Abstract
Background
The bile acid-activated receptors, including the membrane G protein–coupled receptor TGR5 and nuclear farnesoid X receptor (FXR), have roles in kidney diseases. In this study, we investigated the role of TGR5 in renal water handling and the underlying molecular mechanisms.
Methods
We used tubule suspensions of inner medullary collecting duct (IMCD) cells from rat kidneys to investigate the effect of TGR5 signaling on aquaporin-2 (AQP2) expression, and examined the in vivo effects of TGR5 in mice with lithium-induced nephrogenic diabetes insipidus (NDI) and Tgr5 knockout (Tgr5−/−) mice.
Results
Activation of TGR5 by lithocholic acid (LCA), an endogenous TGR5 ligand, or INT-777, a synthetic TGR5-specific agonist, induced AQP2 expression and intracellular trafficking in rat IMCD cells via a cAMP-protein kinase A signaling pathway. In mice with NDI, dietary supplementation with LCA markedly decreased urine output and increased urine osmolality, which was associated with significantly upregulated AQP2 expression in the kidney inner medulla. Supplementation with endogenous FXR agonist had no effect. In primary IMCD suspensions from lithium-treated rats, treatment with INT-767 (FXR and TGR5 dual agonist) or INT-777, but not INT-747 (FXR agonist), increased AQP2 expression. Tgr5−/− mice exhibited an attenuated ability to concentrate urine in response to dehydration, which was associated with decreased AQP2 expression in the kidney inner medulla. In lithium-treated Tgr5−/− mice, LCA treatment failed to prevent reduction of AQP2 expression.
Conclusions
TGR5 stimulation increases renal AQP2 expression and improves impaired urinary concentration in lithium-induced NDI. TGR5 is thus involved in regulating water metabolism in the kidney.
Keywords: TGR5, AQP2, cAMP, lithium
Bile acids are products of cholesterol metabolism, which are classically known for facilitating the digestion and absorption of dietary lipids in the small intestine. Two primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), synthesized in the liver, are either conjugated with taurine/glycine or secreted into intestines where secondary bile acids, such as lithocholic acid (LCA) and deoxycholic acid, are formed.1,2 It is well established that bile acids bind to and activate two receptors, the nuclear farnesoid X receptor (FXR) and the membrane G protein–coupled receptor (GPCR) TGR5 (also known as GPBAR1 or GPR131). Among all the unconjugated bile acids, LCA is a potent activator of TGR5 whereas CDCA is an activator for FXR.3 Besides the endogenous agonists, more selective synthetic ligands for FXR or TGR5, such as INT-767, INT-777, and INT-747, are widely used to identify the specific effects of these two receptors. It has been demonstrated that TGR5 is widely expressed in many tissues, including the intestine, gallbladder, brain, and kidney.4 Like many other GPCRs, TGR5 regulates the cAMP-protein kinase A (PKA) signaling pathway. Activation of TGR5 increases intracellular cAMP to stimulate cAMP-dependent PKA, which activates cAMP response element binding protein (CREB), initiating target gene transcription. The activation of TGR5 by bile acids or synthetic ligands is reported to play a role in metabolic homeostasis, including alleviating inflammatory responses, improving glucose tolerance in obesity and diabetic mice,5,6 and stimulating energy expenditure in brown adipose tissues.7,8
Aquaporin-2 (AQP2) is an important water channel protein critically involved in urine concentration and water homeostasis in the body. AQP2 is localized at the principal cells of collecting ducts in the kidney and is mainly regulated by arginine vasopressin (AVP). Increased circulating AVP induces protein expression, phosphorylation, and intracellular trafficking of AQP2, leading to water transport across the epithelium in the collecting ducts. This results in increased osmotic water permeability and urinary concentration.9 Abnormal AQP2 expression in the kidney is the primary cause of acquired forms of nephrogenic diabetes insipidus (NDI),10 such as sustained lithium intake, which is associated with downregulation of AQP2 protein expression in the collecting ducts and impaired urinary concentration ability.11–13
Recently, a study has shown that mice with FXR deficiency developed impaired urine concentration, which was associated with decreased expression of AQP2 in the kidney.14 Interestingly, in fxr−/− mice, CDCA, an FXR agonist with weak TGR5 affinity, still mildly improved urine concentration defect, indicating that other factors were also involved.14 Although abundant expression of TGR5 in the kidney is observed,6,15 whether TGR5 participates in the regulation of renal water handling remains unknown.
In this study, we demonstrated that bile acid receptor TGR5 stimulation triggers transmembrane water transport and upregulates renal AQP2 expression via a cAMP-PKA signaling pathway. In mice with lithium-induced NDI, TGR5 activation upregulates AQP2 expression and improves urinary concentration defect. TGR5 deletion was associated with attenuated urine concentration ability and decreased AQP2 gene and protein expression in mice.
Methods
Materials
For semiquantitative immunoblotting and immunofluorescence analysis, previously characterized affinity-purified polyclonal antibodies to AQP2 were used.16 For immunofluorescence analysis in the kidney, rabbit anti-TGR5 was purchased from R&D Systems (for mouse) and mouse anti-TGR5 was purchased from Thermo Fisher Scientific (for human), mouse anti-AQP2 was purchased from Santa Cruz Biotechnology. Rabbit anti-CREB and phospho-CREB (ser133), and rabbit anti-GSK3β and phospho-GSK3β (ser9) were purchased from Cell Signaling Technology. Antibodies to pS256-AQP2 and AVPR-V2 were purchased from Abcam. Mouse anti–β-actin was purchased from Sigma-Aldrich. The horseradish peroxidase–conjugated secondary antibodies were obtained from Dako. Lithium chloride, 1-deamino-8-D-arginine vasopressin (dDAVP), H89, MDL12330A, LCA, and CDCA were purchased from Sigma-Aldrich. Tolvaptan, INT-767, INT7–777, and INT-747 were purchased from MedChemExpress Co.
Animals
All animal procedures were approved by the Animal Care and Use Committee of Sun Yat-sen University (Ethics Committee of ZSSOM on Laboratory Animal Care No. 2016-048; Guangzhou, China). Mice with TGR5 gene deletion on a background of C57BL/6 were obtained from Guangdong Engineering and Technology Research Center for Disease-Model Animals, Sun Yat-sen University. Male wild-type mice and tgr5−/− mice (8–12 weeks old) were maintained on a 12-hour light/dark cycle at 24°C, and received food and water ad libitum during the experiment. For identification of the genotypes, DNA extracted from tails of both tgr5+/+ and tgr5−/− mice were amplified by PCR and then used for agarose gel electrophoresis or DNA sequence analysis.
Lithium Diet and Bile Acids Treatment
Protocol 1
Male Wistar rats were fed a lithium diet (40 mmol/kg dry food) or normal diet for 10 days. Inner medullary collecting duct (IMCD) suspensions were prepared from these rats.
Protocol 2
Male wild-type C57BL/6 mice or tgr5−/− mice were randomly divided into four groups: chow diet group (the control), lithium diet group, lithium plus 0.2% LCA group, or lithium plus 0.5% CDCA group. The lithium diet was a mice chow diet supplemented with lithium chloride 40 mmol/kg of dry food for the first 4 days and 20 mmol/kg of dry food for three more days; 0.2% LCA and 0.5% CDCA were mixed into the lithium diet for 7 days.
Protocol 3
Male wild-type C57BL/6 mice (n=20) were randomly divided into four groups: chow diet group (the control), lithium diet group, lithium plus 0.3% LCA group, or lithium plus 0.5% CDCA group. Mice were fed a lithium diet (40 mmol/kg of dry food) for 14 days and 0.3% LCA or 0.5% CDCA was supplemented into the lithium diet for the last 5 days.
On the final day (i.e., when mice were euthanized), 24-hour urine samples, blood samples, and the kidneys of mice were collected and prepared for measurement of physiologic parameters, protein and mRNA abundance, and histologic analysis.
Water Deprivation in Wild-Type and tgr5−/− Mice
Both tgr5+/+ mice and tgr5−/− mice were divided into two groups (control group and water deprivation groups). All animals were acclimatized to metabolic cages for 3 days. Mice were deprived of water for 36 hours but had free access to chow diets. At the end of the experiment, urine samples, blood samples, and kidneys were collected.
IMCD Tubule Preparation and Treatments
Rat IMCD suspensions were prepared as previously described.16 Primary IMCD cells were pretreated with or without MDL-12330A, H89, or tolvaptan for 30 minutes, then incubated with different concentration of LCA, CDCA, INT-767, INT-777, INT-747 or a vehicle for 6 hours. IMCD cells prepared from mice were treated with LCA for 6 hours. After the incubation, protein was collected in radioimmunoprecipitation buffer with proteinase cocktails, and the samples were used for immunoblotting, as described previously.16 The experiment was repeated three times.
Immunofluorescence Microscopy in Primary Cultured IMCD Cells
Rat IMCD suspensions were treated with hyperosmotic DMEM/F12 medium containing 10% FBS, 100 U/ml penicillin Gm and 100 U/ml streptomycin sulfate. In order to maintain AQP2 expression, the cells were grown on 24-mm transwell plates with 0.4 μm pores (3450; Corning) and dDAVP was added. When at 70%–80% confluence, the cells were switched to hyperosmotic medium without FBS for 6 hours before the experiments began. IMCD cells were then incubated with LCA (10 µM) or CDCA (100 µM) for 1 hour. After incubation, IMCD cells were washed twice with ice-cold PBS and fixed with 4% paraformaldehyde. Immunofluorescence was performed for examining AQP2 expression and trafficking in IMCD cells.
Measurement of Cell Water Volume Changes by Fluorescence Self-Quenching
The measurement of cell volume changes (Vt/V0) was performed as previously demonstrated.17 In brief, rat primary IMCD cells were seeded on collagen-coated 35-mm glass-bottom dishes in hypertonic medium for 3 days and starved in serum-free medium for another 24 hours. IMCD cells were then incubated with vehicle, dDAVP (10−9 M), LCA (10 µM), or CDCA (100 µM) for 6 hours (Figure 1H). The measurement of cell volume changes was performed as previously demonstrated (see Supplemental Material for details).17 Data were obtained from six independent experiments in all groups. The total number of cells examined was 70: 10 for the vehicle-treated group, 20 for the dDAVP (10−9 M)-treated group, 20 for the LCA (10 µM)-treated group, and 20 for the CDCA (100 µM)-treated group.
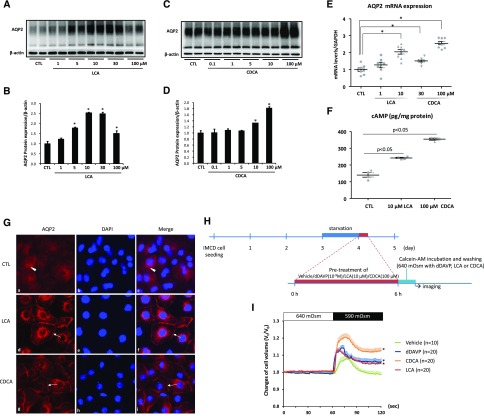
Figure 1.
LCA induced AQP2 expression and apical membrane accumulation, and facilitated transmembrane water transport in rat IMCD cells. (A–E) Primary IMCD suspensions were treated with LCA or CDCA at different concentrations for 6 hours. AQP2 protein expression and mRNA levels were detected by (A–D) Western blot and (E) RT-PCR analysis, respectively; n=6 or 8 biologically independent samples in each group. (F) Both LCA (10 μM) and CDCA (100 μM) treatment increased cAMP levels in IMCD cell lysate; n=4 biologically independent samples in each group. (G) Immunofluorescence of AQP2 showed that in primary cultured IMCD cells, LCA (10 μM) and CDCA (100 μM) treatment for 1 hour promoted apical membrane abundance of AQP2 (arrows); n=6 biologically independent samples in each groups (magnification ×400). (H and I) Measurement of relative changes in cell volume by fluorescence self-quenching in primary cultured IMCD cells. A protocol of cell volume measurement was shown (H) and relative changes in cell volume were measured by fluorescence self-quenching (I). After LCA (10 μM), CDCA (100 μM) or dDAVP (10−9 M) was added, and relative changes in IMCD cell volume were significantly increased when extracellular osmolality was changed from 640 to 590 mOsm/kg˙H2O. n indicates biologically independent samples in each group. Data are shown as mean±SEM; *P<0.05 compared with CTL. CTL, control.
Statistical Analyses
Results are presented as the means±SEM. Data were analyzed by one-way ANOVA and Newman–Keuls tests for multiple comparisons. Statistical significance was accepted at the P<0.05 level.
More methods for other measurements and analysis are in the Supplemental Material.
Results
TGR5 Stimulation Induced Upregulation of AQP2 Protein Expression via cAMP-PKA Signaling Pathway in IMCD Cells
To investigate the effects of TGR5 and FXR on renal AQP2 expression, tubule suspensions of the IMCD of rat kidneys were treated with an endogenous TGR5 agonist LCA or an FXR agonist CDCA for 6 hours. Western blot analysis showed both LCA and CDCA treatment increased the expression of AQP2 protein (Figure 1, A–D). LCA had the most potent effect on AQP2 expression at the concentration of 10 µM; for CDCA, 100 µM showed the strongest effects. These two doses were used in the following experiments. RT-PCR showed increased AQP2 mRNA levels induced by LCA or CDCA treatment (Figure 1E). LCA and CDCA induced an elevated cAMP concentration in the cell lysates (Figure 1F). Immunofluorescence demonstrated that AQP2 staining was dispersed throughout the cytoplasm (arrowhead) in the primary cultured IMCD cells. Treatment with LCA or CDCA for 1 hour caused a marked increase in AQP2 staining intensity in the plasma membranes (arrows) and certain intracellular compartments (Figure 1G). The observed changes of AQP2 protein abundance induced by TGR5 or FXR were further supported by the fluorescence quenching analysis, which measured the cell volume changes in rat primary IMCD cells. When extracellular osmolality was changed from 640 to 590 mOsm/Kg˙H2O, the calculated “cell volume changes” of IMCD cells pretreated with LCA or CDCA (Figure 1H) were rapidly and significantly increased compared with the vehicle-treated group (Figure 1I). This finding was similar to the dDAVP-treated IMCD cells, which were the positive control (Figure 1I). These data suggest that TGR5 activation by LCA increases AQP2 protein expression and trafficking, and potentially promotes transmembrane water transport in IMCD cells.
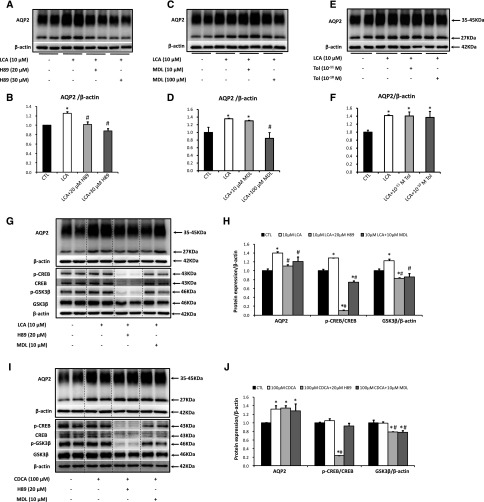
To further examine the role of cAMP-PKA pathway in the regulation of AQP2 protein expression by TGR5, rat IMCD suspensions were pretreated with either adenylyl cyclase (AC) inhibitor MDL12330A or PKA inhibitor H89 for 30 minutes, before incubation with LCA or CDCA. Increased expression of AQP2 induced by LCA was effectively attenuated by either MDL12330A or H89 treatment (Figure 2, A–D), indicating that LCA activated AC and elevated intracellular cAMP production, which subsequently activated PKA pathway and thus upregulated AQP2 expression. Consistent with this, the increased ratio of p-CREB/CREB protein induced by LCA was also significantly attenuated after MDL12330A or H89 treatments (Figure 2, G and H). Vasopressin type 2 receptor (V2R) antagonist tolvaptan, on the other hand, failed to inhibit LCA-induced AQP2 protein expression (Figure 2, E and F), indicating that a vasopressin-independent signaling pathway was involved. Interestingly, neither MDL12330A nor H89 were able to attenuate the increased AQP2 protein expression triggered by CDCA (Figure 2, I and J), although CDCA also increased intracellular cAMP levels in IMCD cells (Figure 1E). As CDCA is the most potent endogenous agonist to FXR, our data support the concept that CDCA stimulates AQP2 expression in IMCD cells directly through FXR-mediated induction of transcription.14 Recent studies have shown that inactivation or deletion of glycogen synthase kinase 3β (GSK3β), a downstream target of CREB signaling, reduced AQP2 expression.18,19 LCA rather than CDCA treatment was associated with increased expression of GSK3β protein (Figure 2, G–J), which was also inhibited by either MDL12330A or H89, indicating the involvement of GSK3β in LCA-induced upregulation of AQP2 in IMCD cells.
Figure 2.
LCA increased AQP2 protein expression via cAMP-PKA signaling pathway. Primary IMCD suspensions were pretreated with DMSO (vehicle), H89 (20, 30 μM), MDL-12330A (10, 100 μM), or tolvaptan (10−11, 10−10 M) for 30 minutes and then treated with LCA (10 μM) or CDCA (100 μM) for 6 hours. Cell lysates were subjected to Western blot analysis for AQP2 (A–F), p-CREB (ser133), CREB, p-GSK3β (ser9), GSK3β or β-actin (G–J); n=6 biologically independent samples in each groups. Data are shown as mean±SEM; *P<0.05 compared with CTL; #P<0.05 compared with either LCA or CDCA. CTL, control; MDL, MDL12330A; Tol, tolvaptan.
To investigate the specific effects of TGR5 or FXR on AQP2 protein expression, a synthetic TGR5 agonist INT-777, the FXR agonist INT-747 and dual TGR5-FXR agonist INT-767 were used. Western blots showed that INT-767, INT-777, and INT-747 upregulated AQP2 protein expression (Figure 3, A–F). Pretreatment with MDL12330A or H89 markedly prevented the upregulation of AQP2 expression induced by INT-767 or INT-777 (Figure 3, G, H, and J), but not by FXR agonist INT-747 in IMCD cells (Figure 3, I and J). Taken together, these findings suggest that activation of TGR5, but not FXR, promotes AQP2 expression via cAMP-PKA pathway in IMCD cells.
Figure 3.
Synthetic TGR5 ligands increased AQP2 protein expression via cAMP-PKA signaling pathway. AQP2 protein expression was examined by Western blot in IMCD suspensions with INT-767 (10, 20 µM), INT-777 (50, 100 µM), or INT-747 (10, 30 µM) treatment for 6 hours (A–F). Primary IMCD suspensions were pretreated with DMSO (vehicle), H89 (10 µM), or MDL-12330A (10 µM) for 30 minutes, and then stimulated with INT-767 (10 µM), INT-777 (100 µM), or INT-747 (10 µM) for 6 hours. Cell lysates were subjected to Western blot analysis for AQP2 (G–J); n=6 biologically independent samples in each groups. Data are shown as mean±SEM; *P<0.05 compared with CTL; #P<0.05 compared with INT-767, INT-777, or INT-747. CTL, control; MDL, MDL12330A.
The Role of TGR5 in Lithium-Induced NDI In Vitro and In Vivo
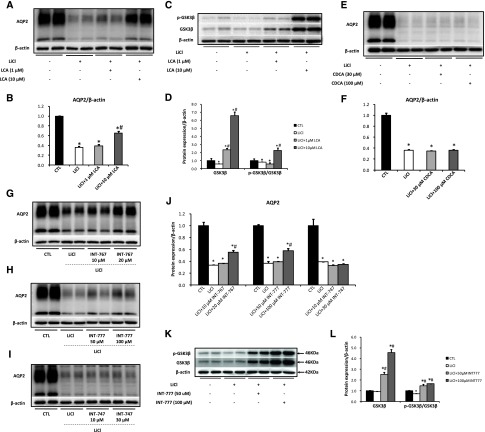
As TGR5 agonists promoted AQP2 protein expression in IMCD cells, we next investigated the potential role of TGR5 in lithium-induced NDI. In IMCD suspensions prepared from lithium-treated rats (protocol 1), AQP2 protein expression was dramatically reduced compared with control, which was markedly improved by LCA (Figure 4, A and B). However, CDCA failed to increase AQP2 expression (Figure 4, E and F). Dual TGR5 and FXR agonist INT-767 or TGR5 agonist INT-777, but not FXR agonist INT-747, significantly increased AQP2 protein expression in IMCD suspensions prepared from lithium-treated rats (Figure 4, G–J). GSK3β plays a crucial role in the progress of lithium-induced NDI.20,21 LCA (Figure 4, C and D) and INT-777 (Figure 4, K and L) treatment significantly increased GSK3β protein expression and the p-GSK3β/GSK3β ratio when compared with lithium group. These results suggest that TGR5 stimulation induces the activation of GSK3β and thus increases AQP2 expression in IMCD cells prepared from lithium-treated rats.
Figure 4.
TGR5 activation prevented lithium-induced downregulation of AQP2 by inducing GSK3β expression. Wistar rats were fed a lithium diet (protocol 1) and kidney inner medulla were harvested, digested, and cultured. The IMCD suspensions prepared from lithium-treated rats were treated with different agonists (LCA: 1, 10 µM; CDCA: 30, 100 µM; INT-767: 10, 20 µM; INT-777: 50, 100 µM; INT-747: 10, 30 µM) for 6 hours and cell lysates were harvested for Western blot analysis; n=6 biologically independent samples in each groups. AQP2 expression was upregulated (A and B) and GSK3β protein expression was induced (C and D) by LCA treatment, but not by CDCA (E and F). INT-767 or INT-777, but not INT-747 treatment increased AQP2 expression (G–J). INT-777 treatment significantly promoted GSK3β protein expression (K and L). Data are shown as mean±SEM; *P<0.05 compared with CTL; #P<0.05 compared with lithium. CTL, control; LiCl, lithium.
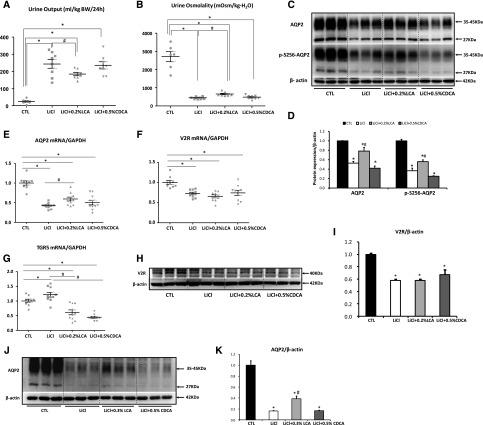
To further confirm the role of TGR5 in vivo, we administrated LCA or CDCA to mice with lithium-induced NDI. In lithium-treated mice (protocol 2), urine output was markedly increased and urine osmolality was dramatically reduced, which both were significantly prevented by LCA, but not CDCA (Figure 5, A and B). Consistently, protein abundance of AQP2 and phosphorylated AQP2 at Ser 256 in the inner medulla was markedly decreased in lithium-treated mice compared with control, which was significantly prevented by LCA, but not CDCA (Figure 5, C and D). Moreover, LCA but not CDCA mildly increased AQP2 mRNA levels in the inner medulla of lithium-treated mice (Figure 5E). Lithium treatment was associated with markedly reduced mRNA (Figure 5F) and protein levels of V2R (Figure 5, H and I) in the inner medulla, which was not prevented by either LCA or CDCA. Interestingly, both LCA and CDCA inhibited increased mRNA levels of TGR5 in the kidneys of lithium-treated mice (Figure 5G). LCA but not CDCA also prevented the reduction of renal AQP2 protein expression in mice with 14-day lithium treatment-induced NDI (protocol 3) (Figure 5, J and K). Taken together, both in vitro and in vivo data from lithium-induced NDI rats and mice suggest that LCA increases AQP2 expression and thus improves urine concentration ability. This regulation is likely attributed to a direct effect of TGR5 activation, independent of V2R.
Figure 5.
LCA increased renal AQP2 expression and improved urine concentration defect in mice with lithium treatment. Mice fed with lithium diet were administrated with 0.2% LCA or 0.5% CDCA for 7 days (protocol 2). LCA administration decreased urine output and increased urine osmolality, whereas CDCA did not show any effects in lithium treated mice (A and B). AQP2 and p-S256 AQP2 expression in the inner medulla were detected by Western blot analysis (C and D). The inner medulla of the kidneys were subjected to quantitative RT-PCR analysis for AQP2, V2R, and TGR5 (E–G) and Western blot analysis for V2R (H and I); n=6–10 biologically independent samples in each group. LCA but not CDCA prevented reduced AQP2 protein expression in mice with lithium treatment for 14 days (J and K) (protocol 3); n=5 biologically independent samples in each group. Data are presented as mean±SEM *P<0.05 versus CTL; #P<0.05 versus lithium. CTL, control, LiCl, lithium, LiCl+0.2% LCA, lithium plus 0.2% LCA; LiCl+0.3% LCA, lithium plus 0.3% LCA; LiCl+0.5% CDCA, lithium plus 0.5% CDCA; V2R, arginine vasopressin type 2 receptor.
TGR5 Gene Deficiency Attenuated Urine Concentration Ability in Mice
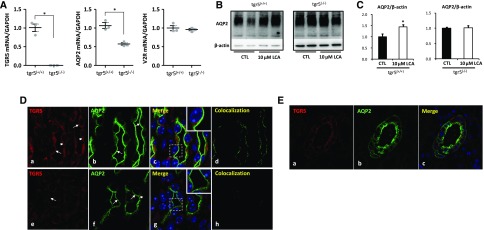
TGR5 gene expression in the kidney has been previously reported.4,22 Global TGR5 gene (Gpbar1) deletion mice were generated in order to examine the role of TGR5 in renal water handling (Supplemental Figure 1). As expected, kidney medullary mRNA expression of TGR5 was completely abolished in tgr5−/− mice (Figure 6A). In the kidney, tgr5−/− mice exhibited significantly reduced AQP2 mRNA, whereas V2R mRNA levels were almost the same between two genotype mice (Figure 6A). LCA was not able to induce upregulation of AQP2 protein in IMCD cells prepared from tgr5−/− mice (Figure 6, B and C). Immunofluorescence demonstrated the presence of TGR5 and AQP2 in the principal cells of the collecting duct in mouse (Figure 6D) and human kidneys (Figure 6E). In IMCD principal cells of tgr5+/+ mice kidneys, TGR5 staining was found both apically and intracellularly (Figure 6D, a–d). TGR5 staining was partially colocalized with AQP2 on the apical plasma membrane. In the kidney of tgr5−/− mice, only faint TGR5 staining was observed and thus no colocalization of TGR5 staining with AQP2 was observed (Figure 6D, e–h). In human kidney sections, both TGR5 and AQP2 were expressed in the cortical collecting duct principal cells (Figure 6E). These findings indicate a potentially physiologic association between TGR5 and AQP2 in the kidney.
Figure 6.
TGR5 gene-deficient mice exhibited reduced renal AQP2 mRNA and protein expression. Quantitative RT-PCR analysis showed a complete suppression of TGR5 mRNA with a decreased mRNA level of AQP2 and unchanged mRNA level of V2R in the inner medulla of tgr5−/− mice compared with tgr5+/+ mice (A); n=3 biologically independent samples in each group. Data are presented as mean±SEM; *P<0.05 versus tgr5+/+ mice. LCA failed to increase AQP2 protein expression in IMCD cells prepared from tgr5−/− mice (B and C); n=4 biologically independent samples in each group. Data are presented as mean±SEM; *P<0.05 versus control. Immunofluorescence showed codistribution of TGR5 (red) and AQP2 (green) in principal cells of IMCDs of tgr5+/+ mouse kidney (D) (a–d). TGR5 labeling of tgr5−/− mice was markedly diminished, although the background fluorescence was retained (D) (e–h). Arrows: apical membrane, arrowheads: intracellular compartments. Magnification 10×63; Original magnification: ×2 (insets). TGR5 and AQP2 were expressed in the cortical collecting ducts in human kidneys (E). Magnification 20×63. CTL, control.
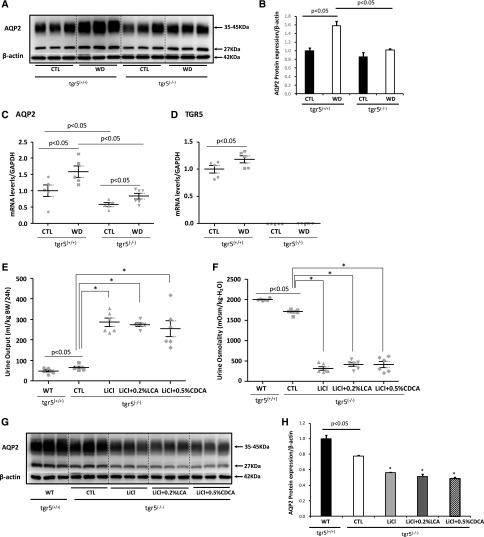
Compared with tgr5+/+ mice, tgr5−/− mice showed mildly increased urine output and decreased urine osmolality (Table 1), which was associated with reduction of AQP2 protein expression in the inner medulla (Figure 7, A and B). To further examine the importance of TGR5 in urine concentration, tgr5−/− and tgr5+/+ mice were subjected to dehydration. After water deprivation for 36 hours, urine output was high and urine osmolality was low in tgr5−/− mice when compared with tgr5+/+ mice (Table 1). Consistent with this, AQP2 protein and mRNA expression were dramatically increased after dehydration in tgr5+/+ mice, but only slightly increased in tgr5−/− mice (Figure 7, A–C). Surprisingly, AVP concentration in 36-hour urine samples was mildly decreased in tgr5−/− mice compared with wild-type mice (Table 1). After deprivation, urine AVP was strikingly increased by about eight-fold in tgr5+/+ mice, but only increased by two-fold in tgr5−/− mice (Table 1).
Table 1.
Physiologic parameters in tgr5+/+ and tgr5−/− mice with or without dehydration
| tgr5+/+Mice | tgr5−/− Mice | |||
|---|---|---|---|---|
| Parameters | CTL | WD 36 h | CTL | WD 36 h |
| UO, ml/kg body wt per 36 h | 61±4 | 24±6a | 95±7a | 46±5b,c |
| Uosm, mOsm/kg˙H2O | 2045±79 | 3817±311a | 1820±68a | 2819±109b,c |
| Posm, mOsm/kg˙H2O | 299±1.5 | 325±1.8a | 308±1.7a | 322±2.3b |
| PNa+, mmol/L | 142±0.8 | 154±1.0a | 147±1.0a | 153±1.2b |
| PCreatinine, mg/L | 9±0.5 | 16±0.5a | 11±0.7a | 14±0.5b,c |
| UAVP, pg/ml | 337±32 | 2655±310a | 202±9a | 413±10b,c |
CTL, control; WD 36 h, water deprivation for 36 hours; UO, urine output; Uosm, urine osmolality; Posm, plasma osmolality; PNa+, plasma sodium concentration; PCreatinine, plasma creatinine; UAVP, urinary AVP concentration;.
P<0.05 compared with control in tgr5+/+ mice.
P<0.05 compared with control in tgr5−/− mice.
P<0.05 compared with WD 36 h in tgr5+/+ mice; n=5–7.
Figure 7.
tgr5−/− mice exhibited impaired urine concentration. tgr5+/+ mice and tgr5−/− mice were subjected to water deprivation for 36 hours. The inner medulla of the kidneys were dissected for Western blotting of AQP2 (A and B) and mRNA analysis of AQP2 and TGR5 (C and D); n=5–6 biologically independent samples in each group. Data are presented as mean±SEM. tgr5−/− mice were fed with lithium diet with or without 0.2% LCA or 0.5% CDCA for 7 days (protocol 2). LCA failed to prevent increased urine output (E), decreased urine osmolality (F), and reduced AQP2 protein expression (G and H) in the inner medulla of tgr5−/− mice treated with lithium; n=6 biologically independent samples in each group. Data are presented as mean±SEM; *P<0.05 versus CTL; #P<0.05 versus LiCl. CTL, control; LiCl, lithium; LiCl+0.2% LCA, lithium plus 0.2% LCA; LiCl+0.5% CDCA, lithium plus 0.5% CDCA; WD, water deprivation; WT, wild-type.
As TGR5 stimulation improved urine concentration and upregulated AQP2 protein expression, we next investigated whether LCA prevented lithium-induced NDI in tgr5−/− mice (protocol 2). Lithium treatment caused markedly increased urine output and dramatically decreased urine osmolality in tgr5−/− mice, which was not ameliorated by either LCA or CDCA treatment (Figure 7, E and F). Western blots demonstrated a markedly reduced AQP2 protein expression in renal inner medulla of tgr5−/− mice after lithium treatment, which could not be prevented by either LCA or CDCA (Figure 7, G and H). These data strongly suggest renal TGR5 could play a role in alleviating the lithium-induced impairment of urine concentration.
Discussion
Our study provides evidence that renal TGR5 plays an important role in regulating water homeostasis. We demonstrated that stimulation of TGR5 in the kidney by endogenous agonist LCA or the synthetic ligand INT-777 markedly facilitated transmembrane water transport and promoted AQP2 gene and protein expression as well as intracellular trafficking in IMCD cells via a cAMP-PKA signaling pathway. LCA attenuated lithium-induced NDI by increasing AQP2 expression in the inner medulla of mice. Mice with TGR5 deficiency displayed attenuated urine concentration ability in response to water deprivation. Interestingly, FXR activation was not capable of preventing lithium-induced NDI, although it upregulated AQP2 expression under normal conditions. These findings reveal a novel role for TGR5 in renal urine concentration and water regulation, and might indicate a potential therapeutic option for NDI.
TGR5 is widely expressed in various human organs including the kidney.4,22,23 In microdissected kidney collecting ducts, TGR5 mRNA level was shown to be the highest in OMCD, followed by IMCD and CCD.15 As seen in other epithelial cells, such as cholangiocytes,24 in the IMCD principal cells of mouse kidneys, TGR5 protein is localized in the apical plasma membrane and also in intracellular compartments, which is coincidently consistent with the distribution of AQP2. The codistribution of TGR5 and AQP2 in the collecting duct provides histologic evidence, which supports the concept that stimulation of TGR5 activates cAMP-PKA signaling pathway and thus regulates AQP2 expression and urine concentration. Our data demonstrated a direct effect of TGR5 on AQP2 protein expression and intracellular trafficking and thus water transmembrane transport. The cAMP-PKA pathway is the principal mediator of AQP2 trafficking and transcription.9,25 In IMCD cells, LCA upregulated AQP2 protein expression and caused enhanced membrane distribution of AQP2, indicating a plasma membrane trafficking from the intracellular compartments. These were associated with increased cAMP concentration in the cell lysates and increased CREB phosphorylation after LCA treatment. Increased AQP2 protein expression was also observed after treatment with synthetic TGR5 agonist INT-777 and dual TGR5-FXR agonist INT-767 in IMCD cells. Importantly, the observed increase in AQP2 expression was markedly inhibited by AC or PKA inhibitors, but not by the V2R antagonist tolvaptan. These data suggest that the activation of TGR5 stimulates cAMP-PKA signaling pathway, causing an upregulation of AQP2 protein, independent of V2R.
Evidence has demonstrated a role of GSK3β in renal water handling.11,18,20 Inactivation or tissue-specific gene deletion of GSK3β in renal collecting ducts can lead to impaired AVP-responsive AQP2 expression and urine concentration in mice, which was associated with reduced AC activity and concomitant decrease in cAMP generation in the kidney.18 A positive cAMP−CREB−GSK3β−cAMP feed-forward mechanism has been recently proposed.19 The observed upregulated GSK3β protein likely underlies the increased AQP2 expression by TGR5 activation. Lithium is known to inhibit GSK3β in the clinical therapeutic range26 and GSK3β is considered as an essential regulator in lithium-induced NDI. Both LCA and INT-777 treatment prevented reduced GSK3β protein expression in IMCD cells prepared from lithium-treated rats. GSK3β is therefore likely a downstream target in TGR5 signaling pathway and TGR5 may potentiate cAMP−CREB−GSK3β−cAMP feed-forward signaling loop, attenuating lithium-induced downregulation of AQP2 in IMCD cells.
TGR5 gene knockout mice display a healthy and fertile phenotype with normal development, although increased enzymes in bile acid metabolism and sex-related metabolic disorders are noted.22,23,27 However, to our knowledge, there is no previous evidence suggesting an involvement of TGR5 in the regulation of water homeostasis in the kidney. In this study, mRNA and protein expression of AQP2 were reduced in the tgr5−/− mice, basally. LCA could not induce AQP2 expression in IMCD cells prepared from tgr5−/− mice. The maximal urinary concentrating capacity and AQP2 protein expression in response to 36 hours of water deprivation in these mice failed to reach levels attained by the wild-type mice. Surprisingly, urinary AVP concentration was slightly, but significantly, lower in tgr5−/− mice than tgr5+/+ mice. Urinary AVP showed a mediated increase after dehydration in tgr5−/− mice, which was not as robust as wild-type mice. The mechanism underlying this is largely unknown; however, it is likely that AVP secretion is at least partially associated with TGR5 receptor activation in the hypothalamic supraoptic nuclei (SON). Early studies have shown a physiologic relationship between AVP and bile acid homeostasis. AVP secretion is stimulated by chronic cholestasis after bile duct ligation in rats28; conversely, cholestasis has been observed in V1a AVP receptor knockout mice.29 Recently, TGR5 is shown expressed in the hypothalamus SON and pituitary gland in human30 and rodents,31 where it plays an important role in activating SON synthesis and AVP secretion.31 In tgr5−/− mice, both bile duct ligation–elicited SON synthesis and blood rise of AVP was much reduced when compared with wild-type mice, although increased plasma bile acid levels were comparable between two types of mice.31 In this study, low urinary AVP levels in tgr5−/− mice may contribute to downregulated protein abundance of AQP2, and thus the increased urine output seen in tgr5−/− mice. However, a direct role of TGR5 in AQP2 regulation cannot be excluded. It must be noted that TGR5 stimulation directly regulates AQP2 expression and trafficking via cAMP-PKA signaling cascade in primary IMCD cells. Moreover, in lithium-treated tgr5−/− mice, LCA failed to increase AQP2 protein and mRNA expression compared with wild-type mice. These findings suggest that TGR5 plays an important role in renal water homeostasis and urine concentration, likely through regulating the expression of AQP2 in kidney collecting ducts.
FXR is ubiquitously distributed in renal tubules and FXR activation upregulates AQP2 transcription mainly via binding to FXR response element site in AQP2 gene promoter.14 This is probably the reason why AC and PKA inhibitors could not suppress an increased AQP2 protein expression induced by CDCA or INT-747. CDCA induced intracellular cAMP concentration in our study and CDCA slightly increased the transcription of AQP2 gene in cultured IMCD cells prepared from fxr−/− mice,14 indicating other mechanisms are probably involved. A very recent study showed the TGR5 promoter has a functional FXR responsive element.32 It is therefore plausible to speculate that FXR induces cAMP production and upregulates AQP2 expression, at least partially, through stimulating TGR5 activation. In contrast to TGR5, FXR activation was not able to rescue decreased AQP2 protein expression, and thus failed to ameliorate polyuria and urine concentration defect in mice with lithium treatment.
To conclude, our studies identify TGR5 and the TGR5-activated cAMP-PKA signaling pathway as unique mechanisms in regulating renal AQP2 expression and urine concentration. These findings may not only help to understand the molecular mechanisms regarding urine concentration, but also provide a possible explanation for increased water retention in some clinical settings, e.g., liver cirrhosis. Our study also indicates that activation of TGR5 may be a novel therapeutic option for some conditions with water loss, such as lithium-induced NDI.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Guangdong Engineering and Technology Research Center for Disease-Model Animals, Sun Yat-Sen University, China, for providing tgr5 gene deletion mice. We thank Dr. George McNamara for assistance in acquiring confocal images at the Ross Fluorescence Imaging Center (Johns Hopkins University). We greatly appreciate Dr. Lisa Bankir’s very constructive suggestions on arginine vasopressin measurement.
S.L., M.Q., Y.K., X.Z., Q.L., S.H., M.H., and H.X. performed the experiments. S.L., C.L., and W.W. designed the study, analyzed and interpreted the results, and wrote and edited the manuscript. M.Q., Y.K., and X.Z. assisted with main experiments. M.R., V.K., H.-J.C., T.-H.K., B.H.B., A.Z.R., and M.L. provided essential reagents and techniques for this study and reviewed the manuscript. H.-J.C. and T.-H.K. performed measurement of cell water volume changes. M.R., V.K., B.H.B., and A.Z.R. performed immunolocalization of TGR5 and AQP2 in the kidneys. S.L., M.Q., Y.K., X.Z., H.-J.C., M.R., B.H.B, Q.L., S.H., M.H., H.X., V.K., T.-H.K., A.Z.R., M.L., C.L., and W.W. approved the final version of manuscript. C.L. and W.W. conceived and supervised the study.
This work was supported by the National Natural Science Foundation of China (grants 81670646 and 81570635), Natural Science Foundation of Guangdong Province (grants 2016A020215034 and 2014A030313168), Natural Science Foundation of Guangzhou (grant 201707010036), and the International Cooperation Program Fund of Sun Yat-Sen University (grant 02300-31145400). This work was also supported by the National Research Foundation of Korea funded by the Ministry of Science, Information and Communication Technology and Future Planning, Korea (grants 2016R1A2B4009365 and 2017R1D1A3B03032262), Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 974, and National Institutes of Health grants R01AG049493, R01DK098336, and R01DK116567.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018030271/-/DCSupplemental.
References
- 1.Hofmann AF: The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159: 2647–2658, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Ridlon JM, Kang DJ, Hylemon PB: Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Asgharpour A, Kumar D, Sanyal A: Bile acids: Emerging role in management of liver diseases. Hepatol Int 9: 527–533, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al.: A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Vallim TQ, Edwards PA: Bile acids have the gall to function as hormones. Cell Metab 10: 162–164, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, et al.: G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol 27: 1362–1378, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, et al.: The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22: 418–426, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Wondisford FE: Unlikely partners in weight loss? Cell Metab 3: 81–82, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Knepper MA, Kwon TH, Nielsen S: Molecular physiology of water balance. N Engl J Med 372: 1349–1358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA: Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: Mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kwon TH, Laursen UH, Marples D, Maunsbach AB, Knepper MA, Frokiaer J, et al.: Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 279: F552–F564, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Huang S, Gao M, Liu J, Jia X, Han Q, et al.: Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci U S A 111: 2277–2282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng P, Lin Y, Wang F, Luo R, Zhang T, Hu S, et al.: 4-PBA improves lithium-induced nephrogenic diabetes insipidus by attenuating ER stress. Am J Physiol Renal Physiol 311: F763–F776, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Lee MS, Choi HJ, Park EJ, Park HJ, Kwon TH: Depletion of vacuolar protein sorting-associated protein 35 is associated with increased lysosomal degradation of aquaporin-2. Am J Physiol Renal Physiol 311: F1294–F1307, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Rao R, Patel S, Hao C, Woodgett J, Harris R: GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21: 428–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakade VR, Tao S, Rajagopal M, Zhou X, Li X, Yu AS, et al.: A cAMP and CREB-mediated feed-forward mechanism regulates GSK3β in polycystic kidney disease. J Mol Cell Biol 8: 464–476, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, et al.: Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kishore BK, Ecelbarger CM: Lithium: A versatile tool for understanding renal physiology. Am J Physiol Renal Physiol 304: F1139–F1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, et al.: Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol 191: 197–205, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, et al.: Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 398: 423–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keitel V, Ullmer C, Häussinger D: The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem 391: 785–789, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F: Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Klein PS, Melton DA: A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 93: 8455–8459, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassileva G, Hu W, Hoos L, Tetzloff G, Yang S, Liu L, et al.: Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J Endocrinol 205: 225–232, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Swain MG, Patchev V, Vergalla J, Chrousos G, Jones EA: Suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress in a rat model of acute cholestasis. J Clin Invest 91: 1903–1908, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiroyama M, Aoyagi T, Fujiwara Y, Birumachi J, Shigematsu Y, Kiwaki K, et al.: Hypermetabolism of fat in V1a vasopressin receptor knockout mice. Mol Endocrinol 21: 247–258, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, et al.: Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Doignon I, Julien B, Serrière-Lanneau V, Garcin I, Alonso G, Nicou A, et al.: Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J Hepatol 54: 481–488, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, et al.: Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem 292: 11055–11069, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.