Abstract
In the present work, the antiglaucoma drug, acetazolamide, was formulated as microsponges in situ gel for ocular drug delivery aiming an improved therapeutic efficacy and reduction in the systemic side effects of oral acetazolamide. The microsponges were prepared by the quasi emulsion solvent diffusion method and were incorporated into 25% pluronic F-127 in situ gel. Ethyl cellulose polymer in different proportions with drug was used to prepare the microsponges. Different parameters were evaluated to select the best formulation. The formula S2 with drug to polymer ratio (2:1) showed high entrapment efficiency of about 82% and mean particle size of about 10 µm with polydispersity index (PDI) of 0.22, which are suitable characters for ocular delivery. The in situ gels were evaluated for physicochemical properties (pH, gelling capacity, gelation time and rheological properties) and in vivo studies. S2 formulation showed higher therapeutic efficacy compared to free drug in gel. It was non irritant to the rabbit's eye. These results indicated that acetazolamide microsponges in situ gel have potential ability for ophthalmic delivery.
Keywords: Microsponges, Acetazolamide, Ophthalmic gels, Intraocular pressure
1. Introduction
Microsponges are polymeric delivery systems composed of porous microspheres of an inert polymer that can entrap active ingredients and control their release (Jadhav et al., 2013, Vyas et al., 2010). They are tiny sponge like spherical particles that consist of myriad of interconnecting voids within a non-collapsible structure with large porous surface. The size of these microsponges can be varied, usually from 5 to 300 µm in diameter (Jadhav et al., 2013, Vyas et al., 2010). Moreover, it may enhance the stability and reduce the side effects of the active ingredients from topical formulation (Amrutiya et al., 2009).
Microsponges are prepared by several methods as emulsion systems and liquid-liquid suspension polymerization methods. Emulsion systems include water in oil in water (w/o/w) emulsion solvent diffusion, oil in oil emulsion solvent diffusion and quasi emulsion solvent diffusion (ESD) method (Srivastava and Pathak, 2012). Quasi emulsion solvent diffusion (ESD) method is the most common emulsion system used with microsponges preparation (Abdelmalak and El-Menshawe, 2012, Çomoğlu et al., 2003, Jain and Singh, 2011, Jelvehgari et al., 2006).
Acetazolamide, a carbonic anhydrase inhibitor, is still the most effective drug for the treatment of glaucoma for many years (Kaur et al., 2000), also it is used in the treatment of various forms of epilepsy and to prevent or ameliorate the symptoms of acute high altitude sickness (Mora et al., 2013). In addition, acetazolamide is used as an adjuvant in brain imaging for identifying ischemic areas (Camargo, 2001). To obtain the desired lowering in intraocular pressure (IOP), large oral doses of acetazolamide are used, which would cause peripheral inhibition of carbonic anhydrase enzyme that is distributed in almost all body organs. This usually results in several side effects, which are not tolerated by most of the patients and hence they discontinue the therapy (Epstein and Grant, 1977). The most common reported side effects are diuresis, gastrointestinal symptoms including cramping, epigastric burning, nausea, diarrhea and metabolic acidosis (Granero et al., 2008). Acetazolamide is available in the market as tablets, capsules and no topical ophthalmic formulation existed due to low permeability coefficient of about (4.1 × 10−6 cm/sec) which limits its ocular bioavailability because of the insufficient amount of the drug reaching the ciliary body (Morsi et al., 2014). Thus, many attempts were made in order to develop an effective topical acetazolamide formulation. These attempts include; formulation of acetazolamide in aqueous solutions containing cyclodextrins in order to increase the aqueous solubility of the drug (Ammar et al., 1998), preparation of high-water-content soft contact lenses soaked in 2.5% and 5% acetazolamide to improve the drug effect (Friedman et al., 1985), preparation of polymeric acetazolamide suspension (10%) with penetration enhancers (Kaur et al., 2000), formulation of acetazolamide liposomal dispersions (El-Gazayerly and Hikal, 1997, Hathout et al., 2007) and niosomal dispersions (Aggarwal et al., 2004, Guinedi et al., 2005). More recent attempts include; incorporation of acetazolamide in dendritic nano-architectures (Mishra and Jain, 2014a) and nanoemulsion formulations (Morsi et al., 2017) However, all these attempts were not devoid of problems such as poor patient compliance, difficulty of insertion as in contact lenses and the reported tissue irritation due to the high concentrations of the drug (5% and 10%) as well as toxicological problems accompanied by penetration enhancers. In addition, the most critical problem regarding nanoemulsion formulations is the toxicity of its components (Saifullah et al., 2016).
One of the main problems encountered with the ophthalmic drug delivery systems is the rapid and extensive precorneal loss caused by the drainage and the high tear fluid turnover (Morsi et al., 2017). To overcome these problems an increase in the contact time between drug and corneal surface is required. In situ gelling systems are viscous liquids, which undergo a sol to gel transition, when applied to human body, due to change in a physicochemical parameter such as temperature, pH or ionic strength (Robinson and Mlynek, 1995). In situ gelling systems allow accurate and reproducible administration of drugs unlike the preformed gels, and are capable of prolonging the residence time to the mucosal surfaces (Krauland et al., 2003).
The aim of this study was to formulate novel acetazolamide loaded microsponges and formulating them into in situ gel for ocular drug delivery, in order to decrease the systemic side effects of acetazolamide and increase patient compliance.
2. Materials and methods
2.1. Materials
Acetazolamide (AZM) (99.9% purity) was obtained from CID Company, Egypt. Ethyl cellulose (EC) polymer (degree of substitution 2.42 to 2.53, viscosity of a 5% w/w solution in 80:20 toluene: ethanol by weight at 25 °C, approx. 14cP) was obtained from BDH chemicals Ltd Poole England. Poly vinyl alcohol (PVA) (M.W = 72000) was obtained from MP Biomedicals, LLC, France. Pluronic® F-127(PF-127) and dialysis membrane (MWCO 12–14 kDa) were obtained from Sigma Aldrich, St. Louis, MO, USA. Triethyl citrate (TEC) was from Alfa Aesar GmbH & Co KG, Germany. Dichloromethane (DCM), sodium chloride (NaCl) and calcium chloride dihydrate (CaCl2·2H2O) were obtained from El Nasr Pharmaceutical Chemicals Co., Egypt. Sodium hydroxide scales (NaOH) was obtained from Iso-chem CO. Egypt. Sodium bicarbonate (NaHCO3) was from El Gomhouria Co. Egypt.
2.2. Methods
2.2.1. Preparation of acetazolamide loaded microsponges
Microsponges were prepared by the quasi emulsion solvent diffusion method (Aldawsari and Badr-Eldin, 2013). Firstly, the organic (internal) phase was prepared by dissolving EC polymer and TEC in 10 ml of DCM. TEC (1% w/v) was used as a plasticizer. Then, the calculated amount of the drug was added in the polymeric solution and was ultrasonicated for 20 min in an ice bath using probe ultrasonicator (Model CPX 400, Cole-Parmer instruments Vernon Hills, Illinois U.S.A) for homogenous dispersion and particle size reduction of the drug. The polymeric solution was then added drop wise to the aqueous solution previously prepared by dissolving PVA (0.5% w/v) in 100 ml distilled water at 70 °C with stirring until it was completely dissolved, then the whole mixture was stirred using overhead stirrer at 3000 rpm for two hours till complete evaporation of the organic solvent and formation of the microsponges. The mixture was left in a refrigerator for 24 h for complete precipitation of the microsponges, then, the microsponges were filtered, washed with small amount of diluted sodium hydroxide to remove any free drug, washed several times with double distilled water and dried in an oven at 40 °C for 48 h, then kept for further studies.
2.2.2. Optimization of the formulation parameters and the processing
Several factors that influence the characteristics of the microsponges were studied, to obtain their effects on production yield, entrapment efficiency, drug loading and particle size of microsponges formulations.
2.2.2.1. Effect of the drug to polymer ratio
Different drug to polymer (AZM: EC) ratios (3:1, 2:1, 1:1, 1:2, 1:3 and 1:4) were investigated to prepare the microsponges formulations. In each formulation, the amounts of DCM (10 ml), PVA (0.5%w/v) and distilled water (100 ml) were kept constant. The microsponges formulations were prepared using overhead stirrer at a stirring speed of 3000 rpm for 2 h. The microsponge formulations possessing drug to polymer ratios 3:1, 2:1, 1:1, 1:2, 1:3 and 1:4 and denominated (S1, S2, S3, S4, S5 and S6 respectively) are shown in Table 1.
Table 1.
Composition of various microsponges formulations.
| Components | Drug: polymer ratio |
|||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | |
| 3:1 | 2:1 | 1:1 | 1:2 | 1:3 | 1:4 | |
| Acetazolamide (mg) | 300 | 200 | 100 | 100 | 100 | 100 |
| Ethyl cellulose (mg) | 100 | 100 | 100 | 200 | 300 | 400 |
| Triethylcitrate (%w/v) | 1 | 1 | 1 | 1 | 1 | 1 |
| Poly vinyl alcohol (%w/v) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Water (ml) | 100 | 100 | 100 | 100 | 100 | 100 |
2.2.2.2. Effect of the volume of the internal phase (DCM)
The effect of the internal phase volume was investigated by using three different volumes of DCM (5, 10 and 20 ml) to choose the optimum one.
2.2.2.3. Effect of the stirring speed and stirring time
Different stirring speeds (500, 1000, 2000 and 3000 rpm) and times of stirring (0.5, 1, 2, 4 and 8 h) were employed for the selected formulation to choose the optimum speed and time.
2.2.2.4. Effect of the amount of the emulsifying agent (PVA)
Different concentrations of PVA; 0.1%, 0.3%, 0.5%, 0.7% and 1.0% w/v were evaluated to study the effect of the amount of the emulsifying agent (PVA) on the microsponges formulations.
2.2.2.5. Effect of ultrasonication time
In order to determine the appropriate ultrasonication time, different times (5, 10 and 20 min) were investigated.
2.2.3. Characterization of the microsponges formulations
2.2.3.1. Particle size and size distribution
A definite dried amount of the prepared microsponges were suspended in water and were sonicated for one minute to prevent aggregation of the microsponges, then, the mean particle size and size distribution were performed for the microsponges formulation using laser scattering particle size distribution analyzer (HORIBA LA-300). Polydispersity index (PDI) was calculated according to the following equation (Iego, 2012):
where the polydispersity index (PDI) = the square of the (standard deviation (σ)/mean particle diameter (d)). The mean PDI obtained by calculating the average of three PDI calculations.
2.2.3.2. Drug loading (DL%) and entrapment efficiency (EE %)
Accurately weighed 50 mg of the drug loaded microsponges were crushed in a mortar, then, transferred to a beaker containing 20 ml of 0.1 M NaOH and stirred on a magnetic stirrer for sufficient time to extract and dissolve the drug, then this solution was filtered using 0.45 µm disc filter and the filtrate was diluted to 100 ml with 0.1 M NaOH solution, then 1 ml was withdrawn, completed to 10 ml with the solution of 0.1 M NaOH and was measured spectrophotometrically at 290 nm against a blank solution similarly treated. The amount of the entrapped drug was calculated using the calibration curve of AZM in 0.1 M NaOH.
The drug loading (DL%) and entrapment efficiency (EE %) were calculated using the following equations (Akash et al., 2013):
2.2.3.3. Determination of percentage yield
The formed microsponges were washed, dried and then were weighed accurately. The yield of microsponges was determined by comparing the whole weight of the formed microsponges against the combined weight of the polymer and drug components.
2.2.3.4. Microsponges surface morphology
The morphology and surface characteristics of the prepared microsponges were studied using the scanning electron microscopy (SEM). One drop of the homogenously suspended microsponges in water was taken and left till complete drying then coated with gold–palladium alloy under vacuum. Coated samples were then examined using SEM.
2.2.3.5. Differential scanning calorimetric (DSC) analysis
Thermal analysis using a DSC apparatus was carried out on AZM, EC polymer, physical mixture of AZM and EC polymer, plain microsponges (microsponges without the drug) and the selected drug-loaded microsponges formulation using a computer-interfaced shimadzu calorimeter (Model DSC-50, Japan). Samples (approximately 4 mg) were accurately weighed and were put in an aluminum pans and sealed. All samples were run at a heating rate of 10 °C/min over a temperature range 25–350 °C and thermograms were obtained.
2.2.4. Development of acetazolamide loaded microsponges in situ gels
PF-127 hydrogel containing AZM loaded microsponges equivalent to 1% w/w of the drug were prepared by the cold method (El-Laithy et al., 2011). Three different concentrations of plain gels (15, 20 and 25% w/v) were prepared and were tested for their gelling capacity. The concentration that had given the best gelling properties was selected as the best and was used for medicated gel formulation. The weighed amount of PF-127 was slowly added to double distilled water with gentle mixing. The mixture was left in refrigerator at 4 °C overnight for complete swelling of the polymer. After the formation of a clear viscous solution, the accurately weighed amount of microsponges was added to the cold solution and were mixed gently with a glass rod. The solution was sonicated for 1 min at 4 °C to form a homogenous gel.
The free drug in gel was also prepared by adding the calculated amount of the drug to a definite volume of double distilled water and then was ultrasonicated for 2–3 min to decrease the size of drug crystals to about 5–10 µm, after that, the calculated amount of PF-127 powder was added to the preformed suspension, mixed gently and left overnight in refrigerator at 4 °C for complete swelling and dissolution of the polymer in water.
2.2.5. Evaluation of the prepared in situ gels containing acetazolamide loaded microsponges
2.2.5.1. Determination of the pH
The pH of the formulations were determined in triplicate using calibrated pH meter. The average reading was recorded.
2.2.5.2. Determination of the gelation time
The gelation time was determined by tube inversion method (Asasutjarit et al., 2011). 2 ml of the prepared gel maintained at 4 °C was placed in a test tube, the test tube was placed in water bath maintained at gelation temperature (35 °C ± 1), the in situ gel was observed for gelation by inverting the test tube at time intervals. The gelation time was determined when there is no flow of the gel upon tube inversion.
2.2.5.3. Determination of the gelling capacity
The gelling capacity was determined by placing a drop of the in situ gel in a test tube containing 2 ml of freshly prepared simulated tear fluid (pH 7.4) equilibrated at 35 ± 1 °C, the time taken for its gelling formation then dissolution of the gel was visually observed and the gelling capacity was evaluated (Qi et al., 2007) as follows:
| (−) | No gelation |
| (+) | The gel formed after few minutes and dissolved rapidly |
| (++) | Immediate gelation and remains for few hours |
| (++ +) | Immediate stiff gelation which remains for extended period of time |
2.2.5.4. Determination of the rheological behavior of the prepared gels
Viscosity of the prepared gels were determined using a Brookfield Programmable Rheometer (Model RVDV-III U) Brookfield Engineering laboratories, INC, Middleboro, MA, USA. The viscosity was determined at different shear rates from 10 to 50 rpm and then in a descending order (from 50 to 10 rpm) keeping a period of 10 s at each rpm. The samples were equilibrated at 35 ± 1 °C prior to each measurement. The viscometer was fitted with T-F spindle 96 and the viscosity was investigated. All measurements were performed in triplicates.
2.2.5.5. In vitro release of drug from microsponges alone and from acetazolamide loaded microsponges in situ gel formulations
In vitro release test was performed in 50 ml of simulated tear fluid (STF) solution (pH 7.4 at 35 ± 1 °C) at 50 rpm using the dialysis method. STF is composed of sodium bicarbonate (0.2%), calcium chloride dihydrate (0.008%) and sodium chloride (0.67%) (Marques et al., 2011). A semipermeable standard cellophane membrane was stretched over the end of a dialysis tube, an accurate weight of the microsponges alone or 0.5 g of each of the prepared microsponges gels (each corresponding to 5 mg of the drug) were placed on the membrane in the dialysis tube, which was suspended so that the membrane was just below the surface of the buffered dialysis solution, the assembly allowed to shake at 50 rpm at a temperature maintained at 35 ± 1 °C). Samples of 2 ml were withdrawn from the release medium in the beaker at different time intervals (0.08, 0.25, 0.5, 1, 2, 3, 4, 5 and 6 h) and were analyzed spectrophotometrically at 265 nm against a blank similarly treated. The withdrawn samples were replaced by equal volumes of the STF solution at the same temperature to maintain sink conditions. The experiment was conducted independently in triplicate.
Kinetic analysis of the in vitro release data was also done in order to determine the drug release mechanism, in vitro release data was fitted to a zero-order (m0–m = Kt), first order (log m = log m0–Kt/2.303) and Higuchi model (m0–m = Kt1/2) where m is the amount of the drug remaining in the formulation at time t and m0 is the initial amount of the drug in the formulation (Aksungur et al., 2011, Ali et al., 2014, Costa and Lobo, 2001). The regression coefficient values (r2) were calculated for all the models. Korsmeyer–Peppas equation (m0–m/m0 = Ktn) was used to study the diffusion mechanism by analyzing the diffusion exponent “n”. If n ≤ 0.45, the release follows fickian mechanism, if 0.5 ≤ n ≤ 0.8, the release follows non fickian mechanism (Varshosaz et al., 2008).
2.2.5.6. In vivo efficacy studies
Three domestic rabbits (average weight is 2.5 Kg) were used for each treatment in a cross-over experiment. The rabbits were placed in individual cages with access to food and water. They were maintained in a 12-hour light/12-hour dark cycle in a temperature controlled room (20–25 °C). The experimental procedures conform to the ethical principles of the Egyptian Research Institute of ophthalmology, Giza, Egypt on the use of animals (Monem et al., 2000). Only animals without any signs of ocular inflammation or other observable ocular abnormalities were included in the study. All animals received the five topical treatments: blank microsponges in situ gel (empty microsponges without drug) to establish the IOP baseline before treatment, S2 formula in situ gel (S2 ISG), S3 formula in situ gel (S3 ISG), free drug suspension and the free drug in gel. A 4-day washout period was allowed between treatments. The IOP was measured using standardized schiotz tonometer. Before taking the measurements, the rabbits, eyes were anaesthetized by instilling 1–2 drops of Benoxinate HCl 0.4% (Benox®) eye drops. A single 50 µl drop of the topical formulae loaded with 1% AZM was instilled into the lower cul-de-sac of the right eye. The left eye received no medication and served as a control. The IOP was measured immediately before administration and at time intervals (0.5, 1, 2, 3, 4 and 5 h) after receiving the medication. The IOP was measured three times at each time interval for the dosed eye and control eye and the means of the readings were taken. The readings were converted into intraocular pressure using the 1955 calibration scale for schiotz tonometer. The change in IOP is expressed as the average difference in IOP (ΔIOP) between the dosed and control eye of the same rabbit using equation (Kaur et al., 2000, Mishra and Jain, 2014b)
2.2.5.7. Ocular irritation test
The test was conducted according to the modified Draize test (Baeyens et al., 2002). All the glassware used in the experiment were sterilized by heating and all formulations were prepared under sterile conditions. Three domestic rabbits were used in this experiment. One drop (50 μl) of the microsponges in situ gel formulation was instilled into the lower cul-de-sac of the right eye of each rabbit. The untreated contra-lateral left eye was used as a control. The eyelids were gently held together for about 10 s to avoid the loss of instilled preparations. Each animal was observed for ocular reactions (redness, swelling discharge, conjunctival chemosis, iris and corneal lesions) at 5, 15, 30 min and 1, 2, 3, 6, 9, 12, 24 h post instillation. The following scores were used to evaluate the irritation (Lallemand et al., 2005). A score of 2 or 3 in any category was considered as an indicator of clinically significant irritation.
| 0: | No redness, no inflammation or excessive tearing |
| 1: | Mild redness with inflammation and slight tearing |
| 2: | Moderate redness with moderate inflammation and excessive tearing |
| 3: | Severe redness with severe inflammation and excessive tearing |
2.2.5.8. Stability studies
The stability studies for the prepared microsponges formulations without the gel (S1-S6) were performed at room temperature (25 °C) for 6 months and the effect on EE% and the mean particle size were noticed. Particle size and size distribution were measured after 3 and 6 months of storage. Selected S2 in situ gel microsponges formulation and the free drug in gel were stored at 4 °C for 8 weeks and were evaluated for pH and drug content to know the stability of microsponges in gel versus free drug in gel. 0.5 g of gel (theoretical drug content was 5 mg) was taken at each time interval and was dissolved in 0.1 M NaOH for extraction of the drug, filtered and then measured spectrophotometrically at 290 nm to determine the drug content.
2.2.5.9. Statistical analysis
Statistical analysis was carried out using GraphPad Prism software version 5. One-way analysis of variance (ANOVA) was used to analyze the differences between experimental groups. Newman-Keuls method was used as a post-hoc test. A probability of less than 0.05 (p < 0.05) was considered statistically significant. All experiments were conducted in triplicate and the results were presented as means ± SD.
3. Results and discussion
3.1. Preparation and characterization of microsponges formulations
AZM microsponges were prepared by the quasi emulsion solvent diffusion method using EC polymer which is biologically inert, non-irritating, non-mutagenic, non-allergenic, non-toxic and non-biodegradable polymer (Parikh, 2010). Quasi emulsion solvent diffusion method seems to be easy, reproducible, rapid and has an advantage of avoiding solvent toxicity (Re and Biscans, 1999).
3.1.1. Effect of the formulation parameters on the mean particle size, PDI, EE% and DL%
3.1.1.1. Effect of the drug to polymer ratio
The effect of the drug to polymer ratio on the production yield, drug content, entrapment efficiency and mean particle size is illustrated in Table 2. The statistical manipulation of the results using GraphPad Prism software version 5, one-way analysis of variance (ANOVA) revealed that the polymer and drug concentration have a great influence on the EE% and DL %. The EE% decreased significantly (P < 0.05) from (92.42 ± 4.7) to (47.59 ± 4.5) as the drug concentration decreased from S1 to S3 formulae, also, increasing the polymer concentration from (1:1) drug : polymer ratio in S3 formula to (1:4) in S6 formula resulted in decreasing of the EE% from 47.59 ± 4.5 to 26.85 ± 6.2. This result could be attributed to the increasing in viscosity due to increasing the polymer concentration which led to formation of more rigid polymer coat and difficulty of drug transfer with more viscous medium resulted in low EE%. This is in agreement with the opinion of (Nadia Morsi, et al) who interpreted that increasing in polymer concentration resulted in low EE% due to formation of compact polymer coat that hindered the drug entrapment (Morsi et al., 2016). The mean particle size of the microsponges ranged from 10.89 µm ± 1.02 for S2 formula to 35.30 µm ± 2.72 for S6 formula. Increasing the polymer concentration was found to significantly increased the particle size (p < 0.05). S3, S5, and S6 formulae were significantly different in particle sizes (P < 0.01). This result may be due to increasing the viscosity of the dispersed phase resulted in formation of large globules which were hard to be divided into smaller particles, hence larger droplets were formed and the mean particle size increased. The small PDI values observed for all formulations which are shown in table 2 indicated that the microsponges were homogenous and had narrow size distribution except S6 formulation which had a high PDI value of about 0.65 ± 0.25.
Table 2.
The effect of the drug to polymer ratio on microsponges formation (Values are the mean ± SD (n = 3)).
| Batch Code | Drug:Polymer ratio | Production yield (%) | Theoretical drug content (%) | Actual drug content (%) | Entrapment efficiency (%) | Mean particle size (µm) | PDI |
|---|---|---|---|---|---|---|---|
| S1 | 3:1 | 58.18 ± 0.47 | 75.00 | 69.32 ± 3.56 | 92.42 ± 4.7 | 17.00 ± 0.45 | 0.28 ± 0.08 |
| S2 | 2:1 | 42.10 ± 3.56 | 66.67 | 54.65 ± 1.37 | 82.02 ± 2.5 | 10.89 ± 1.02 | 0.22 ± 0.12 |
| S3 | 1:1 | 31.33 ± 7.85 | 50.00 | 23.80 ± 1.85 | 47.59 ± 4.5 | 11.10 ± 0.60 | 0.40 ± 0.07 |
| S4 | 1:2 | 47.75 ± 9.55 | 33.33 | 13.50 ± 2.05 | 40.51 ± 7.5 | 14.03 ± 0.78 | 0.15 ± 0.03 |
| S5 | 1:3 | 25.50 ± 1.77 | 25.00 | 6.82 ± 2.18 | 27.28 ± 8.7 | 16.99 ± 2.21 | 0.20 ± 0.06 |
| S6 | 1:4 | 29.80 ± 1.98 | 20.00 | 5.37 ± 1.25 | 26.85 ± 6.2 | 35.30 ± 2.72 | 0.65 ± 0.25 |
3.1.1.2. Effect of the volume of the internal phase
The results in Table 3 show that increasing the volume of DCM decreases the mean particle size of the microsponges. The same finding was reported by (Nokhodchi et al., 2005). It was found that there was significant decrease in the mean particle size of S8 and S2 compared to S7 (P < 0.001). This could be explained in terms of viscosity of the internal phase; decreasing the volume of DCM resulted in higher viscosity of the internal phase, leading to the formation of large droplets when added to the external aqueous phase that probably need more energy to be divided into smaller particles and the mean particle size increased. Significant decreasing in the values of EE% were observed in S8 formula using 20 ml DCM compared to S2 and S7 (P < 0.001). The microsponges with better entrapment efficiency were produced when 5 and 10 ml of DCM were used. 10 ml of DCM was chosen as the optimum volume due to highest production yield and the least particle size produced which is suitable for ophthalmic administration.
Table 3.
The effect of the volume of DCM on microsponges formation (Values are the mean ± SD (n = 3)).
| Batch Code | Volume of DCM (ml) | Production yield (%) | Actual drug content (%) | Entrapment efficiency (%) | Mean particle size (µm) | PDI |
|---|---|---|---|---|---|---|
| S7 | 5 | 35.32 ± 2.15 | 55.49 ± 2.28 | 83.23 ± 3.42 | 25.61 ± 3.42 | 0.26 ± 0.08 |
| S2 | 10 | 44.02 ± 3.56 | 54.65 ± 1.37 | 82.02 ± 2.51 | 10.89 ± 2.51 | 0.22 ± 0.12 |
| S8 | 20 | 25.24 ± 0.37 | 27.50 ± 0.95 | 41.24 ± 1.43 | 11.65 ± 1.43 | 0.22 ± 0.01 |
3.1.1.3. Effect of the stirring speed and stirring time
The stirring speed was found to have the greatest influence on microsponges particles size as shown in Table 4. The mean particle size was found to be significantly decreased (P < 0.05) from (296.79 µm ± 0.22) to (10.89 µm ± 1.02) by increasing the stirring speed from 500 to 3000 rpm. High stirring speed led to more breaking down of droplets and decreasing coalescence resulted in the formation of smaller microsponges with particle size of 10 µm which is appropriate size for ophthalmic administration (Rajasekaran et al., 2010, Rathore and Nema, 2009, Tangri and Khurana, 2011). Stirring rate of 500 rpm was proved to give the highest yield (73.32% ± 3.31) and highest EE% (94.96 ± 6.03) but the mean particle size was about (296.79 µm ± 0.22) which is suitable for microsonges intended for oral preparations. Increasing the stirring time didn't significantly affect the EE% or the mean particle size of the microsponges (P > 0.05) as shown in Table 5. 30 min stirring time was not enough for formation of microsponges due to incomplete evaporation of DCM. Two hours of stirring time was chosen as appropriate time for formation of microsponges and ensure the complete evaporation of DCM
Table 4.
The effect of stirring speed on microsponges formation (Values are the mean ± SD (n = 3)).
| Batch Code | Stirring speed (rpm) | Production yield (%) | Actual drug content (%) | Entrapment efficiency (%) | Mean particle size (µm) | PDI |
|---|---|---|---|---|---|---|
| S9 | 500 | 73.32 ± 3.31 | 63.29 ± 4.23 | 94.96 ± 6.03 | 296.79 ± 0.22 | 0.12 ± 0.02 |
| S10 | 1000 | 61.77 ± 1.26 | 59.44 ± 0.16 | 88.88 ± 0.2 | 96.96 ± 7.98 | 0.17 ± 0.06 |
| S11 | 2000 | 51.16 ± 1.17 | 61.05 ± 1.06 | 91.58 ± 1.57 | 34.61 ± 4.18 | 0.12 ± 0.02 |
| S2 | 3000 | 42.45 ± 3.56 | 54.65 ± 1.37 | 82.02 ± 2.51 | 10.89 ± 1.02 | 0.22 ± 0.12 |
Table 5.
The effect of stirring time on microsponges formation (Values are the mean ± SD (n = 3)).
| Batch code | Time of stirring (hs) | Production yield (%) | Actual drug content (%) | Entrapment efficiency (%) | Mean particle size (µm) |
|---|---|---|---|---|---|
| S12 | 1 | 40.68 ± 2.58 | 52.36 ± 2.44 | 78.54 ± 3.66 | 13.04 ± 1.45 |
| S2 | 2 | 42.45 ± 3.32 | 54.65 ± 1.37 | 82.02 ± 2.12 | 10.89 ± 1.02 |
| S13 | 4 | 39.85 ± 3.21 | 53.77 ± 2.91 | 80.65 ± 4.36 | 10.22 ± 1.98 |
| S14 | 8 | 47.97 ± 5.36 | 57.13 ± 2.21 | 85.69 ± 3.32 | 11.21 ± 0.78 |
3.1.1.4. Effect of the amount of emulsifying agent (PVA)
The effect of the amount of emulsifying agent on microsponges formulations is shown in Table 6. There were no significant differences in EE% by using different concentrations of PVA (P > 0.05), also it was observed that as the amount of PVA increased from 0.1 to 0.5%w/v, significant decrease in the mean particle size was observed (P < 0.05), which was in agreement of the opinion of (Vysloužil J, et al) who reported that an excessive increase of PVA concentration can lead to lower particle size (Vysloužil et al., 2014). In another report, increasing PVA concentration ensured better system stabilization against coalescence of the emulsion and therefore led to formation of smaller microparticles (Yang et al., 2001). Increasing the PVA concentration above 0.5% w/v showed a random effect on particle size. So, the concentration of 0.5% w/v of PVA was selected as an optimum concentration as it produced the least particle size (10.89 ± 1.02) with good PDI and EE%.
Table 6.
The effect of the amount of emulsifying agent on microsponges formation. (Values are the mean ± SD (n = 3)).
| Batch code | Amount of PVA (%w/v) | Production yield (%) | Actual drug content (%) | Entrapment efficiency (%) | Mean particle size (µm) | PDI |
|---|---|---|---|---|---|---|
| S15 | 0.1 | 37.87 ± 2.87 | 59.71 ± 2.35 | 89.60 ± 3.50 | 22.0 ± 3.21 | 0.71 ± 0.58 |
| S16 | 0.3 | 50.0 ± 9.30 | 60.03 ± 1.06 | 90.08 ± 1.56 | 17.12 ± 0.25 | 0.31 ± 0.04 |
| S2 | 0.5 | 42.45 ± 3.56 | 54.65 ± 1.37 | 82.02 ± 2.51 | 10.89 ± 0.72 | 0.22 ± 0.12 |
| S17 | 0.7 | 48.05 ± 3.32 | 59.15 ± 0.62 | 88.73 ± 0.92 | 17.55 ± 1.32 | 0.25 ± 0.09 |
| S18 | 1 | 57.83 ± 0.17 | 54.70 ± 3.31 | 82.07 ± 4.95 | 12.44 ± 1.74 | 0.26 ± 0.08 |
3.1.1.5. Effect of the ultrasonication time
Table 7 shows the effect of ultrasonication time on microsponges formation. Increase the sonication time from 5 to 20 min led to significant increase in the EE% (P < 0.01), this may be due to more size reduction of the drug particles by increasing the sonication time which improved the drug entrapment through microsponges. Also, it resulted in decreasing in the mean particle size of microsponges.
Table 7.
The effect of the ultrasonication time on microsponges formation (Values are the mean ± SD (n = 3)).
| Batch code | Ultrasonication time (min.) | Production yield (%) | Theoretical drug content (%) | Actual drug content (%) | EE% | Mean particle size (µm) | PDI |
|---|---|---|---|---|---|---|---|
| S19 | 5 | 38.35 ± 1.65 | 66.67 | 45.77 ± 1.50 | 68.73 ± 2.25 | 19.11 ± 0.13 | 0.28 ± 0.03 |
| S20 | 10 | 32.16 ± 3.50 | 66.67 | 48.49 ± 2.02 | 72.82 ± 3.04 | 19.35 ± 0.69 | 0.31 ± 0.05 |
| S2 | 20 | 42.45 ± 3.56 | 66.67 | 54.65 ± 1.68 | 82.02 ± 2.51 | 10.89 ± 1.02 | 0.22 ± 0.12 |
According to the results of the optimization studies, the optimum formulation parameters for microsponges preparation with quasi-emulsion solvent diffusion method to obtain the smallest particle size suitable for ophthalmic administration are shown in Table 8.
Table 8.
Optimum parameters for microsponges preparation with quasi-emulsion solvent diffusion method to obtain the size suitable for ophthalmic administration.
| Specification | Optimum value |
|---|---|
| Drug: polymer ratio | 2:1 |
| Conc of PVA (%w/v) | 0.5 |
| Volume of organic solvent (ml) | 10 |
| Volume of water in external phase (ml) | 100 |
| Ultrasonication time (minutes) | 20 |
| Stirring speed (rpm) | 3000 |
| Stirring time (hs) | 2 |
3.1.2. Characterization of microsponges formulations
3.1.2.1. Microsponges surface morphology
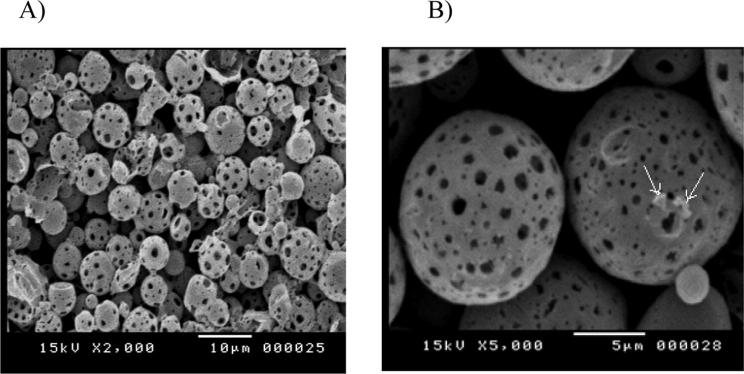
Fig. 1 shows the scanning electron micrographs of microsponges formulation S2. The figure clearly demonstrates that the particles are within the size range of 10 µm and that they are hollow with spherical shape and porous surface. Minor drug particles were also adsorped on the surface of microsponges as shown in Fig. 1B.
Fig. 1.
Scanning electron micrographs of microsponges formulation S2.
3.1.2.2. Differential scanning calorimetric (DSC) analysis
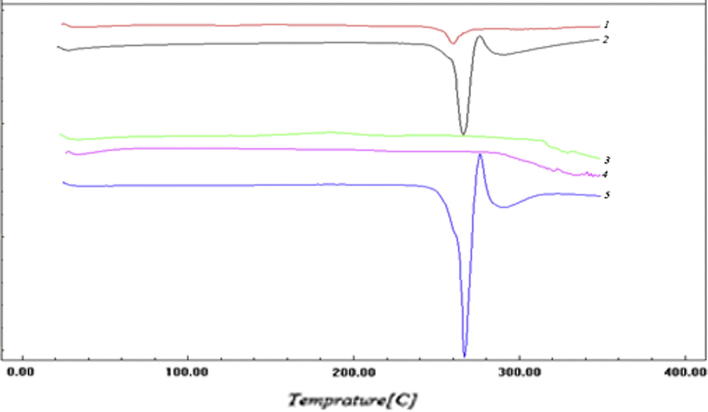
DSC studies were carried out to confirm compatibility between the drug and the polymer. The thermal behavior of the drug, EC polymer, physical mixture of drug & EC polymer, plain formula (pure microsponges without drug) and selected formula S2 are presented in Fig. 2. The thermograms showed a sharp endothermic peak at 260 °C corresponding to the melting point of AZM in the crystalline form. The DSC curves of the physical mixture of drug and polymer and S2 formula exhibited the same characteristic peak of the drug. The results indicate that the drug is intact and preserves the peak of its melting point at 260 °C. Also it is indicative of the compatibility between the drug and the polymer, and the suitability of the preparation process. The small intensity of the endothermic peak in S2 formula compared to the physical mixture indicates the good entrapment of the drug inside the voids of the microsponges. No new peaks were observed in the melting point range as observed in the DSC thermograms of microsponges formulation.
Fig. 2.
DSC thermograms of (1) S2 formula, (2) Physical mixture of drug and EC polymer, (3) Plain formula (EC microsponges without drug), (4) EC polymer, (5) Pure AZM.
3.1.2.3. In-vitro release of acetazolamide from microsponges without the gel
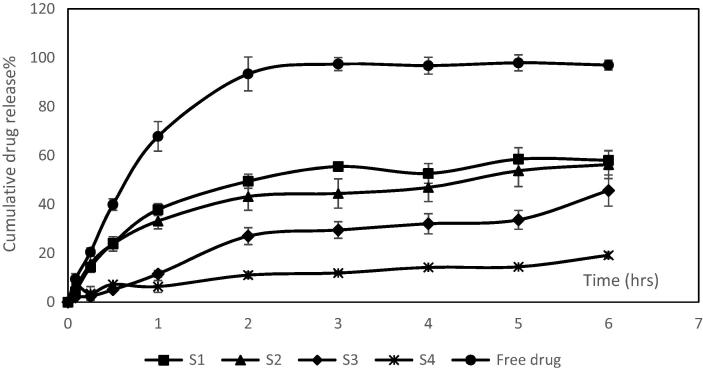
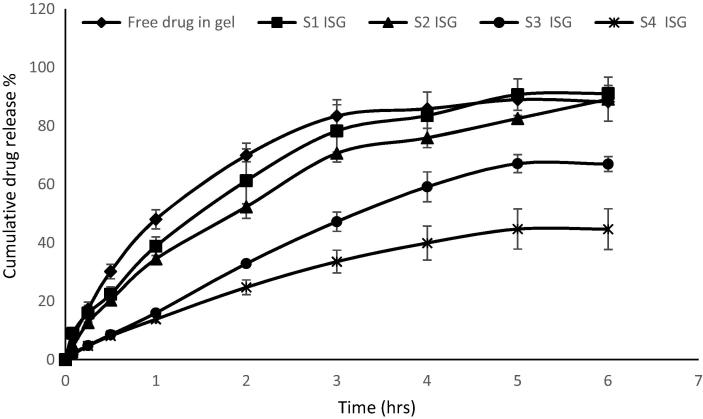
S1, S2, S3 and S4 formulations were chosen for release studies due to their small particle size and high to moderate EE%. The in vitro release studies of these formulations compared to the release of free drug are shown in Fig. 3. The free drug had shown to exhibit a significant higher and faster release (P < 0.001) than from microsponges formulations. The free drug showed about 67.8% of cumulative release after one hour whereas the microsponges formulations showed 6.36–37.87% drug release after one hour. After 2 h, almost all free drug was released (about 93.38%) from the membrane. EC polymer was found to retard the release of the drug from microsponges to a large extent, also increasing the polymer concentration with respect to drug was found to retard the drug release as shown in S4 formula. This could be attributed to that the mirosponges retarded the drug release due to inclusion of the drug within the voids of the mirosponges. These voids acted as a drug reservoir and prolonged the release. Increase the polymer concentration led to increase the wall thickness and the size of the prepared microsponges, resulted in the reduction of surface area and retardation of the drug release from the microsponges. Also, the slow drug release from microsponges may be attributed to the hydrophobic and floating properties of the microsponges leading to reduction of the drug release.
Fig. 3.
In vitro release profiles of AZM from S1, S2, S3 and S4 microsponges formulations compared to free drug release.
3.2. Evaluation of the prepared gels containing acetazolamide microsponges
3.2.1. Determination of the pH
The ideal pH for an ophthalmic preparation should be in the range of 7.2 ± 0.2 (Ammar et al., 2009). The pH values of the prepared gel formulations were measured and found to be in the range of 6.64 to 7.14 (Table 9). However, the limited buffering capacity of the tears is able to adjust the pH values to the physiological pH if it ranged from 3.5 to 8.5 (Fialho and Silva‐Cunha, 2004). Therefore the prepared microsponge gels are adequate for ocular application because they were not buffered and could be adjusted to the physiological pH values by tears.
Table 9.
Evaluation of the in situ gels containing AZM loaded microsponges (Value are the mean ± SD (n = 3)).
| Batch Code | pH measurement | Gellation time (s) | Gelling capacity |
|---|---|---|---|
| S1 ISG | 7.14 ± 0.11 | 22.33 ± 0.58 | ++ |
| S2 ISG | 7.01 ± 0.03 | 24.67 ± 2.89 | ++ |
| S3 ISG | 6.64 ± 0.12 | 23.00 ± 3.61 | ++ |
| S4 ISG | 6.98 ± 0.01 | 17.00 ± 2.65 | ++ |
3.2.2. Determination of the gelation time
The ideal in situ gelling system is the system which is gelled rapidly on exposure to body temperature to prevent its quick removal by tear fluid (Venkatesh et al., 2013). S1, S2 and S3 in situ gels formulations (S1 ISG, S2 ISG and S3 ISG respectively) showed quick gelation time of about 22 to 24 s as presented in Table 9, It was noticed that S4 in situ gel (S4 ISG) showed shorter gelation time of about 17 s which may be due to the large amount of microsponges used during preparation because the EE% of S4 formula was low (40.5% only) compared to S2 formula (82%).
3.2.3. Determination of the gelling capacity
It was observed that (15%w/v PF-127) showed no gelation (“−” grade of gelling capacity) at physiological temperature (35 °C). The (20% w/v PF-127) showed “+” grade of gelling capacity which was formed after few minutes and dissolved rapidly. The (25%w/v PF-127) showed “++” grade of gelling capacity which is reported as the most satisfactory grade (Song et al., 2013), so, this concentration was used for different microsponges gel formulations due to its best gelling capacity characters. The results of the gelling capacity of different microsponges gel formulations are shown in Table 9, it was noticed that all medicated in situ gel formulations were gelled immediately when exposed to STF at 35 ± 1 °C and it retained for about 4 to 5 h.
3.2.4. Determination of the rheological behavior of the prepared gels
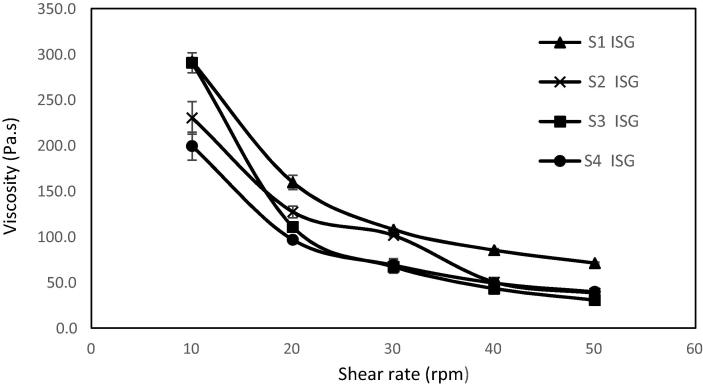
The rheological behavior of the in situ gel formulations (S1 ISG-S4 ISG) is cited in Fig. 4. It was found that the formulations exhibited pseudoplastic flow characteristics (shear thinning systems); the viscosity was increased at low shear rates and decreased under conditions of high shear rates. An advantage of shear thinning formulations is that they have a high viscosity in the open eye, stabilizing the tear film. When blinking occurs, such polymers thin, preventing the feeling of irritation that would occur with high viscosity Newtonian fluid (Fiscella, 2008) and thus allow a good distribution of the formulation over the surface of the eye.
Fig. 4.
The rheological profile of the medicated in situ gel formulations.
3.2.5. In-vitro release of acetazolamide from in-situ gel formulations
Fig. 5 shows the in vitro release profile of AZM from in situ gel formulations S1 ISG to S4 ISG compared to the release of free drug in gel. Incorporation of the medicated microsponges in pluronic gels enhanced the drug release as PF-127 gel decreased the hydrophobic characteristics of the microsponges. This is because PF-127 is non-ionic polymeric surfactant (Schmolka, 1972) which led to increase in the wettability of the microsponges. S1 ISG and S2 ISG formulations showed superior drug release compared to other formulations. S4 ISG formula showed the slowest drug release due to increasing the concentration of EC polymer which retarded drug release as discussed before in the in-vitro release of AZM from microsponges alone.
Fig. 5.
In vitro release profile of AZM from S1 ISG, S2 ISG, S3 ISG and S4 ISG formulations compared to the release of free drug in gel.
3.2.6. Kinetic analysis of the in vitro release data
In order to obtain the mechanism of drug release, the data was fitted according to different release models and the correlation coefficients (r2) were calculated and shown in Table 10. Almost the drug release from the microsponges in situ gels followed Higuchi diffusion model. The diffusion exponent “n” of the Korsmeyer–Peppas equation was 0.5 ≤ n ≤ 0.8 which indicated anomalous diffusion or Non-Fickian diffusion mechanism.
Table 10.
Kinetic models of AZM release from different microsponges in situ gels.
| Batch code | Zero order (r2) | First order (r2) | Higuchi model (r2) | Korsmeyer–Peppas (n) |
|---|---|---|---|---|
| S1 ISG | 0.9768 | 0.9968 | 0.9952 | 0.58 |
| S2 ISG | 0.9780 | 0.9956 | 0.9973 | 0.69 |
| S3 ISG | 0.9800 | 0.9908 | 0.9908 | 0.89 |
| S4 ISG | 0.9733 | 0.9835 | 0.9940 | 0.74 |
3.2.7. In vivo efficacy studies
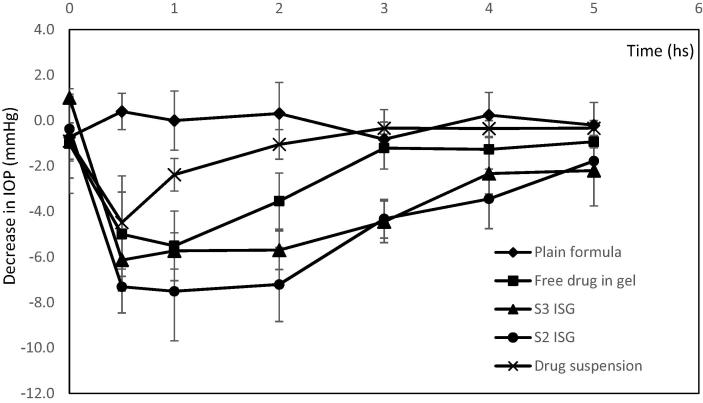
The results of the in vivo efficacy studies are shown in Fig. 6. The results showed that the free drug suspension showed rapid reduction in the IOP values (about −4.5) after half an hour from instilling the drug into the eye and the effect was rapidly depleted within one hour. The rapid and weak effect of the drug suspension was attributed to the rapid washing out of the instilled eye drops and the low permeability coefficient of AZM (4.1 × 10−6 cm/sec). There was significant decrease in the IOP values with S2 and S3 formulae versus plain formula at all measurement times (p < 0.05). The free drug in gel showed significant decrease in the IOP values than plain formula after the first 2 h only (p < 0.01). S2 ISG formula showed significant decrease in the values of IOP after 2 and 3 h compared to free drug in gel (p < 0.05), also S3 ISG formula showed significant decrease in the IOP values after 3 h (p < 0.01) compared to free drug in gel. It was observed that S2 ISG formula exhibited the maximum and fastest decrease in the IOP values (−7.3 after 0.5 h) while S3 ISG formula showed decrease in the IOP values of about (−6.3) compared to free drug in gel (the maximal decrease was −5.5 after 1 h). Although, the in vitro release of free drug in gel was higher, its in vivo therapeutic efficacy was lower than S2 and S3 formula in gel due to the low permeability coefficient of AZM which limits its ocular bioavailability. The instillation of the free drug in gel into rabbit's eye released the drug rapidly till one hour and affected the IOP, then, the amount of the drug decreased and accordingly, the influence on IOP decreased. On the contrary, in case of formulae S2 and S3, the release of AZM was continued from the microsponges which gave higher ocular permeability (due to inclusion into microsponges which allowed more residence time) and so, the effect on IOP was higher. The effect of S2 ISG and S3 ISG formulations was remained for about 5 h while the effect of the free drug suspension and free drug in gel remained only for 2 and 3 h respectively.
Fig. 6.
Changes of IOP values with time in the treated normal rabbits for S2 ISG and S3 ISG formulae compared to free drug in gel and drug suspension. (Note that: The negative sign indicates that the IOP values decreased below than the normal level).
It was found that there were no changes in the values of IOP observed in the untreated eye during the period of treatment indicating that the formulations produced the effect due to a local action not due to the systemic absorption of the drug.
3.2.8. Ocular irritation test
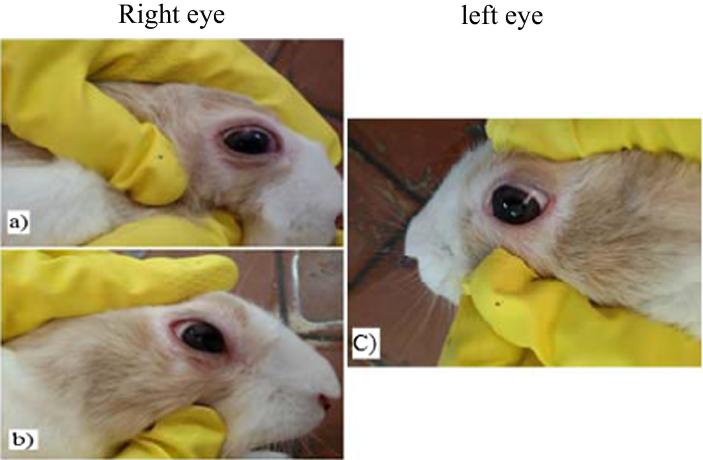
No signs of ocular irritation such as redness, tearing or swelling were observed in S2 ISG formula indicating that it is not irritant. However, S3 ISG formula showed slight redness and tearing which disappeared after 30 min as shown in Fig. 7. It may be due to the high microsponges content during gel preparation because of the low drug EE% in S3 compared to S2 formula.
Fig. 7.
Ocular irritation test of S3 ISG formula after (a) 15 min, (b) 30 min) of the treated right eye compared to (c) left untreated (control) eye.
3.2.9. Stability studies
The results of stability studies of the microsponges without the gel are shown in Table 11. It was observed that there were no significant changes in the EE (%) or the mean particle size of the microsponges (S1-S6) over the whole period of storage. It was concluded that the formulation of microsponges alone were stable for 6 months of storage at room temperature (25 °C). Table 12 shows the results of stability studies of the selected S2 formula in gel (S2 ISG) compared to free drug in gel at 4 °C. It is observed that the drug content (%) for the free drug in gel decreased by about 12.7% and 23.77% after 2 weeks and 8 weeks of storage respectively. The selected S2 ISG showed a decrease in drug content by about 2.9% only and 19.1% after 4 weeks and 8 weeks of storage respectively. It is concluded that the incorporation of the drug into the microsponges increases the stability of the drug in gel till 4 weeks of storage at a temperature of 4 °C. Concerning the pH, it is noticed that there were no any significant changes in the pH values for the two formulations studied over the whole period of storage.
Table 11.
The entrapment efficiencies (%) and mean particle size after storage of the microsponges without the gel (S1-S6).
| Parameters | Formulation code | Sampling time |
||||||
|---|---|---|---|---|---|---|---|---|
| Initial | 1 month | 2 months | 3 months | 4 months | 5 month | 6 months | ||
| EE (%) | S1 | 92.42 ± 4.7 | 95.82 | 91.65 | 92.58 | 90.33 | 92.82 | 90.88 |
| S2 | 82.02 ± 2.5 | 82.71 | 84.55 | 82.60 | 83.29 | 81.35 | 82.26 | |
| S3 | 47.59 ± 4.5 | 50.63 | 45.72 | 50.23 | 48.50 | 47.60 | 48.94 | |
| S4 | 40.51 ± 7.5 | 41.84 | 42.26 | 35.82 | 36.22 | 41.39 | 40.48 | |
| S5 | 27.28 ± 8.7 | 26.60 | 21.22 | 26.25 | 30.65 | 27.86 | 27.52 | |
| S6 | 26.85 ± 6.2 | 26.20 | 21.80 | 25.48 | 27.60 | 23.21 | 23.76 | |
| Mean particle size (µm) | S1 | 17.00 ± 0.45 | 17.28 | 19.71 | ||||
| S2 | 10.89 ± 1.02 | 11.05 | 11.71 | |||||
| S3 | 11.10 ± 0.60 | 12.59 | 12.63 | |||||
| S4 | 14.03 ± 0.78 | 13.91 | 15.19 | |||||
| S5 | 16.99 ± 2.21 | 15.99 | 15.63 | |||||
| S6 | 35.30 ± 2.72 | 35.83 | 37.57 | |||||
Table 12.
Stability studies of S2 ISG formula compared to free AZM in gel.
| Parameters | Formulation code | Sampling time |
||||
|---|---|---|---|---|---|---|
| Initial | 1 week | 2 weeks | 4 weeks | 8 weeks | ||
| Drug content (%) | S2 ISG | 99.8 | 98.70 | 97.60 | 97.18 | 80.99 |
| Free drug in gel | 100 | 97.80 | 87.30 | 80.23 | 76.23 | |
| pH measurement | S2 ISG | 7.01 ± 0.03 | 7.20 | 7.12 | 6.91 | 6.92 |
| Free drug in gel | 6.86 ± 0.2 | 6.78 | 6.94 | 6.93 | 7.20 | |
4. Conclusion
Stable acetazolamide microsponges were successfully prepared by the quasi emulsion solvent diffusion method. The microsponges were spherical porous particles as shown by SEM. S2 formula showed a mean particle size of about 10 µm which is suitable for ocular administration. The microsponges were incorporated into (25% w/v) pluronic F-127 in situ gels. The prepared gels exhibited pseudoplastic rheological properties which is more comfortable to the eyes. The in vitro release kinetics of the gel formulations followed Higuchi diffusion model. The optimized S2 formula in gel could significantly decrease the IOP in rabbits' eyes causing no irritation. Acetazolamide microsponges in situ gel formulations could be successfully used for topical ocular administration for the treatment of glaucoma and avoiding the systemic side effects accompanied by oral acetazolamide.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Manar M. Obiedallah, Email: Manarhamed9053@yahoo.com.
A.M. Abdel-Mageed, Email: Razakmh48@yahoo.com.
Tahani H. Elfaham, Email: Telfaham@aun.edu.eg.
References
- Abdelmalak N.S., El-Menshawe S.F. A new topical fluconazole microsponge loaded hydrogel: preparation and characterization. Int. J. Pharm. Pharm. Sci. 2012;4(1):460–469. [Google Scholar]
- Aggarwal D., Garg A., Kaur I.P. Development of a topical niosomal preparation of acetazolamide: preparation and evaluation. J. Pharm. Pharmacol. 2004;56(12):1509–1517. doi: 10.1211/0022357044896. [DOI] [PubMed] [Google Scholar]
- Akash M., Iqbal F., Raza M., Rehman K., Ahmed S. Characterization of ethylcellulose and hydroxypropyl methylcellulose microspheres for controlled release of Flurbiprofen. J. Pharm. Drug Deliv. Res. 2013;2(1):2. [Google Scholar]
- Aksungur P., Demirbilek M., Denkbaş E.B., Vandervoort J., Ludwig A., Ünlü N. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake, and kinetic studies. J. Controlled Release. 2011;151(3):286–294. doi: 10.1016/j.jconrel.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Aldawsari H., Badr-Eldin S.M. Microsponges as promising vehicle for drug delivery and targeting: preparation, characterization and applications. African J. Pharm. Pharmacol. 2013;7(17):873–881. [Google Scholar]
- Ali J., Bhatnagar A., Kumar N., Ali A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int. J. Biol. Macromol. 2014;65:479–491. doi: 10.1016/j.ijbiomac.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Ammar H., El-Nahhas S., Khalil R. Cyclodextrins in acetazolamide eye drop formulations. Die Pharmazie. 1998;53(8):559–562. [PubMed] [Google Scholar]
- Ammar H.O., Salama H., Ghorab M., Mahmoud A. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS Pharm. Sci. Tech. 2009;10(3):808. doi: 10.1208/s12249-009-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrutiya N., Bajaj A., Madan M. Development of microsponges for topical delivery of mupirocin. AAPS Pharm. Sci. Tech. 2009;10(2):402–409. doi: 10.1208/s12249-009-9220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asasutjarit R., Thanasanchokpibull S., Fuongfuchat A., Veeranondha S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int. J. Pharmaceut. 2011;411(1):128–135. doi: 10.1016/j.ijpharm.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Baeyens V., Felt-Baeyens O., Rougier S., Pheulpin S., Boisrame B., Gurny R. Clinical evaluation of bioadhesive ophthalmic drug inserts (BODI®) for the treatment of external ocular infections in dogs. J. Controlled Release. 2002;85(1):163–168. doi: 10.1016/s0168-3659(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Camargo E.E. Brain SPECT in neurology and psychiatry. J. Nucl. Med. 2001;42(4):611–623. [PubMed] [Google Scholar]
- Çomoğlu T., Gönül N., Baykara T. Preparation and in vitro evaluation of modified release ketoprofen microsponges. Il Farmaco. 2003;58(2):101–106. doi: 10.1016/s0014-827x(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Costa P., Lobo J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharmaceut. Sci. 2001;13(2):123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- El-Gazayerly O.N., Hikal A.H. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int. J. Pharmaceut. 1997;158(2):121–127. [Google Scholar]
- El-Laithy H., Nesseem D., Shoukry M. Evaluation of two in situ gelling systems for ocular delivery of Moxifloxacin: in vitro and in vivo studies. J. Chem. Pharmaceut. Res. 2011;3(2):66–79. [Google Scholar]
- Epstein D.L., Grant W.M. Carbonic anhydrase inhibitor side effects: serum chemical analysis. Arch. Ophthalmol. 1977;95(8):1378–1382. doi: 10.1001/archopht.1977.04450080088009. [DOI] [PubMed] [Google Scholar]
- Fialho S.L., Silva-Cunha D. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004;32(6):626–632. doi: 10.1111/j.1442-9071.2004.00914.x. [DOI] [PubMed] [Google Scholar]
- Fiscella R.G. Butterworth-Heinemann; Saint Louis: 2008. Chapter 2 - Ophthalmic Drug Formulations, Clinical Ocular Pharmacology (Fifth Edition) pp. 17–37. [Google Scholar]
- Friedman Z., Allen R.C., Raph S.M. Topical acetazolamide and methazolamide delivered by contact lenses. Arch. Ophthalmol. 1985;103(7):963–966. doi: 10.1001/archopht.1985.01050070089036. [DOI] [PubMed] [Google Scholar]
- Granero G., Longhi M., Becker C., Junginger H., Kopp S., Midha K., Shah V., Stavchansky S., Dressman J., Barends D. Biowaiver monographs for immediate release solid oral dosage forms: Acetazolamide. J. Pharmaceut. Sci. 2008;97(9):3691–3699. doi: 10.1002/jps.21282. [DOI] [PubMed] [Google Scholar]
- Guinedi A.S., Mortada N.D., Mansour S., Hathout R.M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharmaceut. 2005;306(1):71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Hathout R.M., Mansour S., Mortada N.D., Guinedi A.S. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharm. Sci. Tech. 2007;8(1):E1–E12. doi: 10.1208/pt0801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iego, S., 2012. A guide to dynamic light scattering measurement and analysis. NanoComposix.
- Jadhav N., Patel V., Mungekar S., Bhamare G., Karpe M., Kadams V. Microsponge delivery system: an updated review, current status and future prospects. J. Sci. Innov. Res. 2013;2(6):1097–1110. [Google Scholar]
- Jain V., Singh R. Design and characterization of colon-specific drug delivery system containing paracetamol microsponges. Arch. Pharmacal. Res. 2011;34(5):733–740. doi: 10.1007/s12272-011-0506-4. [DOI] [PubMed] [Google Scholar]
- Jelvehgari M., Siahi-Shadbad M.R., Azarmi S., Martin G.P., Nokhodchi A. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int. J. Pharmaceut. 2006;308(1–2):124–132. doi: 10.1016/j.ijpharm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Kaur I.P., Singh M., Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int. J. Pharmaceut. 2000;199(2):119–127. doi: 10.1016/s0378-5173(00)00359-8. [DOI] [PubMed] [Google Scholar]
- Krauland A.H., Leitner V.M., Bernkop-Schnürch A. Improvement in the in situ gelling properties of deacetylated gellan gum by the immobilization of thiol groups. J. Pharmaceut. Sci. 2003;92(6):1234–1241. doi: 10.1002/jps.10371. [DOI] [PubMed] [Google Scholar]
- Lallemand F., Furrer P., Felt-Baeyens O., Gex-Fabry M., Dumont J.-M., Besseghir K., Gurny R. A novel water-soluble cyclosporine A prodrug: ocular tolerance and in vivo kinetics. Int. J. Pharmaceut. 2005;295(1):7–14. doi: 10.1016/j.ijpharm.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Marques M.R., Loebenberg R., Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28. [Google Scholar]
- Mishra V., Jain N.K. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharmaceut. 2014;461(1–2):380–390. doi: 10.1016/j.ijpharm.2013.11.043. [DOI] [PubMed] [Google Scholar]
- Mishra V., Jain N. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharmaceut. 2014;461(1):380–390. doi: 10.1016/j.ijpharm.2013.11.043. [DOI] [PubMed] [Google Scholar]
- Monem A.S., Ali F.M., Ismail M.W. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int. J. Pharmaceut. 2000;198(1):29–38. doi: 10.1016/s0378-5173(99)00348-8. [DOI] [PubMed] [Google Scholar]
- Mora M.J., Tartara L.I., Onnainty R., Palma S.D., Longhi M.R., Granero G.E. Characterization, dissolution and in vivo evaluation of solid acetazolamide complexes. Carbohydrate Polym. 2013;98(1):380–390. doi: 10.1016/j.carbpol.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Morsi N., Mohamed M., Refai H., El Sorogy H. Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int. J. Pharm. Pharmaceut. Sci. 2014;6(11):227–236. [Google Scholar]
- Morsi N., Ghorab D., Refai H., Teba H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int. J. Pharmaceut. 2016;506(1–2):57–67. doi: 10.1016/j.ijpharm.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Morsi N., Ibrahim M., Refai H., El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur. J. Pharmaceut. Sci. 2017;104:302–314. doi: 10.1016/j.ejps.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Nokhodchi A., Jelveghari M., Siahi M.-R., Dastmalchi S. The effect of formulation type on the release of benzoyl peroxide from microsponges. Iranian J. Pharmaceut. Sci. 2005;1(3):131–142. [Google Scholar]
- Parikh B. Microsponge as novel topical drug delivery system. J. Global Pharma Technol. 2010;2(1) [Google Scholar]
- Qi H., Chen W., Huang C., Li L., Chen C., Li W., Wu C. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int. J. Pharmaceut. 2007;337(1):178–187. doi: 10.1016/j.ijpharm.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Rajasekaran A., Kumaran K., Preetha J.P., Karthika K. A comparative review on conventional and advanced ocular drug delivery formulations. Int. J. Pharm. Tech. Res. 2010;2(1):668–674. [Google Scholar]
- Rathore K., Nema R. An insight into ophthalmic drug delivery system. Int. J. Pharm. Sci. Drug Res. 2009;1(1):1–5. [Google Scholar]
- Re M., Biscans B. Preparation of microspheres of ketoprofen with acrylic polymers by a quasi-emulsion solvent diffusion method. Powder Technol. 1999;101(2):120–133. [Google Scholar]
- Robinson J.R., Mlynek G.M. Bioadhesive and phase-change polymers for ocular drug delivery. Adv. Drug Deliv. Rev. 1995;16(1):45–50. [Google Scholar]
- Saifullah M., Ahsan A., Shishir M.R.I. 12 - Production, stability and application of micro- and nanoemulsion in food production and the food processing industry A2 - Grumezescu, Alexandru Mihai Emulsions. Acad. Press. 2016:405–442. [Google Scholar]
- Schmolka I.R. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. Part A. 1972;6(6):571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- Song J., Bi H., Xie X., Guo J., Wang X., Liu D. Preparation and evaluation of sinomenine hydrochloride in situ gel for uveitis treatment. Int. Immunopharmacol. 2013;17(1):99–107. doi: 10.1016/j.intimp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Pathak K. Microsponges: a futuristic approach for oral drug delivery. Expert Opin. Drug Deliv. 2012;9(7):863–878. doi: 10.1517/17425247.2012.693072. [DOI] [PubMed] [Google Scholar]
- Tangri P., Khurana S. Basics of ocular drug delivery systems. Int. J. Res. Pharmaceut. Biomed. Sci. 2011;2(4):1541–1552. [Google Scholar]
- Varshosaz J., Tabbakhian M., Salmani Z. Designing of a thermosensitive chitosan/poloxamer in situ gel for ocular delivery of ciprofloxacin. Open Drug Deliv. J. 2008;2(1) [Google Scholar]
- Venkatesh M., Purohit K., Kumar P. Development and evaluation of chitosan based thermosensitive in situ gels of pilocarpine. Int. J. Pharm. Pharm. Sci. 2013;1(5):164–169. [Google Scholar]
- Vyas L., Tapar K., Laddha B., Lahoti A., Nema R. Formulation and development of anti-blemish preparations using microsponge technology. J. Chem. Pharm. Res. 2010;2(5):562–571. [Google Scholar]
- Vysloužil J., Doležel P., Kejdušová M., Mašková E., Mašek J., Lukáč R., Košťál V., Vetchý D., Dvořáčková K. Influence of different formulations and process parameters during the preparation of drug-loaded PLGA microspheres evaluated by multivariate data analysis. Acta Pharmaceutica. 2014;64(4):403–417. doi: 10.2478/acph-2014-0032. [DOI] [PubMed] [Google Scholar]
- Yang Y.-Y., Chung T.-S., Ng N.P. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22(3):231–241. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]