A post-functionalization protocol was used for the synthesis of two new tris-hybrid Al-centred Anderson-type polyoxomolybdates with indometacin or cinnamic acid.
Keywords: polyoxomolybdate, organic–inorganic hybrids, alkoxylation, post-functionalization, hexamolybdoaluminate, crystal structure, indometacin, cinnamic acid, antibacterial activity
Abstract
The single-side Al-centred tris-functionalized hybrid organic–inorganic Anderson polyoxomolybdates (C16H36N)3[Al(OH)3Mo6O18(OCH2)3CNH(C10H8O)]·C9H7N·4CH3OH·5H2O (AlMo6-NH-Cin; Cin is cinnamic acid, C10H9O2) and (C16H36N)3[Al(OH)3Mo6O18(OCH2)3CNH(C19H15ClNO3)]·9H2O (AlMo6-NH-Indo; Indo is indometacin, C19H16ClNO4) have been prepared in a mild three-step synthesis and structurally characterized by single-crystal X-ray diffraction, 1H NMR and IR spectroscopies and elemental analysis. Both AlMo6-NH-Cin and AlMo6-NH-Indo crystallize in the orthorhombic space group Pbca. The antibacterial activities of AlMo6-NH-Cin and AlMo6-NH-Indo against the Gram-negative human mucosal pathogen Moraxella catarrhalis were investigated by determination of the minimum inhibitory concentration, which is 32 µg ml−1 for AlMo6-NH-Cin and 256 µg ml−1 for AlMo6-NH-Indo.
Introduction
Polyoxometalates (POMs), an exceptional class of metal–oxide clusters with various compositions, exhibit an oxygen-rich surface with strong coordination potential (Pope, 1983 ▸). They have attracted much attention owing to their unique catalytic (Wang & Yang, 2015 ▸), redox (Gumerova & Rompel, 2018 ▸), magnetic (Clemente-Juan et al., 2012 ▸) and bioactive properties (Bijelic & Rompel, 2015 ▸, 2017 ▸; Molitor et al., 2017 ▸; Fu et al., 2015 ▸; Bijelic et al., 2018a ▸,b ▸) and constitute promising building blocks for advanced materials. Recently, increasing effort has been devoted to the introduction of organic and metal–organic units into the metal oxide frameworks in order to functionalize POM materials (Dolbecq et al., 2010 ▸). Among the various synthetic strategies for the organic functionalization of POMs, alkoxylation has gained much attention due to the diversity and tunability of alkoxyl ligands, especially when using the disk-shaped Anderson-type anions [Xn +Hm M 6O24](12–n–m)– (M = Mo6+ and W6+; X = heteroatom, e.g. Te6+ and I7+ for A-type with m = 0, or Al3+ and Ni2+ for B-type with m = 6), with a wide spectrum of central heteroatoms (Blazevic & Rompel, 2016 ▸; Zhang et al., 2018 ▸). In particular, after Hasenknopf et al. (2002 ▸) had pioneered and established the synthesis of tris-derivatives of Anderson polyoxomolybdates (POMos), this archetype has been widely used as starting materials for the attachment of various tris [tris(hydroxymethyl)methane]-based organic ligands [RC(CH2OH)3, denoted R-Tris]. If the R group itself is reactive (–NH2, –CH2OH etc.), post-functionalization with a variety of organic molecules, including ligands containing aromatic units (Al-Sayed et al., 2015 ▸) or alkyl chains (Rosnes et al., 2013 ▸) via imine, amide or ester-bond formation, is possible. The resulting hybrid materials were used in supramolecular self-assembly (Macdonell et al., 2015 ▸) or for the formation of metal–organic frameworks (MOFs; Li et al., 2016 ▸). Major application fields are bio-inorganic (Yvon et al., 2014 ▸), nano-structured (Song et al., 2009 ▸), energy storage (Ji et al., 2015 ▸), optical (Boulmier et al., 2018 ▸) and photochemical (Schaming et al., 2010 ▸) materials.
Herein, two biologically active molecules, namely indometacin and cinnamic acid, were used to post-functionalize the Al-centred Anderson anion [Al(OH)3Mo6O18(OCH2)3CNH2]3− (Wu et al., 2011 ▸) via amidation reaction, resulting in two novel single-side grafted hybrid organic–inorganic Anderson-type POMos, namely (TBA)3[Al(OH)3Mo6O18(OCH2)3CNH(C10H8O)]·C9H7N·4CH3OH·5H2O (AlMo6-NH-Cin; Cin is cinnamic acid and TBA is tetrabutylammonium) and (TBA)3[Al(OH)3Mo6O18(OCH2)3CNH(C19H15ClNO3)]·9H2O (AlMo6-NH-Indo; Indo is indometacin). Both compounds were structurally characterized by single-crystal X-ray diffraction, IR spectroscopy and elemental analysis. Their antibacterial activity against Moraxella catarrhalis was investigated by determination of the minimum inhibitory concentration (MIC).
Experimental
Synthesis and crystallization
Synthesis of AlMo6-NH-Indo
Na3(H2O)6[Al(OH)6Mo6O18]·2H2O (AlMo6) was prepared according to a published procedure (Shivaiah & Das, 2005 ▸). The single-side attachment of Tris-NH2 to AlMo6 was achieved through a modified published procedure (Wu et al., 2011 ▸). AlMo6 (3.84 g, 3.28 mmol) was dissolved in water (20.5 ml) and heated to reflux, when Tris-NH2 (0.735 g, 6.02 mmol) was added. After refluxing for 3 h, the solvent was removed by vacuum. The white powder obtained was redissolved with deionized H2O and then centrifuged to remove unreacted educts. Tetrabutylammonium bromide (TBABr) (4.12 g, 12.8 mmol) was added to the solution and a white precipitate appeared. In order to functionalize AlMo6-NH2 with indometacin, a mixture of indometacin (0.172 g, 0.500 mmol), AlMo6-NH2 (1.05 g, 0.519 mmol) and EEDQ (N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline; 0.143 g, 0.570 mmol) in CH3CN (9.00 ml) was stirred at 323 K for 24 h. The solvent was collected and removed by vacuum. The remaining yellow solid was redissolved in an MeOH–H2O mixture (2:1 v/v), followed by the addition of TBABr (0.5 g). After several weeks, crystals suitable for single-crystal X-ray diffraction were obtained [yield 2.3 g, 32% (based on Mo)]. FT–IR (cm−1): 324 (s), 368 (s), 395 (m), 442 (s), 484 (s), 505 (m), 534 (m, sh), 567 (m), 611 (m), 650 (s), 736 (m), 754 (m), 796 (m), 833 (w), 850 (m), 897 (s), 918 (s), 939 (s), 1012 (w, sh), 1027 (m), 1053 (m), 1072 (m), 1091 (m), 1122 (m), 1151 (m), 1174 (w), 1224 (m), 1290 (w), 1315 (m), 1336 (w, sh), 1361 (m), 1369 (m), 1396 (sh), 1458 (m), 1479 (m), 1552 (m), 1564 (m), 1610 (m), 1677 (m), 2871 (m), 2933 (m), 2960 (m), 3081 (w), 3322 (m, br). Elemental analysis for C71H145AlClMo6N5O36 (calculated) (%): C 38.38 (37.27), H 6.75 (6.61), N 3.06 (3.06), Cl 1.29 (1.55), O 22.3 (25.17). 1H NMR (500.32 MHz, CD3CN, 298 K): δ 0.96 (t, 36H), 1.34 (m, 24H), 1.59 (m, 24H), 3.53 (m, 24H), 3.62 (s, 2H), 3.81 (s, 3H), 2.15 (s, 3H), 6.66 (dd, 1H), 6.85 (d, 1H), 6.96 (d, 1H), 7.02 (d, 1H), 7.55 (d, 2H), 7.64 (d, 2H), 64 (s, 6H in CH2—μ3-O groups).
Synthesis of AlMo6-NH-Cin
The preparation of AlMo6-NH-Cin was similar to that of AlMo6-NH-Indo, except that cinnamic acid (0.074 g, 0.500 mmol) was used instead of indometacin [yield 1.9 g, 27% (based on Mo)]. FT–IR (cm−1): 324 (s), 368 (s), 441 (s), 482 (s), 503 (m), 518 (m), 534 (m), 565 (m), 578 (m), 607 (m), 649 (s), 832 (w), 898 (s), 916 (s), 939 (s), 983 (w), 1035 (m), 1060 (m), 1112 (w), 1118 (m), 1153 (m), 1193 (w), 1228 (m), 1284 (w), 1323 (w), 1348 (m), 1380 (m), 1458 (m), 1479 (m), 1548 (m, br), 1575 (w), 1627 (m), 1668 (m), 1720 (w), 1731 (w, sh), 2874 (m), 2933 (m), 2960 (m), 3062 (w), 3290 (m, br), 3404 (m, br). Elemental analysis for C73H154AlMo6N5O32.6 (calculated) (%): C 36.48 (36.92), H 6.50 (7.00), N 2.92 (3.16), O 22.16 (23.10). 1H NMR (500.32 MHz, CD3CN, 298 K): δ 0.98 (t, 36H), 1.37 (m, 24H in TBA), 1.61 (m, 24H), 3.11 (d, 12H), 3.51 (m, 24H), 5.12 (q, 4H), 6.46 (d, 1H), 7.37 (d, 1H), 7.47–7.91 (m, 5H), 64 (s, 6H in CH2—μ3-O groups).
IR spectroscopy
Both compounds were identified by IR measurements on a Bruker Vertex70 IR Spectrometer equipped with a single-reflection diamond-ATR (attenuated total reflectance) unit in the range 4000–300 cm−1.
1H NMR
NMR spectra were recorded on a Bruker FT–NMR Avance III 500 MHz instrument at 500.32 (1H) MHz in CD3CN at ambient temperature. Chemical shifts were referenced relative to the solvent signal for 1H nucleus.
Elemental analysis
The determination of C/H/N/O/Cl was carried out using an ‘EA 1108 CHNS-O’ elemental analyzer by Carlo Erba Instruments at the Mikroanalytisches Laboratorium, University of Vienna.
MIC determination
Minimum inhibitory concentrations (MICs) were determined by the broth microdilution method according to guidelines of the Clinical Laboratory Standards Institute (Wikler, 2009 ▸). Double dilutions of tested compounds in 96-well microtiter plates were prepared in the concentration range 1–256 µg ml−1. M. catarrhalis (ATCC 23246) was grown on Columbia agar with 5% defibrinated sheep blood. Inocula were prepared by the direct colony suspension method and plates were inoculated with 5 × 10−4 CFU per well. Results were determined by visual inspection after 20–22 h of incubation at 310 K in ambient air. Testing was performed by the standard broth microdilution method with azithromycin (Lode et al., 1996 ▸) as the reference antibiotic to assess test validity. MIC determinatiom was performed at the School of Medicine, University of Zagreb, Croatia.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. The positions of the independent H atoms were obtained by difference Fourier techniques and were refined with free isotropic displacement parameters. Fixed isotropic displacement parameters for all H atoms with a value equal to 1.5U eq of the corresponding OH or H2O group atom were assigned. Restrained distances for D—H bonds were applied to avoid short D—H⋯H—D interactions. In the case of disordered groups, some bonds were added to or deleted from the connectivity array.
Table 1. Experimental details.
| AlMo6-NH-Cin | AlMo6-NH-Indo | |
|---|---|---|
| Crystal data | ||
| Chemical formula | (C16H36N)3[Al(OH)3Mo6O18(OCH2)3CNH(C10H8O)]·C9H7N·4CH3OH·5H2O | (C16H36N)3[Al(OH)3Mo6O18(OCH2)3CNH(C19H15ClNO3)]·9H2O |
| M r | 2227.19 | 2288.02 |
| Crystal system, space group | Orthorhombic, P b c a | Orthorhombic, P b c a |
| Temperature (K) | 100 | 200 |
| a, b, c (Å) | 16.1062 (17), 26.512 (3), 45.569 (5) | 21.8904 (6), 23.9848 (6), 37.719 (1) |
| V (Å3) | 19458 (3) | 19803.9 (9) |
| Z | 8 | 8 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.84 | 0.85 |
| Crystal size (mm) | 0.23 × 0.15 × 0.03 | 0.15 × 0.12 × 0.05 |
| Data collection | ||
| Diffractometer | Bruker APEXII CCD | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2013 ▸) | Multi-scan (SADABS; Bruker, 2013 ▸) |
| T min, T max | 0.666, 0.746 | 0.678, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 293657, 17800, 15423 | 374956, 18115, 15743 |
| R int | 0.066 | 0.050 |
| (sin θ/λ)max (Å−1) | 0.602 | 0.602 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.066, 0.144, 1.24 | 0.029, 0.075, 1.06 |
| No. of reflections | 17800 | 18115 |
| No. of parameters | 1129 | 1166 |
| No. of restraints | 39 | 53 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 1.27, −1.04 | 1.18, −0.64 |
Results and discussion
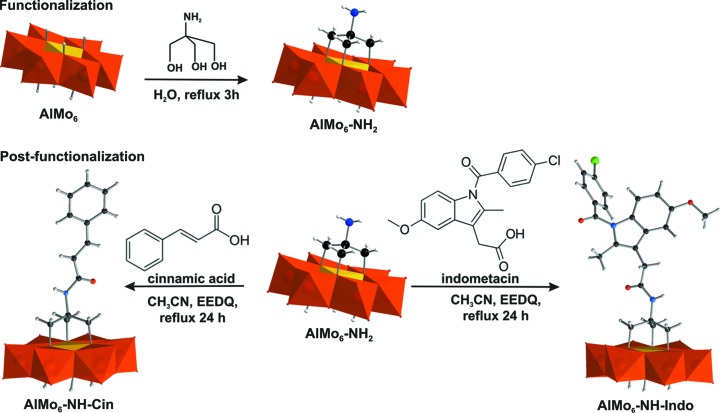
AlMo6-NH-Cin and AlMo6-NH-Indo were prepared via post-functionalization by pre-forming the hybrid cluster AlMo6-NH2 which was modified by amidation reactions (Fig. 1 ▸). The fact that single-side grafted anions were obtained supports an earlier theory claiming that the aqueous solvent is a key factor for the formation of single-sided Anderson derivatives (Wu et al., 2011 ▸; Blazevic et al., 2015 ▸; Gumerova et al., 2016 ▸).
Figure 1.
Functionalization of [Al(OH)6Mo6O18]3− (AlMo6) with the Tris-NH2 ligand, followed by further post-functionalization of [Al(OH)3Mo6O18(OCH2)3CNH2]3− (AlMo6-NH2) with indometacin or cinnamic acid, respectively. EEDQ is N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. Colour code: {MoO6} octahedra orange and {AlO6} octahedra yellow, with C atoms black, N blue, Cl green, H grey and O red.
X-ray crystallographic analysis shows that the asymmetric units in AlMo6-NH-Cin and AlMo6-NH-Indo consist of the hybrid Anderson anion, three TBA counter-cations, solvent molecules and, in the case of AlMo6-NH-Cin, one molecule of quinoline as a by-product from EEDQ decomposition. The structural analysis revealed that both compounds crystallize in the orthorhombic space group Pbca. AlMo6-NH-Cin and AlMo6-NH-Indo both show the characteristic Anderson-type structure, with a central {AlO6} octahedron surrounded by six edge-shared {MoO6} octahedra that form a planar array of distorted octahedra (Fig. 1 ▸). Three different coordination modes of O atoms are found in the structure: six triple-bridged O atoms (denoted μ3-O) connect the heteroatom and two Mo atoms, six double-bridged O atoms (denoted μ2-O) connect two Mo atoms and two terminal O atoms (denoted Ot) are connected to each of the six Mo atoms. The bond lengths of the three different binding modes are summarized in Table 2 ▸ and are in good agreement with other tris-functionalized Anderson POMos (Wu et al., 2011 ▸; Al-Sayed et al., 2015 ▸; Blazevic et al., 2015 ▸).
Table 2. Selected bond lengths (Å) in AlMo6—NH-Cin and AlMo6—NH-Indo .
| AlMo6—NH-Cin | AlMo6—NH-Indo | |
|---|---|---|
| Mo—μ3-O | 2.291 (4)–2.391 (4) | 2.3068 (19)–2.3632 (18) |
| Mo—μ2-O | 1.910 (4)–1.941 (5) | 1.912 (2)–1.944 (2) |
| Mo—Ot | 1.694 (5)–1.722 (5) | 1.696 (2)–1.711 (2) |
| Al—μ3-O | 1.864 (5)–1.923 (4) | 1.863 (2)–1.927 (2) |
The tris-ligand caps one side of the planar hexagon by binding to three μ3-O atoms of the {AlO6} fragment, whereas on the other side of AlMo6-NH-Cin and AlMo6-NH-Indo, the respective μ3-O atoms according to BVS calculations [−1.16 (O2), −1.20 (O4) and −1.19 (O6) for AlMo6-NH-Indo, and −1.15 (O1), −1.16 (O3) and −1.18 (O5) for AlMo6-NH-Indo, calculated according to (Brown & Altermatt, 1985 ▸)] are protonated.
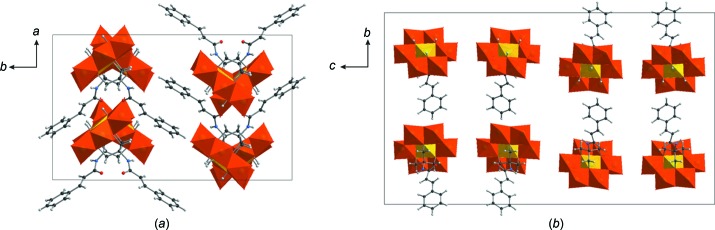
The crystal packing of AlMo6-NH-Cin can be described as alternate layers of POMo anions and TBA counter-cations, which are repeated along the c axis (Fig. 2 ▸). The orientations of the hybrid polyanions along the c and b axes also alternate with an angle of approximately 85° between the planes of the inorganic Anderson ‘disks’ (Fig. 2 ▸ a). The attached ligands are turned towards each other along the bc plane. The distances between the inorganic POMo skeletons along the a axis are around 9.5 Å, and around 14 Å along the b axis. All four lattice water molecules are situated in front of the undecorated side of the anion and form strong intermolecular hydrogen bonds with μ3-O—H fragments, with short distances in the region 1.85–1.94 Å.
Figure 2.
The crystal packing of AlMo6-NH-Cin, viewed along (a) the c axis and (b) the a axis. The TBA counter-cations and the solvent molecules have been omitted for clarity. Colour code: {MoO6} octahedra orange and {AlO6} octahedra yellow, with C atoms black, N blue, H grey and O red.
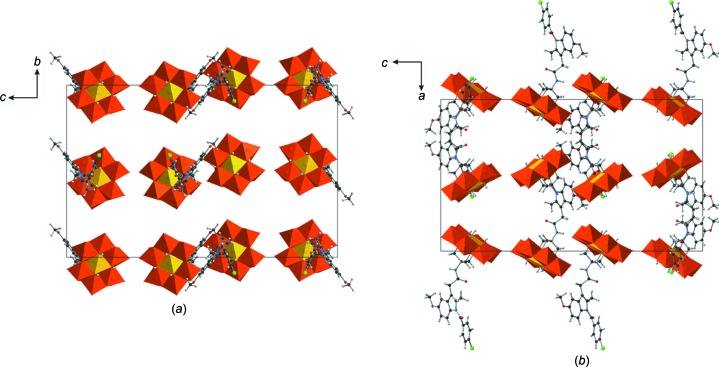
The crystal packing of AlMo6-NH-Indo is similar to that of AlMo6-NH-Cin and can be described as alternate layers of POMo anions and TBA counter-cations, which are repeated along the a axis (Fig. 3 ▸). The orientation of the hybrid polyanions along the c and b axes is the same, with the grafted sides turned in different directions (Fig. 3 ▸ b). The distances between inorganic POMos along the a axis are around 12 Å, around 11 Å along the b axis and approximately 5 Å along the c axis. Six of nine lattice water molecules are situated in front of the unfunctionalized side and form strong intermolecular hydrogen bonds with μ3-O—H fragments and Ot atoms, with distances in the range 1.86–2.08 Å. The crystallographic refinement results for both AlMo6-NH-Cin and AlMo6-NH-Indo suggest no π–π interactions between the aromatic ring and the C=C double bond based on geometry and separation.
Figure 3.
The crystal packing of AlMo6-NH-Indo, viewed along (a) the a axis and (b) the b axis. The TBA counter-cations and the solvent molecules have been omitted for clarity. Colour code: {MoO6} octahedra orange and {AlO6} octahedra yellow, with C atoms black, N blue, H grey and O red.
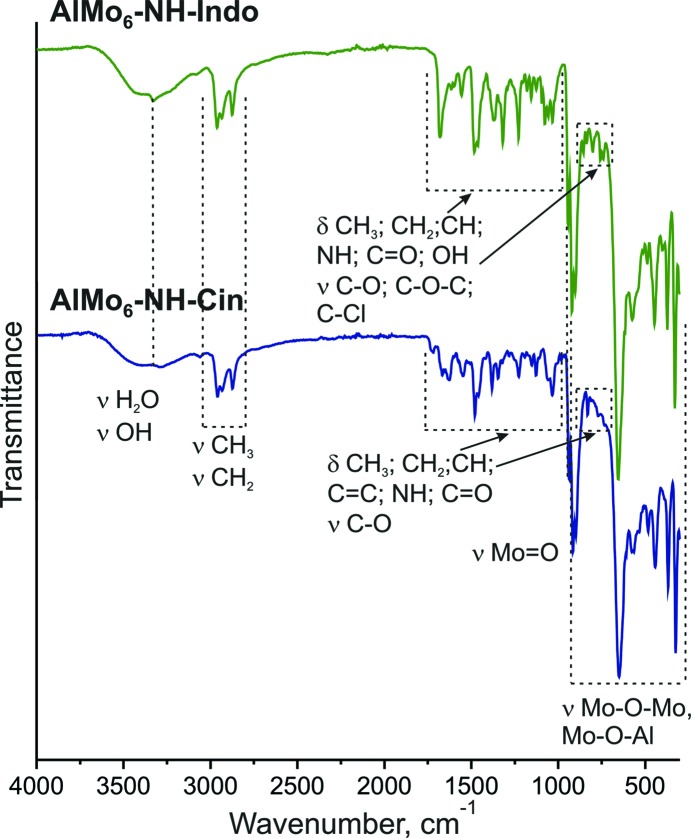
The IR spectra of AlMo6-NH-Cin and AlMo6-NH-Indo (Fig. 4 ▸) are typical for Anderson-type POMos and the characteristic peaks of the core structure are all in agreement with the peaks observed in the spectrum of Na3(H2O)6[Al(OH)6Mo6O18]·2H2O (Shivaiah & Das, 2005 ▸). The stretching vibrations of the terminal Mo=O units appear at 939 cm−1, whereas the peaks in the region from 300 to 920 cm−1 correspond to the antisymmetric and symmetric deformation vibrations of the Mo—O—Mo and Mo—O—Al bridging fragments. The peaks appearing in the region 1030–1125 cm−1 could be assigned to C—O stretching vibrations, indicating the successful grafting of the tris ligands.
Figure 4.
The IR spectra of AlMo6-NH-Cin and AlMo6-NH-Indo in the region from 4000 to 300 cm−1.
The antibacterial activities of AlMo6-NH-Cin and AlMo6-NH-Indo against the Gram-negative human mucosal pathogen Moraxella catarrhalis (Karalus & Campagnari, 2000 ▸) were investigated by determination of the minimum inhibitory concentration (MIC). AlMo6-NH-Cin shows a higher activity, with MIC values of 32 µg ml−1, while AlMo6-NH-Indo shows an MIC value of 256 µg ml−1. The MIC values for both compounds are much higher than for the clinically applied drug azithromycin, which has an MIC value of 0.06 µg ml−1. Taking into account that AlMo6-NH-Cin and AlMo6-NH-Indo have the same inorganic POMo part, counter-cations and net charge, it can be assumed that their antibacterial activities differs only due to the organic ligands attached. It is known that cinnamic acid and its derivatives exhibit antimicrobial activity against pathogenic and spoilage bacteria (Sova, 2012 ▸), indometacin in its turn, as a nonsteroidal anti-inflammatory drug (Lucas, 2016 ▸), showed bacteriostatic activity against Helicobacter pylori (Shirin et al., 2006 ▸), whereas pure inorganic Ni- and Te-centred Anderson-type POMos and POTs are inactive (MIC > 256 µg ml−1) against M. catarrhalis (Gumerova et al., 2018 ▸). Thereby, the activity of AlMo6-NH-Cin is caused by the synergistic effect of AlMo6 and cinnamic acid, which is not the case for AlMo6-NH-Indo. The preliminary results obtained here show that not only does the activity of the attached ligand play a role, but also synergism with POMs strongly influences the properties of the hybrid compounds.
Conclusion
The success in synthesizing AlMo6-NH-Cin and AlMo6-NH-Indo shows the versatility and reproducibility of the post-functionalization protocol for the alkoxylation of Anderson POMs. The attachment of bioactive ligands makes the hybrid Anderson POMos reported herein potentially superior to pure inorganic structures for antibacterial applications.
Supplementary Material
Crystal structure: contains datablock(s) global, mo_ambl235_pbca, taco104_0m. DOI: 10.1107/S2053229618012536/jr3025sup1.cif
Structure factors: contains datablock(s) mo_ambl235_pbca. DOI: 10.1107/S2053229618012536/jr3025mo_ambl235_pbcasup4.hkl
Structure factors: contains datablock(s) taco104_0m. DOI: 10.1107/S2053229618012536/jr3025taco104_0msup3.hkl
Acknowledgments
We are grateful to Dr Hana Čipčić-Paljetak and Professor Dr Donatella Verbanac for the antibacterial investigations at the Center for Translational and Clinical Research, Croatian Center of Excellence for Reproductive and Regenerative Medicine, School of Medicine, University of Zagreb, Croatia. We thank Ricarda Bugl for the support with NMR spectroscopy measurements at the NMR Centre, Core facilities service of University of Vienna, and Elias Tanuhadi, MSc, for proofreading the manuscript.
Funding Statement
This work was funded by Austrian Science Fund grants M2203 and P27534.
References
- Al-Sayed, E., Blazevic, A., Roller, A. & Rompel, A. (2015). Chem. Eur. J. 21, 17800–17807. [DOI] [PMC free article] [PubMed]
- Bijelic, A., Aureliano, M. & Rompel, A. (2018a). Angew. Chem. Int. Ed. In the press. doi:10.1002/anie.201803868.
- Bijelic, A., Aureliano, M. & Rompel, A. (2018b). Chem. Commun 54, 1153–1169. [DOI] [PMC free article] [PubMed]
- Bijelic, A. & Rompel, A. (2015). Coord. Chem. Rev. 299, 22–38. [DOI] [PMC free article] [PubMed]
- Bijelic, A. & Rompel, A. (2017). Acc. Chem. Res 50, 1441–1448. [DOI] [PMC free article] [PubMed]
- Blazevic, A., Al-Sayed, E., Roller, A., Giester, G. & Rompel, A. (2015). Chem. Eur. J. 21, 17800–17807.
- Blazevic, A. & Rompel, A. (2016). Coord. Chem. Rev. 307, 42–64.
- Boulmier, A., Vacher, A., Zang, D., Yang, S., Saad, A., Marrot, J., Oms, O., Mialane, P., Ledoux, I., Ruhlmann, L., Lorcy, D. & Dolbecq, A. (2018). Inorg. Chem. 57, 3742–3752. [DOI] [PubMed]
- Brown, I. D. & Altermatt, D. (1985). Acta Cryst. B41, 244–247.
- Bruker (2013). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Clemente-Juan, J. M., Coronado, E. & Gaita-Ariño, A. (2012). Chem. Soc. Rev. 41, 7464–7478. [DOI] [PubMed]
- Dolbecq, A., Dumas, E., Mayer, C. R. & Mialane, P. (2010). Chem. Rev 110, 6009–6048. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Fu, L., Gao, H., Yan, M., Shouzhu, L., Li, X., Dai, Z. & Liu, S. (2015). Small, 11, 2938–2945. [DOI] [PubMed]
- Gumerova, N. I., Al-Sayed, E., Krivosudský, L., Čipčić-Paljetak, H., Verbanac, D. & Rompel, A. (2018). Front. Chem. 6, article No. 336. [DOI] [PMC free article] [PubMed]
- Gumerova, N. I., Roller, A. & Rompel, A. (2016). Eur. J. Inorg. Chem. pp. 5507–5511.
- Gumerova, N. I. & Rompel, A. (2018). Nat. Rev. Chem. 2, article No. 0112.
- Hasenknopf, B., Delmont, R., Herson, P. & Gouzerh, P. (2002). Eur. J. Inorg. Chem. pp. 1081–1087
- Ji, Y., Hu, J., Huang, L., Chen, W., Streb, C. & Song, Y.-F. (2015). Chem. Eur. J. 21, 6469–6474. [DOI] [PubMed]
- Karalus, R. & Campagnari, A. (2000). Microbes Infect. 2, 547–559. [DOI] [PubMed]
- Li, X.-X., Wang, Y.-X., Wang, R.-H., Cui, C.-Y., Tian, C.-B. & Yang, G.-Y. (2016). Angew. Chem. Int. Ed. 55, 6462–6466. [DOI] [PubMed]
- Lode, H., Borner, K., Koeppe, P. & Schaberg, T. (1996). J. Antimicrob. Chemother. 37, 1–8. [DOI] [PubMed]
- Lucas, S. (2016). Headache: J. Head Face Pain, 56, 436–446.
- Macdonell, A., Johnson, N. A. B., Surman, A. J. & Cronin, L. (2015). J. Am. Chem. Soc. 137, 5662–5665. [DOI] [PubMed]
- Molitor, C., Bijelic, A. & Rompel, A. (2017). IUCrJ, 4, 734–740. [DOI] [PMC free article] [PubMed]
- Pope, M. (1983). In Heteropoly and Isopoly Oxometalates. Berlin: Springer.
- Rosnes, M. H., Musumeci, C., Yvon, C., Macdonell, A., Pradeep, C. P., Sartorio, C., Long, D.-L., Pignataro, B. & Cronin, L. (2013). Small, 9, 2316–2324. [DOI] [PubMed]
- Schaming, D., Allain, C., Farha, R., Goldmann, M., Lobstein, S., Giraudeau, A., Hasenknopf, B. & Ruhlmann, L. (2010). Langmuir, 26, 5101–5109. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Shirin, H., Moss, S. F., Kancherla, S., Kancherla, K., Holt, P. R., Weinstein, I. B. & Sordillo, E. M. (2006). J. Gastroenterol. Hepatol. 21, 1388–1393. [DOI] [PubMed]
- Shivaiah, V. & Das, S. (2005). J. Chem. Sci. 117, 227–233.
- Song, Y.-F., McMillan, N., Long, D.-L., Kane, S., Malm, J., Riehle, M. O., Pradeep, C. P., Gadegaard, N. & Cronin, L. (2009). J. Am. Chem. Soc. 131, 1340–1341. [DOI] [PubMed]
- Sova, M. (2012). Mini Rev. Med. Chem. 12, 749–767. [DOI] [PubMed]
- Wang, S.-S. & Yang, G.-Y. (2015). Chem. Rev. 115, 4893–4962. [DOI] [PubMed]
- Wikler, M. A. (2009). Approved Standard M7-A8. https://clsi.org/standards/products/microbiology/documents/m07/.
- Wu, P., Yin, P., Zhang, J., Hao, J., Xiao, Z. & Wei, Y. (2011). Chem. Eur. J. 17, 12002–12005. [DOI] [PubMed]
- Yvon, C., Surman, A. J., Hutin, M., Alex, J., Smith, B. O., Long, D.-L. & Cronin, L. (2014). Angew. Chem. Int. Ed. 53, 3336–3341. [DOI] [PubMed]
- Zhang, J., Huang, Y., Li, G. & Wei, Y. (2018). Coord. Chem. Rev. In the press. doi: 10.1016/j.ccr.2017.10.025.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, mo_ambl235_pbca, taco104_0m. DOI: 10.1107/S2053229618012536/jr3025sup1.cif
Structure factors: contains datablock(s) mo_ambl235_pbca. DOI: 10.1107/S2053229618012536/jr3025mo_ambl235_pbcasup4.hkl
Structure factors: contains datablock(s) taco104_0m. DOI: 10.1107/S2053229618012536/jr3025taco104_0msup3.hkl