Abstract
Infectious diseases are associated with disruption of host homeostasis. This can be triggered directly by pathogens or indirectly by host immune-driven resistance mechanisms. Disease tolerance is a defense strategy against infection that sustains host homeostasis, without exerting a direct negative impact on pathogens. The mechanisms governing disease tolerance encompass host metabolic responses that maintain vital homeostatic parameters within a range compatible with survival. Central to this defense strategy is the host's ability to sense and adapt to variations in nutrients, such as iron and glucose. Here we address how host responses regulating iron and glucose metabolism interact to establish disease tolerance and possibly modulate resistance to infection.
Keywords: iron metabolism, glucose metabolism, anorexia of infection, disease tolerance, nutritional immunity
Introduction
Avoidance, resistance, and disease tolerance are evolutionarily conserved defense strategies that limit the negative impact of pathogens on host health and fitness (1). Avoidance limits exposure to exogenous pathogens and resistance expels, neutralizes or destroys invading pathogens, while disease tolerance acts without interfering directly with pathogens (1, 2) (Figure 1).
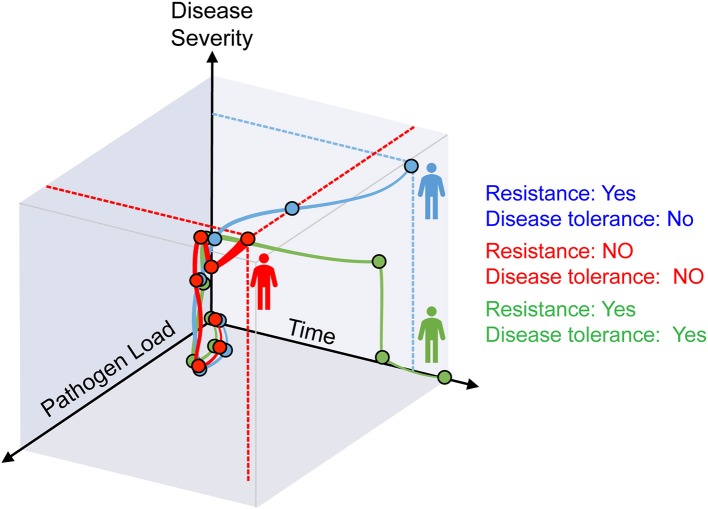
Figure 1.
Resistance and disease tolerance to infection. As host pathogen load increases during infection, disease symptoms become apparent and give rise to the clinical signs of infectious diseases. After an initial phase where both pathogen load and disease severity increase, the three possible outcomes are: (i) host homeostasis prevails based on resistance and disease tolerance mechanisms that eliminate pathogens and sustain vital metabolic outputs (green), (ii) resistance mechanisms reduce pathogen load but tissue damage control mechanisms fail to establish disease tolerance, compromising host homeostasis (blue); (iii) resistance mechanisms fail to control pathogen burden and tissue damage control mechanisms fail to establish diseases tolerance, compromising host homeostasis (red).
Disease tolerance relies on stress and damage responses that confer tissue damage control (3), that is, support the functional output of host tissues as a means to maintain vital homeostatic parameters within a range compatible with survival to infection (2, 4, 5). Stress and damage responses sense and react to variations in environmental cues or to damage imposed to cellular macromolecules and organelles, respectively (3). These are essential to provide metabolic adaptation to the stress and damage imposed directly by pathogens or indirectly by immune driven resistance mechanisms (3, 4).
Infections can impose a distinctive host behavioral pattern referred to as sickness behavior (6, 7). This encompasses anorexia, characterized by a reduction of food intake, possibly aimed at limiting nutrient availability to invading pathogens (8, 9) (Figure 2). While protective against some classes of pathogens (10–12), anorexia of infection carries a high evolutionary trade-off in that nutrient deprivation can compromise host homeostasis. For example, reduced iron intake in response to infection can lead to anemia of chronic disease (13), while reduced glucose intake can lead to hypoglycemia (10, 14, 15). Here we explore how regulation of host iron and glucose metabolisms impact on the establishment of disease tolerance and possibly on resistance to infection.
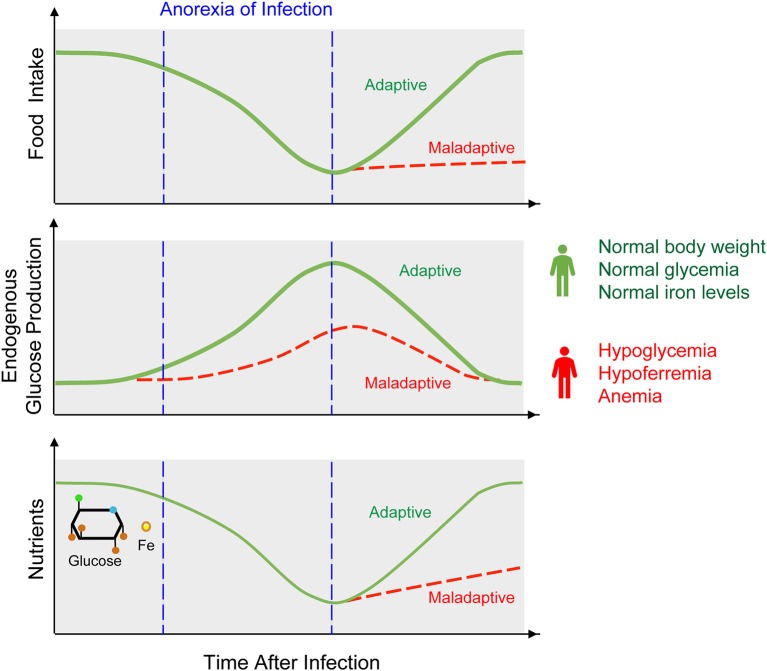
Figure 2.
Anorexia of infection, metabolic adaptation, and outcome of infection. Anorexia is a hallmark of sickness behavior that consists on a transient reduction of food intake. Anorexia of infection probably limits pathogens from accessing nutrients, such as glucose or iron. This defense strategy however, cannot be sustained indefinitely as to avoid the development of hypoglycemia, hypoferremia, and anemia, eventually culminating in death of the infected host. Therefore, anorexia of infection must be coupled to a host metabolic response that regulates endogenous production of nutrients, such as illustrated for example for hepatic glucose production. This metabolic response is essential to establish disease tolerance to infection and may also impact on resistance to infection.
Iron metabolism and disease tolerance
Iron is the most abundant transition metal present on Earth and perhaps for this reason was co-opted early in evolution to catalyze vital redox-based reactions in most living organisms, from prokaryotes to eukaryotes (16). Like other divalent metals, iron can shift between reduced (ferrous; Fe2+) and oxidized (ferric; Fe3+) or even higher oxidation states (ferryl; Fe4+), via reversible exchange of electrons with electrophilic or nucleophilic molecules, respectively. In doing so, iron is at the center stage of a variety of vital biological processes, including the transport and storage of gaseous molecules, energy production, as well as other components of cellular metabolism (17, 18). Probably due to its essential role in supporting these vital functions, microbial pathogens evolved multiple strategies to acquire iron from their hosts, while infected hosts co-evolved to limit iron availability to pathogens (18–22). This evolutionarily conserved defense strategy against infection is referred to as nutritional immunity (23).
Regulation of host iron metabolism in response to infection
Nutritional immunity is directed at inhibiting pathogens growth, via opposing mechanisms that limit nutrients' availability to intracellular or extracellular pathogens (18–22). Defense strategies limiting iron availability to intracellular pathogens rely on systemic inhibition of iron cellular import and can lead to hyperferremia (18–22). In contrast, defense strategies limiting iron availability to extracellular pathogens rely on cellular iron import mechanisms that promote cellular iron overload and hypoferremia (18–22). If uncontrolled, this can lead to the production of reactive oxygen species (ROS) via the Haber–Weiss–Fenton sequence (24), oxidizing and eventually damaging cellular macromolecules and organelles (22). In support of this notion, patients with genetic disorders characterized by cellular iron overload, such as hereditary hemochromatosis, are highly susceptible to a range of infections (25).
Regulation of iron metabolism confers tissue damage control
Disruption of host iron homeostasis is a hallmark of many infectious diseases (18, 22), as illustrated for example in malaria, the disease caused by Plasmodium spp. infection (26–28), polymicrobial sepsis (14, 29), tuberculosis caused by Mycobacterium tuberculosis (30, 31) or acquired immune deficiency syndrome, caused by human immunodeficiency virus (HIV) infection (31). Regulation of host iron metabolism is critical to confer tissue damage control, and in doing so, establishes disease tolerance to infection, as demonstrated for example for malaria (32) or polymicrobial sepsis (14).
The majority of the iron present in mammals exists in the form of heme (17, 33, 34), a tetrapyrrole ring that binds a central iron atom through different nitrogen atoms (34, 35). Heme is used essentially as a prosthetic group of hemoproteins, such as hemoglobin, myoglobin, or cytochrome c, where iron is deployed to exchange and store gaseous molecules or to transport electrons, respectively (33, 34). The largest pool of heme in mammals is found within hemoglobin in red blood cells (RBC), a prime target for invading pathogens in their search for iron (22, 33). As such, RBC lysis is a recurrent event associated with infection leading to the release of hemoglobin into plasma (17, 22, 36–38). Extracellular hemoglobin disassembles and auto-oxidizes, releasing its non-covalently bound prosthetic heme groups (33, 38) (Figure 3). This can lead to the generation of labile heme, that is, heme loosely bound to plasma acceptor proteins, macromolecules or low molecular weight ligands that fail to control its redox activity (36, 39). As it becomes bioavailable, a fraction of the labile heme in plasma acts in a pathogenic manner, compromising the establishment of disease tolerance to infection, as illustrated for malaria (38, 40, 41) or polymicrobial sepsis (14, 29).
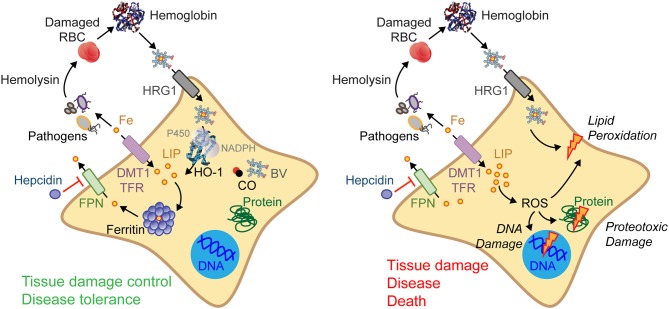
Figure 3.
Regulation of cellular iron metabolism in response to infection. Several resistance mechanisms may be used to restrict extracellular pathogens from accessing iron. For example, host cells can import heme/iron via heme transporters, such as the heme responsive gene 1 (HRG1), or via iron transporters, such as the divalent metal transporter-1 (DMT1) or the transferrin (TF)-transferrin receptor (TFR) that uptakes iron-TF complexes. Intracellular heme is catabolized by HO-1, generating iron, biliverdin (BV), and carbon monoxide (CO) (left). Hepcidin prevents cellular iron export via ferroportin (FPN) and as such LIP arising from heme catabolism must be stored by ferritin. These mechanisms are essential to confer tissue damage control and establish disease tolerance to systemic infections (left). When these protective mechanisms fail (right) intracellular heme and LIP increases promoting the generation of ROS, damaging DNA, proteins, and lipids. Ultimately this can compromise tissue damage control and the establishment of disease tolerance to infection (right).
Labile heme can also compromise resistance to infection via mechanisms inhibiting macrophage phagocytosis and impairing bacterial clearance (42) or mechanisms inducing macrophages to undergo programmed cell death (43). Moreover, labile heme can also be scavenged directly by bacterial pathogens, as demonstrated in the case of Staphylococcus aureus (44) or Citrobacter rodentium (45), promoting pathogen growth and compromising host resistance to infection (21, 46).
The pathological effects of labile heme are countered by host defense mechanisms that converge at the level of heme catabolism and storage of the iron extracted from heme (33, 34, 47). Under physiological conditions heme is catabolized by heme oxygenase-1 and -2 (HO-1 and HO-2), which cleave the tetrapyrrole ring, generating equimolar amounts of iron, carbon monoxide, and biliverdin (48). Upon infection, the stress-responsive HO-1 becomes the rate limiting enzyme in heme catabolism (33), playing a critical role in the establishment of disease tolerance to systemic infections, as illustrated for malaria (40, 41, 49) or polymicrobial sepsis (29).
The iron extracted via heme catabolism by heme oxygenases, integrates the cellular labile iron pool (LIP), becoming available to pathogens while catalyzing the production of ROS via the Haber–Weiss–Fenton sequence (24) (Figure 3). The pro-oxidant effects associated with excess heme catabolism and LIP overload are countered via the induction of cellular iron export by the solute carrier family 40 member 1 (SLC40A1), also known as ferroportin 1 (FPN1) (17, 22). Once excreted, iron is captured in plasma by transferrin (17, 22, 50) and delivered, via the transferrin receptor, to erythropoietic precursors where iron is required to support heme and hemoglobin synthesis (17, 22).
To prevent overt accumulation of extracellular iron, ferroportin expression and activity are downregulated by hepcidin, an acute-phase 25-amino acid peptide encoded by the HAMP gene (51, 52). In support of this notion, hepcidin accumulates in plasma in response to infection, inhibiting ferroportin expression/activity and impairing cellular iron export (51, 52). This can lead to cellular LIP accumulation, a potentially deleterious effect countered via iron storage and neutralization by ferritin (47, 53, 54).
Ferritin is a multimeric complex composed of ferritin heavy (heart) chain (FTH) and light (liver) chain (FTL) (47, 53, 54) (Figure 3). The ferroxidase activity of FTH converts pro-oxidant Fe2+ into nucleated Fe3+ (47, 53, 54), preventing LIP from participating in Haber–Weiss–Fenton sequence (24), limiting ROS generation and avoiding oxidative damage (17, 33). Supporting this notion, ferritin is essential to enforce tissue damage control and to establish disease tolerance to malaria (32) and to polymicrobial sepsis (14) (Figure 3). This protective effect depends on the ferroxidase activity of FTH, suggesting that iron conversion to its oxidized form (Fe3+) and subsequent incorporation into ferritin, are critical to establish disease tolerance to infection.
A significant proportion of ferritin is secreted (55), suggesting that the protective effects of ferritin are not restricted to its intracellular functions. In keeping with this notion, soluble ferritin is protective against Escherichia coli infection (56), and acts therapeutically to establish disease tolerance to polymicrobial sepsis (14). This argues for a role of extracellular ferritin as soluble iron chelator/transporter enforcing the establishment of disease tolerance to infection. Unexpectedly, the protective effects of ferritin extends beyond its antioxidant role, in that ferritin also controls glucose metabolism (14).
Glucose metabolism and disease tolerance
Glucose is a key nutrient for most living organisms, acting both as a metabolic fuel for ATP production via glycolysis or mitochondrial electron transport and as a biosynthetic intermediate for amino acid, lipid, and nucleic acid synthesis (57). While glucose intake from food allows for systemic delivery, glucose can also be synthesized endogenously from glucose precursors via gluconeogenesis or glycogenolysis in the liver, kidneys, or intestine (58). Glucose uptake from diet and its endogenous synthesis are tightly regulated to maintain blood glucose levels within a homeostatic range (5, 59). Enforcing this homeostatic range is particularly challenging during an infection (5), given that pathogens and their hosts often compete for this nutrient. Similar to iron, the infected host evolved strategies to limit glucose availability to pathogens, while maintaining glucose levels within a range compatible with survival. One of the strategies limiting glucose availability to pathogens relies on reducing glucose and glucose precursors intake from diet, via anorexia of infection. This is probably a component of nutritional immunity conferring resistance against pathogens (8–12, 60) (Figure 2).
Glucose availability in response to infection
The impact of anorexia of infection on the outcome of infectious diseases varies widely depending on the host and pathogen species (9–12, 61). In fruit flies, anorexia of infection promotes the establishment of disease tolerance to Salmonella Typhimurium infection, while compromising resistance to Listeria monocytogenes infection (11). In mice, anorexia of infection is protective against L. monocytogenes (10, 12), but deleterious against influenza virus infections (10, 62). Anorexia of infection also impacts on the outcome of gastrointestinal parasitic infections (60, 61), reducing body weight upon Nippostrongylus brasiliensis infection (63), while increasing immunopathology in response to Trichostrongylus colubriformis infection (64).
Mechanisms regulating anorexia of infection are not clearly established (9, 10, 61), but certainly encompass pathogen sensing via host pattern recognition receptors (PRR) (9). Signaling downstream of PRR elicits the production of interleukins (IL), such as IL-1, IL-6, IL-8, or tumor necrosis factor (TNF), which signal systemically to induce anorexia of infection as well as to regulate glucose metabolism (9). One of the mechanisms via which this occurs involves the secretion of leptin by adipose tissue (9, 65), an hormone that signals in the central nervous system (CNS) to reduce food intake and regulate energy consumption (66).
Pathogens can modulate anorexia of infection directly to promote their survival and/or transmission (9, 10, 61, 67). For example, S. Typhimurium inhibits PRR activation/signaling, reducing IL-1β secretion in the gut and increasing food consumption, as well as blood glucose levels (67). This reduces S. Typhimurium virulence and promotes host disease tolerance, while increasing Salmonella transmission (67), most likely as an evolutionary trade-off. The nematode N. brasiliensis also induces anorexia, via the regulation CNS signaling (68, 69), even though the exact mechanism by which this occurs has not been established.
Anorexia of infection is also associated with reduction in caloric intake, i.e., caloric restriction, which can per se modulate the outcome of infection (70). For example, caloric restriction increases susceptibility to polymicrobial (71) and viral infections (10, 72), while reducing Plasmodium virulence and promoting survival to malaria (73).
Although protective against bacterial infections (8, 9) mechanisms reducing blood glucose levels must be tightly regulated to prevent the development of lethal hypoglycemia. In support of this notion, inhibition of hepatic glucose production in mice carrying a liver-specific deletion of glucose 6 phosphatase 1 (g6pc1) compromises disease tolerance to polymicrobial infections (14). This suggests that while reducing blood glucose levels can be protective against bacterial infections (8, 9), endogenous glucose production is required to prevent the development of lethal hypoglycemia and establish disease tolerance to polymicrobial sepsis (14).
Impact of metabolic diseases on infection
The impact of glucose metabolism on the outcome of infectious diseases is illustrated by the effect of metabolic diseases, such as obesity or diabetes, on the outcome of infections. For example, hyperglycemia in diabetic rodents is associated with increased susceptibility to polymicrobial sepsis (74, 75) as well to L. monocytogenes (76) or M. tuberculosis (77) infections. Moreover, hyperglycemia promotes intestinal permeability and increases susceptibility to bacterial infection in mice (78). This pathogenic effect is mediated via glucose import by intestinal epithelial cells, disrupting the functional integrity of the gut epithelium via a mechanism that interferes with epithelial tight and adherens junctions (78). Despite this experimental evidence, whether deregulation of glucose homeostasis impacts on the outcome of bacterial infections in humans remains unclear. For example, clinical evidence suggests that diabetes mellitus is not a major risk factor for sepsis severity (79), while both hyperglycemia and hypoglycemia are major risk factors for sepsis mortality (80, 81). Of note, rodents develop hypoglycemia rather than hyperglycemia in response to bacterial infections (14, 15, 82, 83). In some cases, hypoglycemia is preceded by a transient state of hyperglycemia, but whether this is triggered by infection and/or other associated experimental procedure is not clear (14, 15).
Glucose control of innate and adaptive immune function
Regulation of host glucose metabolism can impact on pathogens directly or indirectly, via modulation of immune-driven resistance mechanisms (84–86). Proliferation, differentiation, and effector function of immune cells is regulated by two major metabolic programs, namely, oxidative phosphorylation, and aerobic glycolysis (84–86). Signaling via PRR in macrophages or dendritic cells shifts metabolic flux from oxidative phosphorylation to aerobic glycolysis, a phenomenon known as the Warburg effect (87). Despite being less energetically effective, glycolysis generates pyruvate, nicotinamide adenine dinucleotide (NADH), and other metabolic intermediates used by major biosynthetic pathways (84, 86). This metabolic shift also promotes the pentose phosphate pathway, generating nicotinamide adenine dinucleotide phosphate (NADPH), a critical component of the NADPH oxidase (NOX) enzyme complexes, generating ROS involved in pathogen killing (84, 86, 88). In contrast to their microbicidal effector functions, other macrophage effector functions promoting tissue healing and regeneration rely primarily on oxidative phosphorylation (86, 88).
A marked increase in aerobic glycolysis is also a hallmark of T cell activation, together with a more modest induction of oxidative phosphorylation (86, 89), presumably accommodating the reduction in oxygen availability that arises during infections (85, 90). This metabolic reprogramming is orchestrated by a complex mechanism involving the store-operated Ca2+ entry (SOCE), a key regulator of cellular calcium signaling (91), the hypoxia-inducible factor 1α (HIF1α), a transcriptional master regulator of hypoxia, as well as the mammalian target of rapamycin complex 1 (mTORC1), a master regulator of cell growth (84, 86, 92). Of note, mTORC1 controls the expression of glycolytic genes in innate and adaptive immune cells via a mechanism involving HIF1α (93–95). The relative impact of these metabolic pathways on the outcome of infections can be illustrated in the context of M. tuberculosis infection, where myeloid HIF1α plays a critical role to induce the Warburg effect (96), supporting resistance to M. tuberculosis (97). Similarly, mice lacking HIF1α in the myeloid compartment also fail to shift to aerobic glycolysis, succumbing to bacterial sepsis (98).
In contrast to effector T cells, memory T cells rely on oxidative phosphorylation to produce energy, using fatty acids to produce acetyl coenzyme A (acetyl-CoA) and fuel the Krebs cycle, via a mechanism known as fatty acid oxidation (FAO) (84–86). Moreover, recent work has shown that FAO in memory T cells, can occur not only via carnitine palmitoyltransferase IA (CPT IA)-dependent, but also independent mechanisms (99), suggesting that memory T cells are able to use a wide range of fatty acids in order to obtain energy. The switch between aerobic glycolysis and oxidative phosphorylation relies on a mechanism involving the transcription repressor Bcl-6 (100), which downregulates glycolytic genes and promotes the T and B cell differentiation toward the memory compartment (101–103). Presumably, the combined effect of reduced glycemia and Bcl-6 expression are likely to promote effector to memory T cell transition in response to infection. Whether glucose availability impacts on immune-driven resistance mechanisms remains, to the best of our knowledge, to be determined.
Cross-talk between iron and glucose metabolism in response to infection
A functional interplay between iron and glucose metabolism has been established primarily in the context of metabolic diseases, such as porphyria (104, 105) or diabetes (106–108). For example, hepatic glucose production induces hepcidin expression (109, 110) (Figure 4A) and inhibits cellular iron export by ferroportin, leading to cellular iron overload and hypoferremia (110). Conversely, cellular iron overload regulates insulin production in pancreatic β-cells (106, 111) and is thought to contribute critically to impair glucose metabolism in diabetic patients (112, 113) (Figure 4B). This interplay is probably operational in other pathologic conditions such as atherosclerosis (107) or β-thalassemia major (114).
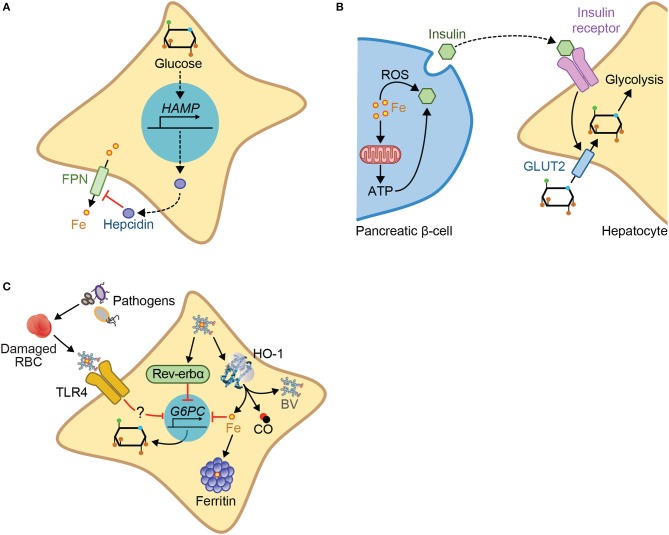
Figure 4.
Mechanisms of iron-glucose metabolism cross-talk. Iron and glucose can cross-talk via different mechanisms: (A) Glucose increases expression of hepcidin, inhibiting cellular iron export via ferroportin. (B) Iron acts via the production of ROS or via mitochondrial respiration and subsequent ATP production, to promote insulin exocytosis by the pancreatic β-cells. Insulin binding to the insulin receptor in target cells, e.g., hepatocytes, promotes cellular glucose import via the glucose transporter 2 (GLUT2), and glycolysis. (C) In the context of infection, glucose metabolism can be regulated by heme via a pathway that involves TLR4, but which has not yet been fully described. Heme interaction with the nuclear receptor Rev-erbα, downregulates the transcription of gluconeogenic genes including G6PC, the enzyme catalyzing the last step of gluconeogenesis. G6PC is also downregulated by iron produced via heme catabolism by HO-1, an inhibitory effect countered by ferritin.
More recently a crosstalk between iron and glucose metabolism has also been established in the context of infections (14, 115, 116). Namely, iron intake from diet leads to decreased pathogen virulence, without interfering with pathogen burden, favoring asymptomatic infection with the enteric pathogen C. rodentium (116). This occurs through a mechanism via which iron intake promotes insulin resistance, reducing glucose uptake by the intestine, and thus promoting glucose availability in the gut, leading to the suppression of virulence factors (116). Deregulation of host iron metabolism in response to polymicrobial infection compromises the establishment of disease tolerance to sepsis, via a mechanism that deregulates glucose metabolism (14, 117), thus also illustrating the crosstalk between iron and glucose. This pathologic mechanism is driven by labile heme, which plays a central role in the pathogenesis of sepsis (29). Namely, labile heme inhibits hepatic G6pase and consequently glucose production leading to hypoglycemia (14) (Figure 4C). This pathogenic effect has been linked functionally to a transcriptional repression of g6pc1 gene (14). In support of this notion, mice lacking hepatic g6pc1 develop lethal hypoglycemia in response to polymicrobial sepsis or heme administration (14). This suggests that hepatic glucose production is required to counter the hypoglycemia induced by labile heme (14). This is also consistent with the notion that deregulation of glucose metabolism plays a central role in pathogenesis of infectious diseases, including sepsis (10, 14, 80, 81, 118). This occurs via a mechanism that is not associated with modulation of host pathogen load (10, 14, 117), demonstrating that regulation of glucose metabolism controls the establishment of disease tolerance to infection (10, 14, 117).
The molecular mechanism via which labile heme induces hypoglycemia is not entirely clear but has been linked to signaling via Toll-like receptor 4 (TLR4) (14) (Figure 4C), a PRR that senses labile heme (119). This is consistent with the induction of hypoglycemia by TLR4 ligands, such as LPS (120). Whether heme sensing by TLR4 mediates the development of hypoglycemia during polymicrobial sepsis was not established. The pathway through which heme represses g6pc1 transcription (14, 121) is likely to involve the heme sensor and transcriptional repressor Rev-erbα (121) (Figure 4C). Whether this mechanism is operational in vivo to repress hepatic glucose production and elicit hypoglycemia in response to infection remains to be established. It is possible as well that TLR4 and Rev-erbα synergize to repress g6pc1 transcription in hepatocytes.
Iron sequestration by ferritin counters heme-driven repression of g6pc1 transcription, suggesting that heme represses g6pc1 transcription via a mechanism involving iron (14). In keeping with this notion, polymicrobial infections in mice are associated with the induction of ferritin in the liver, which is essential to sustain hepatic glucose-6-phosphatase (G6Pase) expression and counter the development of lethal hypoglycemia (14, 122) (Figure 4C). Whether iron accumulation in hepatocytes synergizes with TLR4 and Rev-erbα to repress g6pc1 transcription remains to be established.
Regulation of hepatic glucose production by ferritin may be part of an adaptive response promoting the development of insulin resistance, presumably countering unfettered cellular glucose utilization in host tissues and allowing to restore normal blood glucose levels (123). This effect of ferritin should also contribute to prevent the development of hypoglycemia in response to infections.
Conclusions and future perpectives
Resistance to infection is generally perceived as the predominant host defense strategy against infection. This dogma has been challenged by the recurrent observation that the severity of infectious diseases can at times be dissociated from host pathogen burden. In the last few years these observations have been interpreted as revealing disease tolerance as a critical host defense strategy against infection. This defense strategy relies on tissue damage control mechanisms controlling the metabolic output of host tissue and maintaining vital homeostatic parameters within a range compatible with host survival. This is illustrated for mechanisms regulating iron and glucose metabolism, which cross-talk to establish disease tolerance to infection. To what extent these tissue damage control mechanisms may be targeted therapeutically remains to be established.
Author contributions
ARC wrote the manuscript with SW and MPS. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all members of the Inflammation group (IGC) for insightful discussions and input. Also thank Rui Martins for help with the illustrations. Support by Fundação para a Ciência e Tecnologia (SFRH/BPD/101608/2014) to ARC and (HMSP-ICT/0018/2011, PTDC/IMI-IMU/5723/2014, LISBOA-01-0145-FEDER-029411) to MPS, European Community 7th Framework (ERC-2011-AdG 294709-DAMAGECONTROL) to MPS, Deutsche Forschungsgemeinschaft (DFG; WE 4971/3) to SW.
References
- 1.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science (2012) 335:335–41. 10.1126/science.1214935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. (2008) 8:8–95. 10.1038/nri2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares MP, Gozzelino R, Weis S. Tissue damage control in disease tolerance. Trends Immunol. (2014) 35:35–94. 10.1016/j.it.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell (2014) 54:54–8. 10.1016/j.molcel.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell (2015) 160:160–27. 10.1016/j.cell.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. (1988) 12:12–37. 10.1016/S0149-7634(88)80004-6 [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:9–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. (1979) 32:32–6. 10.1093/ajcn/32.3.593 [DOI] [PubMed] [Google Scholar]
- 9.Langhans W. Anorexia of infection: current prospects. Nutrition (2000) 16:16–1005. 10.1016/S0899-9007(00)00421-4 [DOI] [PubMed] [Google Scholar]
- 10.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell (2016) 166:166–25 e12. 10.1016/j.cell.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. (2009) 7:7. 10.1371/journal.pbio.1000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing EJ, Young JB. Acute starvation protects mice against Listeria monocytogenes. Infect Immun. (1980) 28:28–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. (2005) 352:352–23. 10.1056/NEJMra041809 [DOI] [PubMed] [Google Scholar]
- 14.Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, et al. Metabolic adaptation establishes disease tolerance to sepsis. Cell (2017) 169:169–75.e14. 10.1016/j.cell.2017.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira FBD, dos Santos C, Bruxel MA, Nunes EA, Spiller F, Rafacho A. Glucose homeostasis in two degrees of sepsis lethality induced by caecum ligation and puncture in mice. Int J Exp Pathol. (2017) 98:98–40. 10.1111/iep.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. (2007) 69:69–85. 10.1146/annurev.physiol.69.031905.164337 [DOI] [PubMed] [Google Scholar]
- 17.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell (2017) 168:168–61. 10.1016/j.cell.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. (2015) 15:15–10. 10.1038/nri3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer LD, Skaar EP. Transition metals and virulence in bacteria. Annu Rev Genet. (2016) 50:50–91. 10.1146/annurev-genet-120215-035146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol. (2012) 10:10–37. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuñez G, Sakamoto K, Soares MP, Núñez G, Sakamoto K, Soares MP. Innate Nutritional Immunity. J Immunol. (2018): 11–8. 10.4049/jimmunol.1800325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares MP, Weiss G. The Iron age of host-microbe interactions. EMBO Rep. (2015) 16:16–500. 10.15252/embr.201540558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg ED. Nutritional immunity. host's attempt to withold iron from microbial invaders. JAMA (1975) 231:231. [DOI] [PubMed] [Google Scholar]
- 24.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. (2001) 33:33–59. 10.1016/S1357-2725(01)00063-2 [DOI] [PubMed] [Google Scholar]
- 25.Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. (2007) 11:11–7. 10.1016/j.ijid.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 26.Prentice AM, Verhoef H, Cerami C. Iron fortification and malaria risk in children. JAMA (2013) 310:310–5. 10.1001/jama.2013.6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabeza GF, Loyevsky M, Gordeuk VR, Weiss G. Iron chelation therapy for malaria: a review. Pharmacol Ther. (1999) 81:81–75. 10.1016/S0163-7258(98)00037-0 [DOI] [PubMed] [Google Scholar]
- 28.Portugal S, Drakesmith H, Mota MM. Superinfection in malaria: plasmodium shows its iron will. EMBO Rep. (2011) 12:12–42. 10.1038/embor.2011.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. (2010) 2:2ra71. 10.1126/scitranslmed.3001118 [DOI] [PubMed] [Google Scholar]
- 30.Banerjee S, Farhana A, Ehtesham NZ, Hasnain SE. Iron acquisition, assimilation and regulation in mycobacteria. Infect Genet Evol. (2011) 11:11–38. 10.1016/j.meegid.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 31.McDermid JM, Hennig BJ, van der Sande M, Hill AV, Whittle HC, Jaye A, et al. Host iron redistribution as a risk factor for incident tuberculosis in HIV infection: an 11-year retrospective cohort study. BMC Infect Dis. (2013) 13:13. 10.1186/1471-2334-13-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gozzelino R, Andrade BB, Larsen R, Luz NF, Vanoaica L, Seixas E, et al. Metabolic adaptation to tissue iron overload confers tolerance to malaria. Cell Host Microbe (2012) 12:12–704. 10.1016/j.chom.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 33.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. (2010) 50:50–54. 10.1146/annurev.pharmtox.010909.105600 [DOI] [PubMed] [Google Scholar]
- 34.Tsiftsoglou AS, Tsamadou AI, Papadopoulou LC. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol Ther. (2006) 111:111–45. 10.1016/j.pharmthera.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 35.Poulos TL. The Janus nature of heme. Nat Prod Rep. (2007) 24:24. 10.1039/b604195g [DOI] [PubMed] [Google Scholar]
- 36.Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Curr Opin Immunol. (2016) 38:38–100. 10.1016/j.coi.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 37.Soares MP, Hamza I. Macrophages and Iron metabolism. Immunity (2016) 44:44–504. 10.1016/j.immuni.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira A, Balla J, Jeney V, Balla G, Soares MP. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med. (2008) 86:86–111. 10.1007/s00109-008-0368-5 [DOI] [PubMed] [Google Scholar]
- 39.Gouveia Z, Carlos AR, Yuan X, Aires-da-Silva F, Stocker R, Maghzal GJ, et al. Characterization of plasma labile heme in hemolytic conditions. FEBS J. (2017) 284:284–301. 10.1111/febs.14192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira A, Marguti I, Bechmann I, Jeney V, Chora A, Palha NR, et al. Sickle hemoglobin confers tolerance to plasmodium infection. Cell (2011) 145:145–409. 10.1016/j.cell.2011.03.049 [DOI] [PubMed] [Google Scholar]
- 41.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. (2007) 13:13–10. 10.1038/nm1586 [DOI] [PubMed] [Google Scholar]
- 42.Martins R, Maier J, Gorki A-D, Huber KVM, Sharif O, Starkl P, et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat Immunol. (2016) 17:17–72. 10.1038/ni.3590 [DOI] [PubMed] [Google Scholar]
- 43.Fortes GB, Alves LS, de Oliveira R, Dutra FF, Rodrigues D, Fernandez PL, et al. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood (2012) 119:119–75. 10.1182/blood-2011-08-375303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-Source preference of staphylococcus aureus infections. Science (2004) 305:305–8. 10.1126/science.1099930 [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto K, Kim Y-G, Hara H, Kamada N, Caballero-Flores G, Tolosano E, et al. IL-22 controls iron-dependent nutritional immunity against systemic bacterial infections. Sci Immunol. (2017) 2:2eaai8371. 10.1126/sciimmunol.aai8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins R, Knapp S. Heme and hemolysis in innate immunity: adding insult to injury. Curr Opin Immunol. (2018) 50:50–20. 10.1016/j.coi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 47.Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. (2014) 20:20–69. 10.1089/ars.2013.5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen R, Gouveia Z, Soares MP, Gozzelino R. Heme cytotoxicity and the pathogenesis of immune-mediated inflammatory diseases. Front Pharmacol. (2012) 3:3. 10.3389/fphar.2012.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci USA. (2009) 106:106–42. 10.1073/pnas.0903419106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lane DJ, Merlot AM, Huang ML, Bae DH, Jansson PJ, Sahni S, et al. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim Biophys Acta (2015) 1853:1853–44. 10.1016/j.bbamcr.2015.01.021 [DOI] [PubMed] [Google Scholar]
- 51.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (2004) 306:306–3. 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 52.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science (2012) 338:338–72. 10.1126/science.1224577 [DOI] [PubMed] [Google Scholar]
- 53.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta (1996) 1275:1275–203. 10.1016/0005-2728(96)00022-9 [DOI] [PubMed] [Google Scholar]
- 54.Arosio P, Elia L, Poli M. Ferritin, cellular iron storage and regulation. IUBMB Life (2017) 69:69–22. 10.1002/iub.1621 [DOI] [PubMed] [Google Scholar]
- 55.Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm. (2011) 118:118–47. 10.1007/s00702-011-0582-0 [DOI] [PubMed] [Google Scholar]
- 56.Lipinski P, Jarzabek Z, Broniek S, Zagulski T. Protective effect of tissue ferritins in experimental Escherichia coli infection of mice in vivo. Int J Exp Pathol. (1991) 72:72–30. [PMC free article] [PubMed] [Google Scholar]
- 57.Navdeep C. Navigating Metabolism Press. 1st ed. Cold Spring Harbor :Laboratory Press; (2015). [Google Scholar]
- 58.Soty M, Gautier-Stein A, Rajas F, Mithieux G. Gut-Brain glucose signaling in energy homeostasis. Cell Metab. (2017) 25:25–42. 10.1016/j.cmet.2017.04.032 [DOI] [PubMed] [Google Scholar]
- 59.Soty M, Penhoat A, Amigo-Correig M, Vinera J, Sardella A, Vullin-Bouilloux F, et al. A gut–brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Mol Metab. (2015) 4:4–17. 10.1016/j.molmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyriazakis II, Tolkamp BJ, Hutchings MR. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim Behav. (1998) 56:56–74. 10.1006/anbe.1998.0761 [DOI] [PubMed] [Google Scholar]
- 61.Colditz IG. Six costs of immunity to gastrointestinal nematode infections. Parasite Immunol. (2008) 30:30–70. 10.1111/j.1365-3024.2007.00964.x [DOI] [PubMed] [Google Scholar]
- 62.Swiergiel AH, Smagin GN, Dunn AJ. Influenza virus infection of mice induces anorexia: comparison with endotoxin and interleukin-1 and the effects of indomethacin. Pharmacol Biochem Behav. (1997) 57:57–96. 10.1016/S0091-3057(96)00335-8 [DOI] [PubMed] [Google Scholar]
- 63.Crompton DWT, Walters DE, Arnold S. Changes in the food intake and body weight of protein-malnourished rats infected with Nippostrongylus brasiliensis (Nematoda). Parasitology (1981) 82:82–38. 10.1017/S0031182000041834 [DOI] [PubMed] [Google Scholar]
- 64.Greer AW, Stankiewicz M, Jay NP, McAnulty RW, Sykes AR. The effect of concurrent corticosteroid induced immuno-suppression and infection with the intestinal parasite Trichostrongylus colubriformis on food intake and utilization in both immunologically naïve and competent sheep. Anim Sci. (2005) 80:80–99. 10.1079/ASC41100089 [DOI] [Google Scholar]
- 65.Sachot C, Poole S, Luheshi GN. Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J Physiol. (2004) 561:561–72. 10.1113/jphysiol.2004.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature (1994) 372:372–32. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 67.Rao S, Schieber AM, O'Connor CP, Leblanc M, Michel D, Ayres JS. Pathogen-Mediated inhibition of anorexia promotes host survival and transmission. Cell (2017) 168:168–16 e12. 10.1016/j.cell.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horbury SR, Mercer JG, Chappell LH. Anorexia induced by the parasitic nematode, Nippostrongylus brasiliensis: effects on NPY and CRF gene expression in the rat hypothalamus. J Endocrinol. (1995) 7:7–73. 10.1111/j.1365-2826.1995.tb00728.x [DOI] [PubMed] [Google Scholar]
- 69.Roberts HC, Hardie LJ, Chappell LH, Mercer JG. Parasite-induced anorexia: leptin, insulin and corticosterone responses to infection with the nematode, Nippostrongylus brasiliensis. Parasitology (1999) 117–23. 10.1017/S0031182098003503 [DOI] [PubMed] [Google Scholar]
- 70.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. (2011) 32:32–221. 10.1016/j.mam.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 71.Sun D, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol. (2001) 8:8–11. 10.1128/CDLI.8.5.1003-1011.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr. (2008) 138:138–75. 10.3945/jn.108.093633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mancio-Silva L, Slavic K, Grilo Ruivo MT, Grosso AR, Modrzynska KK, Vera IM, et al. Nutrient sensing modulates malaria parasite virulence. Nature (2017) 547:547–6. 10.1038/nature23009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Filgueiras LR Jr, Martins JO, Serezani CH, Capelozzi VL, Montes MBA, Jancar S. Sepsis-induced Acute Lung Injury (ALI) is milder in diabetic rats and correlates with impaired NFkB activation. PLoS ONE (2012) 7:7. 10.1371/journal.pone.0044987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiller F, Carlos D, Souto FO, de Freitas A, Soares FS, Vieira SM, et al. α1-Acid glycoprotein decreases neutrophil migration and increases susceptibility to sepsis in diabetic mice. Diabetes (2012) 61:61–91. 10.2337/db11-0825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, et al. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes (2005) 54:54–9. 10.2337/diabetes.54.1.182 [DOI] [PubMed] [Google Scholar]
- 77.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. (2007) 37:37–24. 10.1165/rcmb.2006-0478OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winer DA, Luck H, Tsai S, Winer S. The intestinal immune system in obesity and insulin resistance. Cell Metab. (2016) 23:23–26. 10.1016/j.cmet.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 79.van Vught LA, Scicluna BP, Hoogendijk AJ, Wiewel MA, Klein Klouwenberg PMC, Cremer OL, et al. Association of diabetes and diabetes treatment with the host response in critically ill sepsis patients. Crit Care (2016) 20:20 10.1186/s13054-016-6571429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller SI, Wallace RJ, Musher DM, Septimus EJ, Kohl S, Baughn RE. Hypoglycemia as a manifestation of sepsis. Am J Med. (1980) 68:68–54. 10.1016/0002-9343(80)90250-8 [DOI] [PubMed] [Google Scholar]
- 81.Van Cromphaut SJ, Vanhorebeek I, Van den Berghe G, Berghe G. Glucose metabolism and insulin resistance in sepsis. Curr Pharm Des. (2008) 14:14–99. 10.2174/138161208784980563 [DOI] [PubMed] [Google Scholar]
- 82.Heuer JG, Bailey DL, Sharma GR, Zhang T, Ding C, Ford A, et al. Cecal ligation and puncture with total parenteral nutrition: a clinically relevant model of the metabolic, hormonal, and inflammatory dysfunction associated with critical illness. J Surg Res. (2004) 121:121–86. 10.1016/j.jss.2004.04.018 [DOI] [PubMed] [Google Scholar]
- 83.Singamsetty S, Shah FA, Guo L, Watanabe Y, McDonald S, Sharma R, Zhang Y, et al. Early initiation of low-level parenteral dextrose induces an accelerated diabetic phenotype in septic C57BL/6J mice. Appl Physiol Nutr Metab. (2016) 41:41–9. 10.1139/apnm-2015-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell (2017) 169:169–86. 10.1016/j.cell.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. (2016) 291:291–10. 10.1074/jbc.R115.693903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. (2016) 16:16–65. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warburg O. On the origin of cancer cells. Science (1956) 123:123–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 88.Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. (2015) 25:25–84. 10.1038/cr.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pearce EL, Poffenberger MC, Chang C-HH, Jones RG, Aghajanirefah A, Matarese F, et al. Fueling immunity: insights into metabolism and lymphocyte function. Science (2013) 342:342 10.1126/science.1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol. (2015) 68:68–9. 10.1016/j.molimm.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 91.Vaeth M, Maus M, Klein-Hessling S, Freinkman E, Yang J, Eckstein M, et al. Store-operated Ca2+ entry controls clonal expansion of T cells through metabolic reprogramming. Immunity (2017) 47:47–79.e6. 10.1016/j.immuni.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell (2017) 168:168–76. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finlay DK, Rosenzweig E, Sinclair L V, Feijoo-Carnero C, Hukelmann JL, Rolf J, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. (2012) 209:209–53. 10.1084/jem.20112607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. (2011) 208:208–76. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1α Is essential for myeloid cell-mediated inflammation. Cell (2003) 112:112 645–57. 10.1016/S0092-8674(03)00154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi L, Salamon H, Eugenin EA, Pine R, Cooper A, Gennaro ML. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci Rep. (2015) 5:5. 10.1038/srep18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Braverman J, Sogi KM, Benjamin D, Nomura DK, Stanley SA. HIF-1α Is an essential mediator of IFN-γ-dependent immunity to mycobacterium tuberculosis. J Immunol. (2016) 197:197–97. 10.4049/jimmunol.1600266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345:345 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. (2018) 28:28–15.e7. 10.1016/j.cmet.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol. (2014) 15:15–64. 10.1038/ni.2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity (2011) 35:35–95. 10.1016/j.immuni.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. (2010) 11:11–20. 10.1038/ni.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. (2002) 3:3–63. 10.1038/ni802 [DOI] [PubMed] [Google Scholar]
- 104.Khadilkar S V, Yadav RS, Patel BA. Porphyrias. In: Neuromuscular Disorders. Singapore: Springer Singapore; (2018). p. 493–502. [Google Scholar]
- 105.Telega GW. Metabolic and genetic liver diseases: porphyrias. In: Saeian K, Shaker R, editors. Liver Disorders. Cham: Springer; (2017). p. 381–7. [Google Scholar]
- 106.Fernandez-Real JM, McClain D, Manco M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care (2015) 38:38–76. 10.2337/dc14-3082 [DOI] [PubMed] [Google Scholar]
- 107.Fernandez-Real JM, Manco M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. (2014) 2:2–26. 10.1016/S2213-8587(13)70174-8 [DOI] [PubMed] [Google Scholar]
- 108.Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab. (2013) 17:17–41. 10.1016/j.cmet.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aigner E, Felder TK, Oberkofler H, Hahne P, Auer S, Soyal S, et al. Glucose acts as a regulator of serum iron by increasing serum hepcidin concentrations. J Nutr Biochem. (2013) 24:24–7. 10.1016/j.jnutbio.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 110.Vecchi C, Montosi G, Garuti C, Corradini E, Sabelli M, Canali S, et al. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology (2014) 146:146–9. 10.1053/j.gastro.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Backe MB, Moen IW, Ellervik C, Hansen JB, Mandrup-Poulsen T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu Rev Nutr. (2016) 36:36–73. 10.1146/annurev-nutr-071715-050939 [DOI] [PubMed] [Google Scholar]
- 112.Tuomainen TP, Nyyssönen K, Salonen R, Tervahauta A, Korpela H, Lakka T, et al. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern finnish men. Diabetes Care (1997) 20:20–8. [DOI] [PubMed] [Google Scholar]
- 113.Fernández-Real JM, López-Bermejo A, Ricart W, Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes (2002) 51:51–54. 10.2337/DIABETES.51.8.2348 [DOI] [PubMed] [Google Scholar]
- 114.De Sanctis V, Soliman A, Yassin M. Iron overload and glucose metabolism in subjects with beta-thalassaemia major: an overview. Curr Diabetes Rev. (2013) 9:9–41. 10.2174/1573399811309040005 [DOI] [PubMed] [Google Scholar]
- 115.Carlos AR, Weis S, Soares MP. Cross-regulation of iron and glucose metabolism in response to infection. Biochemistry (2017) 56:56–4. 10.1021/acs.biochem.7b00728 [DOI] [PubMed] [Google Scholar]
- 116.Sanchez KK, Chen GY, Schieber AMP, Redford SE, Shokhirev MN, Leblanc M, et al. Cooperative metabolic adaptations in the host can favor asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell (2018) 175:175–58.e15. 10.1016/j.cell.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lecube A, Hernández C, Genescà J, Simó R. Glucose abnormalities in patients with hepatitis C virus infection: epidemiology and pathogenesis. Diabetes Care (2006) 29:29–9. 10.2337/diacare.2951140 [DOI] [PubMed] [Google Scholar]
- 118.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. (2013) 5:5ra95. 10.1126/scitranslmed.3005893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. (2007) 282:282–9. 10.1074/jbc.M610737200 [DOI] [PubMed] [Google Scholar]
- 120.Raetzsch CF, Brooks NL, Alderman JM, Moore KS, Hosick PA, Klebanov S, et al. Lipopolysaccharide inhibition of glucose production through the Toll-like receptor-4, myeloid differentiation factor 88, and nuclear factor kappa b pathway. Hepatology (2009) 50:50–600. 10.1002/hep.22999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science (2007) 318:318–9. 10.1126/science.1150179 [DOI] [PubMed] [Google Scholar]
- 122.Deutschman CS, Andrejko KM, Haber BA, Bellin L, Elenko E, Harrison R, et al. Sepsis-induced depression of rat glucose-6-phosphatase gene expression and activity. Am J Physiol. (1997) 273:273–18. 10.1152/ajpregu.1997.273.5.R1709 [DOI] [PubMed] [Google Scholar]
- 123.Yki-järvinen H, Sammalkorpi K, Koivisto VA, Nikkilä EA. Severity, duration, and mechanisms of insulin resistance during acute infections*. J Clin Endocrinol Metab. (1989) 69:69–23. [DOI] [PubMed] [Google Scholar]