Abstract
Background
Goodpasture syndrome (GP) is a pulmonary-renal syndrome characterized by autoantibodies directed against the NC1 domains of collagen IV in the glomerular and alveolar basement membranes. Exposure of the cryptic epitope is thought to occur via disruption of sulfilimine crosslinks in the NC1 domain that are formed by peroxidasin-dependent production of hypobromous acid. Peroxidasin, a heme peroxidase, has significant structural overlap with myeloperoxidase (MPO), and MPO-ANCA is present both before and at GP diagnosis in some patients. We determined whether autoantibodies directed against peroxidasin are also detected in GP.
Methods
We used ELISA and competitive binding assays to assess the presence and specificity of autoantibodies in serum from patients with GP and healthy controls. Peroxidasin activity was fluorometrically measured in the presence of partially purified IgG from patients or controls. Clinical disease severity was gauged by Birmingham Vasculitis Activity Score.
Results
We detected anti-peroxidasin autoantibodies in the serum of patients with GP before and at clinical presentation. Enriched anti-peroxidasin antibodies inhibited peroxidasin-mediated hypobromous acid production in vitro. The anti-peroxidasin antibodies recognized peroxidasin but not soluble MPO. However, these antibodies did crossreact with MPO coated on the polystyrene plates used for ELISAs. Finally, peroxidasin-specific antibodies were also found in serum from patients with anti-MPO vasculitis and were associated with significantly more active clinical disease.
Conclusions
Anti-peroxidasin antibodies, which would previously have been mischaracterized, are associated with pulmonary-renal syndromes, both before and during active disease, and may be involved in disease activity and pathogenesis in some patients.
Keywords: anti-GBM disease, ANCA, extracellular matrix, glomerulonephritis, Goodpasture-s syndrome, vasculitis
The pulmonary-renal syndromes Goodpasture (GP) disease and ANCA-associated vasculitis are deadly autoimmune conditions characterized by rapidly progressive glomerulonephritis and pulmonary hemorrhage.1 Although these pulmonary-renal syndromes share clinical features, they differ significantly in antigen specificity. GP is defined by antibodies directed against the α3 and α5 chains of collagen IV2–5 in alveolar and glomerular basement membranes, whereas ANCA-associated vasculitis predominately recognizes myeloperoxidase (MPO) or proteinase-3.6 Intriguingly, GP cohorts have an epidemiologic coincidence of anti-MPO antibodies, with “double-positive” (anti-α3 and anti-MPO) patients accounting for 10%–38% of GP cases.7,8 Although differences in clinical outcomes and Non-Collagenous 1 (NC1) domain autoantibody specificity have been suggested between single- (anti-α3 only) and double-positive patients,7–9 a large, multicenter study found a clinically hybrid phenotype among these patients.10 In a unique prediagnosis study of patients with GP, up to 80% of GP cases were found to have anti-MPO antibody recognition preceding active disease at some point. In these patients with a clear antecedent antibody, anti-MPO always became detectable before the anti-GBM antibody.11
A key element in GP pathogenesis is thought to be disruption of normal sulfilimine crosslinking (S=N) between methionine and lysine in the NC1 domain of type IV collagen.5 Peroxidasin, a heme peroxidase with 48% sequence identity with MPO through the peroxidase domain,12 was recently identified as the enzyme responsible for the formation of the S=N crosslink through in vivo generation of hypobromous acid (HOBr).13,14 Because the S=N bond modulates GP antibody recognition and grants immune privilege and resistance to proteolysis in vitro,15–17 we hypothesized that autoantibodies recognizing peroxidasin might exist within the GP population, particularly within the double-positive group, and possibly in other anti–MPO-related vasculitis within pulmonary-renal syndromes.
Herein, we tested time of GP diagnosis, prediagnostic GP, MPO-ANCA, PR3-ANCA, and drug-induced ANCA cohorts for peroxidasin antibodies. By ELISA and competition binding, anti-peroxidasin antibodies were identified both before and during active disease in patients with GP. The anti-peroxidasin autoantibodies functionally inhibited HOBr production in vitro, which is required for extracellular collagen IV crosslinking. The peroxidasin antibodies were shown to be distinct from anti-MPO and account for sizable proportion of ELISA-based “anti-MPO” positivity. Finally, peroxidasin antibodies were also found in MPO-ANCA vasculitis and correlated with higher Birmingham Vasculitis Activity Score.
Methods
Additional detailed experimental methods appear in the Supplemental Material.
Study Cohorts
In this study, three separate, independent cohorts were used to test for the presence of anti-peroxidasin antibodies. All samples within cohorts were obtained under the appropriate institutional review board for each respective institution (Vanderbilt, University of North Carolina at Chapel Hill, and the Department of Defense Serum Repository [DoDSR]). Appropriate material transfer agreements were also instituted between institutions for the transfer of samples. The Vanderbilt GP cohort consisted of 24 samples (either sera or first-treatment plasmapheresis fluid). The prediagnosis GP cohort from the DoDSR consisted of six prediagnosis samples (four time points per sample; 24 total individual samples) and three time of acquisition-, age-, race-, and sex-matched control samples (18 total individuals, 72 separate samples). A schematic detailing sample acquisition and timing in the DoDSR cohort appears in Supplemental Figure 1. The University of North Carolina, Chapel Hill cohort tested consisted of 59 MPO-ANCA, 10 PR3-ANCA, and six drug-induced vasculitis samples in addition to independent controls. Supplemental Table 1 contains demographic information for these cohorts.
Preparation of Antigens
Recombinant human peroxidasin was expressed in HEK293 cells and purified as described previously.13,14 Human MPO was purchased from Athens Research and Technology (Athens, GA). Both peroxidasin and MPO were checked for activity upon thawing by using a tetramethylbenzidine peroxidase assay for maximum consistency. α3 and α5 collagen IV NC1 domains were recombinantly expressed in HEK293 cells and purified exactly as described.5
Detection of Antigens by ELISA
All assays were run in 384-well format with 50 µl volumes for coating and incubation with human sera, and development. We used 100 µl volumes for blocking and wash steps. Peroxidasin (4 nM, 2.5 µg/ml), MPO (4 nM, 0.8 µg/ml), α3(IV), and α5(IV) NC1 were applied immediately after dilution of the antigen in Tris-buffered saline and coated overnight at 4°C on polystyrene 384-well plates (Nunc). Wash steps were then performed on a BioTek ELx50 plate washer. Plates were blocked with 1% BSA (Fraction V–RIA grade; Sigma Aldrich, St. Louis, MO). Human sera or plasmapheresis fluid was then diluted as defined in each experiment (1:100 for primary screen, 1:500 for most subsequent experiments) in 0.1% BSA in Tris-buffered saline with 0.05% v/v Tween-20. Alkaline phosphatase–conjugated secondary antibodies (Sigma Aldrich) were used and then developed using p-nitrophenyl phosphate as the alkaline phosphatase substrate and the absorbance at 405 nm was determined (SpectraMax Plus 384 Microplate Reader; Molecular Devices, Sunnyvale, CA). All samples were run in duplicate. The initial screen of the DoDSR cohort was run on the same day in four consecutive plates with control samples repeated across plates to insure internal consistency. Blank wells coated in BSA and without primary antibody or sera were used as blanks for background correction.
Competition ELISA
Antigens were coated and plates blocked as described above. Sera were diluted 1:500 with varying amounts of identical MPO or peroxidasin and allowed to preincubate for 12 hours at 4°C before application to plates for 1 hour before washing. Secondary antibody application and ELISA assay development proceeded as previously detailed.
Peroxidasin Inhibition Kinetics
Peroxidasin was prepared as previously described.13,14 Antibodies and peroxidasin were premixed (500 pM peroxidasin, 218 µM whole patient IgG [437.5-fold molecular excess], 100 µM NaBr, 140 mM NaCl) and added to a 96-well black-walled plate for fluorimetry using a GloMax Discover fluorimeter equipped with a sample injector (Promega Corporation). Aminophenyl fluorescein (APF; AAT BioQuest, Sunnyvale, CA) (10 µM APF final concentration) was injected and an initial time point measured. Because hypohalous acids react with APF to form fluorescein, an excitation wavelength was set to 470–490 nm with an emission filter set between 500 and 500 nm. Next, initial rates were measured after the addition of 7.5 µM H2O2 (final concentration) using the sample injector, with serial readings every 30 seconds. All patient and control samples were run on the same plate, at the same time, with the same reagent batches to ensure maximum consistency in the rate measurement.
Statistical Analyses
Analysis performed in this work was completed in GraphPad Prism v. 5.00 for Windows (GraphPad Software, San Diego, CA) and SPSS v. 22 (IBM). All statistical tests between groups were analyzed using nonparametric measures indicated unless the data were found to be normal. Thresholds for positivity for antibodies were uniformly set as >3 SD above the control mean. All relevant statistical tests performed referenced with data in figure legends.
Results
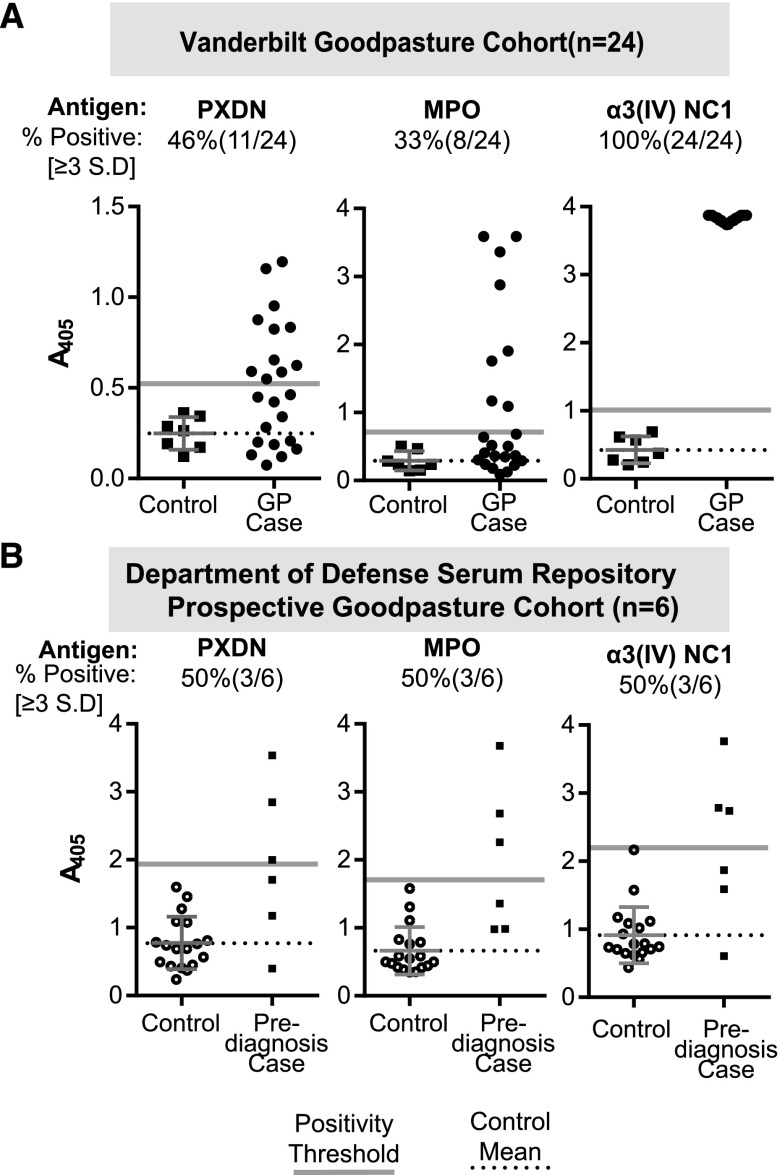
We first tested the stored sera and plasmapheresis effluent of 24 patients with GP and seven healthy controls for peroxidasin immunoreactivity with an ELISA (Figure 1A). Among this cohort, 46% of patients had peroxidasin antibodies, 33% had MPO-ANCA, and 100% had GP autoantibodies. These results demonstrate that there is IgG recognizing peroxidasin as an antigen at diagnosis in a subpopulation of patients with GP.
Figure 1.
Antibodies recognizing peroxidasin (PXDN) are present in patients with GP before and at the time of diagnosis. (A) ELISA data for specified native recombinant antigens from 1:100 dilutions of either serum or early plasmapheresis fluid at the time of presentation. Positivity threshold was determined from the healthy control mean (n=7) plus 3 SD for each antigen. Dotted reference line represents control mean for the specified antigen, and gray line represents positivity threshold. (B) ELISA data for specified native recombinant antigens from 1:100 dilutions for each individual serum sample; maximal values from each patient and control are plotted, regardless of time of draw. A schematic of sample timing appears in Supplemental Figure 1, and each patient’s specific time-course appears in Supplemental Figures 2 and 3. Positivity thresholds derived from all control samples. Controls plotted with mean. Dotted reference line represents control mean for the specified antigen, and gray line represents positivity threshold (+3 SD from control mean).
We then tested the serial prediagnostic DoDSR samples of six patients with biopsy-proven GP and 24 age-, sex-, race-, and age of serum sample-matched controls (4:1) for peroxidasin antibodies. No samples were available at diagnosis for these patients. The demographic overview and sample-draw timing schematic are presented in Figure 1 and Supplemental Table 1. The highest prediagnostic antibody level for each patient was used for preliminary analysis. Similar to the diagnostic cohort, 50% (three out of six) of patients had elevated prediagnostic peroxidasin antibodies compared with none of the controls (P=0.005) (Figure 1B). To more comprehensively analyze this data longitudinally, we included all control samples to recalculate thresholds for peroxidasin, MPO, and α3 (Supplemental Figures 2 and 3). Two patients (4X and 6X) demonstrated obviously elevated peroxidasin immunoreactivity over the timeframe represented (5.6–1.6 and 6.6–1.6 years prediagnosis, respectively) (Supplemental Figure 2). Like the other prediagnostic GP cohort,11 the majority of this cohort (66%; four out of six) had elevated prediagnostic MPO-ANCA reactivity (P=0.007) (Figure 1B, Supplemental Figure 2). Anti-α3 antibodies were also elevated in patients above that of controls (P=0.02) (Figure 1B), and were positive in four out of six patients longitudinally, when compared with the time of acquisition–matched samples, with two patients (2X and 3X) demonstrating a sharp increase in reactivity in the most disease-proximate samples (Supplemental Figure 3). Because these are prediagnosis samples, we tested for the presence of IgM recognizing these antigens to potentially detect an evolving autoimmune response. There was a trend of elevated anti-α3 IgM at approximately 1000 days prediagnosis in three out of six patients (Supplemental Figure 3). There was no elevation in IgM recognizing peroxidasin above control values in any case, and only anti-MPO IgM in one patient (1×) who also demonstrated strong anti-MPO IgG (data not shown). Unfortunately, because of heterogeneity of sample timing between patients, it is difficult to make strong conclusions about the temporality of these antibody responses related to GP clinical disease onset. However, when combined with data from the Vanderbilt cohort, this second independent and uniquely prospective GP cohort corroborates IgG recognition of peroxidasin in patients with GP, validating peroxidasin as an autoantigen in this population both before and at the time of active disease.
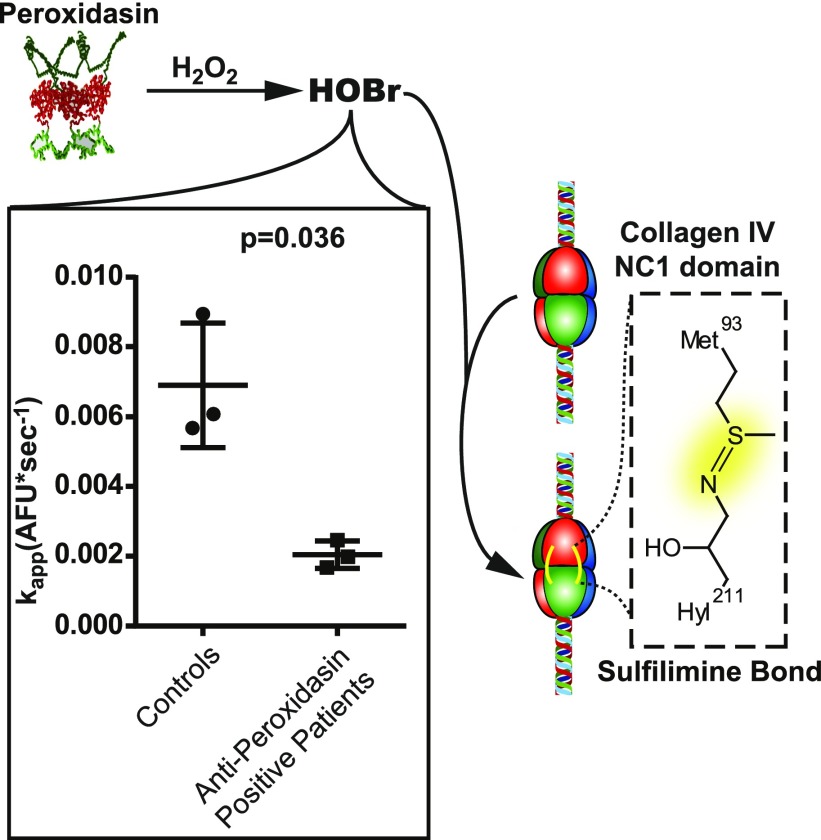
Peroxidasin-mediated HOBr production is essential for sulfilimine bond formation within the NC1 hexamer. Therefore, we next sought to determine if IgG from anti–peroxidasin-positive patients could functionally inhibit peroxidasin HOBr production in vitro, enabling assessment of potential anti-peroxidasin antibody–mediated disruption of basement membrane synthesis or regeneration in vivo.14,18,19 Using a fluorogenic reporter of hypohalous acid production (APF),20 there was a significant 70.3% reduction in apparent rate of oxidant production by peroxidasin in the presence of partially purified IgG from anti–peroxidasin-positive patients (mean difference 4.85×10−3 AFU*s−1; 95% confidence interval, 9.02×10−3 to 0.6×10−3) derived from three separate patients with GP compared with matched controls (Figure 2). The ability of anti-peroxidasin antibodies to inhibit the enzymatic activity of peroxidasin offers the additional possibility that these antibodies could play a feed-forward role in crescent formation during renal injury by inhibiting appropriate reestablishment of the basement membrane, potentially exacerbating the initial insult.
Figure 2.
Peroxidasin is inhibited by patient anti-peroxidasin antibodies in vitro. APF-based peroxidase activity assay (500 pM peroxidasin, 218 µM whole patient IgG [437.5-fold molecular excess], 100 µM NaBr, 140 mM NaCl, 10 µM APF) with initial rates measured after the addition of 7.5 µM H2O2 in the presence of partially purified IgG from anti–peroxidasin-positive patients or matched controls demonstrating significant inhibition of activity by anti-peroxidasin antibodies (t test used, variances found to not significantly differ). AFU, arbitrary fluorescence units.
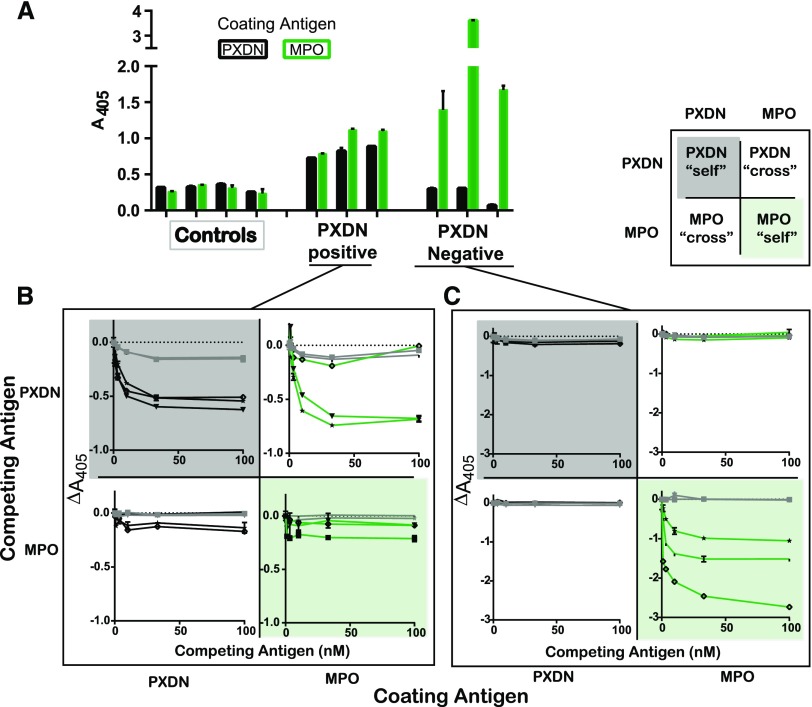
The structural overlap of MPO and peroxidasin, as well as the diagnostic and prediagnostic coincidence of the antibodies, creates a requirement for examination of antibody specificity. To examine this issue, ELISA assays were performed with peroxidasin and MPO coated at the same molarity. Results are shown in Figure 3. Patients could be characterized as belonging to one of two groups: (1) those with similar peroxidasin and MPO immunoreactivity or (2) those who were peroxidasin-negative, but highly MPO-positive. We used competition binding in a 2×2 combinatorial method with MPO and peroxidasin to dissect these groups with representative patients from both cohorts. Patients who demonstrated peroxidasin reactivity could have both the peroxidasin and MPO reactivity competed away by peroxidasin in a dose-dependent manner (Figure 3B), whereas soluble MPO only modestly competed away either peroxidasin or MPO reactivity in this group. This result indicates that antibodies in this group are specific to peroxidasin, and crossreact only with coated, not native, MPO. The nonequivalence of coated and soluble MPO for recognition within this group is consistent with conformation changes upon direct coating of antigens directly to hydrophobic surfaces.21 In the other group, patients that demonstrated no serologic reactivity to peroxidasin but robust reactivity to MPO could have MPO reactivity competed away by soluble MPO, but not peroxidasin (Figure 3C), indicating that these are MPO-specific antibodies in these patients. These results show that peroxidasin-specific antibodies are part of the autoimmune milieu in a subset of patients with GP, and that these anti–peroxidasin-positive patients would be mischaracterized as anti–MPO-positive under standard clinical laboratory conditions.22,23
Figure 3.
The subset of patients with GP recognizing peroxidasin (PXDN) has anti–peroxidasin-specific antibodies. Peroxidasin-positive, MPO-positive, and matched controls were tested for antigen specificity using competition ELISA. Antigens were coated (2.8 nM) and native, soluble peroxidasin or MPO were preincubated with 1:500 dilutions of patient sera for 12 hours at 4°C before exposure to coated antigens. Error bars represent the SEM for duplicates of each condition. Gray lines indicate controls, black lines indicate patients with peroxidasin as coated antigen, green lines indicate patients with MPO as coated antigen. Refer to key in the top right of the figure for quick reference regarding the type of competition binding being tested. “self” refers to same antigen competition and “cross” refers to the other antigen. Each line/shape combination refers to an individual patient. (A) Binding to coated antigens in the absence of any competing protein. (B) Peroxidasin-positive patients have peroxidasin and MPO recognition inhibited by peroxidasin, but not MPO, indicating the presence of peroxidasin-specific antibodies. Of note, the top right quadrant demonstrates peroxidasin’s ability to compete away MPO recognition. (C) MPO-only positive patients have MPO binding, which is unaffected by soluble peroxidasin, but completely inhibited by soluble MPO, indicating that these antibodies are MPO specific.
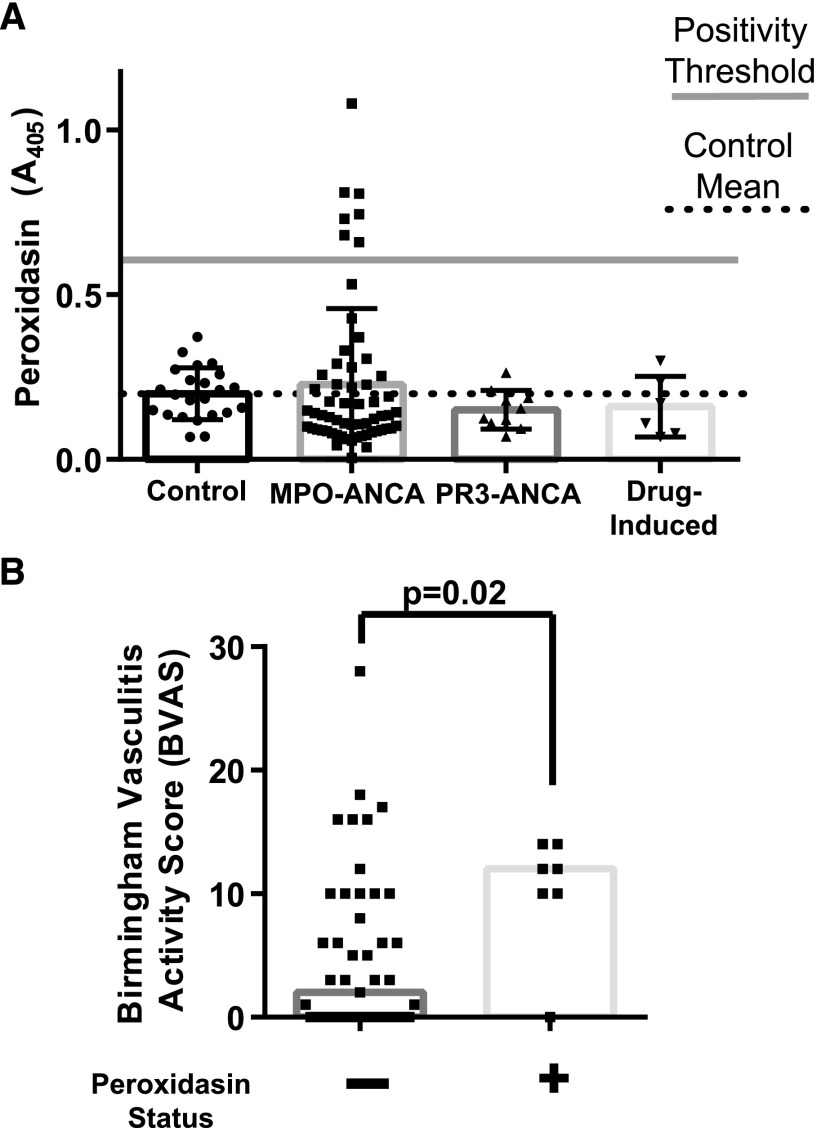
To assess if anti-peroxidasin antibodies were part of the broader vasculitis milieu, sera from patients with MPO-ANCA, PR3-ANCA, and drug-induced ANCA vasculitis were tested by ELISA. Interestingly, peroxidasin-positive patients were found only in the MPO-ANCA vasculitis group (eight out of 59; 13.5%). Disease controls demonstrated no more reactivity than healthy controls (Figure 4A). The specificity profile of the peroxidasin antibodies in these patients were tested and demonstrated the presence of both anti–MPO- and anti–peroxidasin-specific antibodies (Supplemental Figure 4), yet there was no significant difference in age or crude disease phenotype (age, sex, or end-organ involvement) (Supplemental Table 2). Because of the clinical characterization of these samples, we were able to assess the degree of clinical disease severity associated with anti-peroxidasin antibodies by assessing the Birmingham Vasculitis Activity Score.24 Patients with anti-peroxidasin antibodies had significantly worse vasculitis activity (Figure 4B; median 12 versus 2; P=0.02), and had a median score above values associated with substantially increased intensive care unit mortality.25
Figure 4.
Anti-peroxidasin antibodies are present in anti-MPO ANCA-associated vasculitis and are significantly associated with worse clinical disease. (A) ELISA data for specified ANCA-associated vasculitis from 1:100 dilutions of serum. Positivity threshold was determined from the control mean (n=23) plus 3 SD. Controls plotted with mean±SD. Dotted reference line represents control mean for the specified antigen, and gray line represents positivity threshold (+3 SD). (B) Birmingham Vasculitis Activity Scores grouped by anti-peroxidasin positivity status for anti-MPO ANCA-associated vasculitis patients (n=54; n=7 anti-peroxidasin positive). Median Birmingham Vasculitis Activity Score for the anti–peroxidasin-positive samples was 12 versus two for the peroxidasin-negative samples. Difference in median tested by Mann–Whitney U test.
Discussion
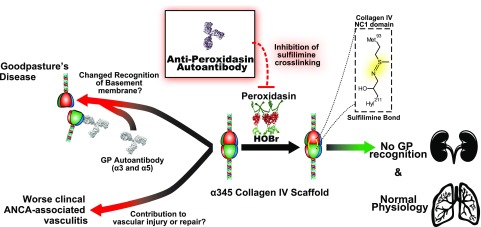
This work demonstrates peroxidasin to be a novel autoantigen within the pulmonary-renal syndrome spectrum of disease. Through examination of a unique cohort composed of serial predisease samples from patients with GP, anti-peroxidasin antibodies were found to coexist with modest anti-α3 antibodies before the onset of fulminate disease. These findings suggest that in this subset of patients, inhibitory anti-peroxidasin antibodies might be part of GP pathogenesis and support the importance of appropriate sulfilimine crosslinking of the collagen IV NC1 domain to prevent pathogenic anti-α3 antibodies from binding (Figure 5).5,16,17 Nevertheless, because GP is a rare disease, multiple hits including genetic26 and environmental factors4 are probably required, and the presence of anti-peroxidasin antibodies in a subset of patients may simply represent an additional hit.
Figure 5.
The potential role of anti-peroxidasin autoantibodies in pulmonary renal syndromes based on enzyme inhibition and sulfilimine cross-linking (S=N) of the basement membrane.
The finding that anti-peroxidasin antibodies crossreact with coated MPO highlights the need to further investigate specific epitope recognition and characteristics in patients currently described as double-positive (anti-MPO and anti-α3), particularly in light of recent studies detailing the hybrid clinical phenotype and potential need for different treatment strategies.10 This crossreactivity with MPO is notable because anti-MPO antibodies are known not to crossreact with closely related eosinophil peroxidase, but have variable recognition on the basis of glycosylation.27,28 There have been conflicting outcomes data for both renal and overall survival in this patient group.7–9,29,30 Re-evaluation on the basis of peroxidasin positivity of clinical differences, presentation, and epitope recognition within this subset of patients with GP is warranted. The further identification of specific anti-peroxidasin antibodies within a subset of more active MPO-ANCA vasculitis raises the possibility that anti-peroxidasin antibodies are a unique serologic marker of disease spanning the pulmonary-renal syndrome spectrum (Figure 5). This is an intuitively appealing hypothesis because of the role of peroxidasin role in the crosslinking of robust vascular collagen IV, which plays an important role in tissue homeostasis31 and potentially disease pathogenesis.
Disclosures
None.
Supplementary Material
Acknowledgments
A.S.M. performed all experiments. A.S.M., V.P., G.B., S.W.O., and B.G.H. designed all Goodpasture disease-related work. A.S.M., J.H., M.F., W.F.P., and R.J.F. conceived and designed all vasculitis-related work. S.W.O., D.J.L., and T.P.B. managed sample acquisition from the Department of Defense. V.P. maintained the Vanderbilt cohort. J.H., M.F., and W.F.P. facilitated sample acquisition from the University of North Carolina, Chapel Hill. Data were analyzed by A.S.M. and reviewed collectively.
This work was supported by National Institutes of Health grants P01-DK058335 (to R.J.F), R01 DK18381 (to B.G.H.), and F30 DK100094 (to A.S.M.), as well as T32 GM07347 (to the Vanderbilt Medical-Scientist Training Program), the Canby Robinson Society, the Shayne Scholarship (both Vanderbilt institutional support of A.S.M.), and K08 DK097306 and the Burroughs-Wellcome Fund Career Award for Medical Scientists (13030995) (to G.B.).
The views expressed in this presentation are those of the authors and do not reflect the official policy of the Department of Defense, or the United States Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Peroxidasin—a Novel Autoantigen in Anti-GBM Disease?” on pages 2605–2607.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018050519/-/DCSupplemental.
References
- 1.Lee RW, D’Cruz DP: Pulmonary renal vasculitis syndromes. Autoimmun Rev 9: 657–660, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Cui Z, Zhao MH, Jia XY, Wang M, Hu SY, Wang SX, et al.: Antibodies to α5 chain of collagen IV are pathogenic in Goodpasture’s disease. J Autoimmun 70: 1–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson BG: The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514–2527, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, et al.: Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennette JC, Falk RJ: Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 10: 463–473, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD: Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int 66: 1535–1540, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Hellmark T, Niles JL, Collins AB, McCluskey RT, Brunmark C: Comparison of anti-GBM antibodies in sera with or without ANCA. J Am Soc Nephrol 8: 376–385, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Hellmark T, Zhao J, Cui Z, Segelmark M, Zhao MH, et al.: Antigen and epitope specificity of anti-glomerular basement membrane antibodies in patients with goodpasture disease with or without anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 18: 1338–1343, 2007 [DOI] [PubMed] [Google Scholar]
- 10.McAdoo SP, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N, et al.: Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int 92: 693–702, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC, et al.: Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol 22: 1946–1952, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soudi M, Zamocky M, Jakopitsch C, Furtmüller PG, Obinger C: Molecular evolution, structure, and function of peroxidasins. Chem Biodivers 9: 1776–1793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, et al.: Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8: 784–790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG: Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157: 1380–1392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, et al.: A sulfilimine bond identified in collagen IV. Science 325: 1230–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanacore R, Pedchenko V, Bhave G, Hudson BG: Sulphilimine cross-links in Goodpasture’s disease. Clin Exp Immunol 164[Suppl 1]: 4–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanacore RM, Ham AJ, Cartailler JP, Sundaramoorthy M, Todd P, Pedchenko V, et al.: A role for collagen IV cross-links in conferring immune privilege to the Goodpasture autoantigen: Structural basis for the crypticity of B cell epitopes. J Biol Chem 283: 22737–22748, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorning D, Vracko R: Renal glomerular basal lamina scaffold: Embryologic development, anatomy, and role in cellular reconstruction of rat glomeruli injured by freezing and thawing. Lab Invest 37: 105–119, 1977 [PubMed] [Google Scholar]
- 19.Vracko R: Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol 77: 314–346, 1974 [PMC free article] [PubMed] [Google Scholar]
- 20.Flemmig J, Zschaler J, Remmler J, Arnhold J: The fluorescein-derived dye aminophenyl fluorescein is a suitable tool to detect hypobromous acid (HOBr)-producing activity in eosinophils. J Biol Chem 287: 27913–27923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler JE, Ni L, Brown WR, Joshi KS, Chang J, Rosenberg B, et al.: The immunochemistry of sandwich ELISAs--VI. Greater than 90% of monoclonal and 75% of polyclonal anti-fluorescyl capture antibodies (CAbs) are denatured by passive adsorption. Mol Immunol 30: 1165–1175, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Csernok E: ANCA testing: The current stage and perspectives. Clin Exp Nephrol 17: 615–618, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Holle JU, Herrmann K, Gross WL, Csernok E: Comparative analysis of different commercial ELISA systems for the detection of anti-neutrophil cytoplasm antibodies in ANCA-associated vasculitides. Clin Exp Rheumatol 30[Suppl 70]: S66–S69, 2012 [PubMed] [Google Scholar]
- 24.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al.: Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 68: 1827–1832, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Biscetti F, Carbonella A, Parisi F, Bosello SL, Schiavon F, Padoan R, et al.: The prognostic significance of the Birmingham Vasculitis Activity Score (BVAS) with systemic vasculitis patients transferred to the intensive care unit (ICU). Medicine (Baltimore) 95: e5506, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi JD, Chang J, O’Sullivan KM, Pedchenko V, Hudson BG, Vandenbark AA, et al.: The HLA-DRB1*15:01-restricted Goodpasture’s T cell epitope induces GN. J Am Soc Nephrol 24: 419–431, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan S, Salapow MA, Breen R, Broide DH: Eosinophil peroxidase differs from neutrophil myeloperoxidase in its ability to bind antineutrophil cytoplasmic antibodies reactive with myeloperoxidase. Int Arch Allergy Immunol 105: 150–154, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Yu JT, Li JN, Wang J, Jia XY, Cui Z, Zhao MH: Deglycosylation of myeloperoxidase uncovers its novel antigenicity. Kidney Int 91: 1410–1419, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Lionaki S, Jennette JC, Falk RJ: Anti-neutrophil cytoplasmic (ANCA) and anti-glomerular basement membrane (GBM) autoantibodies in necrotizing and crescentic glomerulonephritis. Semin Immunopathol 29: 459–474, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW: Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis 46: 253–262, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Murakami M, Simons M: Regulation of vascular integrity. J Mol Med (Berl) 87: 571–582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.