Abstract
Over the last decades, a growing spectrum of monogenic disorders of human magnesium homeostasis has been clinically characterized, and genetic studies in affected individuals have identified important molecular components of cellular and epithelial magnesium transport. Here, we describe three infants who are from non-consanguineous families and who presented with a disease phenotype consisting of generalized seizures in infancy, severe hypomagnesemia, and renal magnesium wasting. Seizures persisted despite magnesium supplementation and were associated with significant intellectual disability. Whole-exome sequencing and conventional Sanger sequencing identified heterozygous de novo mutations in the catalytic Na+, K+-ATPase α1 subunit (ATP1A1). Functional characterization of mutant Na+, K+-ATPase α1 subunits in heterologous expression systems revealed not only a loss of Na+, K+-ATPase function but also abnormal cation permeabilities, which led to membrane depolarization and possibly aggravated the effect of the loss of physiological pump activity. These findings underline the indispensable role of the α1 isoform of the Na+, K+-ATPase for renal-tubular magnesium handling and cellular ion homeostasis, as well as maintenance of physiologic neuronal activity.
Keywords: ATP1A1, Na-K ATPase, α1 subunit, hypomagnesemia, seizures, intellectual disability
Main Text
Magnesium is essential for numerous cellular processes, including energy metabolism, protein and nucleic acid synthesis, and the maintenance of the electrical potential of nervous tissues and cell membranes. Genetic investigations in children with inherited forms of hypomagnesemia could identify critical components of epithelial magnesium transport at the molecular level.1 Affected children commonly present with seizures, muscle spasms, or tetany. In the majority of cases, magnesium supplementation leads to relief of clinical symptoms and allows for a normal motor and cognitive development despite persistence of subnormal serum magnesium levels. In contrast with this favorable clinical course, we noted a small group of nine children who, despite appropriate magnesium supplementation, experienced prolonged and repeated seizure activity associated with severe intellectual disability. Of this cohort, two individuals had previously been diagnosed with hypomagnesemia, seizures, and mental retardation [HOMGSMR, MIM: 616418] due to bi-allelic mutations in CNNM2 [MIM: 607803].2 Here, we identified heterozygous de novo mutations in ATP1A1 [MIM: 182310] (RefSeqGene: NG_047036, GenBank: NM_000701), encoding the α1 isoform of Na+, K+-ATPase, in three children from this cohort. Data on clinical symptoms and biochemical measures at the time of disease manifestation were collected retrospectively from medical charts. Affected children with ATP1A1 mutations were clinically reevaluated during follow-up, and biochemical data were obtained.
The three infants initially presented between 6 days and 6 months of age with generalized convulsions (Table 1, for the full dataset please refer to Table S1 in the Supplemental Data). At the time of manifestation, severe hypomagnesemia (0.30–0.36 mmol/L) was noted. Calculation of urinary fractional excretion rates of magnesium indicated massive renal magnesium wasting. Although urinary calcium excretion was not uniformly elevated, initial renal ultrasound examinations indicated medullary hyperechogenicity compatible with incipient nephrocalcinosis in individuals B-II-1 and C-II-2. All children were treated with antiepileptic drugs and received intravenous magnesium followed by ongoing oral supplementation. However, seizure activity persisted with frequent generalized seizures and repeated status epilepticus despite amelioration of serum magnesium levels. After a status epilepticus with both tonic-clonic seizure activity and hypoxemia for more than one hour, the cerebral magnetic resonance imaging (MRI) of individual A-II-1 showed bilateral parietooccipital cortical and subcortical diffusion restriction compatible with hypoxic ischemic encephalopathy. This resulted in a marked developmental setback and impaired vision. Follow-up MRI showed cerebral volume loss. All three children uniformly had significant global developmental delay, displayed limited motor skills, and spoke only in single words. Two individuals (B-II-1 and C-II-2) showed clinical features compatible with an autism spectrum disorder. MRI examinations revealed cerebral volume loss in individual C-II-2 also. Blood-pressure measurements repeatedly demonstrated normotension in all children, and cardiac examinations performed in individuals A-II-1 and C-II-2 were unremarkable. Individuals B-II-1 and C-II-2 exhibited significant polyuria of 4–8 ml/kg/h, but renal concentrating ability remained at least partially intact (random urine osmolalities of >400 mosmol/L). Although laboratory analyses did not reveal renal salt wasting or a significant activation of the renin-aldosterone system and serum potassium levels were mostly within the reference range, all affected individuals exhibited repeated episodes with significant hypokalemia (S-K+ of 2.1 to 2.6 mmol/L). Calculation of the transtubular potassium gradient during hypokalemic episodes indicated renal potassium wasting. The most recent laboratory examinations in individuals A-II-1 and B-II-1 demonstrated persisting hypomagnesemia despite high doses of oral magnesium supplementation, and fractional urinary excretion rates of magnesium confirmed major renal magnesium wasting. Parents of all three children were clinically unaffected and showed normal serum magnesium levels.
Table 1.
Overview of Clinical Characteristics and Genotypes
|
Individual |
|||
|---|---|---|---|
| A-II-1 | B-II-1 | C-II-2 | |
| Demographics | |||
| Origin | of European descent | of European descent | First Nations Canadian |
| Gender | female | female | male |
| Age at manifestation | 6 months | 2 months | 6 days |
| First symptom | generalized seizures | generalized seizures | generalized seizures |
| Initial Laboratory Findings | |||
| S-Mg (mmol/L) (0.75–1.1) | 0.36 | 0.35 | 0.30 |
| FE-Mg (%) (3-5%) | 26.0 | 33.8 | nd |
| Most Recent Findings | |||
| Age at last follow-up | 4 years | 10 years | 6 years |
| S-Mg (mmol/L) (0.75.-1.1) | 0.57 | 0.28 | 0.62 |
| FE-Mg (%) (3-5%) | 15.3 | 27.0 | 21.3 |
| seizure activity | repeated status epilepticus | monthly seizures | frequent seizures, repeated status epilepticus |
| neurological outcome | global developmental delay, hyperactive behavior | global developmental delay, suspected autism spectrum disorder | global developmental delay, speech delay, diagnosis of severe autism, self-biting behavior |
| ATP1A1 Mutations | |||
| nucleotide level | c.905T>C | c.907G>C | c.2576T>G |
| protein level | p.Leu302Arg | p.Gly303Arg | p.Met859Arg |
Extraction of DNA from whole blood was performed according to standard protocols. All genetic studies were approved by the respective ethics committees of the involved centers. The parents provided written informed consent. The clinical phenotype initially suggested the diagnosis of hypomagnesemia with secondary hypocalcemia (HSH) [HOMG1, MIM: 602014]; however, mutations in TRPM6 [MIM: 607009] were excluded.3, 4 Under the assumption of an unknown disease phenotype, we performed whole-exome sequencing in individual A-II-1 as well as the family C trio in order to identify the underlying genetic defect. Details on target enrichment, sequencing, and data analysis are provided in the Supplemental Data. We focused on missense, nonsense, splice-site, and frameshift variants upon all modes of inheritance. After performing sequential filtering and keeping variants predicted as pathogenic, we could identify no common gene with homozygous or compound-heterozygous variants in the two affected individuals. In contrast, both were found to carry a single heterozygous mutation, c.905T>G (p.Leu302Arg) and c. 2576T>G (p.Met859Arg) in ATP1A1, respectively. In silico analyses predicted the variants to be pathogenic; they were predicted to affect highly conserved amino acid residues of the Na+, K+-ATPase α1 protein (Figure 1, Table S2). None of the identified mutations is listed in publicly available exome databases, i.e., ExAC and gnomAD browsers. Identified mutations were confirmed by Sanger sequencing (details of primers are available on request); the trio analysis of family C exomes as well as sequencing of the parents of individual A-II-1 demonstrated that both mutations occurred de novo. We could not identify any additional gene with heterozygous variants shared by both affected individuals (A-II-1 and C-II-2). Therefore, we do not have any genetic evidence that the phenotype observed here results from additive effects of variants in a gene other than ATP1A1. Subsequently, ATP1A1 screening by conventional Sanger sequencing revealed that a third variant, c.907G>C (p.Gly303Arg), existed in a heterozygous state in individual B-II-1 but was not found in either parent and also occurred de novo. In families A and B, paternity was confirmed by analysis of seven independent polymorphic microsatellite markers. Paternity in family C was confirmed by segregation analysis of rare variants identified in the trio exome.
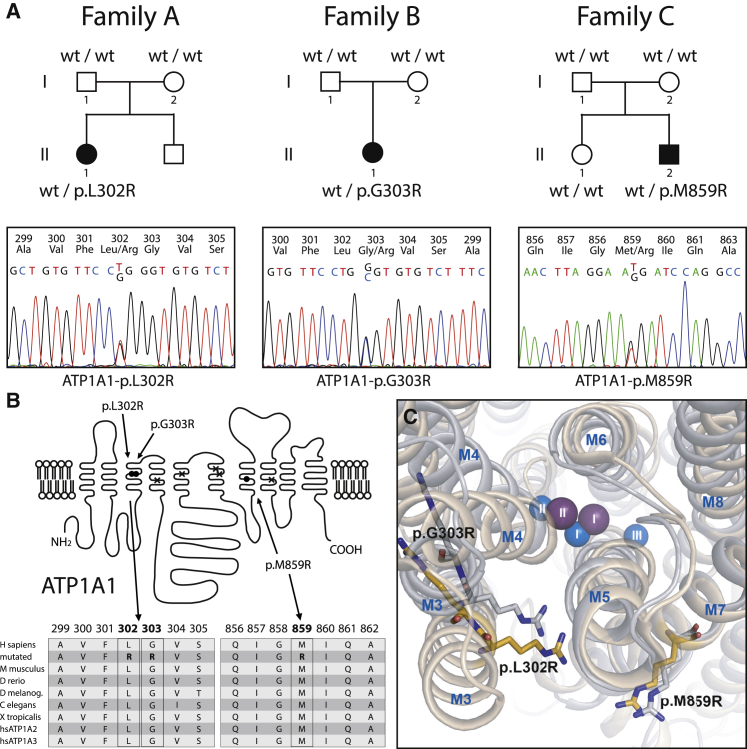
Figure 1.
Family Pedigrees, Electropherograms of Identified ATP1A1 Mutations, and Localization of Mutations Within the ATP1A1 Protein (α1 Subunit of Na+, K+-ATPase) with Multiple Sequence Alignment
(A) Heterozygous ATP1A1 mutations p.Leu302Arg (p.L302R), p.Gly303Arg (p.G303R), and p.Met859Arg (p.M859R) identified in the three individuals (A-II-1, B-II-1, and C-II-2) were not present in unaffected parents but occurred de novo.
(B) Whereas adjacent amino acid residues Leu302 and Gly303 are located within the third transmembrane domain, M859 lies within the seventh transmembrane helix of the encoded α1 subunit of Na+, K+-ATPase (filled circles). Crosses indicate ion-binding carboxylate residues (Glu334 in M4; Glu786 in M5; Asp811 and Asp815 in M6; and Asp933 in M8). A multiple-sequence alignment (RefSeq NP_000692, UniProt P05023) of ATP1A1 amino acid residues surrounding mutated positions p.302, p.303, and p.859, respectively (bold), is shown. All three positions are highly conserved between species and between α subunit homologs ATP1A1, ATP1A2, and ATP1A3.
(C) Structural location of the arginine residues in mutants p.Leu302Arg, p.Gly303Arg, and p.Met859Arg. The arginines were inserted into the atomic models derived from crystal structures with Na+ or K+ bound. The central transmembrane domain of the α1 subunit, consisting of helices M3 to M8 with bound ions (Na+ blue, K+ purple, numbering according to conventional nomenclature) is shown as seen from the extracellular surface. The bulky arginines seem to be able to disturb the ion-binding sites I and II. The p.Gly303Arg arginine is predicted to collide with M4, whereas the p.Leu302Arg arginine most likely collides with M5, particularly in the K+-bound form. Finally, the p.Met859Arg arginine might interact with an M7 glycine essential to the M7 kink that makes room for the binding of the K+ ions.
For biochemical studies, mutations were introduced into full-length cDNA encoding the ouabain-insensitive rat α1 isoform of Na+, K+-ATPase and expressed in COS-1 cells. Ouabain selection was used for obtaining stable viable cell lines.5 However, although COS cells transfected with wild-type rat Atp1a1 grew normally, several attempts to keep COS cells growing after transfection with mutant rat Atp1a1 cDNA and under ouabain selection failed; this indicates that, in contrast to the wild-type enzyme, none of the three mutants (p.Leu302Arg, p.Gly303Arg, or p.Met859Arg) was able to carry out the Na+ and K+ transport required to support cell growth.
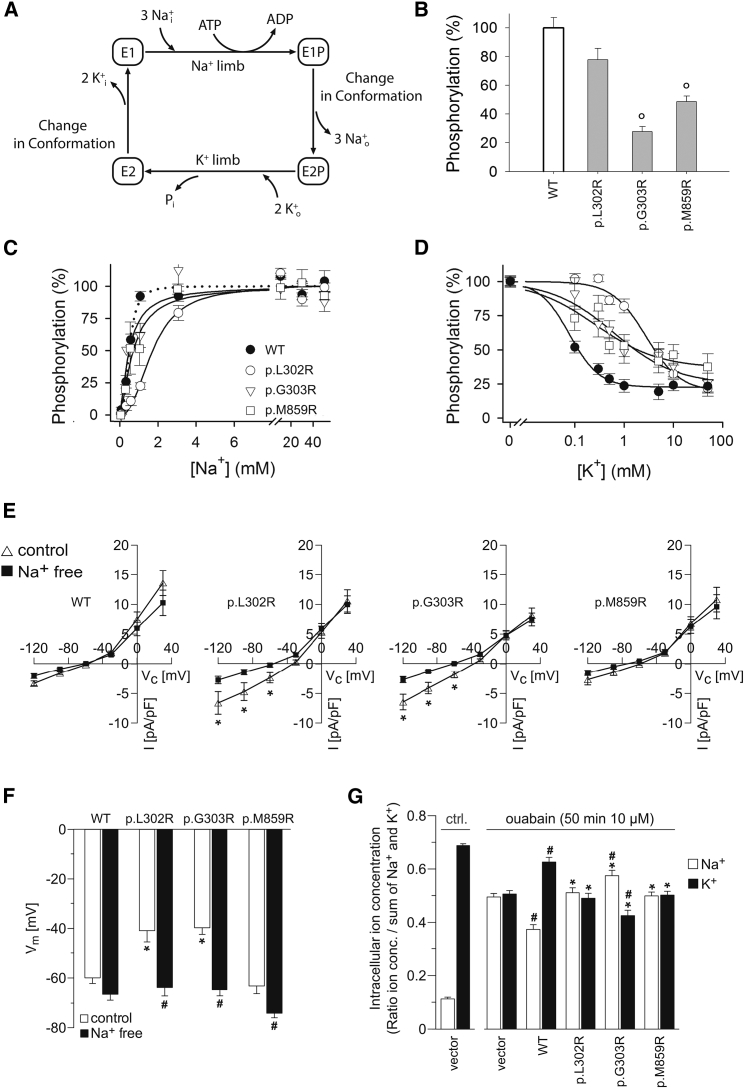
Therefore, transient expression was performed in the presence of siRNA to knock down endogenous Na+, K+-ATPase.6 Leaky plasma membranes were assayed functionally by previously described methods7 (details are provided in the Supplemental Data). Na+, K+-ATPase pump function follows the Post-Albers reaction cycle (Figure 2A), starting with phosphorylation after binding of intracellular Na+, which facilitates “pumping” of Na+ to the outside, before binding of K+, which, after dephosphorylation of the enzyme, is “pumped” into the cell. Measurements of phosphorylation under maximal conditions where the Na+ sites were saturated and dephosphorylation was blocked with oligomycin demonstrated that all three mutants became phosphorylated, indicating that they were expressed, although at a significantly reduced level for p.Gly303Arg and p.Met859Arg, and that they were able to perform the Na+ limb of the Post-Albers cycle (Figure 2B). A significantly reduced Na+ affinity was, however, seen for the p.Leu302Arg mutant. The other two mutants showed reduced cooperativity of Na+ binding, as deduced from Hill coefficients (Figure 2C and Figure S1). The K+ affinity was also significantly reduced for p.Leu302Arg, whereas the other two mutants showed less effect on K+ affinity but reduced cooperativity of K+ binding (Figure 2D). Determination of the E1P/E2P distribution by taking advantage of the ADP sensitivity of the phosphoenzyme showed no reduction of the level of E2P (Figure S2), indicating that the defective K+ binding is a direct effect on K+ interaction with the E2P state rather than a defect in the critical conformational change from E1P to E2P of Na+, K+-ATPase.
Figure 2.
Functional Characterization of Mutant ATP1A1 in Transfected Cells
Mutations corresponding to human ATP1A1 p.Leu302Arg (p.L302R), p.Gly303Arg (p.G303R), and p.Met859Arg (p.M859R) were introduced in rat α1 Na+, K+-ATPase (rat Atp1a1), which is insensitive to the inhibitor ouabain.
(A) Post-Albers scheme of the Na+, K+-ATPase reaction cycle.
(B) Transient expression of mutant enzymes with concomitant siRNA-mediated knockdown of endogenous Na+, K+-ATPase. All three mutants were phosphorylated with [γ-32P]-ATP in the Na+ reaction, indicating expression of mutant proteins and the ability to perform the Na+ limb of the Post-Albers reaction cycle (maximal phosphorylation signals relative to WT), albeit at a significantly reduced expression and/or phosphorylation level relative to that of WT, for p.Gly303Arg and p.Met859Arg (“o” = p < 0.001 for p.Gly303Arg and p.Met859Arg by a one-way ANOVA test; p = 0.027 for p.Leu302Arg; n = 3–5).
(C) Na+ dependence of phosphorylation showing a significant 3.5-fold reduced affinity for Na+ for p.Leu302Arg relative to WT, whereas the affinity was WT-like for p.Gly303Arg and p.Met859Arg, but with reduced cooperativity (see curves separated in different panels with statistics in Figure S1).
(D) K+-sensitivity of the Na+, K+-ATPase phosphoenzyme intermediate. Symbols are the same as in (C). K+ interaction was assessed by the ability of K+ to inhibit phosphorylation. The Hill equation for inhibition was used for data fitting.6 The cooperativity was WT-like for p.Leu302Arg (Hill coefficient 1.3–1.4), whereas the apparent affinity for K+ of this mutant was reduced significantly (p < 0.001 by a one-way ANOVA test, n = 8), 36-fold relative to WT. For p.Gly303Arg and p.Met859Arg, the Hill coefficients were only 0.6–0.7, indicating loss of cooperativity between the two K+ sites.
(E) Whole-cell currents of adrenal NCI-H295R cells expressing wild-type (WT) or different mutant (p.Leu302Arg, p.Gly303Arg, p.Met859Arg) ouabain-insensitive versions of rat Atp1a1. Compared to WT cells, mutant Atp1a1-expressing cells (except for mutant p.Met859Arg) displayed an abnormal current which was reduced after removal of Na+, indicating an abnormal Na+ permeability as causative for abnormal inward currents of Na+ ions in mutant cells.
(F) Mutant-expressing NCI-H295R cells (except for mutant p.Met859Arg) had a depolarized membrane potential under control conditions but were hyperpolarized to the level of WT cells after removal of extracellular Na+.
(G) Intracellular Na+ and K+ contents in cell lysates of HEK293 cells under control conditions and after treatment with the Na+, K+-ATPase inhibitor ouabain. Ouabain treatment strongly increased intracellular Na+ and decreased intracellular K+ in non-transfected cells. Expression of WT rat Atp1a1 significantly attenuated these changes of intracellular Na+ and K+, whereas this was not the case for all mutant-expressing cells. Expression of the p.Gly303Arg mutant increased Na+ and decreased K+ even more in comparison to vector control cells. n = 7–9 per group.
(B–G) (all data are presented as means ± SEM).
Next, electroporation was used for transfecting adrenocortical carcinoma NCI-H295R cells (Cell Line Service) as well as HEK293 cells with plasmids containing full-length cDNA sequences encoding wild-type or mutant ouabain-resistant rat Atp1a1. Transfected cells were identified with anti-CD8-coated dynabeads (Life Technologies). Whole-cell patch-clamp recordings were performed as described.8 For the determination of intracellular Na+ and K+ contents, flame photometry was used.8 Intracellular Na+ and K+ content was measured under control conditions and after treatment with 10 μM ouabain-inhibiting endogenous human Na+, K+-ATPase. Patch-clamp analyses of NCI-H295R cells expressing mutant ATP1A1 constructs p.Leu302Arg and p.Gly303Arg revealed an abnormal Na+ permeability compared to that of wild-type cells, as manifested by Na+-dependent inward currents at negative voltages (Figure 2E). Moreover, cells expressing mutant ATP1A1 p.Leu302Arg and p.Gly303Arg also showed a depolarized membrane potential in the presence of Na+, but upon removal of extracellular Na+, the membrane potential was hyperpolarized to the level of wild-type-expressing cells (Figure 2F). In these experiments, cells expressing the mutant p.Met859Arg were indistinguishable from wild-type-ATP1A1-expressing cells. Inhibition of endogenous Na+, K+-ATPase by ouabain produced pronounced changes in intracellular Na+ and K+ in HEK293 cells (Figure 2G). These changes were significantly attenuated after expression of wild-type rat Atp1a1, whereas all three mutants failed to compensate for the block of endogenous ATP1A1. Expression of mutant p.Gly303Arg led to even more pronounced disturbances of intracellular Na+ and K+ content compared to that in non-transfected cells, possibly reflecting abnormal ion fluxes (Figure 2G). Analyses of intracellular pH levels revealed an abnormal H+ permeability and significant changes of intracellular pH upon alteration of extracellular pH for mutant p.Leu302Arg (Figure S3).
The Na+, K+-ATPase is an integral membrane protein that catalyzes the transport of three Na+ ions out of and two K+ ions into the cell at the expense of one molecule of ATP. It generates the electrochemical driving force that powers essential functions, such as neuronal firing, muscle contraction, and transepithelial ion transport. The Na+, K+-ATPase is a heterodimer consisting of α and β subunits and is complemented by auxiliary FXYD proteins.9 The catalytic α subunit binds translocating Na+ and K+ as well as ATP, coupling ionic movements across the cell membrane to ATP hydrolysis.10 In mammals, there exist four α isoforms that are expressed in a developmental- and tissue-specific manner, suggesting specific functional roles (α1–α4, ATP1A1–ATP1A4).11, 12 The ubiquitously expressed α1 subunit (ATP1A1) represents the major α isoform in the kidney and is present in virtually all cell types and structures of the central nervous system (CNS).11
Others studied the effects of a targeted disruption of α1 (ATP1A1) almost 20 years ago in mice; the focus then was on the cardiac phenotype.13 Bi-allelic knock-out of ATP1A1 led to early fetal lethality, suggesting that complete loss of ATP1A1 function is not compatible with life. In contrast, heterozygous mice were fertile and generally healthy; however, they exhibited a hypocontractile cardiac phenotype mimicking cardiac glycoside toxicity.13 The severe phenotype in the affected children differs from these heterozygous (Atp1a1+/−) mice. The cardiac examination of the affected children did not reveal any pathologic findings, possibly indicating inter-species differences or suggesting compensatory mechanisms in the human heart. Seizures and hypomagnesemia have not been reported in (Atp1a1+/−) mice; however, they represent pivotal findings in the affected children.
Hypomagnesemia in the affected individuals presented here is caused by massive renal Mg2+ wasting. Within the nephron, the distal convoluted tubule (DCT) represents the segment mediating active transcellular Mg2+ transport. Here, the basolaterally expressed Na+, K+-ATPase establishes favorable electrochemical gradients for apical cation influx through Mg2+-permeable ion channels and also provides the exit mechanism for reabsorbed Na+ ions. The DCT exhibits the highest density and activity of the Na+, K+-ATPase; α1 (ATP1A1) represents the predominating α isoform.14 The critical role of Na+, K+-ATPase activity for Mg2+ reabsorption in the DCT has already been highlighted by the discovery of genetic defects in its auxiliary γ subunit (encoded by FXYD2 [MIM: 601814]) in persons with dominant hypomagnesemia [HOMG2, MIM: 154020],15 as well as by the hypomagnesemia observed in individuals with SeSAME (EAST) syndrome [SESAMES, MIM: 612780].16 This autosomal-recessive disorder is caused by mutations in KCNJ10 [MIM: 602208], encoding the Kir4.1 potassium channel that is co-expressed basolaterally in the DCT and recycles K+ as a prerequisite for maintenance of Na+, K+-ATPase activity.
Despite considerable variability, serum Mg2+ levels in the affected children remained persistently low during follow-up despite a high dose oral Mg2+ supplementation (Table 1). Individuals with genetic defects in TRPM6 or FXYD2 also present with seizures; however, in these individuals, Mg2+ supplementation usually leads to a rapid cessation of epileptic activity, and physical and mental development are generally undisturbed even though Mg2+ levels remain subnormal.17 In contrast, the children with ATP1A1 defects exhibited persistent seizures and uniformly developed significant intellectual disability. Therefore, the neurologic phenotype has to be considered as a primary feature of ATP1A1 deficiency due to a genuine disturbance of Na+, K+-ATPase function in the CNS. Here, α1 is ubiquitously expressed and thought to maintain neuronal housekeeping functions, whereas the α3 isoform potentially acts as a reserve pump that is only required during phases of increased intracellular Na+ concentrations, i.e., after repeated action potentials.11, 18 Expression of the α2 isoform is restricted to astrocytes and developing neurons. In the CNS, constant Na+, K+-ATPase activity is required for generating the resting membrane potential and for buffering and clearance of extracellular K+ transients during neuronal activity.19 Decreases in Na+, K+-ATPase activity have been detected in animal models of epilepsy and in forms of human myoclonus epilepsy and mitochondrial disorders.20, 21, 22 Moreover, pharmacologic inhibition of neuronal Na+, K+-ATPase by cardiac glycosides provokes seizures in rats,23 and changes in membrane potential and epileptic bursting activity were detected after the blocking of Na+, K+-ATPase activity by ouabain in vitro.24
Very recently, germline mutations in ATP1A1 have been reported in individuals with Charcot-Marie-Tooth type 2 disease (CMT2) [CMT2DD, MIM: 618036].25 Combining extensive data from different exome sequencing projects, the authors identified heterozygous ATP1A1 missense mutations in seven CMT2-affected families. Segregation analyses were compatible with dominant inheritance. In accordance with the peripheral nervous system (PNS) phenotype, strong α1 expression was demonstrated in axolemma and myelin sheaths of sensory and motor neurons. The identified missense mutations affect conserved amino acid residues in different regions of the α1 protein. Interestingly, a mutational clustering is observed within the helical linker region (residues 592–608). Mutations in this region have been shown to reduce the rate of E1P to E2P conformational transition during the Post-Albers reaction cycle, but without completely blocking the conversion.26 Ouabain survival assays demonstrated a significant decrease in cell viability, and functional studies in Xenopus oocytes showed a significant Na+-current reduction compatible with a deleterious effect on Na+, K+-ATPase ion-transport function.25
In contrast to findings in these previously reported CMT2-affected families, the clinical evaluation of the affected children with de novo ATP1A1 mutations did not reveal any signs of peripheral neuropathy. This phenotypic discrepancy could possibly be attributed to the relatively young age of the children; the age of onset of clinical symptoms in the previously reported CMT2-affected families varied between 8 and 50 years of age.25 Conversely, data on serum magnesium levels as well as a potential concomitant epileptic phenotype were not reported in the CMT2-affected families. Yet, the authors detail migraine headaches in one CMT2-affected family and explicitly note the possibility of combined CNS and PNS symptoms and an expanding spectrum of phenotypes associated with ATP1A1 mutations. Clearly, the predominant renal and CNS symptoms as well as the unfavorable clinical course of the children presented here with profound intellectual disability indicate a distinct clinical entity caused by ATP1A1 defects.
Such a pleiotropic effect with variable phenotypes has already been described for mutations in the Na+, K+-ATPase homologs α2 and α3 (ATP1A2 [MIM: 182340] and ATP1A3 [MIM: 182350]). The disease spectrum caused by germline mutations in these two isoforms comprises familial forms of migraine (MHP2, MIM: 602481), alternating hemiplegia of childhood (AHC1 [MIM: 104290] and AHC2, [614820]), and rapid-onset dystonia parkinsonism (DYT12, MIM: 128235), as well as CAPOS syndrome [CAPOS, MIM: 601338], a complex neurological disorder comprising cerebellar ataxia, optic atrophy, and sensorineural hearing loss.27, 28, 29, 30 Interestingly, similar or even identical ATP1A3 mutations are associated with variable phenotypes, indicating that genetic, epigenetic, or environmental modifiers might play a role. Functional analyses of mutated ATP1A2 co-expressed with wild-type constructs suggested a functional haploinsufficiency rather than a dominant-negative effect.27 Established mouse models for both genes recapitulate critical features of the human phenotype.21, 31 Interestingly, a knock-in mouse model with the engineered ATP1A2 variant p.Gly301Arg, which is orthologous to the ATP1A1 p.Gly303Arg variant identified in individual B-II-1, was produced recently, and heterozygous mice recapitulated the severe human MHP2 phenotype associated with this mutation.32 Together, the in vitro and animal data argue for a complete loss of function of the mutated allele.32, 33, 34
Seizures, cognitive deficits, developmental delay, and psychiatric manifestations are known co-morbidities of the mentioned disorders.29, 35 Therefore, impaired Na+, K+-ATPase function in the CNS might cause epileptic seizures regardless of the specific α isoform affected. In line with this assumption, even small reductions in Na+, K+-ATPase pump activity were shown to be able to trigger epileptic seizures.36
The ATP1A1 mutations identified here affect amino acid residues in the vicinity of the Na+ and K+ ion-binding residues of the Na+, K+-ATPase enzyme (Figures 1B and 1C). In line with the structural analyses, our functional studies indicate a disturbed Na+ and K+ binding, resulting in a loss of ATPase function; namely, Na+ and K+ transport activities were severely impaired in all three mutants Furthermore, a reduced expression level (particularly for p.Gly303Arg, cf. Figure 2B) could play a role in pathogenesis. Notably, p.Leu302Arg showed a strong effect on the affinity for both Na+ and K+, whereas p.Gly303Arg and p.Met859Arg mutants disrupted cooperativity between the sites for both Na+ and K+, suggesting a defective function of the so-called sites I and II that interact sequentially with Na+ and K+ during the pump cycle. Moreover, leak currents, and abnormal H+ permeabilities were observed for mutant p.Gly303Arg upon overexpression of p.Leu302Arg. These abnormal ion fluxes that transform the Na+, K+-ATPase into a passive ion channel might add to the pathogenesis of the severe phenotype reported here and could explain the phenotypic differences between the affected children and (Atp1a1+/−) mice, which lack one copy of Atp1a1, as well as CMT2-affected individuals who harbor ATP1A1 missense mutations impairing the E1P to E2P conformational transition during the Post-Albers reaction cycle.
Interestingly, a similar neomorphic effect with inward leak currents carried by H+ and Na+ has also been postulated for somatic ATP1A1 mutations identified in aldosterone-producing adenomas (APAs) in individuals with arterial hypertension.6, 37 Because of these genetic and functional similarities, we also specifically evaluated the affected children for the presence of primary aldosteronism. Though we observed episodes of hypokalemia and elevated serum bicarbonate concentrations, typical APA findings such as suppressed renin levels or increased blood pressure were lacking in the young children presented here.
In conclusion, we describe a clinical entity comprising hypomagnesemia, refractory seizures, and severe intellectual disability associated with heterozygous de novo mutations in ATP1A1, encoding the α1 subunit of Na+, K+-ATPase. ATP1A1 defects should be suspected in hypomagnesemic individuals presenting with seizures and developmental delay, especially if the epilepsy does not respond to an amelioration of hypomagnesemia. Our observations demonstrate further genetic heterogeneity among renal magnesium wasting disorders and underline the crucial role of basolateral Na+, K+-ATPase for tubular magnesium reabsorption. Furthermore, these findings illustrate the critical role of the α1 subunit of Na+, K+-ATPase for the maintenance of ionic gradients, the generation of resting membrane potential, and the termination of neuronal activity in the central nervous system; they also illustrate the pleiotropic effects associated with this subunit’s dysfunction. Understanding the molecular basis provides a platform for further studies on the pathogenesis and potential treatment of hypomagnesemia, epilepsy, and intellectual disability.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We are grateful to the children and their families for their participation in this study. We would like to acknowledge the following clinicians and laboratory scientists involved in the management of the children and molecular studies: Xiaohua Han, Ruth Giesbrecht, Dora Pak, Michelle Higginson, Aisha Ghani, Colin J. Ross, and Wyeth W. Wasserman. Individual C-II-2 was part of the Treatable Intellectual Disability Endeavour (TIDE) with funding support from the B.C Children’s Hospital Foundation as “1st Collaborative Area of Innovation” (www.tidebc.org); Genome British Columbia (grant number SOF-195); the Canadian Institutes of Health Research (grant number 301221); and informatics infrastructure supported by Genome British Columbia and Genome Canada (ABC4DE Project). C.D.M.v.K. is a recipient of the Michael Smith Foundation for Health Foundation Research Scholar Award. The work was supported by grants to B.V. from the Lundbeck Foundation (grant number R223-2016-595) and the Danish Medical Research Council (grant number 7016-00193B). Support to D.B. and R.K. was provided by Kids Kidney Research; Kidney Research UK; St Peter’s Trust for Kidney Bladder and Prostate Research; The David and Elaine Potter Foundation; and the European Union, 7th Framework Program (grant agreement 2012-305608 “European Consortium for High-Throughput Research in Rare Kidney Diseases (EURenOmics).” S.B. and R.W. were supported by the “Deutsche Forschungsgemeinschaft” (BA4436/2-1 to S.B. and SFB699 to R.W.). K.P.S. and M.K. received support from the Hans-Joachim-Bodlee Foundation.
Published: November 1, 2018
Footnotes
Supplemental Data include three figures, supplemental Methods and Results, and two tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.10.004.
Web Resources
CADD (Combined Annotation Dependent Depletion), https://cadd.gs.washington.edu/
ExAC (Exome Aggregation Consortium), http://exac.broadinstitute.org
GnomAD (Genome Aggregation Database), http://gnomad.broadinstitute.org
OMIM (Online Mendelian Inheritance in Man), http://www.omim.org
PolyPhen2, http://genetics.bwh.harvard.edu/pph2
SIFT (Sorting Intolerant From Tolerant), http://sift.bii.a-star.edu.sg
Supplemental Data
References
- 1.Viering D.H.H.M., de Baaij J.H.F., Walsh S.B., Kleta R., Bockenhauer D. Genetic causes of hypomagnesemia, a clinical overview. Pediatr. Nephrol. 2017;32:1123–1135. doi: 10.1007/s00467-016-3416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arjona F.J., de Baaij J.H., Schlingmann K.P., Lameris A.L., van Wijk E., Flik G., Regele S., Korenke G.C., Neophytou B., Rust S. CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet. 2014;10:e1004267. doi: 10.1371/journal.pgen.1004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlingmann K.P., Weber S., Peters M., Niemann Nejsum L., Vitzthum H., Klingel K., Kratz M., Haddad E., Ristoff E., Dinour D. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 4.Walder R.Y., Landau D., Meyer P., Shalev H., Tsolia M., Borochowitz Z., Boettger M.B., Beck G.E., Englehardt R.K., Carmi R., Sheffield V.C. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 5.Vilsen B. Glutamate 329 located in the fourth transmembrane segment of the alpha-subunit of the rat kidney Na+,K+-ATPase is not an essential residue for active transport of sodium and potassium ions. Biochemistry. 1993;32:13340–13349. doi: 10.1021/bi00211a048. [DOI] [PubMed] [Google Scholar]
- 6.Beuschlein F., Boulkroun S., Osswald A., Wieland T., Nielsen H.N., Lichtenauer U.D., Penton D., Schack V.R., Amar L., Fischer E. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013;45:440–444. doi: 10.1038/ng.2550. e1–e2. [DOI] [PubMed] [Google Scholar]
- 7.Toustrup-Jensen M., Hauge M., Vilsen B. Mutational effects on conformational changes of the dephospho- and phospho-forms of the Na+,K+-ATPase. Biochemistry. 2001;40:5521–5532. doi: 10.1021/bi002367m. [DOI] [PubMed] [Google Scholar]
- 8.Tauber P., Aichinger B., Christ C., Stindl J., Rhayem Y., Beuschlein F., Warth R., Bandulik S. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca(2+)-ATPase ATP2B3. Endocrinology. 2016;157:2489–2499. doi: 10.1210/en.2015-2029. [DOI] [PubMed] [Google Scholar]
- 9.Sweadner K.J., Arystarkhova E., Donnet C., Wetzel R.K. FXYD proteins as regulators of the Na,K-ATPase in the kidney. Ann. N Y Acad. Sci. 2003;986:382–387. doi: 10.1111/j.1749-6632.2003.tb07218.x. [DOI] [PubMed] [Google Scholar]
- 10.Post R.L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 1972;247:6530–6540. [PubMed] [Google Scholar]
- 11.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Lingrel J.B. Na,K-ATPase: isoform structure, function, and expression. J. Bioenerg. Biomembr. 1992;24:263–270. doi: 10.1007/BF00768847. [DOI] [PubMed] [Google Scholar]
- 13.James P.F., Grupp I.L., Grupp G., Woo A.L., Askew G.R., Croyle M.L., Walsh R.A., Lingrel J.B. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol. Cell. 1999;3:555–563. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 14.Lücking K., Nielsen J.M., Pedersen P.A., Jørgensen P.L. Na-K-ATPase isoform (alpha 3, alpha 2, alpha 1) abundance in rat kidney estimated by competitive RT-PCR and ouabain binding. Am. J. Physiol. 1996;271:F253–F260. doi: 10.1152/ajprenal.1996.271.2.F253. [DOI] [PubMed] [Google Scholar]
- 15.Meij I.C., Koenderink J.B., van Bokhoven H., Assink K.F., Groenestege W.T., de Pont J.J., Bindels R.J., Monnens L.A., van den Heuvel L.P., Knoers N.V. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat. Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 16.Bockenhauer D., Feather S., Stanescu H.C., Bandulik S., Zdebik A.A., Reichold M., Tobin J., Lieberer E., Sterner C., Landoure G. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N. Engl. J. Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlingmann K.P., Sassen M.C., Weber S., Pechmann U., Kusch K., Pelken L., Lotan D., Syrrou M., Prebble J.J., Cole D.E. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J. Am. Soc. Nephrol. 2005;16:3061–3069. doi: 10.1681/ASN.2004110989. [DOI] [PubMed] [Google Scholar]
- 18.Munzer J.S., Daly S.E., Jewell-Motz E.A., Lingrel J.B., Blostein R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. J. Biol. Chem. 1994;269:16668–16676. [PubMed] [Google Scholar]
- 19.Larsen B.R., Stoica A., MacAulay N. Managing brain extracellular K(+) during neuronal activity: the physiological role of the Na(+)/K(+)-ATPase subunit isoforms. Front. Physiol. 2016;7:141. doi: 10.3389/fphys.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquezan B.P., Funck V.R., Oliveira C.V., Pereira L.M., Araújo S.M., Zarzecki M.S., Royes L.F., Furian A.F., Oliveira M.S. Pentylenetetrazol-induced seizures are associated with Na+,K+-ATPase activity decrease and alpha subunit phosphorylation state in the mice cerebral cortex. Epilepsy Res. 2013;105:396–400. doi: 10.1016/j.eplepsyres.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Clapcote S.J., Duffy S., Xie G., Kirshenbaum G., Bechard A.R., Rodacker Schack V., Petersen J., Sinai L., Saab B.J., Lerch J.P. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc. Natl. Acad. Sci. USA. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindoff L.A., Engelsen B.A. Mitochondrial diseases and epilepsy. Epilepsia. 2012;53(Suppl 4):92–97. doi: 10.1111/j.1528-1167.2012.03618.x. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson J., St Pierre T., Minnich J., Barbeau A. Seizures in rats associated with divalent cation inhibition of NA + -K + -ATP’ase. Can. J. Biochem. 1971;49:1217–1224. doi: 10.1139/o71-175. [DOI] [PubMed] [Google Scholar]
- 24.Vaillend C., Mason S.E., Cuttle M.F., Alger B.E. Mechanisms of neuronal hyperexcitability caused by partial inhibition of Na+-K+-ATPases in the rat CA1 hippocampal region. J. Neurophysiol. 2002;88:2963–2978. doi: 10.1152/jn.00244.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lassuthova P., Rebelo A.P., Ravenscroft G., Lamont P.J., Davis M.R., Manganelli F., Feely S.M., Bacon C., Brožková D.S., Haberlova J. Mutations in ATP1A1 Cause Dominant Charcot-Marie-Tooth Type 2. Am. J. Hum. Genet. 2018;102:505–514. doi: 10.1016/j.ajhg.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke D.M., Loo T.W., MacLennan D.H. Functional consequences of alterations to amino acids located in the nucleotide binding domain of the Ca2(+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1990;265:22223–22227. [PubMed] [Google Scholar]
- 27.De Fusco M., Marconi R., Silvestri L., Atorino L., Rampoldi L., Morgante L., Ballabio A., Aridon P., Casari G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 28.de Carvalho Aguiar P., Sweadner K.J., Penniston J.T., Zaremba J., Liu L., Caton M., Linazasoro G., Borg M., Tijssen M.A., Bressman S.B. Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Heinzen E.L., Arzimanoglou A., Brashear A., Clapcote S.J., Gurrieri F., Goldstein D.B., Jóhannesson S.H., Mikati M.A., Neville B., Nicole S., ATP1A3 Working Group Distinct neurological disorders with ATP1A3 mutations. Lancet Neurol. 2014;13:503–514. doi: 10.1016/S1474-4422(14)70011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demos M.K., van Karnebeek C.D., Ross C.J., Adam S., Shen Y., Zhan S.H., Shyr C., Horvath G., Suri M., Fryer A., FORGE Canada Consortium A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet J. Rare Dis. 2014;9:15. doi: 10.1186/1750-1172-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gritz S.M., Radcliffe R.A. Genetic effects of ATP1A2 in familial hemiplegic migraine type II and animal models. Hum. Genomics. 2013;7:8. doi: 10.1186/1479-7364-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bøttger P., Glerup S., Gesslein B., Illarionova N.B., Isaksen T.J., Heuck A., Clausen B.H., Füchtbauer E.M., Gramsbergen J.B., Gunnarson E. Glutamate-system defects behind psychiatric manifestations in a familial hemiplegic migraine type 2 disease-mutation mouse model. Sci. Rep. 2016;6:22047. doi: 10.1038/srep22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spadaro M., Ursu S., Lehmann-Horn F., Veneziano L., Antonini G., Giunti P., Frontali M., Jurkat-Rott K. A G301R Na+/K+ -ATPase mutation causes familial hemiplegic migraine type 2 with cerebellar signs. Neurogenetics. 2004;5:177–185. doi: 10.1007/s10048-004-0183-2. [DOI] [PubMed] [Google Scholar]
- 34.Santoro L., Manganelli F., Fortunato M.R., Soldovieri M.V., Ambrosino P., Iodice R., Pisciotta C., Tessa A., Santorelli F., Taglialatela M. A new Italian FHM2 family: clinical aspects and functional analysis of the disease-associated mutation. Cephalalgia. 2011;31:808–819. doi: 10.1177/0333102411399351. [DOI] [PubMed] [Google Scholar]
- 35.Deprez L., Weckhuysen S., Peeters K., Deconinck T., Claeys K.G., Claes L.R., Suls A., Van Dyck T., Palmini A., Matthijs G. Epilepsy as part of the phenotype associated with ATP1A2 mutations. Epilepsia. 2008;49:500–508. doi: 10.1111/j.1528-1167.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan G.P., Filatov G., Shilnikov A., Bazhenov M. Electrogenic properties of the Na+/K+ ATPase control transitions between normal and pathological brain states. J. Neurophysiol. 2015;113:3356–3374. doi: 10.1152/jn.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azizan E.A., Poulsen H., Tuluc P., Zhou J., Clausen M.V., Lieb A., Maniero C., Garg S., Bochukova E.G., Zhao W. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.