Abstract
Background
The individual course of CKD may vary, and improved methods for identifying which patients will experience short-term eGFR loss are needed. Assessing urinary Dickkopf-3 (DKK3), a stress-induced tubular epithelia–derived profibrotic glycoprotein, may provide information about ongoing tubulointerstitial fibrosis and short-term eGFR loss.
Methods
To investigate urinary DKK3’s potential as a biomarker of short-term eGFR loss (over 12 months), we prospectively assessed eGFR and urinary DKK3 levels in patients with CKD of various etiologies at baseline and annual follow-ups. We also measured urinary DKK3 in a general population sample and patients with diagnostic kidney biopsies or IgA nephropathy under treatment.
Results
Median urinary DKK3-to-creatinine concentration at baseline was significantly higher in patients with CKD than the general population sample (431 versus 33 pg/mg). In the CKD cohort, having a urinary DKK3-to-creatinine level >4000 pg/mg was independently and significantly associated after multiple adjustments with mean annual decline in eGFR of 7.6% over 12 months. Urinary DKK3 significantly improved prediction of kidney function decline compared with eGFR or albuminuria alone. Urinary DKK3-to-creatinine levels were related to the extent of tubulointerstitial fibrosis in kidney biopsies. In patients with IgA nephropathy, a rise in urinary DKK3 was associated with significant eGFR decline within 6 months, whereas stable or decreasing urinary DKK3 indicated a more favorable course.
Conclusions
Urinary DKK3 levels identify patients at high risk for eGFR decline over the next 12 months regardless of the cause of kidney injury and beyond established biomarkers, potentially providing a tool to monitor CKD progression and assess effects of interventions.
Keywords: chronic kidney disease, clinical nephrology, IgA nephropathy, interstitial fibrosis, tubule cells, progression of chronic renal failure
Visual Abstract
Ongoing renal interstitial fibrosis and tubular atrophy, also referred to as tubulointerstitial fibrosis, are common pathologic hallmarks of etiologically different CKD entities that finally result in organ failure.1–3 This has been shown for primary kidney diseases, such as IgA nephropathy (IgAN), as well as for diabetic and/or hypertensive kidney injury.4–6 Particularly in diabetic kidney disease, the view has been focused on changes in glomerular structure and function as major determinants of renal prognosis.4 However, recent experimental and clinical data highlight the role of tubular epithelial cell hypoxia and phenotypic transformation with initiation of tubulointerstitial fibrosis in the pathogenesis of progressive CKD.1,4 Nevertheless, precise definition of progressive tubulointerstitial fibrosis in patients with diagnosed CKD is not even possible by histopathologic examination of a kidney biopsy, because this only reflects the present status at the time of biopsy. Moreover, biomarkers for this specific pathologic course are lacking so far.
We have recently identified Dickkopf-3 (DKK3) as a stress-induced, renal tubular epithelia–derived, secreted glycoprotein (molecular mass, 38 kDa) that induces tubulointerstitial fibrosis through its action on the canonical Wnt/β-catenin signaling pathway.7 Genetic abrogation as well as antibody-mediated abrogation of DKK3 led to significantly decreased interstitial matrix accumulation, reduced tubular atrophy, and hence, preserved kidney function in various CKD mouse models.7 Notably, the profibrotic effects of DKK3 in the kidney were independent from the cause of initial damage. Because DKK3 is secreted into the urine under tubular stress conditions, it may also serve as a noninvasive diagnostic biomarker for short-term eGFR loss. In contrast to biomarkers, such as neutrophil gelatinase–associated lipocalin (NGAL), that reflect a static snapshot of tubular injury, urinary DKK3 provides an integral picture of the dynamic process of tubular stress and ensuing tubulointerstitial fibrosis independent of the injury reason. This is pertinent, because accurate identification of patients who at high risk for short-term eGFR decline is still challenging.8
Established biomarkers for prediction of CKD progression are eGFR and albuminuria. In the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, patients with CKD of different etiologies are categorized as having low, moderate, high, or very high risk for kidney disease progression according to their (baseline) eGFR and albuminuria.9 However, in 1.7 million participants from 35 cohorts with 12,344 ESRD events, CKD progression was highly variable even in patients within the same KDIGO risk category.10 In addition, recent studies revealed that, for example, in patients with diabetes, advanced CKD (i.e., eGFR<60 ml/min per 1.73 m2) may be present even in the absence of higher-grade albuminuria.11–14 Therefore, biomarkers that indicate eGFR loss are highly desirable.15–17
The main objective of this study was to examine the association between urinary DKK3 and the change of eGFR in patients with CKD of various etiologies as a noninvasive tool to identify patients at high risk for short-term eGFR decline. We also tested the hypothesis that measurement of urinary DKK3 may be helpful to monitor individual kidney disease activity in patients under treatment (the STOP IgAN Trial).
Methods
General Population (the I LIKE HOMe Study)
In total, 481 current/former employees of the Saarland University Medical Center in Homburg/Saar, Germany were recruited. Written informed consent was obtained from all participants. The study protocol was approved by the local ethics committee. Participants were excluded when they had a pathologic urine sediment (e.g., significant hematuria and/or leukocyturia, macroalbuminuria [i.e., albumin-to-creatinine ratio in spot urine >300 mg/g], and/or eGFR<60 ml/min per 1.73 m2). Additional exclusion criteria were active tumor disease, inflammatory or autoimmune disease requiring systemic immunosuppressive treatment, or a serum high-sensitivity C-reactive protein >20 mg/L. A standardized questionnaire was used to record a history of smoking, diabetes mellitus, current medication, and cardiovascular comorbidity. Participants with self-reported diabetes mellitus, a fasting blood glucose level of >126 mg/dl , and/or current use of hypoglycemic medication were categorized as diabetic. Blood and spot urine samples were obtained after overnight fasting, and samples were immediately processed and stored at −80°C until analyses.
Patients from the CKD Cohort (the CARE FOR HOMe Study)
The ongoing CARE FOR HOMe Study continuously recruits clinically stable patients with confirmed CKD from the German states of Saarland and Rhineland-Palatinate who are referred to the outpatient clinic of the Saarland University Medical Center. The study was approved by the local ethics committee, and all participants provided written informed consent. The study design has been published in detail before.18 In brief, inclusion criteria are the presence of CKD stages 2–4 in accordance with the KDIGO guidelines9 and age ≥18 years old. Main exclusion criteria are active malignancy, clinically apparent infections, active immunosuppressive therapy, kidney transplant, and AKI (defined as increase in plasma creatinine >50% within the preceding 4 weeks).
The last data evaluation was done in July 2017. Until then, 575 participants had been enrolled with a mean follow-up of 5.1±2.1 years. All participants were invited for annual follow-up examinations for assessment of kidney disease progression. Blood and spot urine samples were obtained after overnight fasting, and samples were immediately processed and stored at −80°C until analyses. eGFR, albuminuria, urinary DKK3, and standard laboratory parameters were assessed at the time of study inclusion and in all available annual samples during follow-up.
Kidney Biopsies Study
Urinary DKK3 levels and the extent of tubulointerstitial fibrosis were determined in patients with different CKD etiologies who consecutively underwent a diagnostic kidney biopsy at the University Hospital Innsbruck in Austria. The use of surplus material from routine diagnostics for research purposes was approved by the local ethics committee. We studied 76 patients in whom a kidney biopsy specimen containing at least eight glomeruli and a ratio of renal medulla to cortex of <0.5 was available at study entry. Blood and spot urine samples were obtained at the time of kidney biopsy after overnight fasting. Samples were immediately processed and stored at −80°C until analyses. The histopathologic diagnoses were idiopathic membranous nephropathy (n=12), IgAN (n=9), FSGS (n=8), membranoproliferative GN (n=7), minimal change disease (n=4), interstitial nephritis (n=4), diabetic nephropathy (n=4), ANCA-associated vasculitis (n=9), lupus nephritis (n=4), and miscellaneous (n=15).
Participants from the STOP IgAN Trial
The study design, patient selection, and results of the STOP IgAN Trial were published in 2015.19 In brief, in this multicenter, randomized, controlled trial, patients with biopsy-proven IgAN entered a 6-month run-in phase, in which supportive care therapy (e.g., strict BP control, including blockade of the renin-angiotensin system) was optimized. Patients who had persistent proteinuria of at least 0.75 g/d were randomly assigned to receive either supportive care alone or supportive care plus immunosuppressive therapy (i.e., corticosteroids, cyclophosphamide, and azathioprine). We studied 96 STOP IgAN participants in whom spot urine samples were available from the 6-month run-in phase and 57 patients in whom spot urine samples were available from the first 6 months of the randomized treatment period. Before enrollment into the STOP IgAN Trial, all patients underwent a kidney biopsy. We correlated urinary DKK3 levels with the extent of tubulointerstitial fibrosis in 43 patients in whom a kidney biopsy specimen containing at least eight glomeruli and a ratio of renal medulla to cortex of <0.5 was available at study entry.
Laboratory Measurements
Standard laboratory parameters and albumin in spot urine samples were measured at the Saarland University Medical Center in Homburg, Germany (the I LIKE HOMe Study, the CARE FOR HOMe Study, and the STOP IgAN Trial) and the University Hospital Innsbruck in Austria (the kidney biopsies study). All plasma creatinine measurements are traceable to an isotope dilution mass spectrometry. In all participants, eGFR was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.9 suPAR was measured in plasma samples of participants of the CARE FOR HOMe Study at baseline using a commercially available ELISA as previously described.20
Measurement of DKK3 in Urine
For the detection of DKK3 in human urine, antibodies against DKK3 were raised in the German Cancer Research Center in Heidelberg, Germany. The hybridomas for these antibodies were deposited at the Leibnitz Institute German Collection of Microorganisms and Cell Cultures in Braunschweig, Germany. On the basis of antibodies produced by these hybridomas, a novel DKK3 ELISA was designed, and the detection conditions were specifically adjusted for recognition of the DKK3 molecule in urine. No interference with other DKK molecules or albumin was observed. The DKK3 antibodies and the test indication are the subject of a patent application. The ELISA is certified for diagnostic use in humans in the European Union (ReFiNE; DiaRen UG, Homburg/Saar, Germany). For diagnostic use, the urine has to be clarified by centrifugation for 10 minutes at approximately 370×g (2000 rpm) to remove debris that may interfere with the measurement. Preferably, the measurement of DKK3 is performed in fresh (morning) urine. Studies have documented that DKK3 is stable in cold stored urine samples (at 4°C) for up to 24 hours (<10% breakdown). Alternatively, urine samples can be immediately frozen at −20°C (for longer periods at −80°C) until they are measured. Up to two cycles of freezing and thawing do not significantly affect the DKK3 recovery rate in urine. The lower detection limit of the assay is 30 pg/ml. The intra-assay test variabilities of repeated urine sample measurements are 3.1% in the lower detection range (approximately 500 pg DKK3 per 1 ml urine) and 3.5% in the higher detection range (approximately 1500 pg DKK3 per 1 ml urine). The values for interassay test variability are 4.7% in the lower detection range and 5.1% in the higher detection range. There is no crossreactivity with other proteins of the DKK family (i.e., DKK1 and DKK2). Urinary DKK3 levels were normalized to urinary creatinine concentrations to account for dilution of the urine. All urine samples were immediately frozen at −80°C after collection. In additional analyses, we observed no relevant degradation of DKK3 during freezing. We could not detect any difference of DKK3 in freshly used urine samples, frozen urine samples, and urine samples after one freeze-thaw cycle. To exclude any bias from different sample processing, DKK3 in all samples from all cohorts presented in the manuscript was measured at one site (Saarland University Medical Center) in a blinded manner.
Histopathologic Examination of Kidney Biopsy Slides
The extent of tubulointerstitial fibrosis on scanned periodic acid–Schiff-stained biopsy sections (3 μm, MIRAX Viewer; Zeiss, Oberkochen, Germany) was morphometrically determined for cortex only and is given as a percentage of the total cortical tubulointerstitium.7 The histopathologic examination of kidney biopsy specimens was done in a blinded fashion in the Department of Cellular and Molecular Pathology, German Cancer Research Center (Heidelberg, Germany).
Statistical Analyses
Continuous data are presented as mean±SD when normally distributed or median and interquartile range for variables with skewed distribution. Categorical data are presented as percentages. Variables with skewed distribution (DKK3 to creatinine, albuminuria, and C-reactive protein) were log transformed. Statistical differences between continuous variables were determined using one-way ANOVA, Kruskal–Wallis test, or chi-squared test for categorical variables.
In the CARE FOR HOMe Study, 575 participants were included in the analyses. On the basis of the annual follow-up, longitudinal data analyses were performed in 1-year blocks during follow-up. We analyzed nonlinear associations between DKK3 to creatinine and eGFR change by using restricted cubic splines of urinary DKK3-to-creatinine levels with four knots (0, 76.4, 441.2, and 6008.5 pg/mg) placed at the fifth, 35th, 65th, and 95th percentiles. Respective restricted cubic spline plots are shown. Analyses were adjusted for age, sex, body mass index (BMI), systolic BP, smoking, diabetes, eGFR, and log albuminuria. On the basis of the visual inspection of the spline plots, we divided urinary DKK3-to-creatinine levels for subsequent analyses into four categories according to the observed annual eGFR changes: ≤200, 201–1000, 1001–4000, and >4000 pg/mg creatinine. Next, we built general estimation equations to determine the association between the aforementioned categories of DKK3-to-creatinine and eGFR changes during the following 12 months and account for correlations between multiple observations. Analyses were adjusted for age, sex, BMI, systolic BP, smoking, diabetes, eGFR, and log albuminuria. Moreover, sensitivity analyses were performed using tertiles or quartiles of DKK3 to creatinine. Least square means of change of eGFR were calculated for categories of urinary DKK3-to-creatinine concentrations.
Furthermore, integrated discrimination improvement (IDI), net reclassification improvement (NRI), and c statistics were calculated for DKK3 added to the eight-variable (i.e., age, sex, eGFR, albuminuria, diabetes, hypertension, calcium, phosphate, bicarbonate, and albumin) kidney failure risk equation as reported by Tangri et al.,21 which is validated to predict the 2- and 5-years risk of ESRD or transplantation. For this purpose, we built four risk categories (>0%, >2%, >5%, and >10% of annual declines of eGFR) as recently reported.21 Exact confidence intervals are reported according to the method described previously.22
Because of the smaller sample size, STOP IgAN Trial participants were dichotomized into two groups at the median of urinary DKK3 to creatinine (i.e., 779 pg/mg). Generalized linear models, including the aforementioned covariates, were built to study the association between DKK3 to creatinine and changes of eGFR during the run-in period. Additionally, restricted cubic splines of DKK3 to creatinine with three knots (90.9, 779.6, and 3121.3 pg/mg) placed at tenth, 50th, and 90th percentiles were plotted. Furthermore, the association between change of DKK3 to creatinine during early treatment phase and change of eGFR was studied. For this purpose, we analyzed nonlinear associations between DKK3 to creatinine and eGFR change by using restricted cubic splines of change of urinary DKK3-to-creatinine levels during the early treatment phase with three knots (−1251, −54.5, and 548 pg/mg) placed at tenth, 50th, and 90th percentiles. Analyses were adjusted for age, sex, BMI, systolic BP, smoking, eGFR, and log albuminuria. Similar to the approach described above, we determined the additive value of DKK3 to a model comprising age, sex, BMI, systolic BP, eGFR, and albuminuria during the run-in phase by calculating IDI, NRI, and c statistics. Because of the smaller sample size, two risk categories (i.e., >0% and >5% declines of eGFR within 6 months) were built.
In the general population study and the CARE FOR HOMe Study, bivariate correlations between urinary DKK3-to-creatinine concentrations and several clinical parameters were reported as Spearman ρ.
A two-sided P value <0.05 was considered statistically significant. Analyses were performed using SPSS version 21.0 and STATA IC 15. Multivariate-adjusted restricted cubic spline analyses were performed using the STATA package “postrcspline.” IDI, NRI, and c statistics were calculated using the STATA package “nriidi.”
Results
Urinary DKK3 Levels in the General Population and Patients with CKD
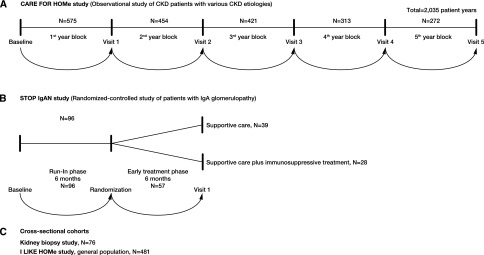
Figure 1 gives an overview on the studies and cohorts included in these analyses.
Figure 1.
Overview of studies and cohorts. (A) CARE FOR HOMe study (CKD patients) comprising 575 participants with annual follow-up (2,035 patient years). (B) STOP IgAN study, randomized-controlled study comprising 96 patients with IgA nephropathy. (C) Cross-sectional cohorts, Kidney Biopsies study comprising 76 patients and I LIKE HOMe study comprising 481 participants of the general population.
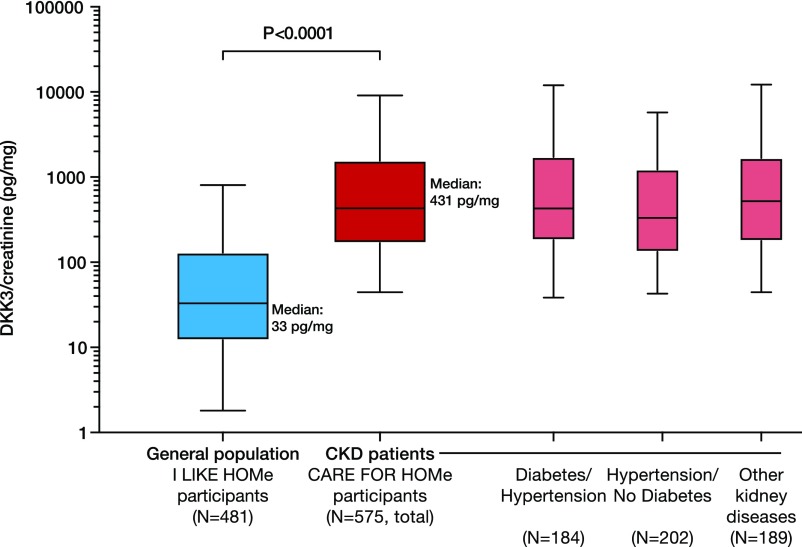
Baseline characteristics of participants of the I LIKE HOMe Study (n=481), representing the general population, and the CARE FOR HOMe Study cohort (n=575), comprising patients with various CKD etiologies, are shown in Supplemental Tables 1–3. Figure 2 shows urinary DKK3-to-creatinine concentrations in subjects from the I LIKE HOMe Study and patients with CKD from the CARE FOR HOMe Study cohort. The distribution of urinary DKK3-to-creatinine levels in the I LIKE HOMe Study participants was skewed, with a median urinary DKK3-to-creatinine concentration of 33 (126) pg/mg. Urinary DKK3 concentrations in the general population were significantly lower compared with those in patients with CKD from the CARE FOR HOMe Study cohort (P<0.001). Multivariate-adjusted means of DKK3 to creatinine also differed significantly between the general population and patients with CKD (P<0.001) (Supplemental Table 4). We found no significant correlations between urinary DKK3-to-creatinine levels and age, sex, eGFR, presence of diabetes, hypertension, or albuminuria in the general population (Supplemental Table 5).
Figure 2.
Urinary Dickkopf-3 (DKK3) is elevated in patients with CKD. Distribution of urinary Dickkopf-3 (DKK3)-to-creatinine levels (on logarithmic scale) in the participants of the I LIKE HOMe Study cohort representing the general population (n=481) compared with patients with CKD from the CARE FOR HOMe Study cohort (n=575), with no relevant overlap between subjects from the general population and patients with CKD. Urinary DKK3-to-creatinine levels were comparable in hypertensive patients with CKD with (n=184) and without (n=202) diabetes and patients with CKD of other etiology (n=189).
Urinary DKK3 and CKD Progression during Longitudinal Follow-Up
In the CARE FOR HOMe Study cohort, higher urinary DKK3 to creatinine was associated with women, lower BMI and eGFR, and higher albumin excretion rate but was not associated with systolic or diastolic BP, smoking, presence of diabetes, or serum C-reactive protein concentrations (Supplemental Tables 3 and 6).
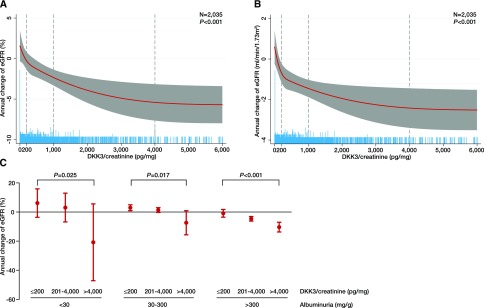
The association between urinary DKK3 to creatinine and change of eGFR over the next risk period was analyzed in 1-year blocks. A total of 2035 person-years were available for analysis on the basis of the annual visits during follow-up. To model the association between urinary DKK3 to creatinine and the subsequent eGFR change, restricted cubic spline regression models were built. Figure 3, A and B shows the restricted cubic spline plots displaying the association between urinary DKK3-to-creatinine levels and the annual change of eGFR. Notably, in patients with urinary DKK3 levels below 200 pg/mg creatinine, eGFR increased or did not change during observation. In contrast, in patients with urinary DKK3 levels exceeding 1000 pg/mg creatinine, eGFR declined.
Figure 3.
DKK3 predicts risk of short-term eGFR loss in patients with CKD. (A and B) Restricted cubic spline plots of the association between annual change of eGFR in (A) percentage and (B) milliliters per minute per 1.73 m2 and urinary Dickkopf-3 (DKK3)-to-creatinine concentrations in participants of the CARE FOR HOMe Study in 1-year blocks with a total of 2035 patient-years available. The red line indicates the estimated change of eGFR with the respective 95% confidence interval (gray area). Dashed vertical lines indicate DKK3-to-creatinine levels used as cutoffs in subsequent analyses. Blue spikes show the distribution of urinary DKK3-to-creatinine concentrations. All plots are adjusted for age, sex, body mass index, systolic BP, diabetes, smoking status, eGFR, and log albuminuria. (C) Annual change of eGFR according to categories of DKK3 to creatinine in patients with albuminuria <30, 30–300, and >300 mg/g. All analyses were adjusted for age, sex, body mass index, systolic BP, diabetes, smoking status, eGFR, and log albuminuria.
On the basis of these plots, urinary DKK3-to-creatinine levels were divided into categories (≤200, 201–1000, 1001–4000, and >4000 pg/mg creatinine), and multivariable-adjusted general estimation equations were built (Table 1). After adjustment for potential confounders, such as age, sex, BMI, systolic BP, smoking, diabetes, eGFR, and albuminuria, urinary DKK3 remained a significant and independent indicator of eGFR decline within the following 12-month period. Adjustment for eGFR (model 2) and albuminuria (model 3) did not attenuate this association, and there was no interaction between DKK3 and albuminuria (Table 1). These findings underline the independent role of urinary DKK3 as a significant marker for CKD progression. Urinary DKK3 concentrations >4000 pg/mg creatinine were associated with a mean eGFR decline of 7.6% (95% confidence interval, −10.8 to −4.3%; P<0.001 versus reference) in the subsequent 12 months. In sensitivity analyses, results were not affected by the selection of different categories of urinary DKK3 to creatinine (Supplemental Table 7) or modified by angiotensin-converting enzyme inhibitor or AT1 receptor blocker therapy (Supplemental Table 8).
Table 1.
Estimated marginal means of annual change of eGFR according to categories of Dickkopf-3–to-creatinine concentrations in urine in participants of the CARE FOR HOMe Study cohort
| Urinary DKK3 to Creatinine, pg/mg | Annual Change of eGFR, % | 95% CI | P Value | Annual Change of eGFR, ml/min | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Crude | ||||||
| ≤200 | 1.3 | −0.2 to 2.8 | Reference | 0.2 | −0.5 to 0.8 | Reference |
| 201–1000 | −1.5 | −2.6 to −0.4 | <0.01 | −1.2 | −1.7 to −0.7 | 0.002 |
| 1001–4000 | −2.3 | −4.1 to −0.6 | 0.003 | −1.5 | −2.3 to −0.7 | 0.002 |
| >4000 | −8.3 | −11.7 to −5.0 | <0.001 | −2.3 | −3.4 to −1.3 | <0.001 |
| Model 1 | ||||||
| ≤200 | 0.8 | −0.9 to 2.6 | Reference | −0.1 | −0.9 to 0.6 | Reference |
| 201–1000 | −2.1 | −3.5 to −0.7 | 0.004 | −1.5 | −2.2 to −0.9 | 0.002 |
| 1001–4000 | −2.7 | −4.7 to −0.7 | <0.01 | −1.6 | −2.5 to −0.7 | <0.01 |
| >4000 | −9.0 | −12.3 to −5.6 | <0.001 | −2.6 | −3.7 to −1.5 | <0.001 |
| Model 2 | ||||||
| ≤200 | 1.6 | −1.4 to 3.5 | Reference | 0.3 | −0.5 to 1.1 | Reference |
| 201–1000 | −1.7 | −3.2 to −0.3 | 0.001 | −1.3 | −2.0 to −0.6 | <0.001 |
| 1001–4000 | −3.1 | −5.2 to −1.0 | <0.001 | −1.8 | −2.8 to −0.9 | <0.001 |
| >4000 | −11.5 | −15.1 to −8.0 | <0.001 | −4.3 | −5.5 to −3.0 | <0.001 |
| Model 3 | ||||||
| ≤200 | 0.9 | −0.9 to 2.8 | Reference | 0.1 | −0.7 to 0.9 | Reference |
| 201–1000 | −1.7 | −3.1 to −0.3 | <0.01 | −1.3 | −2.0 to −0.6 | 0.002 |
| 1001–4000 | −2.4 | −4.5 to −0.3 | 0.01 | −1.6 | −2.5 to −0.7 | 0.002 |
| >4000 | −7.6 | −10.8 to −4.3 | <0.001 | −2.8 | −4.1 to −1.6 | <0.001 |
Model 1: adjusted for age, sex, body mass index, systolic BP, diabetes, and smoking. Model 2: model 1 adjusted for eGFR. Model 3: model 2 adjusted for log albuminuria. P=0.21 for the interaction between log albuminuria and DKK3 to creatinine. DKK3, Dickkopf-3; 95% CI, 95% confidence interval.
Furthermore, higher urinary DKK3 to creatinine was associated with significantly greater loss of eGFR within all categories of albuminuria (Figure 3C) and independent of the underlying kidney disease (Supplemental Figure 1).
To test the predictive value of urinary DKK3, we added DKK3 to a model comprising the eight-variable kidney failure risk equation as reported by Tangri et al.21 Notably, addition of DKK3 to the kidney failure risk equation increased IDI, NRI, and c statistics (Supplemental Table 9, Table 2). Moreover, we compared the predictive value of DKK3 with suPAR, which has been recently reported to predict CKD progression during long-term follow-up.23 In contrast to DKK3, plasma suPAR levels did not predict eGFR changes during a 12-month interval (Supplemental Table 10), and addition of suPAR to a model comprising DKK3 did not improve prediction of CKD progression (Supplemental Table 11).
Table 2.
Integrated discrimination improvement and net reclassification improvement by addition of urinary Dickkopf-3 to creatinine to the eight-variable kidney failure risk equation as reported by Tangri et al.21 in predicting >0%, >5%, and >10% decreases of eGFR in participants of the CARE FOR HOMe Study
| Outcome | IDI | 95% CI | P Value | NRI | 95% CI | P Value |
|---|---|---|---|---|---|---|
| >0% decrease of eGFR | 0.014 | 0.01 to 0.02 | <0.001 | 0.082 | 0.02 to 0.14 | <0.01 |
| >2% decrease of eGFR | 0.009 | 0.01 to 0.01 | <0.001 | 0.067 | 0.01 to 0.12 | 0.02 |
| >5% decrease of eGFR | 0.014 | 0.01 to 0.02 | <0.001 | 0.106 | 0.06 to 0.16 | <0.001 |
| >10% decrease of eGFR | 0.013 | 0.01 to 0.02 | <0.001 | 0.045 | 0.01 to 0.08 | <0.01 |
IDI, integrated discrimination improvement; 95% CI, 95% confidence interval; NRI, net reclassification improvement.
Urinary DKK3 and Tubulointerstitial Fibrosis
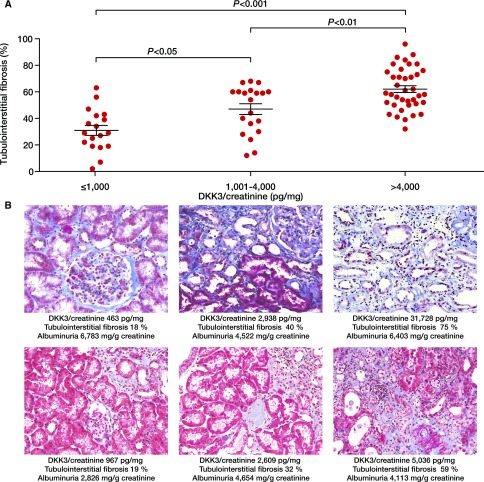
The association between urinary DKK3 concentrations and tubulointerstitial fibrosis in kidney biopsies was first analyzed in 76 patients who underwent a diagnostic kidney biopsy at the University Hospital Innsbruck, Austria (Supplemental Table 12). Elevated DKK3-to-creatinine in urine was significantly associated with a higher degree of tubulointerstitial fibrosis in the biopsy specimens of both patients with glomerular diseases and patients with interstitial kidney diseases (Figure 4A). Figure 4B shows representative kidney biopsy specimens of patients with various CKD etiology and different urinary DKK3-to-creatinine levels. Notably, the association of DKK3 and tubulointerstitial fibrosis was independent of eGFR, albuminuria, or type of kidney disease (Supplemental Table 13). In addition, Supplemental Figure 2 shows the extent of tubulointerstitial fibrosis in kidney biopsy specimens of STOP IgAN Trial participants before study inclusion according to their urinary DKK3-to-creatinine levels.
Figure 4.
DKK3 associates with higher tubulointerstitial fibrosis. (A) Tubulointerstitial fibrosis in patients who underwent diagnostic kidney biopsies according to urinary Dickkopf-3 (DKK3)-to-creatinine concentrations. (B) Representative kidney biopsy specimens from three patients with membranous nephropathy (upper panels) and three patients with focal segmental sclerosis (lower panels) according to urinary DKK3-to-creatinine concentrations.
Urinary DKK3 and CKD Progression in the STOP IgAN Trial
To further validate urinary DKK3 as a biomarker of ongoing CKD progression, we measured urinary DKK3 levels in all available urine samples from participants of the 6-month run-in phase (n=96) and the 6-month early randomized treatment phase of the STOP IgAN Trial (n=57).19 Characteristics of this STOP IgAN Trial subcohort at enrollment are shown in Supplemental Tables 14 and 15.
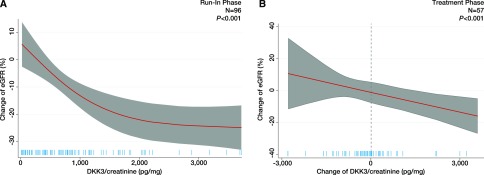
During the run-in phase, urinary DKK3 >779 pg/mg creatinine (i.e., median) was associated with a mean eGFR decline of 19.1% (95% confidence interval, −24.2 to −14.0%; P<0.001 versus reference) after adjustment for potential confounders, including baseline eGFR and albuminuria (Supplemental Table 16). This result was independent of the DKK3 cutoff chosen (Supplemental Table 17). Figure 5A shows the restricted cubic spline plots displaying the association between urinary DKK3-to-creatinine levels and the change of eGFR. Also, in participants of the STOP IgAN Trial, addition of DKK3 to a model comprising eGFR and albuminuria significantly increased IDI, NRI, and c statistics (Supplemental Table 18).
Figure 5.
DKK3 predicts eGFR loss in patients with IgA nephropathy. (A) Restricted cubic spline plot of the association between change of eGFR and urinary Dickkopf-3 (DKK3)-to-creatinine concentrations during the run-in phase in participants of the STOP IgAN Trial adjusted for age, sex, body mass index, systolic BP, smoking status, eGFR, and log albuminuria. (B) Restricted cubic spline plot of the association between change of eGFR (percentage) and change of urinary DKK3 to creatinine during early treatment phase in participants of the STOP IgAN Trial adjusted for age, sex, body mass index, systolic BP, smoking status, eGFR, and log albuminuria.
After the run-in period, all participants of the STOP IgAN Trial who had persistent proteinuria of at least 0.75 g/d despite optimized supportive therapy were randomly assigned to receive either supportive care alone or supportive care plus immunosuppressive therapy. We assumed that immunosuppressive treatment may modulate stress-induced DKK3 tubular epithelial secretion and thus, interfere with the ability of urinary DKK3 to indicate actual progression. Indeed, patients under immunosuppressive therapy showed a trend toward a greater reduction of DKK3 compared with those receiving supportive care only (Supplemental Figure 3). Therefore, we examined the association between changes in urinary DKK3 levels during the first 6 months of the treatment phase and changes of eGFR during this period in all STOP IgAN Trial participants, regardless of randomization. As presented in Figure 5B and Supplemental Table 19, a rise in urinary DKK3-to-creatinine concentrations during the treatment phase was associated with a significant (P=0.02) eGFR decline, whereas stable or decreasing urinary DKK3-to-creatinine levels indicated a more favorable course of kidney function. There was no interaction between the randomization to the two intervention arms and urinary DKK3-to-creatinine levels. In contrast to urinary DKK3-to-creatinine concentration, albuminuria was not significantly related to changes of eGFR (Supplemental Table 19).
Discussion
In this study, the association between urinary DKK3 and short-term eGFR loss in patients with CKD was assessed. Independent of CKD etiology, urinary DKK3 reliably discriminated patients with CKD with a subsequent decline of eGFR from those with subsequently stable kidney function within 12 months. This was particularly the case in patients without albuminuria.
It is assumed that, beyond the age of 40 years old, the annual GFR loss related to the aging process is approximately 0.7 ml/min per 1.73 m2.24 Thereby, hypertension and diabetes represent the most important and frequent risk factors for CKD progression.9,25 However, even in subjects in whom both risk factors are present, the course of CKD may be variable.10 The CARE FOR HOMe Study mainly comprises patients with diabetic and hypertensive kidney injury. Exactly in those patients who are commonly seen in primary care, we identified a strong association between urinary DKK3 levels and eGFR loss within 12 months. DKK3 levels above 4000 pg/mg creatinine were related to a mean annual eGFR loss of 7.6%, which definitely exceeds the rate of age-related loss of kidney function.
Currently, prediction of eGFR loss during long-term follow-up is attempted by the use of kidney failure risk equations including (baseline) eGFR and albuminuria as well as various clinical and biochemical variables.9,21 Although refining of these equations with inclusion of more putative progression factors can improve their accuracy, the individual CKD course is variable and difficult to predict by general equations, particularly under disease-modifying interventions. This can be well observed in the early treatment phase of the STOP IgAN Trial. Here, a carefully selected cohort of patients with IgAN displayed a highly unpredictable course of kidney function within 6 months. Nevertheless, changes in urinary DKK3-to-creatinine concentrations helped to identify those patients with fast eGFR decline (i.e., rising urinary DKK3-to-creatinine levels were accompanied by a significant eGFR decline during this period). In contrast, eGFR remained stable or even slightly increased in patients in whom urinary DKK3-to-creatinine levels did not change substantially or even decreased. On the basis of these observations, we suggest that urinary DKK3 not only is a biomarker for short-term eGFR loss but may be involved in its pathogenesis as indicated by experimental studies.7
Previous studies have shown associations between biomarkers of tubular injury, such as NGAL and kidney injury molecule-1, and loss of kidney function in patients with CKD, but their clinical usefulness has been challenged.26–28 In the prospective Chronic Renal Insufficiency Cohort Study of 2466 patients with CKD with various etiologies and at different CKD stages, neither kidney injury molecule-1 nor NGAL were independently associated with the rate of disease progression, and none of the biomarkers tested improved the risk prediction in comparison with a clinical model that included eGFR and the urinary albumin-to-creatinine ratio.26 Additionally, suPAR, which has been recently shown to be associated with eGFR loss during a longer time period (i.e., 1337 days), did not predict short-term decline of eGFR in our study.20
In the STOP IgAN Trial, changes in urinary DKK3 were independently associated with changes in eGFR, even after adjustment for albuminuria or randomization to the treatment arms. This finding is of particular interest, because the development of albuminuria—a putative indicator of glomerular damage—and increased tubular secretion of DKK3 in the urine may not be related to the same pathophysiologic mechanism. This is further corroborated by the CARE FOR HOMe Study, where increased urinary DKK3 indicated significant loss of kidney function, even in the absence of albuminuria. Therefore, it might be inferred that persistently elevated urinary DKK3 levels indicate ongoing tubular “stress,” leading to progressive tubulointerstitial fibrosis independent of eGFR and albuminuria, and the type of kidney disease. Indeed, DKK3 as a member of Wnt pathway modulators29 has been shown to be a stress-induced, tubular epithelial cell–derived regulator of kidney fibrosis in different CKD mouse models.7 Mechanistically, DKK3 deficiency caused diminished canonical Wnt/β-catenin signaling in tubular epithelial cells, which was accompanied by an antifibrogenic inflammatory response within the injured kidney. These data highlight a crucial role of the Wnt/β-catenin pathway in renal tubulointerstitial injury30 and thereby, DKK3 in declining eGFR within 12 months.31
In most subjects of the general population, urinary DKK3 concentrations were below 200 pg/mg creatinine and hence, much lower than in patients with CKD, although our general population cohort comprised subjects with arterial hypertension and/or diabetes. Nevertheless, we detected urinary DKK3 levels above 1000 pg/mg creatinine in only a minority of them. We speculate that unknown conditions or yet unidentified kidney injury might be the source of increased urinary DKK3 concentrations. Future studies have to examine whether DKK3 might also allow for identification of subjects with clinically nonapparent kidney injury, which cannot be detected by currently available laboratory parameters, and the temporal relationship between changes in urinary DKK3 and alterations of eGFR. All participants in these studies were of Caucasian ancestry, and therefore, the applicability of our results to other races is limited. Moreover, additional studies are necessary to examine whether DKK3-directed therapeutic interventions may improve the outcome in patients with progressive CKD.
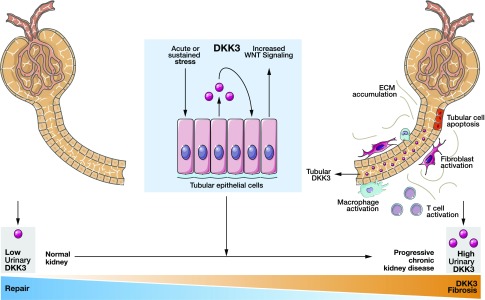
In summary, measurement of DKK3 in urine represents a novel tool for the identification of patients at high risk for short-term eGFR loss regardless of the cause of kidney injury and beyond currently established biomarkers (Figure 6). Therefore, DKK3 may improve the management of patients with CKD as a personalized medicine approach, because urinary DKK3-to-creatinine levels could be used as a tool to monitor, and if necessary, also intensify therapeutic intervention to halt CKD progression. These include general measures, such as effective BP and blood glucose control,32,33 dietary salt restriction,34 and cessation of smoking, and specific interventions, such as high-dose treatment with inhibitors of the renin-angiotensin system including mineral-corticoid receptor inhibitors.35
Figure 6.
Summarizing figure. Main concept of the work. DKK3, Dickkopf-3; ECM; WNT.
Disclosures
D.F. is associated with DiaRen UG.
Supplementary Material
Acknowledgments
We thank Prof. Dr. B. Arnold, Dr. G. Moldenhauer, and Dr. M. Meister from the Department of Molecular Immunology, German Cancer Research Center (Heidelberg, Germany) for their contribution to the development of the Dickkopf-3 ELISA.
S.Z., J.F., D.F., and T.S. are supported by Deutsche Forschungsgemeinschaft (DFG) grant SFB TRR 219 “Mechanisms of cardiovascular complications in CKD.” S.Z. is supported by the Else-Kroener Fresenius Foundation. H.-J.G. is supported by DFG grant SFB 1118 “Diabetes.”
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018040405/-/DCSupplemental.
References
- 1.Liu BC, Tang TT, Lv LL, Lan HY: Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int 93: 568–579, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Schnaper HW: The tubulointerstitial pathophysiology of progressive kidney disease. Adv Chronic Kidney Dis 24: 107–116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farris AB, Colvin RB: Renal interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol Hypertens 21: 289–300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert RE: Proximal tubulopathy: Prime mover and key therapeutic target in diabetic kidney disease. Diabetes 66: 791–800, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Yu SM, Bonventre JV: Acute kidney injury and progression of diabetic kidney disease. Adv Chronic Kidney Dis 25: 166–180, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seccia TM, Caroccia B, Calò LA: Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J Hypertens 35: 205–212, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Federico G, Meister M, Mathow D, Heine GH, Moldenhauer G, Popovic ZV, et al.: Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 1: e84916, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, et al.: ISN Global Kidney Health Summit participants : Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 390: 1888–1917, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Andrassy KM: Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease.’ Kidney Int 84(3): 622–623, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al.: Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, et al.: ERA-EDTA diabesity working group : Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol 3: 382–391, 2015 [DOI] [PubMed] [Google Scholar]
- 12.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G: Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 27: 195–200, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Bash LD, Selvin E, Steffes M, Coresh J, Astor BC: Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med 168: 2440–2447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer H, Boucher RE, Leehey D, Fried L, Wei G, Greene T, et al.: Increasing mortality in adults with diabetes and low estimated glomerular filtration rate in the absence of albuminuria. Diabetes Care 41: 775–781, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brosius FC, Pennathur S: How to find a prognostic biomarker for progressive diabetic nephropathy. Kidney Int 83: 996–998, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration : Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol 2: 634–647, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Chang A, Rostand SG, Hebert L, Appel LJ, Astor BC, et al.: A within-patient analysis for time-varying risk factors of CKD progression. J Am Soc Nephrol 25: 606–613, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolignano D, Lennartz S, Leonardis D, D’Arrigo G, Tripepi R, Emrich IE, et al.: High estimated pulmonary artery systolic pressure predicts adverse cardiovascular outcomes in stage 2-4 chronic kidney disease. Kidney Int 88: 130–136, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al.: STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Hayek SS, Quyyumi AA, Reiser J: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 374: 891, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, et al.: CKD Prognosis Consortium : Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. JAMA 315: 164–174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman M, Bryant G: Statistics with Confidence, London, BMJ Books, 2005 [Google Scholar]
- 23.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al.: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denic A, Glassock RJ, Rule AD: Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 23: 19–28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, et al.: workshop participants : Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20: 1199–1209, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Hsu CY, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, et al.: CRIC Study Investigators; CKD Biomarkers Consortium : Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int 91: 196–203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, et al.: Chronic Renal Insufficiency Cohort (CRIC) study investigators : Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou LT, Lv LL, Pan MM, Cao YH, Liu H, Feng Y, et al.: Are urinary tubular injury markers useful in chronic kidney disease? A systematic review and meta analysis. PLoS One 11: e0167334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gröne EF, Federico G, Nelson PJ, Arnold B, Gröne HJ: The hormetic functions of Wnt pathways in tubular injury. Pflugers Arch 469: 899–906, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Tan RJ, Fu H, Liu Y: Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab Invest 96: 156–167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Shi S, Senthilnathan S, Yu J, Wu E, Bergmann C, et al.: Genetic variation of DKK3 may modify renal disease severity in ADPKD. J Am Soc Nephrol 21: 1510–1520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, et al.: Collaborators on Trials of Lowering Glucose (CONTROL) group : Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: A meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 5: 431–437, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V: Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 28: 368–375, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, et al.: Chronic Renal Insufficiency Cohort Study Investigators : Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27: 1202–1212, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P: Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 23: 165–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.