Abstract
Background
Older people with Intellectual Disability (ID) have a high prevalence of gastrointestinal conditions such as Gastro-Oesophageal Reflux Disease (GORD). However, despite this, information about treatment, in particular the use of Proton Pump Inhibitors (PPIs), in this population is sparse and limited.
Objective
To investigate the prevalence and pattern of PPI use among older people with ID.
Method
Data on PPI use and key demographics was analysed from Wave 2 (2013/2014) of IDS-TILDA, a nationally representative longitudinal study of 677 participants aged 40 years and above in Ireland. Descriptive statistics, bivariate analyses and binary logistic regression were carried out.
Results
Just over a quarter, 27.9% (n = 189), of participants reported use of PPIs, and 53.4% (n = 101) were female. The largest proportion of PPI users (53.4%) were aged between 50 and 64 yrs. Most of the PPIs were used in maximum doses (66.7%). However only 43.9% of PPI users had an indication for PPI use (GORD, stomach ulcer or/and an NSAID use), and further 13.2% were also taking an antiplatelet agent. Use among those in residential care homes (54.3%) was much higher than for those living independently or with family (7%). PPI use among those who have severe/profound ID was 25% higher than those with mild ID. Information about the length of PPI use was missing for 31.2%, but of those with data, just over half recorded using the PPIs for more than a year. Apart from an indication, the factors associated with PPI use were older ages (≥50 years), severe/profound level of ID.
Conclusion
PPI use among older people with intellectual disability is prevalent and frequently long term, often without a clear indication. PPI use especially among those with severe/profound ID and those who live in residential care homes, could predispose these individuals to additional comorbidities and in order to avoid inappropriate long term of use regular review is required.
Keywords: Older people, Intellectual disability, Proton pump inhibitors, Medicine use, Inappropriate, Polypharmacy
1. Introduction
Older people are at increased risk of many Gastrointestinal (GI) diseases such as GORD (Franceschi et al., 2009). Unlike younger adults, older people may experience non-specific symptoms and more severe oesophagitis which may be associated with complications such as erosive oesophagitis, Barrett’s oesophagus, or extra-oesophageal complications such as pulmonary aspiration (Chait, 2010, Pilotto et al., 2006). Treatment of GORD in older people is challenging as they are often have multiple comorbidities and polypharmacy. The mainstay and mostly effective treatment are the PPIs (Metz, 2004). The major indications for PPI use are GORD, oesophagitis, stomach ulceration, and treatment of H-pylori infection and concomitantly with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) to protect the GI from damage or ulceration. In addition, PPIs are prescribed off label for patients taking antiplatelet drugs who are considered to be at risk of GI damage (Abraham et al., 2010).
Intellectual disability is “a disability which originates before the age 18 and characterized by significant limitations in both intellectual functioning and in adaptive behaviour, which covers many everyday social and practical skills” (AAIDD, 2017). ‘Intellectual Disability’ was formerly described as ‘Mental Retardation’ in the United States (Schalock et al., 2007), while the term Learning Disability is preferred in the United Kingdom (Emerson and Heslop, 2010) and Developmental Disabilities in Canada (Sullivan et al., 2011). The causes behind ID varies and include genetic (X-linked, other chromosomal), metabolic, teratogenic, central nervous system defects, birth defects, neonatal, perinatal, causes that are multifactorial, and causes of no known etiology (Einfeld and Emerson, 2009, Ellison et al., 2013). Level of ID is classified based on Intelligence Quotient (IQ) scores as follows; mild (50–55 to approx. 70), moderate (35–40 to 50–55) and severe/profound (below 35–40) (APA, 2013).
Although life expectancy for older people with ID has risen (Coppus, 2013, Ng et al., 2015, Patja et al., 2000), they still experience a higher mortality rate compared to the general population (Heslop and Glover, 2015, Lauer and McCallion, 2015, McCarron et al., 2015). People with ID, have been estimated to have twice as many health problems as among those without ID and to receive four times as many repeat prescriptions (Sandberg et al., 2016, Straetmans et al., 2007, van Schrojenstein Lantman-De Valk et al., 2000). They commonly have epilepsy, sensory impairments, osteoporosis, schizophrenia, dementia, musculoskeletal problems, and nutritional problems (May and Kennedy, 2010). GORD, H-pylori infection, dysphagia and constipation are most frequently GI health issues among adults and older adult with ID (Böhmer et al., 2002, De Veer et al., 2008, Haveman et al., 2010, Hermans and Evenhuis, 2014, van Timmeren et al., 2016, Wallace et al., 2004a, Wallace et al., 2004b).
The use of PPIs in the general older population is well documented, with an overall prevalence ranging from 23% to 79% (Burdsall et al., 2013, George et al., 2008, Haenisch et al., 2015, Jarchow-MacDonald and Mangoni, 2013, Nishtala and Soo, 2015). Prevalence of PPI use is also reported to range from 27% to 79.7% among old people who are admitted to long-term facilities or nursing homes (Burdsall et al., 2013, de Souto Barreto et al., 2013, Rane et al., 2016). In clinical settings such as geriatric units, PPI uses range from 33 to 41% (Corsonello et al., 2014, Jarchow-MacDonald and Mangoni, 2013). One study reported a PPI prevalence of 97.4% and 98% for older people at hospital admission and discharge, respectively (Pasina et al., 2011). As the use of PPIs, particularly their long-term use has increased, there has been growing concern about side effects and associated costs (Cahir et al., 2010, Heidelbaugh et al., 2012).
Two studies of adult and older adult with ID, reported use of drugs for stomach ulcer or GORD as 31% and 52% respectively (Charlot et al., 2011, van der Heide et al., 2009), and one study reported that omeprazole, the only PPI then available, was used by 43% of institutionalized people with ID (Böhmer et al., 1997). In Wave 1 data from the Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing (IDS-TILDA) (2011), the reported prevalence of PPI use was 21.7%, and 44% of those who had excessive polypharmacy (10 + medicines) were exposed to PPIs (O'Dwyer et al., 2016). Inappropriate prescribing or a non-evidenced based use of PPIs is considered in the absence of the following indication: treatment of GORD, erosive oesophagitis, peptic ulcer disease, long term NSAIDs use for patients with history or at high risk of GI complications and for eradication of H-pylori (Yadlapati and Kahrilas, 2017). Furthermore, in the absence of risk factors, full therapeutic doses for more than eight weeks are considered inappropriate (AGS, 2015). Studies, including some that have been conducted in Ireland, have reported that the highest prevalence of potentially inappropriate prescribing was among PPIs (Cahir et al., 2010, Hamilton et al., 2011, Ryan et al., 2009). This was confirmed in The Irish Longitudinal Study of Ageing (TILDA), that included 8175 subjects (aged ≥ 50 years, without ID) where PPI use was found as potentially inappropriate in 17.2% and this significantly increased to 21.9% (p < 0.0001) after follow-up at two years (Moriarty et al., 2015a).
In this study, the primary aim was to determine the prevalence of PPI use among older adults with ID and to more specifically examine; (1) the dosage regimen, length of use and the recorded indications; (2) the patterns of use with respect to clinical and demographic factors, place of residence and level of ID; (3) to determine the clinical and demographic factors associated with PPI use.
2. Methodology
Data for this study was drawn from Wave 2 (2013/2014) of the nationally representative longitudinal study (IDS-TILDA) (McCarron et al., 2011). The study includes older people with ID aged 40 and above who have registered in the national intellectual disability database. It is exploring the ageing profile, physical and behavioural health (including medication use), health service needs, psychological health, social networks, living situations, community participation and employment. This study has been previously described in detail elsewhere (McCarron, 2011, McCarron et al., 2014). In this study, we have used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting in observational cross sectional studies (Vandenbroucke et al., 2014).
2.1. Participants
From wave 1 (n = 753), 6% (n = 45) subjects did not participate in Wave 2, and of these 75.5% (n = 34) had died (Fig. 1) for a 94% (n = 708) response rate. Those with missing medicines data were excluded and the final number of participants was 677 (95%).
Fig. 1.
Flow of participants from Wave one to Wave two IDS-TILDA.
2.2. Data collection and categorization
The data collection process has been described comprehensively elsewhere (McCarron et al., 2014). In brief, data collection in Wave 2 comprised a Pre-Interview Questionnaire (PIQ), an extensive face to face Computer Assisted Personal Interview (CAPI) and health assessments. The PIQ was sent to each participant at least a one week before the interview and gathered data on demographics, health status, health care utilization and medication use. Data collected by the PIQ were then confirmed during the interview. The vast majority of subjects completed PIQ and CAPI of Wave 2 (98.7%, n = 699). PIQ only was completed by 0.28% (n = 2). Different interviewing styles were used during the CAPI, depending on the participant’s communication ability and ID level. A respondent-only interview was undertaken directly with the subjects, a proxy interview was completed with a family member or carer most familiar with the subject, or an interview conducted with participant was supported by a proxy.
2.2.1. Medicine information
In the PIQ, participants and/or proxy were asked to record all medicines taken on a regular basis (e.g., ‘daily or weekly’). This included any kind of medicine being used: prescribed, over the counter, and any supplements. The information reported involved medicine name (generic or brand), full dosage regimen and date of first prescription. Medicine data was then confirmed by the interviewers at the time of the in-person interview and was further checked independently by two pharmacists (H. Alm. and A.B.). Medicines were classified according to the WHO Anatomical Therapeutic Chemical (ATC) system using a seven digit code (WHO, 2016). Exposure to PPIs was the primary outcome of interest. Five PPIs were authorised in Ireland for adults at the time of the study; omeprazole, lansoprazole, esomeprazole, pantoprazole and rabeprazole. Doses were stratified as low to medium and high dose based on guidance in the Summary of Product Characteristics approved by the national Irish medicines regulator, the Health Products Regulatory Authority (HPRA, 2014), the British National Formulary (BNF, 2015), and cross-checked with the published criteria for medicines use in older people - STOPP/START which are extensively used in clinical practice in Ireland and the UK (Cahir et al., 2010, Gallagher et al., 2008, Moriarty et al., 2016, Parsons et al., 2012). For older people, PPI doses considered as low to medium were 15 mg for lansoprazole, 10–20 mg for omeprazole, 20 mg esomeprazole, and 20 mg for pantoprazole and 10 mg for rabeprazole. Doses considered as maximum were 30 mg for lansoprazole, 40 mg for omeprazole, 40 mg for esomeprazole, 40 mg for pantoprazole or 20 mg for rabeprazole. Any dose beyond the maximum was categorised as a dose that exceeded the maximum.
The length of PPI exposure was stratified into a ‘less than a year’ and ‘more than a year’. Data collection of Wave 2 was between May 2013 and February 2014. Based on this range, all documented prescription dates for each PPI agent was checked along with the participants’ data collection time individually and any date before May 2012, was considered as (≥year) length of use.
The number of medicines (excluding supplements) that were used concurrently by each participant was assessed to reflect the polypharmacy status. The number of medicines used were stratified into three categories: zero to four - no polypharmacy, from five to nine medicines - polypharmacy, and ten medicines or more - excessive polypharmacy (Fulton and Riley Allen, 2005, Richardson, 2012).
2.2.2. GI conditions and PPI indications
Participants were asked if they had ever been diagnosed by a doctor with any GI conditions. GORD and stomach ulcers that are treated mainly using PPIs were of particular interest. In addition, the use of NSAIDs, another licensed indication for PPIs (HPRA, 2014) was also collected, along with data on those using antiplatelets since they may be at a high risk of GI bleeding and may be prescribed PPIs concurrently (Abraham et al., 2010).
2.2.3. Other covariates
Demographic co-variates included level of ID, classified into mild, moderate and severe/profound; place of residence, classed as those who live independently or with family, those who live in a community group home and those who resided in residential nursing homes.
2.3. Statistical analysis
Data were analysed using the statistics package SPSS version 22. Demographic characteristics of the cohort were expressed as frequencies and percentages (with Wilson 95% confidence intervals for proportions). A p-value of < 0.05 was considered statistically significant. A binary logistic regression was applied to the dependent variable, PPI users or PPI non-users to determine factors associated with PPI utilisation. Cross tabulation and chi-squared statistics were used to compare potential independent variables with the dependent variable (PPI use). In the regression, six variables were included. Four of these variables were demographic characteristics: gender, age, level of ID and place of residence. Two of the variables were associated with PPI indication: any of licensed indications for PPI use in Ireland (included as one variable) and antiplatelet use (included as separate variable). Variable categories that had small numbers in subgroups were collapsed; for example, PPI users who lived independently or with family (n = 13) were collapsed with PPI users who live in community groups (n = 71) into one group. Finally, only those with a verified level of ID (n = 623) were included in the regression. Before applying the regression, multicollinearity between the independent variables was tested, by examining the Spearman’s correlation coefficient and Variance Inflation Factor (VIF). According to Dancey and Reidy's categorization of Spearman’s correlation coefficient, a value of coefficient ≤ 0.39, suggests the relevant variables will be weakly correlated (Dancey, 2004) and thus we considered them to be of no concern. In addition, the VIF was investigated for all the variables, with a cut-off value ≥2 considered as correlated (Kutner, 2004, Neter, 1996). All the variables were entered in the regression model simultaneously. Results were expressed as odds ratio (OR) with the corresponding 95% confidence interval and significance level of <0.05. The minimum sample size needed for the regression model according to Peduzzi (Peduzzi et al., 1996) was calculated using the equation: N = 10 k/p, where p is the smallest of the proportions of negative or positive cases in the population, k is the number of independent variables. For the regression model, there were 6 covariates and the proportion of positive cases (as PPI use) was 0.27, therefore a minimum sample size (N) of 222 was needed. Given there were data from 623 individuals available for regression analyses, sample size was adequate.
3. Results
3.1. Demographic characteristics of the cohort
As illustrated in Table 1, more than the half of the cohort (56.3%, n = 381) were female. Half of the sample were aged between 50 and 65 years (51%, n = 346) and those aged 65 years and over represented 21% (n = 142). Most participants (46%; n = 287) had a moderate level of ID. About 44% (n = 298) lived in community group homes, almost as many 40.8% (n = 276) in residential care.
Table 1.
Baseline demographic characteristics of the eligible subjects (n = 677).
| Characteristic | Total 677 % (95%CI) (n) |
|---|---|
| Gender | |
| Male | 43.7% (40.0–47.4) (296) |
| Female | 56.3% (52.5–59.9) (381) |
| Age (n = 677) | |
| 44–49 | 28% (24.6–31.4) (189) |
| 50–64 | 51% (47.3–54.8) (346) |
| 65+ | 21% (18.0–24.2) (142) |
| Level of ID (Missing = 53) | |
| Mild | 24% (20.8–27.5) (150) |
| Moderate | 46% (42.1–49.9) (287) |
| Severe/Profound | 30% (29.9–33.6) (187) |
| Type of residence (Missing = 1) | |
| Independent/Family | 15% (12.5–17.9) (102) |
| Community group home | 44% (40.3–47.8) (298) |
| Residential care | 40.8% (37.1–44.5) (276) |
Significance level <0.05.
3.2. PPI users and their demographics
Among the total population, 28%(n = 189) reported the use of PPIs. There were significant differences between PPI users and non-users (except for gender and NSAID use) as illustrated in Table 2; among PPI users over half were aged between 50 and 64 years old (n = 101), and 30.2% (n = 57) were aged over 65 (p < 0.001); and there were significantly more people with a severe to profound ID level (p < 0.001). There was a significant association between place of residence and exposure to PPIs; half of the PPI users resided in a residential care settings (n = 102), 38.8% (n = 73) lived in community group homes (p < 0.001).
Table 2.
Bivariate analysis for PPI and non-PPI users.
| Category | PPI users (n = 189) % (95%CI) (n) |
PPI non-users (n = 488) % (95%CI) (n) | p-value |
|---|---|---|---|
| Age (years) | <0.001 | ||
| 40–49 | 16.4% (11.8–22.3) (31) | 32.4% (28.4–36.7) (158) | |
| 50–64 | 53.4% (46.3–60.4) (101) | 50.2% (45.8–54.6) (245) | |
| 65+ | 30.2% (24.1–37) (57) | 17.4% (14.3–21) (85) | |
| Gender | .354 | ||
| Male | 46.6% (39.6–53.7) (88) | 42.6% (38.3–47.1) (208) | |
| Female | 53.4% (46.3–60.4) (101) | 57.4% (52.9–61.7) (280) | |
| Level of ID | (Missing = 13) | (Missing = 40) | |
| Mild | 15.9% (11.2–22) (28) | 27.2% (23.3–31.5) (122) | <0.001 |
| Moderate | 43.2% (36.1–50.6) (76) | 47.1% (42.5–51.7) (211) | |
| Severe/Profound | 40.9% (33.9–48.3) (72) | 25.7% (21.8–29.9) (115) | |
| Place of residence | (Missing = 1) | ||
| Independent/family/Community group homes | 45.7% (38.8–52.9) (86) | 64.3% (60.0–68.5) (314) | <0.001 |
| Residential settings | 54.3% (47.1–61.2) (102) | 35.7% (31.5–40.0) (174) | |
| Number of Medicines | <0.001 | ||
| ≤ 4 (n = 256) | 14.8% (10.5–20.6) (28) | 46.7% (42.3–51.2) (228) | |
| Polypharmacy (5–9) (n = 258) | 39.7% (33.0–46.8) (75) | 37.5% (33.3–41.9) (183) | |
| Excessive polypharmacy (10+) (n = 163) | 45.5% (38.6–52.6) (86) | 15.8% (12.8–19.3) (77) | |
| PPI-licensed indications | |||
| Reported diagnosis of GORD | (Missing = 8) | <0.001 | |
| Yes (n = 69) | 30.7% (24.6–37.6) (58) | 2.3% (1.3–4.1) (11) | |
| No (n = 600) | 69.3% (62.4–75.4) (131) | 97.7% (95.9–98.7) (469) | |
| Reported diagnosis of stomach ulcer | (Missing = 8) | <0.001 | |
| Yes (n = 35) | 14.8% (10.5–20.6) (28) | 1.5% (0.7–3.0) (7) | |
| No (n = 634) | 85.2% (79.4–89.5) (161) | 98.5% (97.0–99.3) (473) | |
| NSAIDs use | 0.174 | ||
| Yes (n = 56) | 10.6% (7.0–15.8) (20) | 7.4% (5.4–1.0) (36) | |
| No (n = 621) | 89.4% (84.2–93.0) (169) | 92.6% (90.0–94.6) (452) | |
| Any of the licensed indications | 43.9% (37.0–51.0) (83) | 10.2% (7.9–13.3) (50) | <0.001 |
| Antiplatelet medicine use | 19% (14.1–25.2) (36) | 9.6% (7.3–12.6) (47) | 0.001 |
Significance level <0.05.
3.3. PPI dosage information and reported indications
Three PPIs accounted for over 80% of reported PPIs; lansoprazole (30.7%, n = 58), esomeprazole (27%, n = 51) and omeprazole (25.4%, n = 48). As may be seen in Table 3, the majority of PPIs were used at the maximum licensed dose (66.7%, n = 124). Eight participants (4.3%) were on PPI doses beyond the maximum licensed dose in Ireland. All users received a PPI daily and this use was for more than a year by over half of the cohort (52.3%, n = 68). Among those who used a PPI at the maximum dose, a majority, 61.8% (n = 42) were using it for more than a year. There was no significant difference between the dose and duration of use (p > 0.05). There were no instances of PPIs being used in conjunction with the antibiotics that form part of the recommended H-pylori eradication regimens in Ireland.
Table 3.
PPI dose versus length of use (n = 189) using a bivariate analysis.
| PPI Length of usea % (95%CI) (n) |
|||||
|---|---|---|---|---|---|
| Category | <1 year | ≥1 year | Missing information | Total | |
| PPI Dosea | Low to Medium | 24.2% (15.2–36.2) (15) | 32.4% (22.4–44.2) (22) | 30.4% (19.9–43.3) (17) | 29% (23–35.9) (54) |
| Maximum | 72.6% (60.4–82.1) (45) | 61.8% (49.9–72.4) (42) | 66.1% (53.0–77.1) (37) | 66.7% (59.6–73.0) (124) | |
| Exceed the maximum | 3.2% (0.9–11.0) (2) | 5.9% (2.3–14.2) (4) | 3.6% (1–12.1) (2) | 4.3% (2.2–8.3) (8) | |
| Missing information | – | – | 5.1% (1.7–13.9) (3) | 1.6% (0.5–4.6) (3) | |
| Total | 47.7% (39.3–56.2) (62) | 52.3% (43.8–60.7) (68) | 31.2% (25–38.1) (59) | 27.9% (24.7–31.4) (189) | |
Significance level <0.05.
p-value of the two categories = .736.
A comparison of PPI users with and without the licensed indications did not show any significant differences between the two groups in their characteristics or in the dose/duration of PPI use (Table 4).
Table 4.
Differences in demographics between PPI users with or without licensed indications (GORD, stomach ulcer and NSAID use).
| Category | PPI users (n = 189)% (95%CI) (n) |
||
|---|---|---|---|
| With licensed indication = 83 | Without licensed indication = 106 | p-value | |
| Gender | 0.91 | ||
| Male | 47% (36.6–57.6) (39) | 46.2% (37.0–55.7) (49) | |
| Female | 53% (42.4–63.4) (44) | 53.8% (44.3–63.0) (57) | |
| Age (years) | 0.31 | ||
| 40–49 | 20.5% (13.2–30.4) (17) | 13.2% (8.0–21.0) (14) | |
| 50–64 | 48.2% (37.8–58.8) (40) | 57.5% (48.0–66.5) (61) | |
| 65+ | 31.3% (22.4–41.9) (26) | 29.2% (21.4–38.5) (31) | |
| Level of ID | (Missing = 5) | (Missing = 8) | |
| Mild | 16.7% (10.0–26.5) (13) | 15.3% (9.5–23.7) (15) | 0.96 |
| Moderate | 42.3% (32.0–53.4) (33) | 43.9% (34.5–53.7) (43) | |
| Severe/Profound | 41% (30.8–52.1) (32) | 40.8% (31.6–50.7) (40) | |
| Place of residence | (Missing = 1) | 0.88 | |
| Independent/ Community group home | 45% (34.8–55.9) (37) | 46.2% (37.0–55.7) (49) | |
| Residential settings | 55% (44.1–65.2) (45) | 53.8% (44.3–63.0) (57) | |
| PPI dose | (Missing = 2) | (Missing = 1) | 0.075 |
| Low – Medium | 21% (13.5–31.1) (17) | 35.2% (26.8–44.7) (37) | |
| Maximum | 72.8% (62.3–81.3) (59) | 62% (52.4–70.6) (65) | |
| Exceed the maximum | 6.2% (2.7–13.6) (5) | 2.9% (1.0–8.1) (3) | |
| Length of use | (Missing = 23) | (Missing = 36) | 0.058 |
| <yr. | 56.7% (44.1–68.4) (34) | 40% (29.3–51.7) (28) | |
| >yr. | 43.3% (31.6–55.9) (26) | 60% (48.3–70.7) (42) | |
Significance level <0.05.
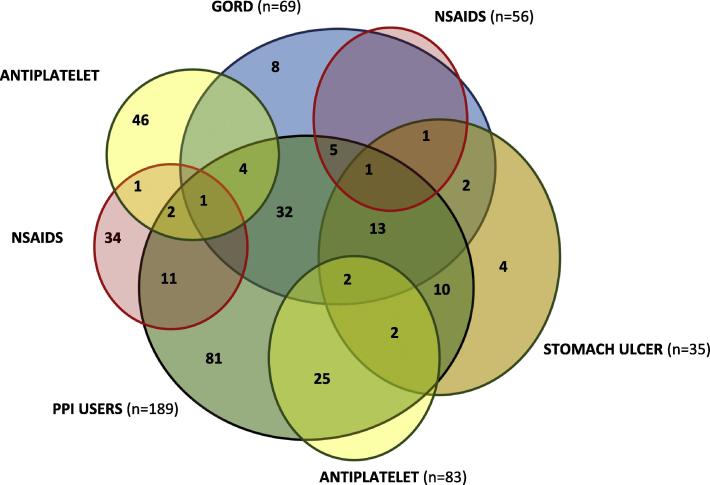
Fig. 2 presents the frequency of use given reported indications and/or antiplatelet use. Those with GORD (with/without stomach ulcers) reported PPI use most frequently. In total, 81 (42.8%) participants were using a PPI without a reported indication. Antiplatelets, primarily aspirin (n = 78), and clopidogrel (n = 6) were reported. There were 46 and 34 participants on antiplatelets and NSAIDs respectively, who did not report the use of a PPI. Those groups were also assessed for any other acid supressing agents and negligible numbers were using an antacid (n = 3) or a Histamine H2 receptor –antagonist (n = 3).
Fig. 2.
Reported indications, antiplatelet use and PPI use expressed by number of participants.
3.4. Demographic patterns in PPI use and dosage
The relationship between PPI use/dosage information and place of residence was examined (data not shown). Differences in PPI use duration across the place of residence were not statistically significant, although the proportions of those who lived in either community group homes (51%) or residential settings (57.3%) using a PPI for more than a year was higher than for those who lived independently or with family (12.5%). The relationship between gender/age group/ level of ID and PPI dosage information was also analysed (data not shown): there were no significant differences between gender/age group and ID level in terms of PPI length of use, although those with severe/profound ID level were using PPI for more than a year at a rate double that for those with mild ID (60.8% vs. 35%, respectively). There were significant differences between age/ID level (but not gender) and PPI doses. Subjects aged over 60 years were using doses beyond the maximum at a higher rate than younger age groups (10.5% vs. 2% and 0%, p = 0.003) and among those with severe/profound ID level (7%) as compared with moderate ID level (2.7%) (p = 0.014).
3.5. Factors associated with PPI use
Results from the binary logistic regression (Table 5) show that neither gender nor living in a residential institution were significantly associated with PPI use after adjusting for confounders. The strongest predictor of PPI use was reporting any one of the licensed indications (GORD, stomach ulcer, NSAID use; OR = 7.18 CI 4.55–11.33; p < 0.001) while receiving an antiplatelet (OR = 2.55 CI 1.47–4.40; p = 0.001), being aged 50–64 years (OR = 2.04 95%CI 1.22–3.39; p = 0.006), being aged ≥65 years (OR = 2.59 95%CI 1.43–4.71; p = 0.002) or having with severe to profound ID (OR = 2.86 95%CI 1.57–5.20; p = 0.001) was so significant.
Table 5.
Factors associated with PPI use among older people with ID, Logistic regression (n = 623).
| Characteristics | OR (95% CI) | p-value |
|---|---|---|
| Gender | ||
| Male | 1.00 | |
| Female | 0.70 (0.47–1.04) | 0.08 |
| Age | ||
| 44–49 years | 1.00 | |
| 50–64 years | 2.04 (1.22–3.39) | 0.006 |
| 65 + years | 2.59 (1.43–4.71) | 0.002 |
| Level of ID | ||
| Mild | 1.00 | |
| Moderate | 1.69 (0.97–2.95) | 0.06 |
| Severe/profound | 2.86 (1.57–5.20) | 0.001 |
| Place of residence | ||
| Independent/Family/Community groups | 1.00 | |
| Residential care settings | 1.41(0.93–2.14) | 0.09 |
| Any PPI indication (NSAIDs use, GORD, Stomach Ulcer) | 7.18 (4.55–11.33) | <0.001 |
| Antiplatelet use | 2.55 (1.47–4.40) | 0.001 |
Reference categories = no PPI use, male, 44–49 years, mild ID level, Independent/Family/Community groups, no any PPI indication, no antiplatelet use. Cox & Snell R Square = .188, Nagelkerke R Square = .27. All significant factors in bold.
4. Discussion
4.1. Statement of principal results
This study examined use of PPIs among a representative sample of older Irish people with ID. The use of PPIs was reported by just over a quarter (27.9%) of the participants, however less than six in ten of those exposed to PPIs reported any indication for PPI use (GORD, stomach ulcer, NSAID use or antiplatelet use). Over half of those exposed to PPIs were aged between 50 and 64 years of age, lived in a residential care home (54.3%). Comparing the prevalence with what has been reported from Wave 1 of this cohort (21.7%) (O'Dwyer et al., 2016), our study shows that there is 6.2% increase in PPI use in 3 years. Two-thirds of PPIs were used at maximum doses and of those who had a documented date of prescribing, just over half recorded use for more than a year. PPI users with an indication did not differ from those without an indication. After adjusting for confounders, factors that were strongly associated with PPI use were older age (≥50 yr.), severe/profound level of intellectual disability, reporting of any of the three authorized PPI indications and antiplatelet use, while place of residence and gender were not significant.
4.2. Comparison with other studies
To our knowledge there are no previous studies of PPI use in older people with ID so direct comparison was not possible. Studies of the issue in the general Irish older population (Moriarty et al., 2015a, O’Sullivan et al., 2013) have reported similar of higher rates of inappropriate PPI use as have international studies (Burdsall et al., 2013, de Souto Barreto et al., 2013, Jarchow-MacDonald and Mangoni, 2013, McDonald et al., 2015a, Rane et al., 2016) but these populations and study characteristics differ substantially from our cohort so that comparisons have limited validity.
Unlike reports for the general older population (Haenisch et al., 2015, Nishtala and Soo, 2015, Rane et al., 2016), there were no differences between males and females in PPI use. Among those who lived in residential care, 37% received a PPI and the proportion of people with severe-profound level of ID taking a PPI was significantly higher than non-users. Furthermore, PPI users with these two characteristics tend to use a PPI for longer duration. Although it might be expected that those with severe and profound ID would live in a residential setting, in Ireland, people with moderate and even mild ID might also live there and so, despite initial indications, after adjusting for covariates, our binary regression did not find that residential care was a predictor for PPI use.
Omeprazole was the most frequently reported agent among those who lived in residential care and although effective it has more potential drug interactions than the other PPIs (Wedemeyer and Blume, 2014). PPI use was also strongly associated with polypharmacy and excessive polypharmacy. In addition, living in residential care facilities was associated with polypharmacy and excessive polypharmacy (O'Dwyer et al., 2016) and this may expose individuals to potentially serious drug-drug interactions (Peklar et al., 2017, Teramura-Grönblad et al., 2016).
4.3. Doses and reported indications of PPIs
Our study illustrates that two-thirds of the reported PPI doses would be considered as maximum in older people and only 4.3% reported doses that exceeded the maximum for older people (esomeprazole 80 mg/day, lansoprazole 60 mg/day, pantoprazole 80 mg/day) (HPRA, 2014). Nevertheless, these high doses predispose this small group of vulnerable people to higher risks and more side effects over time (Masclee et al., 2014).
In this study PPI use was associated with one of the three licensed indications in 44% of participants, and even with the addition of the pragmatic indication of antiplatelet use, for almost half of those reporting PPI use, there was no recorded indication. The indications in this study are self- reported by participants, or by a proxy in some situations. Therefore, it is possible that some of the subjects/proxies were unaware of why the medicine was being taken and/or the related medical reason. Nevertheless, a previous study of people with ID, found poor documentation of indications for drugs to treat stomach ulcer and GORD (van der Heide et al., 2009). The absence of this information and/or the lack of awareness, suggests that the care processes for GI disorders are of poor quality and that evaluating PPI use is not a high priority. This may account for the lack of differences between PPI users with and without a recorded indication.
In contrast to those PPI users without a recorded indication, there were 5% (n = 34) of participants receiving NSAIDs who were not receiving a PPI (nor any other agent for gastro-protection), this is classed as a potential prescribing omission according to Assessing Care of Vulnerable Elders (ACOVE) indicators (Wenger et al., 2001). Furthermore, among the cohort, 6.8% (n = 46) of participants were taking antiplatelets without also taking a PPI. Individuals who are on antiplatelet are at higher risk for GI bleeding (McQuaid and Laine, 2006) and old age renders them more vulnerable (Kim, 2012). However, further assessment is required to determine the need for a PPI, the balance of benefit and risk associated with use of both groups of drugs, and the potential drug-drug and drug-supplement interactions since almost half of PPI users are exposed to excessive polypharmacy (Abraham et al., 2010, Peklar et al., 2017, Scott et al., 2014).
4.4. Long term use and associated risks
This study highlights that the use of PPIs for over one year among old people with ID is common (52.8%). It was not possible in our cohort to estimate what proportion of the remaining 62 participants who had taken a PPI for less than one year had nevertheless been taking them for longer than the recommended 8–12 week period. An Irish retrospective population-based cohort study used the pharmacy claims database and reported that older people were the majority of those who used a PPI for >3 month (Cahir et al., 2012). Additionally, a repeated cross sectional study, used the same data source and showed that long term PPI use (>8 weeks) at high dose increased from 0.8% in 1997 to 23.8% in 2012 (Moriarty et al., 2015b).
The long term or inappropriate use of PPI has raised concerns regarding their side effects. A number of studies of the general older population have reported a link between long term PPI use and risks of osteoporosis or fractures (Cai et al., 2015, Metz, 2008, Sugiyama et al., 2016, Zhou et al., 2016). A meta-analysis of general population studies (Yu et al., 2011) and a specific examination of people ageing with ID (Burke et al., 2016) concur that there is a significant relationship between PPI use and the risk of fracture. Other risks from PPI use must also be considered including Clostridium difficile infection (Leonard et al., 2007, McDonald et al., 2015b), community-acquired pneumonia (Lambert et al., 2015) vitamin and mineral deficiency - especially vitamin B12 and magnesium (Heidelbaugh, 2013) and dementia (Haenisch et al., 2015).
4.5. Clinical relevance of study findings
PPI use should be periodically evaluated every three months for their continued need among older people with ID, especially those with severe or profound ID (Sullivan et al., 2011). Assessment of need in older people is based on the recording of symptoms and invasive investigations. However, people with ID may not be able to communicate their symptoms or to tolerate investigations especially those with a severe or profound level of ID (Böhmer et al., 2002, De Veer et al., 2008). Trained and experienced health care professionals are required to assess and monitor people with ID effectively and consistently, particularly as they age (Macchini et al., 2011, Wallace et al., 2004a). For the safe use of a PPI the lowest effective dose, for the shortest possible duration is recommended. Continued PPI use is indicated for severe esophagitis, chronic use of NSAID or documented evidence of bleeding ulcers (Farrell et al., 2017), but nevertheless periodic re-evaluation is needed to titrate to the lowest effective PPI dose (Freedberg et al., 2017). Alternatives to long term use include intermittent use and de-prescribing strategies like step-down dosing or symptom-driven (i.e. on demand) dosing in the general elderly population (Farrell et al., 2017, Iwakiri et al., 2016, Kapadia, 2015, Pace et al., 2007) should be investigated in people with ID to reduce their long-term exposure to PPIs. Comprehensive guidelines for treatment of acid-related health conditions need to be created specifically for people with ID to address their needs. These guidelines should not only include treatment approaches but also the criteria for diagnosis and review to ensure that PPI prescribing occurs once the long term of use and associated risks of these medicines has been assessed.
4.6. Strengths and limitations
This study has a number of key strengths: First, to the best of our knowledge, it is the first investigation of the prevalence, pattern, dose regimen, and predictors associated with PPI use among older people with ID. The study used a rigorously drawn representative sample, which means the results may be generalized to the ID population in Ireland. The large sample size also provided power to the statistical analysis. Next, the accuracy of the subject’s medical information was strengthened by cross-checking, and accuracy in the medicine use information was improved using two pharmacists to independently inspect and code the information. Finally, comprehensive information was available in relation to medicine doses, frequency, length of exposure and a wide range of covariates, facilitating both a thorough dosage regimen analysis and the investigation of PPI use-associated factors.
Among the study limitations, there were instances of missing PPI duration of use data and information about self-reported/or diagnosis of H-pylori infection/or esophagitis was not captured, meaning it was not possible to verify if some PPI use was to manage this health issue. Similarly, no measures of PPI side effects were available.
5. Conclusion
PPI use among older people with ID is prevalent and frequently at a maximum dose, over a long term, and often without a clear indication. The pattern of long term PPI use without an indication, of no distinguishing features between those with and without an indication and the substantial group of participants taking an NSAID or an antiplatelet and so potentially needing, but not receiving a PPI, suggests that the regular review of GI disorders and their management is not routine, or is inadequate. This is a major concern as people with ID are particularly susceptible to GI disorders that may be chronic and in many cases progressive (Böhmer et al., 2002, Macchini et al., 2011) Therefore, under-diagnosis and under-treatment, as well as unnecessary treatment have a significant potential to harm and to reduce the quality of life of this vulnerable and dependent population. The quality of care that people with ID who have GI disorders needs to be assessed and measures taken to review the care needs of the most vulnerable and of those exposed to the greatest risk of harm. Building upon these findings, tailored de-prescribing approaches for people with ID should be developed and evaluated.
Acknowledgments
The authors would like to thank the people with ID who participated in this study, their families, the services provider, and the IDS-TILDA team for their support.
They would also like to acknowledge funding for the IDS-TILDA study from the Health Research Board (HRB) and the Department of Health. The Authors would like to thank Anne Belton (A.B.) for her assistance in data coding and checking.
Funding
The IDS-TILDA study is funded by the Department of Health and managed by the Health Research Board. The lead author (H.ALM.) received funding for the PhD from the Saudi Ministry of Education, King Abdullah Scholarship. The funding bodies have no influence in the study design or in writing of the manuscript.
Conflict of interest
None declared.
Ethics approval
The IDS-TILDA study was ethically approved by the Faculty of Health Sciences Ethics Committee, and 138 Intellectual Disability Service Providers.
Footnotes
Peer review under responsibility of King Saud University.
References
- AAIDD. 2017. American Association of Intellectual and Developmental Disabilities [Online]. Available: <https://aaidd.org/intellectual-disability/definition#.WbkkSdOGPL8> (Accessed 12/9/2017).
- Abraham N.S., Hlatky M.A., Antman E.M., Bhatt D.L., Bjorkman D.J., Clark C.B., Furberg C.D., Johnson D.A., Kahi C.J., Laine L. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J. Am. Coll. Cardiol. 2010;56:2051–2066. doi: 10.1016/j.jacc.2010.09.010. [DOI] [PubMed] [Google Scholar]
- AGS American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2015;63:2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- APA 2013. Diagnostic and statistical manual of mental disorders (DSM-5®), American Psychiatric Associatin [DOI] [PubMed]
- BNF 2015. British National Formulary, British Medical Association and Royal Pharmaceutical Society.
- Böhmer C.J., Niezen-de Boer M.C., Klinkenberg-Knol E.C., Nadorp J.H., Meuwissen S.G. Gastro-oesophageal reflux disease in institutionalised intellectually disabled individuals. Neth. J. Med. 1997;51:134–139. doi: 10.1016/s0300-2977(97)00055-7. [DOI] [PubMed] [Google Scholar]
- Böhmer C.J.M., Klinkenberg-Knol E.C., Niezen-De Boer M.C. Prevalence, diagnosis and treatment of gastro-oesophageal reflux disease in institutionalised persons with an intellectual disability. J. Intellect. Develop. Disabil. 2002;27:92–105. [Google Scholar]
- Burdsall D.P., Flores H.C., Krueger J., Garretson S., Gorbien M.J., Iacch A., Dobbs V., Homa T. Use of proton pump inhibitors with lack of diagnostic indications in 22 Midwestern US skilled nursing facilities. J. Am. Med. Direct. Assoc. 2013;14:429–432. doi: 10.1016/j.jamda.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Burke, E., Carroll, R., McCallion, P., Walsh, J., McCarron, M., 2016. An investigation of the contributing factors that increase the risk of osteoporosis and osteopenia among adults with an intellectual disability. Age and ageing. Oxford Univ Press Great Clarendon St, Oxford Ox2 6dp, England, 6-6.
- Cahir C., Fahey T., Teeling M., Teljeur C., Feely J., Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Brit. J. Clin. Pharmacol. 2010;69:543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir C., Fahey T., Tilson L., Teljeur C., Bennett K. Proton pump inhibitors: potential cost reductions by applying prescribing guidelines. BMC Health Serv. Res. 2012;12:408. doi: 10.1186/1472-6963-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., Feng W., Jiang Q. Acid-suppressive medications and risk of fracture: an updated meta-analysis. Int. J. Clin. Exp. Med. 2015;8:8893–8904. [PMC free article] [PubMed] [Google Scholar]
- Chait M.M. Gastroesophageal reflux disease: important considerations for the older patients. World J. Gastrointest. Endosc. 2010;2:388–396. doi: 10.4253/wjge.v2.i12.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlot L., Abend S., Ravin P., Mastis K., Hunt A., Deutsch C. Non-psychiatric health problems among psychiatric inpatients with intellectual disabilities. J. Intellect. Disabil. Res. 2011;55:199–209. doi: 10.1111/j.1365-2788.2010.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppus A.M.W. People with intellectual disability: what do we know about adulthood and life expectancy? Develop. Disabil. Res. Rev. 2013;18:6–16. doi: 10.1002/ddrr.1123. [DOI] [PubMed] [Google Scholar]
- Corsonello A., Maggio M., Fusco S., Adamo B., Amantea D., Pedone C., Garasto S., Ceda G.P., Corica F., Lattanzio F. Proton pump inhibitors and functional decline in older adults discharged from acute care hospitals. J. Am. Geriatr. Soc. 2014;62:1110–1115. doi: 10.1111/jgs.12826. [DOI] [PubMed] [Google Scholar]
- Dancey C.P. third ed. Pearson/Prentice Hall; Harlow: 2004. Statistics without maths for psychology : using SPSS for Windows. [Google Scholar]
- de Souto Barreto P., Lapeyre-Mestre M., Mathieu C., Piau C., Bouget C., Cayla F., Vellas B., Rolland Y. Prevalence and associations of the use of proton-pump inhibitors in nursing homes: a cross-sectional study. J. Am. Med. Direct. Assoc. 2013;14:265–269. doi: 10.1016/j.jamda.2012.10.018. [DOI] [PubMed] [Google Scholar]
- De Veer A.J.E., Bos J.T., Boer R.C.N.-D., Böhmer C.J.M., Francke A.L. Symptoms of gastroesophageal reflux disease in severely mentally retarded people: a systematic review. BMC Gastroenterol. 2008;8:23. doi: 10.1186/1471-230X-8-23. 23-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld S., Emerson E. fifth ed. Rutter's Child and Adolescent Psychiatry; 2009. Intellectual disability; pp. 820–840. [Google Scholar]
- Ellison J.W., Rosenfeld J.A., Shaffer L.G. Genetic basis of intellectual disability. Annu. Rev. Med. 2013;64:441–450. doi: 10.1146/annurev-med-042711-140053. [DOI] [PubMed] [Google Scholar]
- Emerson, E., Heslop, P., 2010. A working definition of learning disabilities. Durham: Improving Health & Lives: Learning Disabilities Observatory.
- Farrell B., Pottie K., Thompson W., Boghossian T., Pizzola L., Rashid F.J., Rojas-Fernandez C., Walsh K., Welch V., Moayyedi P. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can. Fam. Physic. 2017;63:354–364. [PMC free article] [PubMed] [Google Scholar]
- Franceschi M., Di Mario F., Leandro G., Maggi S., Pilotto A. Acid-related disorders in the elderly. Best Pract. Res. Clin. Gastroenterol. 2009;23:839–848. doi: 10.1016/j.bpg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Freedberg D.E., Kim L.S., Yang Y.X. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterol. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Fulton M.M., Riley Allen E. Polypharmacy in the elderly: a literature review. J. Am. Acad. Nurse Pract. 2005;17:123–132. doi: 10.1111/j.1041-2972.2005.0020.x. [DOI] [PubMed] [Google Scholar]
- Gallagher P., Ryan C., Byrne S., Kennedy J., O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int. J. Clin. Pharmacol. Ther. 2008;46 doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- George C.J., Korc B., Ross J.S. Appropriate proton pump inhibitor use among older adults: a retrospective chart review. Am. J. Geriat. Pharmacoth. 2008;6:249–254. doi: 10.1016/j.amjopharm.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Haenisch B., von Holt K., Wiese B., Prokein J., Lange C., Ernst A., Brettschneider C., König H.-H., Werle J., Weyerer S. Risk of dementia in elderly patients with the use of proton pump inhibitors. European archives of psychiatry and clinical neuroscience. 2015;265:419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- Hamilton H., Gallagher P., Ryan C., Byrne S., O’Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch. Internal Med. 2011;171:1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]
- Haveman M., Heller T., Lee L., Maaskant M., Shooshtari S., Strydom A. Major health risks in aging persons with intellectual disabilities: an overview of recent studies. J. Policy Pract. Intellect. Disabil. 2010;7:59–69. [Google Scholar]
- Heidelbaugh J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Therapeut. Adv. Drug Saf. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelbaugh J.J., Kim A.H., Chang R., Walker P.C. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap. Adv. Gastroenterol. 2012;5:219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans H., Evenhuis H.M. Multimorbidity in older adults with intellectual disabilities. Res. Dev. Disabil. 2014;35:776–783. doi: 10.1016/j.ridd.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Heslop P., Glover G. Mortality of people with intellectual disabilities in England: a comparison of data from existing sources. J. Appl. Res. Intellect. Disabil. 2015;28:414–422. doi: 10.1111/jar.12192. [DOI] [PubMed] [Google Scholar]
- HPRA. 2014. Health Products Regulatory Authority [Online]. Available: <https://www.hpra.ie/> [Accessed 13/5/2016.
- Iwakiri K., Kinoshita Y., Habu Y., Oshima T., Manabe N., Fujiwara Y., Nagahara A., Kawamura O., Iwakiri R., Ozawa S. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J. Gastroenterol. 2016;51:751–767. doi: 10.1007/s00535-016-1227-8. [DOI] [PubMed] [Google Scholar]
- Jarchow-MacDonald A.A., Mangoni A.A. Prescribing patterns of proton pump inhibitors in older hospitalized patients in a Scottish health board. Geriatr. Gerontol. Int. 2013;13:1002–1009. doi: 10.1111/ggi.12047. [DOI] [PubMed] [Google Scholar]
- Kapadia, S., 2015. Implications of Long-term Proton Pump Inhibitor Use: Promoting Step-Down Therapy for Management of Gastro-esophageal Reflux Disease in the Outpatient Setting. Family Medicine Block Clerkship, Student Projects. 58. [Online]. Available: <http://scholarworks.uvm.edu/fmclerk/58/> (Accessed 1/10/2017).
- Kim J. Management and prevention of upper GI bleeding. Gastroenterol. Nutr. 2012:7–26. [Google Scholar]
- Kutner M. McGraw-Hill Education; Boston; London: 2004. Applied Linear Regression Models. [Google Scholar]
- Lambert A.A., Lam J.O., Paik J.J., Ugarte-Gil C., Drummond M.B., Crowell T.A. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004. doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer E., McCallion P. Mortality of people with intellectual and developmental disabilities from select us state disability service systems and medical claims data. J. Appl. Res. Intellect. Disabil. 2015;28:394–405. doi: 10.1111/jar.12191. [DOI] [PubMed] [Google Scholar]
- Leonard, J., Marshall, J.K., Moayyedi, P., 2007. Systematic review of the risk of enteric infection in patients taking acid suppression. Am. J. Gastroenterol., 102, 2047-56; quiz 2057. [DOI] [PubMed]
- Macchini F., Leva E., Torricelli M., Valade A. Treating acid reflux disease in patients with Down syndrome: pharmacological and physiological approaches. Clin. Exp. Gastroenterol. 2011;4:19–22. doi: 10.2147/CEG.S15872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclee G.M., Sturkenboom M.C., Kuipers E.J. A benefit-risk assessment of the use of proton pump inhibitors in the elderly. Drugs Aging. 2014;31:263–282. doi: 10.1007/s40266-014-0166-4. [DOI] [PubMed] [Google Scholar]
- May M.E., Kennedy C.H. Health and problem behavior among people with intellectual disabilities. Behav. Anal. Pract. 2010;3:4–12. doi: 10.1007/BF03391759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron M. School of Nursing and Midwifery; Trinity College Dublin: 2011. Growing older with an intellectual disability in Ireland 2011: First results from the Intellectual Disability Supplement to the Irish Longitudinal Study on Ageing (IDS-TILDA) [Google Scholar]
- McCarron M., Carroll R., Kelly C., McCallion P. Mortality Rates in the General Irish Population compared to those with an intellectual disability from 2003 to 2012. J. Appl. Res. Intellect. Disabil. 2015;28:406–413. doi: 10.1111/jar.12194. [DOI] [PubMed] [Google Scholar]
- McCarron, M., McCallion, P., Carroll, R., Burke, E., Cleary, E., McCausland, D., McGlinchy, E., O’Donovan, M.-A., Mulryan, N., Shivers, C., 2014. Advancing years, Different challenges: wave 2 IDS-TILDA: findings on the ageing of people with an intellectual disability: an intellectual disability supplement to the Irish Longitudinal Study on Ageing.
- McCarron, M., Swinburne, J., Burke, E., M., E., Mulryan, N., . Andrews, V., Foran, S., McCallion, P., 2011. Growing Older with an Intellectual Disability in Ireland 201: First Results from The Intellectual Disability Supplement of The Irish Longitudinal Study on Ageing: An Accessible Report. Dublin: School of Nursing & Midwifery, Trinity College Dublin.
- McDonald E.G., Jones J., Green L., Jayaraman D., Lee T.C. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J. Hospital Med. 2015;10:281–286. doi: 10.1002/jhm.2330. [DOI] [PubMed] [Google Scholar]
- McDonald E.G., Milligan J., Frenette C., Lee T.C. Continuous proton pump inhibitor therapy and the associated risk of recurrent clostridium difficile infection. JAMA Intern. Med. 2015;175:784–791. doi: 10.1001/jamainternmed.2015.42. [DOI] [PubMed] [Google Scholar]
- McQuaid K.R., Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am. J. Med. 2006;119:624–638. doi: 10.1016/j.amjmed.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Metz D.C. Managing gastroesophageal reflux disease for the lifetime of the patient: evaluating the long-term options. Am. J. Med. 2004;117(Suppl 5A):49s–55s. doi: 10.1016/j.amjmed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Metz D.C. Long-term use of proton-pump inhibitor therapy. Gastroenterol. Hepatol. 2008;4:322–325. [PMC free article] [PubMed] [Google Scholar]
- Moriarty F., Bennett K., Cahir C., Fahey T. Characterizing potentially inappropriate prescribing of proton pump inhibitors in older people in primary care in Ireland from 1997 to 2012. J. Am. Geriatr. Soc. 2016;64:e291–e296. doi: 10.1111/jgs.14528. [DOI] [PubMed] [Google Scholar]
- Moriarty F., Bennett K., Fahey T., Kenny R.A., Cahir C. Longitudinal prevalence of potentially inappropriate medicines and potential prescribing omissions in a cohort of community-dwelling older people. Eur. J. Clin. Pharmacol. 2015;71:473–482. doi: 10.1007/s00228-015-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty F., Hardy C., Bennett K., Smith S.M., Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ open. 2015;5:e008656. doi: 10.1136/bmjopen-2015-008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter, J., 1996. Applied Linear Regression Models, Chicago, Ill, London, Irwin.
- Ng N., Sandberg M., Ahlström G. Prevalence of older people with intellectual disability in Sweden: a spatial epidemiological analysis. J. Intellect. Disabil. Res. 2015;59:1155–1167. doi: 10.1111/jir.12219. [DOI] [PubMed] [Google Scholar]
- Nishtala P., Soo L. Proton pump inhibitors utilisation in older people in New Zealand from 2005 to 2013. Internal Med. J. 2015;45:624–629. doi: 10.1111/imj.12757. [DOI] [PubMed] [Google Scholar]
- O'Dwyer M., Peklar J., McCallion P., McCarron M., Henman M.C. Factors associated with polypharmacy and excessive polypharmacy in older people with intellectual disability differ from the general population: a cross-sectional observational nationwide study. BMJ Open. 2016;6:e010505. doi: 10.1136/bmjopen-2015-010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D.P., O’Mahony D., Parsons C., Hughes C., Murphy K., Patterson S., Byrne S. A prevalence study of potentially inappropriate prescribing in Irish long-term care residents. Drugs Aging. 2013;30:39–49. doi: 10.1007/s40266-012-0039-7. [DOI] [PubMed] [Google Scholar]
- Pace F., Tonini M., Pallotta S., Molteni P., Porro G. Systematic review: maintenance treatment of gastro-oesophageal reflux disease with proton pump inhibitors taken ‘on-demand’. Aliment. Pharmacol. Therapeut. 2007;26:195–204. doi: 10.1111/j.1365-2036.2007.03381.x. [DOI] [PubMed] [Google Scholar]
- Parsons C., Johnston S., Mathie E., Baron N., Machen I., Amador S., Goodman C. Potentially inappropriate prescribing in older people with dementia in care homes. Drugs Aging. 2012;29:143–155. doi: 10.2165/11598560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pasina L., Nobili A., Tettamanti M., Salerno F., Corrao S., Marengoni A., Iorio A., Marcucci M., Mannucci P., Investigators R. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. Eur. J. Internal Med. 2011;22:205–210. doi: 10.1016/j.ejim.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Patja K., Iivanainen M., Vesala H., Oksanen H., Ruoppila I. Life expectancy of people with intellectual disability: a 35-year follow-up study. J. Intellect. Disabil. Res. 2000;44(Pt 5):591–599. doi: 10.1046/j.1365-2788.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Peklar, J., Kos, M., O’Dwyer, M., McCarron, M., McCallion, P., Kenny, R.A., Henman, M.C., 2017. Medication and supplement use in older people with and without intellectual disability: an observational, cross-sectional study. PloS One, 12. [DOI] [PMC free article] [PubMed]
- Pilotto A., Franceschi M., Leandro G., Scarcelli C., D'Ambrosio L.P., Seripa D., Perri F., Niro V., Paris F., Andriulli A., Di Mario F. Clinical features of reflux esophagitis in older people: a study of 840 consecutive patients. J. Am. Geriatr. Soc. 2006;54:1537–1542. doi: 10.1111/j.1532-5415.2006.00899.x. [DOI] [PubMed] [Google Scholar]
- Rane P.P., Guha S., Chatterjee S., Aparasu R.R. Prevalence and predictors of non-evidence based proton pump inhibitor use among elderly nursing home residents in the US. Res. Soc. Administ. Pharm. 2016 doi: 10.1016/j.sapharm.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Richardson, K., M. P., Peklar, J., Galvin, R., Bennett, K., Kenny, R.A., 2012. Polypharmacy in adults over 50 in Ireland: Opportunities for cost savings and improved healthcare. The Irish Longitudinal Study on Ageing TILDA.
- Ryan C., O'Mahony D., Kennedy J., Weedle P., Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Brit. J. Clin. Pharmacol. 2009;68:936–947. doi: 10.1111/j.1365-2125.2009.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Ahlstrom G., Axmon A., Kristensson J. Somatic healthcare utilisation patterns among older people with intellectual disability: an 11-year register study. BMC Health Serv. Res. 2016;16:642. doi: 10.1186/s12913-016-1880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalock R.L., Luckasson R.A., Shogren K.A. The renaming of mental retardation: understanding the change to the term intellectual disability. Intellect. Develop. Disabil. 2007;45:116–124. doi: 10.1352/1934-9556(2007)45[116:TROMRU]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Scott S.A., Owusu Obeng A., Hulot J.S. Antiplatelet drug interactions with proton pump inhibitors. Exp. Opin. Drug Metab. Toxicol. 2014;10:175–189. doi: 10.1517/17425255.2014.856883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straetmans, J.M., van Schrojenstein Lantman-de Valk, H.M., Schellevis, F.G., Dinant, G.J., 2007. Health problems of people with intellectual disabilities: the impact for general practice. Br. J. Gen. Pract., vol. 57, pp. 64–66. [PMC free article] [PubMed]
- Sugiyama T., Torio T., Miyajima T., Kim Y., Oda H. Calcium, proton pump inhibitors, and fracture risk. Osteoporos. Int. 2016;27:349–350. doi: 10.1007/s00198-015-3403-8. [DOI] [PubMed] [Google Scholar]
- Sullivan W.F., Berg J.M., Bradley E., Cheetham T., Denton R., Heng J., Hennen B., Joyce D., Kelly M., Korossy M. Primary care of adults with developmental disabilities: Canadian consensus guidelines. Can. Fam. Physic. 2011;57:541–553. [PMC free article] [PubMed] [Google Scholar]
- Teramura-Grönblad M., Raivio M., Savikko N., Muurinen S., Soini H., Suominen M., Pitkälä K. Potentially severe drug–drug interactions among older people and associations in assisted living facilities in Finland: a cross-sectional study. Scand. J. Prim. Health Care. 2016;34:250–257. doi: 10.1080/02813432.2016.1207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide D.C., van der Putten A.A.J., van den Berg P.B., Taxis K., Vlaskamp C. The documentation of health problems in relation to prescribed medication in people with profound intellectual and multiple disabilities. J. Intellect. Disabil. Res. 2009;53:161–168. doi: 10.1111/j.1365-2788.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- van Schrojenstein Lantman-De Valk, H.M., Metsemakers, J.F., Haveman, M.J., Crebolder, H.F., 2000. Health problems in people with intellectual disability in general practice: a comparative study. Fam. Pract., vol. 17, 405–407. [DOI] [PubMed]
- van Timmeren, E.A., van der Putten, A.A., van Schrojenstein Lantman-de Valk, H.M., van der Schans, C.P., Waninge, A., 2016. Prevalence of reported physical health problems in people with severe or profound intellectual and motor disabilities: a cross-sectional study of medical records and care plans. J. Intellect. Disabil. Res, vol. 60, pp. 1109–1118. [DOI] [PubMed]
- Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., Poole C., Schlesselman J.J., Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int. J. Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Wallace R.A., Schluter P.J., Duff M., Ouellette-Kuntz H., Webb P.M., Scheepers M. A review of the risk factors for, consequences, diagnosis, and management of helicobacter pylori in adults with intellectual disabilities. J. Policy Pract. Intellect. Disabil. 2004;1:147–163. [Google Scholar]
- Wallace R.A., Schluter P.J., Webb P.M. Recurrence of Helicobacter pylori infection in adults with intellectual disability. Internal Med. J. 2004;34:132–133. doi: 10.1111/j.1444-0903.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- Wedemeyer R.-S., Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37:201–211. doi: 10.1007/s40264-014-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger N.S., Shekelle P.G., Davidoff F., Mulrow C. ACOVE quality indicators. Ann. Intern. Med. 2001;135:653–667. doi: 10.7326/0003-4819-135-8_part_2-200110161-00004. [DOI] [PubMed] [Google Scholar]
- WHO, 2016. WHO Centre for Drug Statistics Methodology [Online]. Available: http://www.whocc.no/atc_ddd_index/ (Accessed 18/3/2016 2016).
- Yadlapati R., Kahrilas P.J. When is proton pump inhibitor use appropriate? BMC Med. 2017;15:36. doi: 10.1186/s12916-017-0804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am. J. Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Huang Y., Li H., Sun W., Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos. Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]