, we have confirmed the presence of increased large elastic artery stiffness. In the hypothesis-generating mechanistic components of the study, we have provided the first direct evidence suggesting vascular oxidative stress and inflammation in humans with ADPKD. An acute infusion of ascorbic acid improved FMDBA in the participants with ADPKD, with no effect in the control group, indicating the presence of vascular oxidative stress. Additionally, endothelial cell protein expression of NF-κB was greater in the ADPKD group compared with controls, providing the first cellular evidence that adults with early-stage ADPKD may have increased vascular inflammation. However, there were not alterations in levels of circulating markers of oxidative stress and bioactive lipid mediators assessed.
We observed 15% greater carotid-femoral PWV in participants with early-stage ADPKD compared with healthy controls. This was likely mediated by an increase in mean arterial BP in the ADPKD group, because the difference no longer persisted after adjusting for mean arterial BP, suggesting that targeting BP in early-stage ADPKD may be effective to reduce carotid-femoral PWV. Notably, this difference was remarkably similar to our previous observation of increased carotid-femoral PWV in children and young adults with ADPKD (14% greater than in healthy controls) (20). In adults with early-stage ADPKD, increased carotid-femoral PWV has been noted in one cross-sectional study (12) but not in other cross-sectional studies (31,32) compared with healthy controls. Interestingly, we also noted greater carotid-radial PWV, consistent with our recent observation in children and young adults (20). This is somewhat surprising, because it is an index of peripheral stiffness, whereas carotid-femoral PWV is a measure of central (aortic) stiffness. Carotid-radial PWV also was noted previously to be nonsignificantly elevated in adults with early-stage ADPKD (32). Additionally, carotid systolic BP was elevated in this study, consistent with higher brachial systolic BP in the participants with ADPKD compared with controls (although still well controlled) as well as our previous findings in children and young adults with ADPKD (20).
FMDBA, a measure of endothelium-dependent dilation, did not significantly differ between participants with ADPKD and healthy controls. This is in contrast to a previous study in middle-aged adults with normal kidney function and ADPKD who showed impaired FMDBA compared with healthy controls, regardless of whether hypertension was present (11). Our group has also previously shown impaired FMDBA in children and young adults with ADPKD compared with age- and sex-matched healthy controls (20). A notable difference in this study is that all participants with ADPKD were taking ACEis/ARBs, which are known to improve FMDBA (33) and thus, may have attenuated the difference in FMDBA between participants with ADPKD and healthy controls. Twenty-two percent of participants were also using a statin (on the basis of results from a previous study in ADPKD [34] rather than for hypercholesterolemia), which has also been shown to improve vascular endothelial function (35). Additionally, there was high variability in FMDBA in the ADPKD group, although we were adequately powered on the basis of previous cross-sectional comparisons to detect a difference between participants with ADPKD and healthy controls. Three other studies also measured flow-mediated dilation (in one study, the radial artery was used) and showed no difference in early-stage participants with ADPKD compared with healthy controls (31,36,37). The absence of hypertension may have contributed to the lack of a statistically significant difference between groups in these studies. Of note, impaired endothelium-dependent dilation of subcutaneous resistance vessels to acetylcholine has also been shown in middle-aged adults with ADPKD (38).
Circulating markers of oxidative stress, inflammation, and bioactive lipid mediators were previously shown to be altered in early-stage ADPKD. Circulating levels of PGs (including 8-isoprostane and PGF2α [13,15]), CRP (13), IL-6 (13), 9-HODE (14,28), 13-HODE (14,28), and oxidized LDL (39) are elevated, and the levels of the antioxidant enzyme SOD are lower (13) in adults with early ADPKD. In this study, plasma level of PGs (8-isoprostane, PGF2α, PGD2, and PGE2) did not differ in participants with ADPKD compared with healthy controls. These results differ from our previously published data from the Halt Progression of Polycystic Kidney Disease (HALT-PKD) Study, which found increased levels of these oxidative stress markers (15). This difference may reflect the tighter blood control at baseline or an earlier stage of disease in this study, because alterations in these markers in the HALT-PKD Study were associated with larger kidney volumes.
We also found no evidence of differences in bioactive lipid mediators, with the exception of lower levels of the proinflammatory lipid peroxidation product 13-HODE in the ADPKD group compared with controls. These data are also in contrast with our previously published data from the HALT-PKD Study (28). It is possible that the response of these lipid mediators differs earlier in the course of ADPKD or that tighter baseline BP control and use of antihypertensive agents resulted in lower baseline inflammation, consistent with a lack of increased levels of CRP and IL-6 in this cohort. Notably, circulating markers of oxidative stress and inflammation may differ from and may not reflect local changes in vasculature.
To this point, we provided the first direct evidence in humans with ADPKD of oxidative stress at the level of vasculature. An acute supraphysiologic infusion of ascorbic acid that produced plasma concentrations known to inhibit superoxide production in vitro (23) improved FMDBA in the ADPKD group but not in healthy controls, indicating the presence of vascular endothelial oxidative stress. Despite a lack of difference in circulating markers of inflammation (hsCRP, IL-6, and bioactive lipid mediators), vascular endothelial cell protein expression of NF-κB was increased in the ADPKD group, consistent with inflammation local to the vasculature. However, there were no differences in endothelial cell IL-6 protein expression, indicating that inflammatory changes in ADPKD may occur farther upstream. Notably, despite a lack of significant difference in FMDBA between groups, vascular inflammation was associated with impaired vascular endothelial dysfunction, because participants with NF-κB expression above the median had significantly lower FMDBA.
Increased oxidative stress and inflammation likely contribute to a decline in NO bioavailability, a common mechanism that contributes to both increased large elastic artery stiffness and endothelial dysfunction (6,40). In subcutaneous resistance vessels collected from adults with ADPKD, impairment in endothelium-dependent dilation is mediated by reduced NO bioavailability (14,41). This is also the case in resistance arteries assessed from a rodent model of ADPKD (42). Additionally, NOS activity is reduced in resistance vessels, and plasma levels and excretion of nitrate/nitrite are reduced in ADPKD (14,37). This may be mediated in part by an increase in plasma asymmetric and symmetric dimethylarginine levels, which inhibit endothelial NO synthase (14,15,39). We have previously observed higher levels of asymmetric and symmetric dimethylarginine as well as homocysteine and S-adenosylhomocysteine in patients with early-stage ADPKD compared with healthy controls (15). In contrast, we observed no difference in PeNOS expression in vascular endothelial cells collected from early-stage participants with ADPKD. However, expression may not reflect eNOS activity, and also, it does not reflect production of NO.
The major strength of this study is that we performed the most comprehensive assessment of physiologic mechanisms contributing to vascular dysfunction to date using novel methodology. We provided direct evidence of oxidative stress and inflammation at the level of the vasculature, including collection of vascular endothelial cells and measurement of FMDBA after acute inhibition of oxidative stress. We also used state-of-the art liquid chromatography-tandem mass spectrometry analyses to assess circulating markers. These assessments were performed in a relatively large number of participants with ADPKD given the comprehensive nature of these measurements.
There are also several notable limitations. Although the acute ascorbic acid infusion indeed improved FMDBA in the ADPKD group but not controls, we failed to show a statistically significant baseline impairment in FMDBA in the patients with ADPKD, somewhat limiting the interpretation of the former results. Given that all participants with ADPKD also had a history of hypertension, it is difficult to separate the contributions of hypertension and ADPKD with regard to contributing mechanisms. However, previous research has shown the presence of vascular dysfunction, even in individuals with ADPKD who are free from hypertension (11,12), and ADPKD plus hypertension is the most common clinical state (18). Other differences between the ADPKD and control groups may also have accounted for the observed results rather than the primary disease process. For example, ages were not perfectly matched; however, age-associated decline in vascular function would not be expected in this young age range (43,44). Additionally, participants with ADPKD were selected for participation in a clinical trial and differed from controls by a diagnosis of hypertension. Importantly, despite any residual group differences, our findings are still clinically important.
Because of the scheduling of visits with national recruitment of participants with ADPKD, it was not possible to perform testing during the same point of the menstrual cycle for all women, which would increase variability in measurements. We also did not have information available regarding left ventricular mass index or ambulatory BP monitoring. Finally, markers of oxidative stress and inflammation provide suggestive evidence but not cause and effect data that oxidative stress and inflammation induce vascular dysfunction in ADPKD, and our results were not consistent across all markers.
In conclusion, we have provided preliminary evidence supporting that oxidative stress and inflammation may be physiologic mechanisms contributing to vascular dysfunction in early-stage ADPKD. Our results also reiterate that arterial stiffness is significantly increased in ADPKD and develops early in the disease course before a decline in kidney function. Interventions targeting these processes in early-stage ADPKD may potentially reduce the overall risk of cardiovascular events and mortality in patients with ADPKD.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK097081. K.L.N. is also supported by NIDDK grant K01DK103678. Additional support was provided by National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Award grant UL1 TR002535. Additional funding was provided by the Zell Family Foundation.
Because M.C. is a Deputy Editor of the Clinical Journal of the American Society of Nephrology, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05850518/-/DCSupplemental.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Fick GM, Johnson AM, Hammond WS, Gabow PA: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Ecder T, Schrier RW: Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol 5: 221–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirson Y: Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 173–180, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Levy D: Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Part I. Aging arteries: A “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Covic A, Gusbeth-Tatomir P, Goldsmith DJ: Arterial stiffness in renal patients: An update. Am J Kidney Dis 45: 965–977, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM: Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension 45: 592–596, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM: Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS: Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, Alisir S, Turgut F, Mercanoglu F, Ecder T: Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 43: 854–860, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kocyigit I, Kaya MG, Orscelik O, Kaya C, Akpek M, Zengin H, Sipahioglu MH, Unal A, Yilmaz MI, Tokgoz B, Oymak O, Axelsson J: Early arterial stiffness and inflammatory bio-markers in normotensive polycystic kidney disease patients. Am J Nephrol 36: 11–18, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M: Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol 6: 7–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q, Wilcox CS: Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis 51: 184–191, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Klawitter J, Reed-Gitomer BY, McFann K, Pennington A, Klawitter J, Abebe KZ, Klepacki J, Cadnapaphornchai MA, Brosnahan G, Chonchol M, Christians U, Schrier RW: Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Renal Physiol 307: F1198–F1206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ecder T, Schrier RW: Hypertension in autosomal-dominant polycystic kidney disease: Early occurrence and unique aspects. J Am Soc Nephrol 12: 194–200, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Harris RA, Nishiyama SK, Wray DW, Richardson RS: Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak KL, Farmer H, Cadnapaphornchai MA, Gitomer B, Chonchol M: Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 32: 342–347, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, Hung AM: IL-1 Inhibition and vascular function in CKD. J Am Soc Nephrol 28: 971–980, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR: Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson TS, Xu A, Vita JA, Keaney JF Jr.: Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 83: 916–922, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Eskurza I, Monahan KD, Robinson JA, Seals DR: Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deurenberg P, Weststrate JA, Seidell JC: Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br J Nutr 65: 105–114, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR: Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Jablonski KL, Decker E, Perrenoud L, Kendrick J, Chonchol M, Seals DR, Jalal D: Assessment of vascular function in patients with chronic kidney disease. J Vis Exp 88: 10.3791/51478, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klawitter J, Klawitter J, McFann K, Pennington AT, Abebe KZ, Brosnahan G, Cadnapaphornchai MA, Chonchol M, Gitomer B, Christians U, Schrier RW: Bioactive lipid mediators in polycystic kidney disease. J Lipid Res 55: 1139–1149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klawitter J, Haschke M, Shokati T, Klawitter J, Christians U: Quantification of 15-F2t-isoprostane in human plasma and urine: Results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun Mass Spectrom 25: 463–468, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension : Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azurmendi PJ, Fraga AR, Galan FM, Kotliar C, Arrizurieta EE, Valdez MG, Forcada PJ, Stefan JS, Martin RS: Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol Dial Transplant 24: 2458–2463, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Borresen ML, Wang D, Strandgaard S: Pulse wave reflection is amplified in normotensive patients with autosomal-dominant polycystic kidney disease and normal renal function. Am J Nephrol 27: 240–246, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A: Effects of antihypertensive drugs on endothelial dysfunction: Clinical implications. Drugs 62: 265–284, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW: Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9: 889–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namli S, Oflaz H, Turgut F, Alisir S, Tufan F, Ucar A, Mercanoglu F, Ecder T: Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail 29: 55–59, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Lorthioir A, Joannidès R, Rémy-Jouet I, Fréguin-Bouilland C, Iacob M, Roche C, Monteil C, Lucas D, Renet S, Audrézet MP, Godin M, Richard V, Thuillez C, Guerrot D, Bellien J: Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int 87: 465–472, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Clausen P, Feldt-Rasmussen B, Iversen J, Lange M, Eidemak I, Strandgaard S: Flow-associated dilatory capacity of the brachial artery is intact in early autosomal dominant polycystic kidney disease. Am J Nephrol 26: 335–339, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Iversen J, Strandgaard S: Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 11: 1371–1376, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Raptis V, Georgianos PI, Sarafidis PA, Sioulis A, Makedou K, Makedou A, Grekas DM, Kapoulas S: Elevated asymmetric dimethylarginine is associated with oxidant stress aggravation in patients with early stage autosomal dominant polycystic kidney disease. Kidney Blood Press Res 38: 72–82, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Chue CD, Townend JN, Steeds RP, Ferro CJ: Arterial stiffness in chronic kidney disease: Causes and consequences. Heart 96: 817–823, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Iversen J, Wilcox CS, Strandgaard S: Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int 64: 1381–1388, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Iversen J, Strandgaard S: Contractility and endothelium-dependent relaxation of resistance vessels in polycystic kidney disease rats. J Vasc Res 36: 502–509, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE: Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Reference Values for Arterial Stiffness’ Collaboration : Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J 31: 2338–2350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.