Abstract
Background
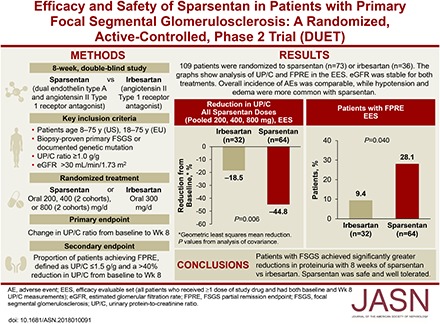
We evaluated and compared the effects of sparsentan, a dual endothelin type A (ETA) and angiotensin II type 1 receptor antagonist, with those of the angiotensin II type 1 receptor antagonist irbesartan in patients with primary FSGS.
Methods
In this phase 2, randomized, double-blind, active-control Efficacy and Safety of Sparsentan (RE-021), a Dual Endothelin Receptor and Angiotensin Receptor Blocker, in Patients with Focal Segmental Glomerulosclerosis (FSGS): A Randomized, Double-blind, Active-Control, Dose-Escalation Study (DUET), patients aged 8–75 years with biopsy-proven FSGS, eGFR>30 ml/min per 1.73 m2, and urinary protein-to-creatinine ratio (UP/C) ≥1.0 g/g received sparsentan (200, 400, or 800 mg/d) or irbesartan (300 mg/d) for 8 weeks, followed by open-label sparsentan only. End points at week 8 were reduction from baseline in UP/C (primary) and proportion of patients achieving FSGS partial remission end point (FPRE) (UP/C: ≤1.5 g/g and >40% reduction [secondary]).
Results
Of 109 patients randomized, 96 received study drugs and had baseline and week 8 UP/C measurements. Sparsentan-treated patients had greater reductions in UP/C than irbesartan-treated patients did when all doses (45% versus 19%; P=0.006) or the 400 and 800 mg doses (47% versus 19%; P=0.01) were pooled for analysis. The FSGS partial remission end point was achieved in 28% of sparsentan-treated and 9% of irbesartan-treated patients (P=0.04). After 8 weeks of treatment, BP was reduced with sparsentan but not irbesartan, and eGFR was stable with both treatments. Overall, the incidence of adverse events was similar between groups. Hypotension and edema were more common among sparsentan-treated patients but did not result in study withdrawals.
Conclusions
Patients with FSGS achieved significantly greater reductions in proteinuria after 8 weeks of sparsentan versus irbesartan. Sparsentan was safe and well tolerated.
Keywords: angiotensin II, endothelin, focal segmental glomerulosclerosis, proteinuria, sparsentan
Visual Abstract
Focal segmental glomerulosclerosis (FSGS) encompasses a heterogeneous group of clinical conditions with defined glomerular histopathology. Patients with FSGS typically present with a variable degree of proteinuria and often nephrotic syndrome.1,2 Primary FSGS has no identifiable cause but may be a consequence of actions of putative circulating permeability factors that damage podocytes.1–5 FSGS may be also a result of genetic abnormalities in podocyte proteins.1–5 In contrast, secondary forms of FSGS are caused by loss of renal parenchyma, metabolic derangements, and other antecedent diseases, drugs, or infections.1 Disease incidence is increasing, and in the United States, nearly 50% of patients with primary FSGS and nephrotic-range proteinuria resistant to treatment will require RRT within 5–10 years of diagnosis.6 FSGS accounts for 5% of adult and 12% of pediatric patients with ESRD.7–9
Current treatment with corticosteroids or other immunomodulating agents is aimed at reducing proteinuria, an independent predictor of renal survival in patients with primary FSGS. These agents are routinely combined with renin-angiotensin system inhibitors (RASIs).10–13 However, use of immunomodulating drugs is often hampered by therapy-limiting side effects.9,12 As a result, the availability of effective and safe, well tolerated drugs to reduce proteinuria is an unmet medical need in primary FSGS.14
Endothelin type A (ETA) receptor antagonists have emerged as promising therapies that may augment RASI actions.15 Preclinical studies have shown that both endothelin (ET) and angiotensin II (AngII) injure podocytes through several molecular mechanisms and small-molecule endothelin receptor antagonists (ERAs) or RASIs ameliorate parenchymal injury and reduce proteinuria in rodent models of FSGS.16,17 In humans, the additive antiproteinuric benefit from combining ERAs and RASIs was demonstrated in diabetic nephropathy.15,18,19
Sparsentan is a first-in-class, orally active, selective antagonist of the angiotensin II type 1 (AT1) receptor and the ETA receptor.17,20 Here we report the outcomes from the double-blind treatment period of the Efficacy and Safety of Sparsentan (RE-021), a Dual Endothelin Receptor and Angiotensin Receptor Blocker, in Patients with Focal Segmental Glomerulosclerosis (FSGS): A Randomized, Double-blind, Active-Control, Dose-Escalation Study (DUET), a phase 2 study of the efficacy and safety of sparsentan compared with irbesartan, to reduce proteinuria in patients with primary FSGS. The hypothesis of the DUET study was that dual blockade of the AT1 and ETA receptors with sparsentan in patients with primary FSGS would reduce proteinuria more than blockade of the AT1 receptor alone (irbesartan).
Methods
A complete description of the DUET study design has been published (Clinicaltrials.gov trial registration: NCT01613118).21 The DUET study, approved as an ancillary study by the Nephrotic Syndrome Study Network cohort study,22 enrolled patients at 44 sites between April of 2014 and April of 2016 in the United States and Europe after institutional review board or ethics committee approvals, in accordance with the Declaration of Helsinki. The methodology is briefly summarized below.
Patients
Eligible patients were aged 8–75 years in the United States and 18–75 years in Europe; all had biopsy-proven FSGS or a disease-causing genetic mutation associated with FSGS, urinary protein-to-creatinine (UP/C) ratio ≥1.0 g/g, and eGFR>30 ml/min per 1.73 m2. Kidney biopsies were performed for clinical indications and processed for light microscopy, immunofluorescence, and electron microscopy on the basis of the availability of tissue for testing. The histologic definition of FSGS was segmental obliteration of glomerular capillaries by extracellular matrix in all cases. Entrapment of plasma proteins as hyalinosis could accompany the sclerosis. Adhesions, or synechiae, could be present between the sclerosing segment and Bowman’s capsule. On electron microscopy, the major finding was extensive effacement of the foot processes without other abnormalities in the glomerular basement membrane. Immunofluorescence could demonstrate segmental staining for Ig M and C3 entrapped in areas of hyalinosis.1 Secondary causes of FSGS were excluded at the discretion of the investigator.
Immunosuppressive medications, except cyclophosphamide and rituximab, were permitted if dosing was stable for 1 month before randomization. The doses of these medications were unchanged during the 8-week, double-blind treatment period.
Study Design and Treatment
After providing informed consent or assent, patients were screened to confirm eligibility and, if necessary, underwent a 2-week washout period to discontinue prescribed AngII-receptor blockers or angiotensin-converting enzyme inhibitors. At week 0, patients were randomized 3:1 through an interactive web response system within sequential dose-escalating, 20-patient cohorts (Figure 1) to receive sparsentan 200, 400 (two cohorts), or 800 (two cohorts) mg/d (Retrophin, Inc., San Diego, CA) or the active control irbesartan 300 mg/d (Bristol-Myers Squibb Sanofi-Synthelabo Partnership, New York, NY). Incremental safety reviews were performed by an independent Data Monitoring Committee (DMC). Initially, only patients aged ≥18 years were enrolled in cohort 1 at the lowest sparsentan dose (200 mg). After eight patients completed 4 weeks of treatment, the DMC performed a safety review and enrollment was opened to patients aged 8–17 years and cohort 2 was opened for randomization of patients to receive sparsentan 400 mg or irbesartan 300 mg. The DMC repeated this process after eight patients completed 4 weeks of treatment at 400 mg, and again after eight patients completed 4 weeks at 800 mg. DMC reviews continued every 6 months. Patients randomized to irbesartan received 150 mg/d for the first week before escalating to 300 mg/d for the remaining 7 weeks. Patients with body wt ≤50 kg received 50% of the assigned study drug doses.
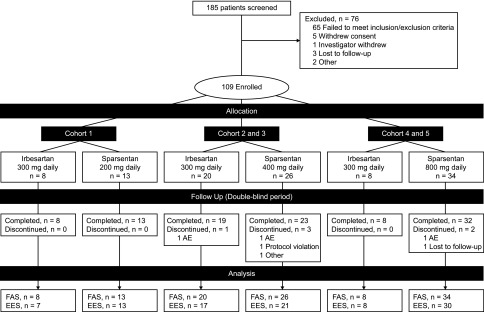
Figure 1.
Patient disposition for the double-blind study period. The prespecified plan for allocation of patients to dose cohorts was 20–40–40 for 200–400–800 mg dose cohorts. The EES population included patients who received at least one dose of study drug and had both baseline and week 8 UP/C assessments. Some patients received half of the assigned nominal dose owing to body wt≤50 kg. AE, adverse event; EES, efficacy evaluable set; FAS, full analysis set.
Investigators, participants, caregivers, and the study sponsor were blinded to treatment allocations until database extraction and unblinding at the completion of the 8-week, double-blind treatment period. After completion of the double-blind treatment period, patients could immediately continue to receive sparsentan in an open-label treatment period for an additional 144 weeks.
The primary end point was change in UP/C ratio from baseline to week 8. The secondary end point was the proportion of patients who achieved the FSGS partial remission end point (FPRE), defined as UP/C≤1.5 g/g and a >40% reduction in UP/C from baseline to week 8.23 Tertiary end points included changes from baseline in BP, eGFR, and select laboratory parameters.
Safety and tolerability were assessed through adverse events (AEs), including severity and relationship to study treatment, physical examinations, and changes in laboratory parameters.
Assessments
Peripheral blood/serum and urine samples were analyzed at a central laboratory. For the primary end point analysis, UP/C was measured in samples from 24-hour urine collections performed before study visits. eGFR was derived using the Modification of Diet in Renal Disease formula for patients aged ≥18 years and the Schwartz formula for patients aged <18 years. BP was measured three times when patients were seated for ≥5 minutes; the mean of the last two readings was recorded.
Statistical Analyses
Statistical analyses were performed using SAS version 9.4. The full analysis set (FAS) was defined as all randomized patients who received at least one dose of study drug and had at least one postbaseline efficacy evaluation. The efficacy evaluable set (EES) included all patients who received at least one dose of study drug and had both baseline and week 8 UP/C measurements. The safety analysis set included all randomized patients who received at least one dose of study drug and had at least one postbaseline safety evaluation. UP/C data were log-transformed before analyses to reduce skewness. Analysis of covariance (ANCOVA) models were fitted to the change from baseline in natural log UP/C, with treatment and dose cohort as main effects and the natural log of baseline UP/C as a covariate. The treatment group geometric mean percent change from baseline in UP/C at 8 weeks and the corresponding 95% confidence interval (95% CI) were calculated by back-transforming least squares means from the ANCOVA models. Treatment groups were compared using the ANCOVA models. Sparsentan dose groups or their combinations were compared with irbesartan in the following prespecified hierarchical order: (1) all sparsentan doses (800, 400, and 200 mg), (2) sparsentan 800 and 400 mg (combined), (3) sparsentan 400 mg, and (4) sparsentan 800 mg.
The proportion of patients achieving FPRE in each sparsentan dose group or their combination was compared with the proportion in the corresponding irbesartan group using the Fisher exact test. Prespecified analyses were performed using data from patient subgroups of the EES population (Supplemental Material).
To address missing values, the EES was used for the analysis of the primary and secondary efficacy end points. In a post hoc sensitivity analysis, change from baseline in natural log UP/C for 13 patients not included in the EES was imputed as zero. In addition, a post hoc mixed-model repeated measures (MMRM) analysis was performed on the basis of FAS with the natural log(UP/C) at week 0 (baseline) and week 8 as the dependent variable, treatment, cohort, visit, and treatment-by-visit interaction as fixed effects, and subject as a random effect. The ratio comparing sparsentan against irbesartan (i.e., [sparsentan week 8/sparsentan week 0]/[irbesartan week 8/irbesartan week 0]) and the corresponding 95% CI are derived from back-transforming the appropriate contrast applied to the estimates from the MMRM.
Safety data were summarized using descriptive statistics and evaluation of 95% CIs.
In addition to descriptive statistics, MMRM, which implicitly impute missing data, were used for analyses of select tertiary end points, namely BP and eGFR.
Results
Patient Disposition and Demographic Characteristics
The study screened 185 patients and enrolled 109 between April of 2014 and April of 2016 (Figure 1) in the FAS, including 23 patients aged 8 to ≤18 years (inclusive) (Table 1). Seventy three patients received sparsentan and 36 patients received irbesartan, of which 94% completed the double-blind period and 88% provided both baseline and week 8 urine samples for determination of the primary end point.
Table 1.
Baseline demographics and characteristics (FAS)
| Characteristics | Irbesartan | Sparsentan, All Doses |
|---|---|---|
| (n=36) | (n=73) | |
| Age, n (%) | ||
| Pediatric, ≥8 to ≤18 yr | 10 (28) | 13 (18) |
| Adult, >18–75 yr | 26 (72) | 60 (82) |
| Sex, n (%) | ||
| Female | 17 (47) | 32 (44) |
| Male | 19 (53) | 41 (56) |
| Race, n (%) | ||
| Asian | 1 (3) | 5 (7) |
| Black | 7 (19) | 8 (11) |
| White | 26 (72) | 57 (78) |
| Other | 2 (6) | 3 (4) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 6 (17) | 14 (19) |
| Non-Hispanic/non-Latino | 30 (83) | 59 (81) |
| Body mass index, kg/m2, mean (SD) | 28.7 (6.4) | 28.4 (6.1) |
| Immunosuppression at baseline, n (%) | 13 (36) | 21 (29) |
| eGFR, ml/min per 1.73 m2, mean (SD) | 74.5 (44.7) | 74.4 (37.3) |
| UP/C ratio, g/g, median (range) | 3.12 (0.9–10.7) | 3.61 (0.4–18.7) |
| ACE inhibitor or ARB use before washout, n (%) | 32 (89) | 59 (81) |
| Use of ≥1 diuretic/antihypertensive agent, n (%) | 20 (56) | 40 (55) |
| Diuretics | 9 (25) | 26 (36) |
| Additional antihypertensive treatments | 16 (44) | 29 (40) |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; FAS, full analysis set; SD, standard deviation; UP/C, urinary protein-to-creatinine ratio.
Baseline characteristics were similar between treatment groups (Table 1), including proportion of patients receiving RASIs before washout, as well as other antihypertensive agents (excluding RASIs and diuretics), and immunosuppressive treatment.
The prespecified plan for allocation of patients was 20–40–40 for the 200–400–800 mg dose cohorts, with a 3:1 randomization of sparsentan to irbesartan within each cohort. The cohort allocation was achieved. After database extraction and unblinding, it was determined that the 3:1 randomization within cohorts was not enforced. Therefore, a retrospective audit of the interactive web response system was arranged, which concluded that there was an incorrect implementation of the intended randomization plan. This error was not caught until the unblinding for the interim analysis, as study blinding was maintained as planned.
Efficacy
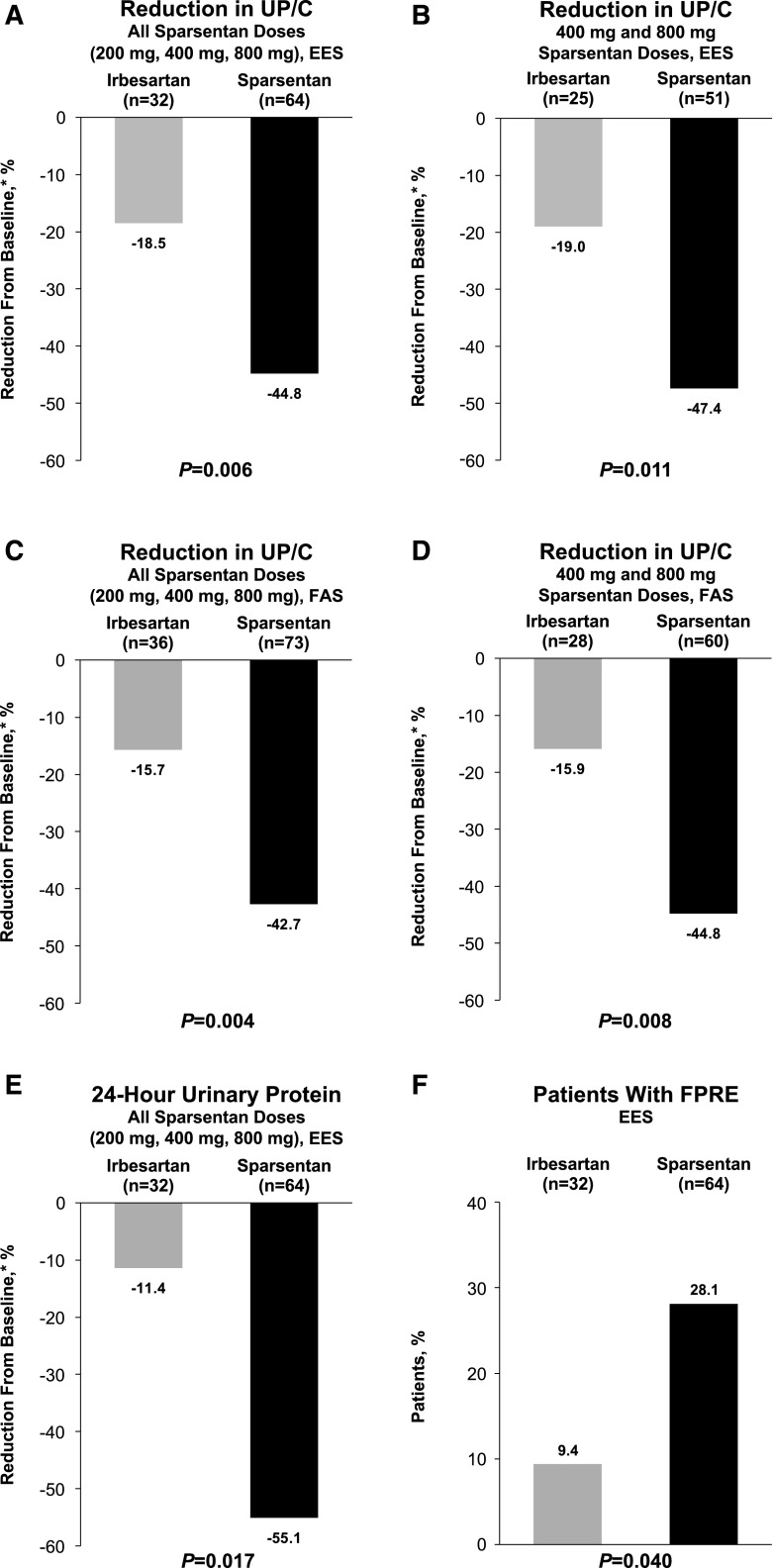
There was a greater reduction in proteinuria among pooled (all doses combined) sparsentan-treated patients (−44.8%; 95% CI, −52.7% to −35.7%) compared with irbesartan-treated patients (−18.5%; 95% CI, −34.6% to 1.7%; P=0.006; Figure 2A) after the 8-week, double-blind treatment period. Significantly greater reductions in UP/C from baseline to week 8 were observed in the pooled higher-dose sparsentan groups (i.e., 400 and 800 mg) compared with the irbesartan group (−47.4% versus −19.0%; P=0.01; Figure 2B, Supplemental Table 1). Although the trend was similar to that in the pooled data analysis, the antiproteinuric effect of individual sparsentan doses was not significantly different from that of irbesartan (Supplemental Table 1). The results were confirmed by post hoc FAS analyses. The results of the MMRM analysis are presented in Supplemental Table 2. The median (range) UP/C calculated on the basis of the FAS are similar to those calculated on the basis of the EES. The ratio of UP/C week 8/baseline for sparsentan is significantly lower than for irbesartan (ratio, 0.69; P=0.01). These findings are consistent with the primary analysis on the basis of EES using ANCOVA. The results were also confirmed by analysis that imputed zero change in proteinuria for 13 patients who were missing baseline or week 8 data (−42.7% versus −15.7% reduction for all doses; P=0.004; Figure 2C; −44.8% versus −15.9% reduction for 400 and 800 mg doses; P=0.008; Figure 2D), as well as analysis of 24-hour urinary protein excretion in the EES (−55.1% versus −11.4% reduction for all doses; P=0.02; Figure 2E).
Figure 2.
Compared with irbesartan, there was a greater reduction in UP/C with sparsentan, and a larger proportion of patients achieved FPRE. The figure illustrates the reduction in UP/C from baseline to week 8 for (A) all sparsentan doses for the EES and (B) 400 and 800 mg sparsentan doses for the EES. Reduction in UP/C from baseline to week 8 for (C) all sparsentan doses for the FAS and (D) 400 and 800 mg doses for the FAS. (E) Reduction in 24-hour urinary protein excretion for the EES. (F) Proportion of patients who achieved FPRE for the EES. *Geometric least squares mean percent change from baseline. P values for changes in UP/C from analysis of covariance. FPRE is defined as UP/C≤1.5 g/g and >40% reduction in UP/C. P value for FPRE obtained using the Fisher exact test. For the FAS analysis, patients with a missing UP/C value were imputed as zero. EES, efficacy evaluable set; FAS, full analysis set; FPRE, FSGS partial remission endpoint; FSGS, focal segmental glomerulosclerosis; UP/C, urinary protein-to-creatinine ratio.
Overall, 28% of sparsentan-treated patients achieved FPRE compared with 9% of irbesartan-treated patients (P=0.04; Figure 2F, Supplemental Table 3). Although not a prespecified analysis, complete remission (UP/C<0.3 g/g) was achieved during the 8-week, double-blind period in three patients randomized to sparsentan with one additional patient reaching UP/C 0.3 g/g. No patients randomized to irbesartan achieved complete remission.
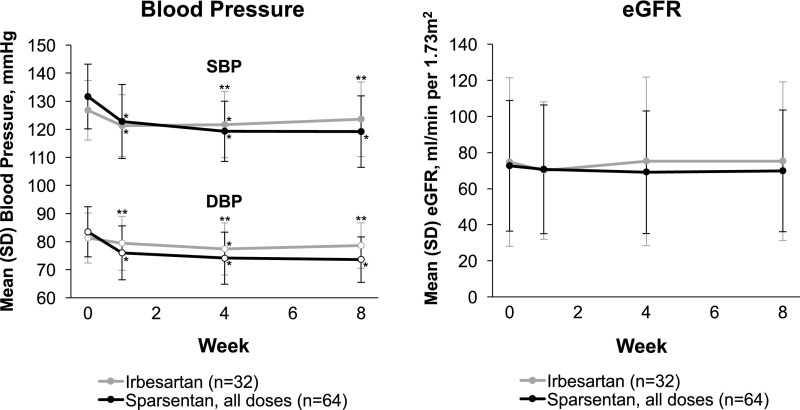
Treatment with sparsentan had a greater effect on BP compared with treatment with irbesartan (Figure 3). From an MMRM model, the difference for sparsentan versus irbesartan in least squares mean change from baseline at week 8 was –7.2 (95% CI, −11.8 to −2.6; P=0.003) for systolic BP and −5.6 (95% CI, −9.0 to −2.2; P=0.002) for diastolic BP. A significantly greater hypotensive effect of sparsentan was also observed in the pooled analysis of data from the 400 and 800 mg groups and the individual 800 mg sparsentan group compared with the irbesartan group (P<0.05), albeit not in the individual sparsentan 400 and 200 mg groups (data not shown).
Figure 3.
Sparsentan had a greater effect on lowering BP compared with irbesartan, while eGFR remained stable in both groups during the double-blind treatment period among patients in the EES. The figure illustrates the analyses of BP and eGFR on the basis of the efficacy evaluable set. eGFR on the basis of the Modification of Diet in Renal Disease formula for patients aged ≥18 years and Schwartz formula for patients aged <18 years. Data were summarized using descriptive statistics and evaluation of 95% CIs. *P<0.05 compared with baseline. **P<0.05 between treatment groups. BP, blood pressure; DBP, diastolic blood pressure; EES, efficacy evaluable set; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SD, standard deviation.
eGFR was similar between treatment groups at baseline and remained stable for sparsentan- and irbesartan-treated patients throughout the double-blind period (Figure 3). From the MMRM model, the difference for sparsentan versus irbesartan in least squares mean change from baseline at week 8 was −4.2 (95% CI, −11.8 to 3.5; P=0.28).
Serum concentrations of albumin and creatinine, as well as liver function tests, remained stable both in sparsentan- and irbesartan-treated patients and were similar in both groups. There was a trend toward increases in serum potassium and decreases in hemoglobin and hematocrit concentrations among sparsentan-treated patients (Supplemental Table 4).
Safety
During the 8-week, double-blind period, the overall incidences of treatment-emergent adverse events (TEAEs), drug-related TEAEs, or serious TEAEs were similar between the sparsentan and irbesartan groups (Table 2). The proportions of study withdrawals because of TEAEs were also similar between treatment groups. No deaths occurred. Compared with irbesartan-treated patients, sparsentan-treated patients experienced more frequent hypotension, dizziness, edema, and gastrointestinal TEAEs such as vomiting, diarrhea, and nausea. In contrast, fatigue, nasal congestion, upper respiratory infections, muscle spasms, and hyperkalemia were more common in the irbesartan-treated patients.
Table 2.
Summary of treatment-emergent adverse events during the double-blind period (FAS)
| Patients, n (%) | ||
|---|---|---|
| AE | Irbesartan | Sparsentan, All Doses |
| (n=36) | (n=73) | |
| Overall incidence of TEAEs | ||
| Any | 26 (72.2) | 56 (76.7) |
| Drug-related | 13 (36.1) | 32 (43.8) |
| Serious | 1 (2.8) | 2 (2.7) |
| Leading to study withdrawal | 1 (2.8) | 2 (2.7) |
| Death | 0 | 0 |
| TEAEs with incidence >5% | ||
| Headache | 7 (19.4) | 14 (19.2) |
| Hypotension/orthostatic hypotension | 3 (8.3) | 12 (16.4) |
| Dizziness | 4 (11.1) | 10 (13.7) |
| Edema/edema peripheral | 1 (2.8) | 9 (12.3) |
| Nausea | 3 (8.3) | 9 (12.3) |
| Diarrhea | 1 (2.8) | 6 (8.2) |
| Vomiting | 1 (2.8) | 6 (8.2) |
| Upper abdominal pain | 2 (5.6) | 4 (5.5) |
| Cough | 2 (5.6) | 3 (4.1) |
| Fatigue | 4 (11.1) | 3 (4.1) |
| Nasal congestion | 4 (11.1) | 2 (2.7) |
| Upper respiratory tract infection | 2 (5.6) | 2 (2.7) |
| Hyperkalemia | 2 (5.6) | 1 (1.4) |
| Muscle spasms | 2 (5.6) | 0 |
AE, adverse event; FAS, full analysis set; TEAEs, treatment-emergent adverse events.
There were no significant changes in severity of edema during the double-blind period (Table 3). There was no indication of changes in body weight or N-terminal pro–B-type natriuretic peptide in either treatment group and no significant differences between groups (Supplemental Table 4). There were also no meaningful between-group differences in overall use and changes in diuretic treatment (Supplemental Tables 5 and 6), although loop diuretics were used more frequently in sparsentan-treated patients than in irbesartan-treated patients.
Table 3.
Severity of edema during the double-blind period (safety analysis set)
| Edema Severity Grade | Patients, n (%) | |||
|---|---|---|---|---|
| Irbesartan (n=36) | Sparsentan, All Doses (n=73) | |||
| Baseline (n=29) | Week 8 (n=28) | Baseline (n=53) | Week 8 (n=60) | |
| 0 | 22 (76) | 24 (86) | 35 (66) | 39 (65) |
| 1+ to 2+ | 6 (21) | 4 (14) | 17 (32) | 18 (30) |
| 3+ to 4+ | 1 (3) | 0 | 1 (2) | 3 (5) |
Outcomes compared using the Fisher exact test. No significant differences were identified.
Subgroup Analyses
Although the trial was not powered for specific subgroup evaluation, analyses were performed to assess antiproteinuric effects of study drugs in patients stratified by factors associated with progression of CKD, including age, race, severity of proteinuria, CKD stage, sex, and presence or absence of hypertension (Supplemental Table 7). There were no statistically significant differences between treatment groups, although within-subgroup observations suggest strong antiproteinuric effects of sparsentan.
Discussion
The DUET study is the largest industry-sponsored, randomized, active-controlled trial in patients with primary FSGS conducted to date. The findings indicate that short-term dual blockade of the AT1 and ETA receptors with sparsentan produced greater reduction in proteinuria than blockade of the AT1 receptor alone. Treatment differences were statistically significant when combining all sparsentan doses and when combining the two higher doses (400 and 800 mg). Moreover, sparsentan was nearly three times as likely to achieve FPRE. The antiproteinuric effect of sparsentan was observed in subgroups defined by age, sex, proteinuria, baseline eGFR, and baseline BP.
Dual AT1-ETA receptor blockade in proteinuric CKD has been studied predominantly in diabetic nephropathy. These studies showed additive effects of ERAs in patients with baseline treatment with RASIs.15,18,19 Experience with ERAs and dual blockade in patients with nondiabetic CKD has been limited. Dhaun et al.24 reported additive effects on proteinuria with the ERA sitaxsentan and RASI therapy in a 6-week study of patients with various primary and secondary nondiabetic glomerulopathies.
AngII and ET-1 affect practically all renal cell types.16,25,26 Overlaps in their pleiotropic actions may have additive effects in the pathogenesis of FSGS, providing a strong rationale for dual blockade.17 Enhanced efficacy of sparsentan compared with irbesartan may reflect the enhanced protective effect on podocytes specifically,24,27 and a spectrum of other nephroprotective antifibrogenic, anti-inflammatory, and antioxidant actions.16,17
Although achieving remission in proteinuria has been shown to be critical for the long-term preservation of kidney function in patients with FSGS, the validity of short-term changes in proteinuria as a surrogate of drug efficacy in delaying/preventing ESRD is still debated.28 Moreover, limitations exist in the use of percentage reductions in proteinuria as an index of drug efficacy. To overcome these limitations, we used FPRE, a novel surrogate end point in FSGS (UP/C reduction by >40% to a value ≤1.5 g/g),23 as a meaningful treatment outcome. As recently published, characterization of FPRE was on the basis of analyses of five well-characterized databases of patients with primary FSGS. Achievement of FPRE in response to a variety of treatments was associated with a significant improvement in renal outcomes compared with patients who did not reach FPRE.23
The improvement in the FPRE rate observed with sparsentan in the double-blind phase of the DUET study suggests that these are clinically meaningful changes in proteinuria with regard to long-term renal outcomes. The ongoing open-label treatment period of the DUET study will evaluate long-term trends in kidney function in sparsentan-treated patients.
Very few serious AEs occurred in this study, and the frequencies were similar between treatment groups. Hypotension, dizziness, and headache were the most frequent TEAEs in sparsentan-treated patients. One patient experienced a two-fold increase in liver function tests that resolved completely within 4 weeks of discontinuation of sparsentan. Other TEAEs, such as fatigue, anemia, and hyperkalemia, were uncommon, were mild to moderate in nature, and did not require study drug discontinuation or study withdrawal. Although sparsentan blocks two signaling systems, the TEAE profile was not additive.
Because fluid retention was a major concern in previous studies with selective ERAs,15,16,19 special attention was paid to indices of fluid retention in the DUET study. Edema-related TEAEs were reported more frequently in sparsentan-treated patients than in irbesartan-treated patients, and mild decreases in hemoglobin or hematocrit were observed in sparsentan-treated patients, suggesting the possibility of hemodilution. Using a semiquantitative scale, we evaluated worsening of edema and additional indirect indices of fluid retention. The proportion of patients receiving sparsentan with mild to moderate edema remained stable, and the proportion with severe edema rose from 2% to 5%. This occurred in parallel with an increase in the use of loop diuretics. However, in nephrotic patients or patients with severe proteinuria, the etiology of edema is multifactorial; therefore, the contribution of ET receptor inhibition to fluid retention is difficult to judge. Patients entered the trial with varying degrees of edema reported as an AE. Moreover, there was no significant change in body weight or N-terminal pro–B-type natriuretic peptide levels from baseline in sparsentan-treated patients. Importantly, no study withdrawals or serious AEs associated with fluid retention occurred. Altogether, during the double-blind period, there were no serious safety signals of concern with respect to fluid retention.
Despite being one of the largest studies in FSGS, a limitation of the DUET study was the number of patients enrolled in each dose cohort. The study did not detect significant differences in antiproteinuric effect between individual dose groups, potentially as a result of small sample sizes. Additionally, exclusion of patients with missing data from the EES also limited the total number of patients included in the analyses, particularly in the higher-dose sparsentan and the irbesartan cohorts. Nevertheless, study patients were representative of the current population of patients with FSGS,22,29 providing confidence in the broad applicability of the findings.
Unfortunately, a departure from the planned 3:1 randomization, caused by flaws in the computerized randomization procedures, led to a 2:1 ratio of sparsentan to irbesartan patients. These problems were not identified until treatment assignment was unblinded. Independent review confirmed that the study blind was maintained until after database extraction. The total DUET study population included only 15 (14%) black patients, thus offering limited opportunity for interpretation of results in this patient subgroup with increased genetic risk for, incidence of, and comorbidities associated with FSGS.12,30,31 Additionally, no effect on eGFR could be detected over the 8-week, double-blind treatment period but is being studied in the ongoing open-label treatment period. Finally, additional factors could have contributed to the greater antiproteinuric effect of sparsentan. These may have included greater reduction in BP with sparsentan versus irbesartan, which was observed in the study, and differences in sodium intake, which were not assessed. Nevertheless, reductions in BP stabilized within 2 weeks after initiation of study treatment. Moreover, available data from 68 patients who were followed for 48 weeks in the open-label treatment period demonstrate a steady rise in the percentage of patients who achieved FPRE, reaching approximately 60% in patients originally randomized to either sparsentan or irbesartan. There was further decline in UP/C during the open-label period that was achieved without parallel changes in BP (data not shown).
Although the study had a high proportion of patients with non-nephrotic-range proteinuria and consequently “mild” FSGS with lower risk of progression to ESRD, many of these patients had a history of overt nephrotic syndrome and were in partial remission on immunosuppressive therapy. We suggest that these patients would also benefit from further reduction of residual proteinuria. The favorable effect of sparsentan in patients with nephrotic-range proteinuria indicates that drug efficacy is not limited to mild cases of primary FSGS (Supplemental Table 7).
Combining two pharmacologic effects in a single molecule has the benefit of reduction in pill burden and potentially improved compliance. Moreover, dual blockade may create synergy and improved efficacy compared with agents that block a single receptor. However, it would be premature to conclude that dual receptor blockade is always superior to single receptor blockade. It will be critical to verify the independent effects of each receptor blocker agent individually before assessing the putative benefit of a dual combined receptor blocking agent.
In conclusion, the results of the 8-week DUET study indicate that dual blockade of the AT1 and ETA receptors by sparsentan (≥400 mg/d) reduces proteinuria significantly more than single blockade of the AT1 receptor by irbesartan (300 mg/d) over 8 weeks of treatment in patients with primary FSGS. However, long-term effects of sparsentan on preservation of kidney function remain to be determined. Sparsentan will be further evaluated in the DUET study open-label treatment period and, in particular, in the phase 3 DUPLEX study (A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS; EudraCT number: 2016-005141-23; US ClinicalTrials.gov identifier: NCT03493685) to determine if it produces sustained reduction in proteinuria and stabilizes kidney function compared with AT1 receptor blockade without undue adverse effects. Positive findings from future, longer-term studies would represent a major advance in the management of FSGS.
Disclosures
A.C., V.K.D., G.G., L.G., B.M., E.M., J.R., and O.Z. have no disclosures to report. S.A. has received consultancy fees and research support from Retrophin, Inc. K.N.C. has received consultancy fees from Mallinckrodt Pharmaceuticals. D.S.G. has received research funding, paid to the University of Michigan, from Bristol-Myers Squibb, Complexa, GSK, NephCure Kidney International, and Retrophin, Inc., and has acted as consultant to Dimerix, Pfizer, Variant, and Janssen through private entity to University of Michigan agreements. J.H. has received consultancy and advisory board honoraria from Mallinckrodt, Aurinia Pharmaceuticals, Variant Pharmaceuticals, and GSK. R.K. is a full-time employee of Retrophin, Inc., and may have an equity or other financial interest in Retrophin, Inc. K.L. has received honoraria for serving as a speaker for Alexion and Mallinckrodt Pharmaceuticals. K.E.M. has a consultancy agreement with Novartis. P.N. has received consultancy fees as a Nephrology Advisory Board member of Retrophin, Inc. T.S. has received research funding from National Institutes of Health, Retrophin, Alexion, Mallinckrodt and Bristol Meyers Squibb. M.S. is an employee of PROMETRIKA, LLC and conducted the analyses under a master service agreement contract with Retrophin, Inc. V.T. has received consultancy fees from AbbVie, Amgen, Bayer, Boehringer-Ingelheim, ChemoCentryx and Fresenius Medical Care. H.T. has received consultancy fees from Kaneka Inc, Otsuka, and ChemoCentryx and was previously a consultant to Genzyme and Optherion. H.T. is an unpaid consultant to Retrophin Inc.
Supplementary Material
Acknowledgments
The authors would like to thank Lana Vegman, Ulysses Diva, Jennifer Hunt, Katherine Lucey, and Meghan McNally of Retrophin, Inc. Lana Vegman provided significant support to the authors in the coordination and development of this manuscript, Ulysses Diva provided statistical support and guidance in addressing journal reviewer comments and Jennifer Hunt, Katherine Lucey, and Meghan McNally made significant contributions by conducting the study and managing data. Writing support for this manuscript was provided by Shelly Asiala-Heckner, PharmD, CMPP, and Lamara D. Shrode, PhD, CMPP, of JB Ashtin, who worked with the authors to develop a first draft and assist with implementing revisions. Editorial support to restyle the manuscript and assist with author-guided revisions was provided by Kristen W. Quinn, Ph.D., of Peloton Advantage, LLC. Funding for writing and editorial support was provided by Retrophin, Inc.
All of the authors critically reviewed the manuscript throughout the editorial process and provided approval of the final manuscript for publication. H.T. and P.N. contributed to the original research project conception and design, data acquisition, and data interpretation. K.N.C. contributed to the original research project conception and design and data acquisition. S.A., A.C., V.K.D., G.G., L.G., D.S.G., J.H., K.L., B.M., E.M., K.E.M., J.R., T.S., V.T, and O.Z. contributed to data acquisition and data interpretation. M.S. contributed to statistical analysis and data interpretation. R.K. contributed to the original research project conception and design and data interpretation. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. Retrophin, Inc., was involved in the trial design and protocol development, provided logistical support, and obtained the data, which were evaluated jointly by the authors and the sponsor. All authors interpreted the data and wrote the manuscript together with the sponsor's medical writing services. All authors accept overall responsibility for the accuracy of the data, its analysis, and this report. This study was supported by Retrophin, Inc., San Diego, CA.
This manuscript was submitted on behalf of the Efficacy and Safety of Sparsentan (RE-021), a Dual Endothelin Receptor and Angiotensin Receptor Blocker, in Patients with Focal Segmental Glomerulosclerosis (FSGS): A Randomized, Double-blind, Active-Control, Dose-Escalation Study (DUET) study group: S.A., Los Angeles Biomedical Research Institute, Torrance, California; Rajendran Alappan, Renal Associates, LLC, Columbus, Georgia; Sanjiv Anand, Utah Kidney Research Center, Salt Lake City, Utah; Nausheen Bano Ali, Southern California Medical Research Center, La Palma, California; Joel Baranski, La Jolla Clinical Research, San Diego, California; John Bissler, Le Bonheur Children’s Hospital, Memphis, Tennessee; Melissa Cadnapaphornchai, Children’s Hospital Colorado, Aurora, Colorado; K.N.C., Mt Sinai School of Medicine, Manhattan, New York; A.C., Lucile Packard Children’s Hospital at Stanford, Palo Alto, California; Nataliya Chorny, Cohen Children’s Medical Center of New York, New Hyde Park, New York; Katherine Dell, The Cleveland Clinic Foundation, Cleveland, Ohio; V.K.D., University of North Carolina Kidney Center, Chapel Hill, North Carolina; Maria Egidi, Azienda Ospedaliero Universitaria Pisana, Pisa, Tuscany, Italy; Matthew Elliott, Metrolina Nephrology Associates, Charlotte, North Carolina; Mohamed El-Shahawy, Academic Medical Research Institute, Los Angeles, California; Ciro Esposito, Instituto Scientifico di Pavia, Pavia, Italy; Daniel Feig, University of Alabama at Birmingham, Birmingham, Alabama; Joseph Flynn, Seattle Children’s Hospital, Seattle, Washington; Alessia Fornoni, University of Miami Miller School of Medicine, Miami, Florida; G.G., Università Cattolica del Sacro Cuore, Fondazione Policlinico A. Gemelli, Rome, Italy; Michael Germain, Renal Transplant Association of New England, Springfield, Massachusetts; L.G., Azienda Ospedaliero Universitaria Policlinico di Bari, Bari, Italy; Keisha Gibson, University of North Carolina Kidney Center, Pediatrics, Chapel Hill, North Carolina; D.S.G., University of Michigan School of Medicine, Ann Arbor, Michigan; Robert Haws, Marshfield Clinic, Marshfield, Wisconsin; J.H., Perelman School of Medicine University of Pennsylvania, Philadelphia, Pennsylvania; Duncan Johnstone, Temple University School of Medicine, Philadelphia, Pennsylvania; Neenoo Khosla, Northshore University Health System, Evanston, Illinois; Nelson Kopyt, Northeast Clinical Research Center, LLC, Bethlehem, Pennsylvania; Jorge Kusnir, Florida Premier Research Institute, LLC, Winter Park, Florida; Pascale Lane, University of Oklahoma, Oklahoma City, Oklahoma; K.L., Joseph M. Sanzari Children’s Hospital (Hackensack), Hackensack, New Jersey; John Mahan, Nationwide Children’s Hospital, Columbus, Ohio; B.M., Colorado Kidney Care, Denver, Colorado; Ellen McCarthy, University of Kansas Medical Center, Kansas City, Kansas; Carlos Mercado, St. George Kidney Care, LLC, Diamond Valley, Utah; K.E.M., Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Enrico Minetti, Azienda Ospedaliero Universitaria Careggi, Firenze, Italy; E.M., Arizona Kidney Disease & Hypertension Centers Medical Research Services, LLC, Phoenix, Arizona; P.N., University of Washington, Seattle, Washington; Carla Nester, University of Iowa Children’s Hospital, Iowa City, Iowa; Alicia Neu, Johns Hopkins University, Baltimore, Maryland; Ana Parades, Miami Children’s Hospital, Miami, Florida; Pablo Pergola, Clinical Advancement Center, San Antonio, Texas; Parthassarathy Raguram, Catholic Health Initiatives Franciscan, Tacoma, Washington; J.R., Columbia University Medical Center, New York, New York; Rupesh Raina, Akron Nephrology Associates, Akron, Ohio; Michelle Rheault, University of Minnesota, Minneapolis, Minnesota; John Robertson, Apex Research of Riverside, Riverside, California; Ivan Rychlik, Faculty Hospital Kralovske Vinohrady, Prague, Czech Republic; Neil Sanghani, Vanderbilt University, Nashville, Tennessee; Ben Sprangers, Universitaire Ziekenhuizen Leuven, Leuven, Belgium; T.S., Children’s Mercy Hospital, Kansas City, Missouri V.T., General Teaching Hospital Prague, Prague, Czech Republic; H.T., New York University Langone Medical Center, New York, New York; Kausik Umanath, Henry Ford Hospital, Detroit, Michigan; Robert Woroniecki, State University of New York Stony Brook, Head of the Harbor, New York; O.Z., New York University Langone Medical Center, New York, New York.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018010091/-/DCSupplemental.
Contributor Information
Collaborators: S.A., Rajendran Alappan, Sanjiv Anand, Nausheen Bano Ali, Joel Baranski, John Bissler, Melissa Cadnapaphornchai, Children’s Hospital Colorado, Aurora, Colorado, K.N.C., Nataliya Chorny, Katherine Dell, V.K.D., Maria Egidi, Matthew Elliott, Mohamed El-Shahawy, Ciro Esposito, Daniel Feig, Joseph Flynn, Alessia Fornoni, Michael Germain, Keisha Gibson, D.S.G., Robert Haws, Duncan Johnstone, Neenoo Khosla, Nelson Kopyt, Jorge Kusnir, Pascale Lane, John Mahan, Ellen McCarthy, Carlos Mercado, K.E.M., Enrico Minetti, Carla Nester, Alicia Neu, Ana Parades, Pablo Pergola, Parthassarathy Raguram, Rupesh Raina, Michelle Rheault, John Robertson, Ivan Rychlik, Neil Sanghani, Ben Sprangers, Kausik Umanath, Robert Woroniecki, and O.Z.
References
- 1.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Zand L, Glassock RJ, De Vriese AS, Sethi S, Fervenza FC: What are we missing in the clinical trials of focal segmental glomerulosclerosis? Nephrol Dial Transplant 32[Suppl 1]: i14–i21, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Messina M, Gallo E, Mella A, Pagani F, Biancone L: Update on the treatment of focal segmental glomerulosclerosis in renal transplantation. World J Transplant 6: 54–68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiser J, Nast CC, Alachkar N: Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis 21: 417–421, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg AZ, Naicker S, Winkler CA, Kopp JB: HIV-associated nephropathies: Epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol 11: 150–160, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kiffel J, Rahimzada Y, Trachtman H: Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis 18: 332–338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. : US Renal Data System 2014 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 66: Svii, S1–S305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spino C, Jahnke JS, Selewski DT, Massengill S, Troost J, Gipson DS: Changing the paradigm for the treatment and development of new therapies for FSGS. Front Pediatr 4: 25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexopoulos E, Stangou M, Papagianni A, Pantzaki A, Papadimitriou M: Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol Dial Transplant 15: 1348–1356, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Cattran DC, Rao P: Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 32: 72–79, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 135, 2012 [Google Scholar]
- 13.Sethna CB, Gipson DS: Treatment of FSGS in children. Adv Chronic Kidney Dis 21: 194–199, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Pullen N, Fornoni A: Drug discovery in focal and segmental glomerulosclerosis. Kidney Int 89: 1211–1220, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, et al.: Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohan DE, Barton M: Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komers R, Plotkin H: Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 310: R877–R884, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, et al.: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014. 24722445 [Google Scholar]
- 19.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, et al.: ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowala MC, Murugesan N, Tellew J, Carlson K, Monshizadegan H, Ryan C, et al.: Novel dual action AT1 and ETA receptor antagonists reduce blood pressure in experimental hypertension. J Pharmacol Exp Ther 309: 275–284, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Komers R, Gipson DS, Nelson P, Adler S, Srivastava T, Derebail VK, et al.: Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: Randomized, controlled trial design (DUET). Kidney Int Rep 2: 654–664, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, et al.: Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troost JP, Trachtman H, Nachman PH, Kretzler M, Spino C, Komers R, et al.: An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 13: 414–421, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhaun N, MacIntyre IM, Kerr D, Melville V, Johnston NR, Haughie S, et al.: Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension 57: 772–779, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Rüster C, Wolf G: Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 22: 1189–1199, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Wennmann DO, Hsu HH, Pavenstädt H: The renin-angiotensin-aldosterone system in podocytes. Semin Nephrol 32: 377–384, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Buelli S, Rosanò L, Gagliardini E, Corna D, Longaretti L, Pezzotta A, et al.: β-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol 25: 523–533, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, et al.: Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 80: 868–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, et al.: A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 78: 698–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woroniecki RP, Ng DK, Limou S, Winkler CA, Reidy KJ, Mitsnefes M, et al.: Renal and cardiovascular morbidities associated with APOL1 status among African-American and Non-African-American children with focal segmental glomerulosclerosis. Front Pediatr 4: 122, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.