Abstract
BACKGROUND:
Metabolites of the tryptophan–kynurenine pathway (i.e., tryptophan, kynurenine, kynurenic acid, quinolinic acid, 3-hydroxyanthranilic) may be associated with diabetes development. Using a case–cohort design nested in the Prevención con Dieta Mediterránea (PREDIMED) study, we studied the associations of baseline and 1-year changes of these metabolites with incident type 2 diabetes (T2D).
METHODS:
Plasma metabolite concentrations were quantified via LC-MS for n = 641 in a randomly selected subcohort and 251 incident cases diagnosed during 3.8 years of median follow-up. Weighted Cox models adjusted for age, sex, body mass index, and other T2D risk factors were used.
RESULTS:
Baseline tryptophan was associated with higher risk of incident T2D (hazard ratio = 1.29; 95% CI, 1.04–1.61 per SD). Positive changes in quinolinic acid from baseline to 1 year were associated with a higher risk of T2D (hazard ratio = 1.39; 95% CI, 1.09–1.77 per SD). Baseline tryptophan and kynurenic acid were directly associated with changes in homeostatic model assessment for insulin resistance (HOMA-IR) from baseline to 1 year. Concurrent changes in kynurenine, quinolinic acid, 3-hydroxyanthranilic acid, and kynurenine/tryptophan ratio were associated with baseline-to-1-year changes in HOMA-IR.
CONCLUSIONS:
Baseline tryptophan and 1-year increases in quinolinic acid were positively associated with incident T2D. Baseline and 1-year changes in tryptophan metabolites predicted changes in HOMA-IR. Tryptophan levels may initially increase and then deplete as diabetes progresses in severity.
Type 2 diabetes (T2D)17 remains a global health concern (1). Recent metabolomics screens have identified tryptophan metabolites as potential biological mediators in the development of T2D (2–5). The majority of free tryptophan in humans is metabolized through the tryptophan–kynurenine pathway (6), producing one equivalent of nicotinamide adenine dinucleotide for each equivalent of tryptophan. Intermediates in this pathway include kynurenine, kynurenic acid, quinolinic acid, and 3-hydroxyanthranilic acid. The conversion of tryptophan to kynurenine is catalyzed by the rate-limiting enzymes indoleamine 2, 3-dioxygenase 1 (IDO1), indoleamine 2, 3-dioxygenase 2 (IDO2), and tryptophan 2, 3-dioxygenase, which are upregulated in T2D patients (7, 8). IDO1 activity may be increased by proinflammatory cytokines, such as interferon; this increase shifts the equilibrium of this pathway downstream away from tryptophan (9, 10). Further downstream conversion of kynurenine to 3-hydroxyanthranilic acid is catalyzed by enzymes dependent on pyridoxal-5-phosphate (P5P) as a cofactor (11), which is deficient in diabetogenic states (12).
Studies of tryptophan metabolism and T2D in human populations are sparse. Cross-sectional studies have identified increased plasma concentrations of kynurenine, 5-hydroxyanthranilic acid, and kynurenic acid in diabetics and insulin-resistant individuals (4, 13) and lower concentrations of tryptophan in nondiabetics (4). The ratio of plasma kynurenine to tryptophan has also been reported to be directly correlated with insulin resistance (14) and T2D (15).These results suggest that tryptophan metabolites may play an important role in T2D pathogenesis.
The current study employs a case–cohort design of participants free of diabetes at baseline, nested within the Prevención con Dieta Mediterránea (PREDIMED) trial. In a previous report (16), we reported that 1-year changes in tryptophan strongly predicted lower risk of incident myocardial infarction and composite cardiovascular disease. Thus, we hypothesized that plasma tryptophan would be inversely associated with T2D. Our primary objectives were to address (a) whether baseline and 1-year changes in plasma tryptophan metabolites (tryptophan, kynurenine, kynurenic acid, quinolinic acid, 3-hydroxyanthranilic acid) were associated with T2D development; (b) whether baseline concentrations of these metabolites were associated with 1-year changes in homeostatic model assessment for insulin resistance (HOMA-IR); and (c) whether a Mediterranean Diet (MedDiet) intervention could offset the harmful consequences of an unfavorable metabolite profile.
Methods
STUDY DESIGN AND POPULATION
The PREDIMED is a primary prevention, large multicenter trial based in Spain (www.predimed.es). The methods of the PREDIMED trial are reported elsewhere (17). Briefly, 7447 participants who were free of cardiovascular disease but at high cardiovascular risk were allocated to 3 groups: (a) a MedDiet supplemented with extra virgin olive oil (MedDiet EVOO); (b) a MedDiet supplemented with mixed nuts (MedDiet nuts); or (c) a control diet in which participants were advised to reduce total fat intake. No calorie restriction or physical activity was advised. Participants were selected to the PREDIMED trial on the basis of either prevalent T2D or 3 or more major cardiovascular risk factors (smoking, hypertension, increased low-density lipoprotein cholesterol, decreased high-density lipoprotein cholesterol, overweight or obesity, or a family history of premature coronary heart disease).
Within the PREDIMED cohort, 3541 participants did not have T2D at baseline. Among nondiabetics at baseline, individuals allocated to the 2 MedDiet groups had a 30% significantly lower risk of T2D than those in the control group (18). The present case–cohort study sample consisted of a random subcohort of 694 nondiabetic participants selected at baseline (approximately 20%) from all T2D-free individuals of the PREDIMED cohort who had available plasma samples and all incident cases (n = 251) of T2D during a median follow-up period of 3.8 years with available samples (samples were unavailable for 22 out of the 273 incident T2D cases occurring in PREDIMED). Institutional Review Boards at each recruitment center approved the study protocol, and participants provided written informed consent.
COVARIATE ASSESSMENT
At baseline and yearly follow-up visits, a questionnaire about lifestyle variables (i.e., diet, physical activity, smoking), history of illnesses, medication use, and family history of disease was given. Physical activity was assessed with the validated Spanish version of the Minnesota Leisure-Time Physical Activity questionnaire (19). Participants were considered to have hypercholesterolemia or hypertension if a physician had previously diagnosed them with the condition and/or they were being treated with cholesterol-lowering or antihypertensive agents, respectively. Trained personnel ascertained anthropometric and blood pressure measurements.
BLOOD SPECIMENS AND METABOLITE PROFILING
Fasting blood samples were collected at baseline, 1 year, and biennial intervals thereafter. Plasma samples among diabetes-free patients at baseline (many of whom would develop incident diabetes later on) were collected to preserve the prospective nature of the study. After an overnight fast, plasma EDTA tubes were collected, and, after centrifugation, aliquots were frozen at 80°. In June 2015, pairs of samples (baseline and first-year visits from each participant) were randomly shipped on dry ice to the Broad Institute for metabolomics analyses.
Tryptophan–kynurenicne metabolites (tryptophan, kynurenine, kynurenic acid, quinolinic acid, 3-hydroxyanthranilic acid) were measured by LC-MS, as previously described (20–22). Kynurenine and quinolinic acid were measured using an LC-MS system that comprised an Aquity UPLC system (Waters and a 5500 QTRAP mass spectrometer, SCIEX). Metabolites were extracted from plasma (30 μL) with 120 μL of 80% methanol containing inosine-15N4, thymine-d4, and glycocholate-d4 internal standards (Cambridge Isotope Laboratories). The samples were centrifuged (10 min, 9000g, 4 °C), and the supernatants (10 μL) were injected directly onto a 150- × 2.0-mm Luna NH2 column (Phenomenex). The column was eluted at a flow rate of 400 μL/min, with initial conditions of 10% mobile phase A (20 mmol/L ammonium acetate and 20 mmol/L ammonium hydroxide in water) and 90% mobile phase B [10 mmol/L ammonium hydroxide in 75:25 (volume:volume) acetonitrile/methanol], followed by a10-minlinear gradient to 100% mobile phase A. Mass spectrometer analyses were carried out by electrospray ionization and selective multiple reaction monitoring scans in the negative ion mode. To create the method, declustering potentials and collision energies were optimized for each metabolite by infusion of reference standards (20). The ion spray voltage was 4.5 kV, and the source temperature was 500 °C. Data were processed and visually inspected for quality of peak integration with MultiQuant software (SCIEX). Tryptophan, kynurenic acid, and 3-hydroxyanthranilic acid were profiled with a Shimadzu Nexera ×2 U-HPLC (Shimadzu Corp.) coupled to a Q Exactive hybrid quadrupole orbitrap mass spectrometer (Thermo Fisher Scientific). Metabolites were extracted from plasma (10 μL) with 90 μL of 74.9: 24.9:0.2 (volume:volume:volume) of acetonitrile: methanol:formic acid–containing stable isotope– labeled internal standards [valine-d8 (Sigma-Aldrich) and phenylalanine-d8 (Cambridge Isotope Laboratories)]. The samples were centrifuged (10 min; 9000g; 4 °C), and supernatants (10 uL) were injected directly onto a 150-× 2-mm, 3-μm Atlantis HILIC column (Waters). The column was eluted isocratically at a flow rate of 250 μL/min with 5% mobile phase A (10 mmol ammonium formate/L and 0.1% formic acid in water) for 0.5 min, followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) over 10 min. Full scan mass spectra 70–800 m/z were acquired in the positive ion and at 70000 resolution and 3-Hz data acquisition rate. Other mass spectrometer settings were as follows: sheath gas, 40; sweep gas, 2; spray voltage, 3.5 kV; capillary temperature, 350 °C; S-lens, 40; heater temperature, 300 °C; microscans, 1; automatic gain control target, 1E6; and maximum ion time, 250 ms. Metabolite identities were confirmed with authentic reference standards. Raw data were processed by TraceFinder 3.1 (Thermo Fisher Scientific) and Progenesis QI (Nonlinear Dynamics).
To enable assessment of data quality and to facilitate data standardization across the analytical queue and sample batches, pairs of pooled plasma reference samples were analyzed at intervals of approximately 20 study samples. One sample from each pair of pooled references served as a passive QC sample to evaluate the analytical reproducibility for measurement of each metabolite, while the other pooled sample was used to standardize data by a “nearest neighbor” approach. Standardized values were calculated with the ratio of the value in each sample over the nearest pooled plasma reference multiplied by the median value measured across the pooled references. After standardization, the CVs measured among 124 pooled QC samples in the negative ion mode method were 10.2% for kynurenine and 17.0% for quinolinic acid. Among 92 pooled QC samples measured in the positive ion mode method, the CVs were 2.2% for tryptophan, 11.2% for kynurenic acid, and 39.4% for 3-hydroxyanthranilic acid.
Fasting glucose was measured with an enzymatic method to convert glucose to 6-phosphogluconate (Advia Chemistry Systems). The intra- and interassay CVs were 1.2% and 1.6%, respectively. Fasting insulin concentrations were measured with an immunoenzymometric assay (Advia Chemistry Systems) with an intra- and inter assay coefficient of variation equal to 3.7% and 4.4%, respectively. Insulin resistance was computed by use of the HOMA-IR method (HOMA-IR = fasting insulin × fasting glucose/405, in which insulin is in μU/mL and glucose is in mg/dL) at baseline and 1 year (23).
ASSESSMENT OF TYPE 2 DIABETES
The PREDIMED protocol originally included T2D as a secondary endpoint. The adjudication of new diagnoses ofincidentcasesofT2Dduringfollow-upwasperformed by the Clinical Endpoint Committee of the PREDIMED, an ad hoc panel of physicians (18). Diagnosis of T2D was considered on the basis of 2 confirmations of fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or 2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) after a 75-g oral glucose load (24). Only confirmed cases were included in the statistical analyses. The Clinical Endpoint Committee was blinded to the intervention group and identities of the patients.
STATISTICAL ANALYSIS
Individual metabolite values were first normalized by a rank-based inverse normal transformation (25). We also considered the ratio of plasma kynurenine to tryptophan (Kyn/Trp ratio) by dividing raw values for each participant and then inverse normalizing the ratio. Quartile cutoff points of each metabolite and Kyn/Trp ratio were generated on the basis of the distributions of metabolites among noncases. We calculated correlations between each inverse normal transformed metabolite under study with Spearman ρ. Baseline-to-1-year changes in plasma metabolite values according to intervention group were calculated by generalized estimating equations with adjustment for covariates and setting recruitment center as a random effect (by LSMEANS with PROC MIXED in SAS).
For the primary analyses, we fitted Cox regression models with the use of Barlow weights (i.e., inverse probability of selection weights) to account for the oversampling of cases and implemented a sandwich variance estimator to correct for correlated risk sets (26). We calculated hazard ratios and their 95% CI in relation to T2D for each metabolite specified as both categorical (quartiles) and continuous (per standard deviation) variables. Follow-up time was defined as the date of enrollment to the date of diagnosis of T2D for cases and to the date of the last visit or the end of the follow-up period for noncases (December 1, 2010). All models were stratified by recruitment center. In Model 1, we adjusted for age (years), sex (male or female), and intervention group (control, MedDiet + EVOO, MedDiet + nuts). In Model 2, we additionally adjusted for smoking status (never, current, or former), body mass index (kg/m2), leisure-time physical activity (metabolic equivalent task, min/day), hypertension (yes or no) dyslipidemia (yes or no), and baseline plasma glucose (with a quadratic term to account for nonlinearity). We conducted a test for linearity by examining an ordinal score based on the median value in each quartile of the metabolites. Two-sided P values were reported according to an α level = 0.05 after adjustment for 6 tests by the False Discovery Rate method (27).
We further examined the associations between 1-year changes in individual metabolites and Kyn/Trp ratio and risk of T2D. We first calculated the difference between baseline and 1-year values and then normalized this difference with the inverse normal transformation. For this analysis, we excluded individuals with <1 year of follow-up, T2D cases diagnosed during the first year of follow-up, or without available samples at 1 year. All change analyses were adjusted by the respective baseline metabolite values. We also tested potential effect modification by age (<65, ≥65 years), sex (male or female), intervention group (control, MedDiet combined for additional power), body mass index (<30, ≥30 kg/m2), smoking (never, former, or current), hypertension (yes or no), dyslipidemia (yes or no) on the relationship between baseline tryptophan and T2D.Likelihood ratio tests were used to assess the significance of interactions between the stratifying variables and baseline tryptophan by use of the difference in number of variables as the appropriate degrees of freedom to report the P for interaction (Pint). Finally, we examined the association between baseline metabolites and 1-year changes of HOMA-IR by using generalized linear models accounting for within recruitment center clustering and adjusting for covariates. Results were reported as the least squares mean differences (MD) (LSMEANS statement in SAS). All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc.).
Results
The final study sample consisted of a random subcohort of 694 nondiabetic participants and 251 incident cases of T2D occurring through a median of 3.8 years of intervention with available samples. Of the 892 unique participants, 641 were included in the subcohort; 53 of the subcohort developed T2D and 198 were incident T2D cases not in the subcohort, for a total of 251 cases. In addition, 663 participants (505 noncases and 157 cases) had samples at 1 year and were included in the 1-year change analyses. Baseline descriptive characteristics of the subcohort according to quartiles of plasma tryptophan are shown in Table 1. Higher concentrations of baseline plasma tryptophan were associated with younger age, male sex, lower prevalence of dyslipidemia, and lower proportion of never-smokers.
Table 1.
Participant’s baseline characteristics of the subcohort (n = 694).a
| Baseline plasma tryptophan |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P valueb | |
| N | 167 | 172 | 174 | 181 | |
| Age, years | 67.5 ± 5.4 | 66.8 ± 6.1 | 66.2 ± 5.6 | 65.6 ± 5.6 | 0.01 |
| Sex, % women | 80.2 | 65.7 | 58.6 | 48.1 | <0.01 |
| Intervention group, % | |||||
| MedDiet + EVOO | 34.1 | 30.8 | 28.7 | 29.3 | 0.08 |
| MedDiet + nuts | 31.7 | 36.1 | 38.5 | 42.0 | |
| Control | 34.1 | 33.1 | 32.8 | 28.7 | |
| Hypertension, % | 86.8 | 93.6 | 91.4 | 91.2 | 0.29 |
| Dyslipidemia, % | 88.0 | 90.7 | 83.3 | 78.5 | <0.01 |
| Smoking, % | |||||
| Never | 70.7 | 59.3 | 55.8 | 58.6 | 0.02 |
| Former | 16.2 | 26.2 | 20.7 | 27.1 | |
| Current | 13.2 | 14.5 | 23.6 | 14.4 | |
| Waist circumference, cm | 99.2 ± 10.5 | 98.9 ± 9.9 | 99.3 ± 11.4 | 100.8 ± 11.0 | 0.35 |
| Leisure-time physical activity (MET), min/d | 198 ± 189 | 250 ± 21 | 263 ± 275 | 240 ± 242 | 0.06 |
| Body mass index, kg/m2 | 30.0 ± 3.6 | 30.0 ± 3.4 | 29.7 ± 3.5 | 30.1 ± 3.8 | 0.61 |
| Total energy intake, kcal/d | 2249 ± 522 | 2270 ± 586 | 2273 ± 540 | 2314 ± 608 | 0.74 |
| MedDietscore (0 to 14 score)c | 8.6 ± 2.0 | 8.5 ± 1.9 | 8.6 ± 2.1 | 8.7 ± 1.9 | 0.68 |
| Baseline glucose, mg/dl | 101.7 ± 15.4 | 100.8 ± 16.0 | 99.1 ± 14.5 | 97.6 ± 15.8 | 0.07 |
Continuous variables ± standard deviation.
Mantel-Haenszel chi-squared P value for categorical variables and ANOVA P values for continuous variables.
MedDiet score was calculated by responses on a 14-item questionnaire. Higher score signifies greater adherence to a Mediterranean diet pattern.
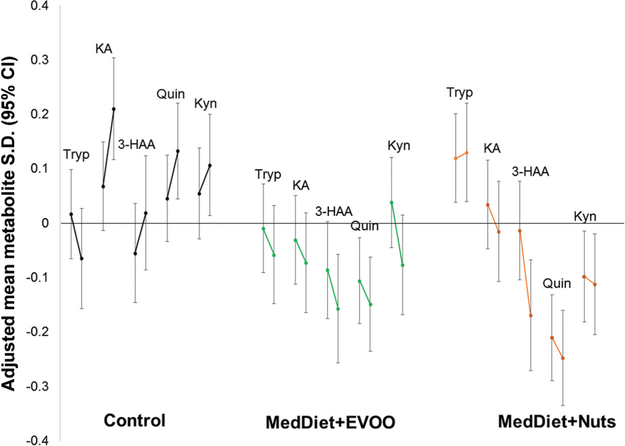
Fig. 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol64/issue8 depicts the correlation matrix of the metabolites under study at baseline. Quinolinic acid was correlated with kynurenine (r = 0.58), while quinolinic acid was not correlated with tryptophan (r = −0.04). In general, all other correlations were moderate (Spearman ρ ranging from 0.10 to 0.33) and significant (P < 0.05). Fig. 1 shows adjusted mean 1-year changes in the metabolites under study by intervention group. Individuals allocated to each of the 2 active intervention arms largely experienced decreases in these plasma metabolites, whereas individuals allocated to the control arm experienced increases in all these plasma metabolites except tryptophan.
Fig. 1.
Least mean squares 1-year changes in plasma metabolites under study according to intervention group.Stratified by recruitment center. Adjusted for age (years), sex (male, female), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia, hypertension, and baseline glucose. KA, kynurenic acid; Quin, quinolinic acid
BASELINE AND 1-YEAR CHANGES IN TRYPTOPHAN METABOLITES AND TYPE 2 DIABETES
Table 2 displays results for the analysis of baseline tryptophan metabolites with incident T2D. In fully adjusted models, baseline tryptophan was prospectively associated with a slightly higher risk of T2D (P trend = 0.045). Associations of changes in metabolites from baseline to 1 year with T2D incidence are shown in Table 3. After adjusting for potential confounders and multiple testing, continuous 1-year changes in quinolinic acid (hazard ratio per SD = 1.39; 95% CI, 1.09–1.77; P 0.047) showed a positive association with T2D incidence.
Table 2.
Hazard ratios (95% CI) for type 2 diabetes by baseline tryptophan metabolites.a
| 251 cases, 694 subcohortb |
|||||||
|---|---|---|---|---|---|---|---|
| Quartiles of baseline metabolites |
|||||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trendc | HR per SD | Pc | |
| Model 1 | |||||||
| Tryptophan | 1.00 (ref) | 1.28(0.80–2.05) | 1.68(1.02–2.75) | 1.18(0.67–2.08) | 0.56 | 1.04 (0.86–1.26) | 0.69 |
| Kynurenine | 1.00 (ref) | 1.07(0.64–1.76) | 1.13(0.70–1.82) | 0.80 (0.48–1.34) | 0.56 | 0.94 (0.79–1.11) | 0.56 |
| Kynurenic acid | 1.00 (ref) | 0.79 (0.48–1.32) | 1.17(0.75–1.84) | 1.21 (0.76–1.92) | 0.56 | 1.10(0.92–1.33) | 0.56 |
| Quinolinic acid | 1.00 (ref) | 0.92 (0.56–1.49) | 0.72 (0.44–1.18) | 0.94 (0.58–1.51) | 0.57 | 0.92 (0.78–1.08) | 0.56 |
| 3−HAA | 1.00 (ref) | 0.93 (0.58–1.49) | 0.80 (0.49–1.30) | 0.69 (0.41–1.14) | 0.56 | 0.91 (0.75–1.10) | 0.56 |
| Kyn/Trp ratio | 1.00 (ref) | 1.23(0.76–1.98) | 1.10(0.66–1.82) | 0.82 (0.49–1.37) | 0.56 | 0.93(0.79–1.10) | 0.56 |
| Model 2 | |||||||
| Tryptophan | 1.00 (ref) | 1.97(1.10–3.52) | 3.26(1.75–6.09) | 2.13(1.12–4.05) | 0.045 | 1.29(1.04–1.61) | 0.12 |
| Kynurenine | 1.00 (ref) | 1.58 (0.87–2.87) | 1.16(0.64–2.08) | 0.90 (0.48–1.69) | 0.71 | 0.99(0.82–1.20) | 0.98 |
| Kynurenic acid | 1.00 (ref) | 0.88 (0.49–1.57) | 0.99 (0.56–1.75) | 1.13(0.65–1.94) | 0.71 | 1.07(0.87–1.31) | 0.98 |
| Quinolinic acid | 1.00 (ref) | 1.12 (0.64–1.95) | 0.85 (0.47–1.54) | 1.02 (0.58–1.79) | 0.80 | 0.97(0.80–1.17) | 0.98 |
| 3−HAA | 1.00 (ref) | 0.91 (0.49–1.67) | 1.07(0.61–1.89) | 0.76 (0.40–1.44) | 0.71 | 1.00(0.80–1.24) | 0.98 |
| Kyn/Trp ratio | 1.00 (ref) | 1.49 (0.85–2.61) | 0.92 (0.49–1.74) | 0.62 (0.33–1.17) | 0.21 | 0.85(0.70–1.03) | 0.50 |
All models stratified by recruitment center. Model 1 adjusted for age (years), sex (male, female), intervention group (control, MedDiet + EVOO, MedDiet + nuts), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalenttasks in minutes/day), dyslipidemia, and hypertension. Model 2 additionally adjusts for baseline glucose and baseline glucose χ baseline glucose.
Fifty-three cases were also in the subcohort (overlapping cases).
Adjusted with the False Discovery Rate method.
Table 3.
Hazard ratios (95% CI) for type 2 diabetes by 1-year changes in tryptophan metabolites.a
| 180 cases 546 subcohortb |
|||||||
|---|---|---|---|---|---|---|---|
| Quartiles of 1-year changes in metabolites |
|||||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trendc | HR per SD | Pc | |
| Model 1 | |||||||
| Tryptophan | 1.00 (ref) | 1.24 (0.68–2.28) | 0.83 (0.43–1.60) | 0.94 (0.48–1.83) | 0.85 | 0.97(0.74–1.26) | 0.96 |
| Kynurenine | 1.00 (ref) | 0.95 (0.52–1.74) | 0.65 (0.33–1.25) | 1.03 (0.58–1.86) | 0.85 | 1.03 (0.83–1.29) | 0.96 |
| Kynurenic acid | 1.00 (ref) | 0.80 (0.44–1.46) | 0.48 (0.25–0.93) | 0.57(0.32–1.03) | 0.12 | 0.81 (0.64–1.03) | 0.24 |
| Quinolinic acid | 1.00 (ref) | 1.68 (0.87–3.23) | 2.17(1.13–4.16) | 1.74 (0.94–3.24) | 0.18 | 1.26(1.02–1.55) | 0.18 |
| 3−HAA | 1.00 (ref) | 0.85 (0.48–1.50) | 0.88 (0.51–1.52) | 1.05 (0.60–1.85) | 0.85 | 1.03 (0.84–1.26) | 0.96 |
| Kyn/Trp ratio | 1.00 (ref) | 1.05 (0.56–1.95) | 0.82 (0.43–1.56) | 1.00 (0.55–1.81) | 0.85 | 1.00 (0.80–1.25) | 0.99 |
| Model 2 | |||||||
| Tryptophan | 1.00 (ref) | 1.03 (0.51–2.09) | 0.95 (0.44–2.02) | 0.64 (0.28–1.44) | 0.56 | 0.92 (0.66–1.26) | 0.88 |
| Kynurenine | 1.00 (ref) | 0.79 (0.38–1.63) | 0.57(0.26–1.24) | 0.89 (0.44–1.81) | 0.73 | 1.05 (0.79–1.39) | 0.88 |
| Kynurenic acid | 1.00 (ref) | 1.15(0.59–2.24) | 0.57(0.25–1.31) | 0.67(0.35–1.29) | 0.27 | 0.85 (0.66–1.10) | 0.63 |
| Quinolinic acid | 1.00 (ref) | 2.47(1.06–5.78) | 2.85(1.27–6.40) | 2.33(1.09–4.96) | 0.18 | 1.39(1.09–1.77) | 0.047 |
| 3−HAA | 1.00 (ref) | 0.87(0.45–1.65) | 0.91 (0.48–1.72) | 0.97(0.47–2.01) | 0.97 | 1.02 (0.79–1.33) | 0.88 |
| Kyn/Trp ratio | 1.00 (ref) | 0.94 (0.46–1.95) | 0.66 (0.31–1.41) | 0.92 (0.45–1.88) | 0.73 | 0.97(0.73–1.29) | 0.88 |
All models stratified by recruitment center. Model 1 adjusted for respective baseline metabolite values (continuous), age (years), sex (male, female), intervention group (control, MedDiet + EVOO, MedDiet + nuts), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalenttasks in minutes/day), dyslipidemia, and hypertension. Model 2 additionally adjusts for baseline glucose and baselineglucose χ baseline glucose.
Forty-one cases were also in the subcohort.
Adjusted with the False Discovery Rate method.
Table 4 shows the associations between baseline metabolitelevelsand1-yearchangesinHOMA-IR.Baseline tryptophan (MD per SD = 0.67; 95% CI, 0.46–0.88) and kynurenic acid (MD per SD = 0.35; 95% CI, 0.23– 0.47) were directly associated with changes in HOMA-IR in fully adjusted models. The baseline Kyn/Trp ratio was inversely associated with HOMA-IR (MD per SD = −0.18; 95% CI, −0.34 to 0.03). Table 5 shows the associations between 1-year changes in metabolites and 1-year changes in HOMA-IR. In fully adjusted models, positive changes in kynurenine (MD per SD = 0.39, 95% CI = 0.17–0.60), quinolinic acid (MD per SD = 0.49; 95% CI, 0.18–0.80), 3-HAA (MD per SD = 0.23; 95% CI, 0.17–0.30), and Kyn/Trp ratio (MD per SD = 0.23; 95% CI, 0.16–0.31) were associated with increases in HOMA-IR by year 1.
Table 4.
Least squares mean differences and associations of 1-year changes in HOMA-IR with baseline metabolite values (n = 217).a
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trendb | Change per SD | Pb | |
|---|---|---|---|---|---|---|---|
| Tryptophan | −0.89 (−1.26, −0.51) | −0.33 (−1.01, 0.35) | 0.42 (0.11,0.73) | 0.53 (0.32,0.75) | 0.24 | 0.67(0.46, 0.88) | <0.001 |
| Kynurenine | −0.31 (−0.90, 0.29) | −0.21 (−1.23, 0.80) | 0.32 (−0.21, 0.84) | 0.20 (−0.45, 0.86) | 0.32 | 0.13 (−0.18, 0.45) | 0.55 |
| Kynurenic acid | −0.53 (−1.28, 0.23) | −0.06 (−0.49, 0.38) | −0.15 (−0.42, 0.13) | 0.65 (−0.11, 1.41) | 0.24 | 0.35 (0.23, 0.47) | <0.001 |
| Quinolinic add | −0.24 (−0.97, 0.49) | 0.36 (−0.56, 1.28) | −0.11 (−0.70, 0.48) | −0.12 (−0.89, 0.64) | 0.96 | −0.01 (−0.37, 0.36) | 0.98 |
| 3-HAA | −0.43 (−1.29, 0.43) | 0.02 (−0.42, 0.46) | 0.53 (0.01, 1.06) | −0.05 (−0.36, 0.26) | 0.24 | 0.08 (−0.14, 0.30) | 0.55 |
| Kyn/Trp ratio | 0.31 (−0.18, 0.80) | −0.17 (−0.52, 0.19) | −0.16 (−0.75, 0.43) | −0.08 (−0.67, 0.51) | 0.53 | −0.18 (−0.34, −0.03) | 0.04 |
All models stratified by recruitment center and adjusted for age (years), sex (male, female), intervention group (control, MedDiet+EVOO, MedDiet+nuts), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia, hypertension, baseline HOMA-IR, and baseline HOMA-IR × baseline HOMA-IR.
Adjusted with the False Discovery Rate method.
Table 5.
Least squares mean differences and associations of 1-year changes in HOMA−IR with 1-year changes in metabolite values (n = 217).
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trendb | Change per SD | Pb | |
|---|---|---|---|---|---|---|---|
| Tryptophan | 0.32 (−0.55, 1.19) | 0.11 (−0.46, 0.69) | −0.08 (−0.56, 0.40) | −0.21 (−0.61,0.19) | 0.43 | −0.15 (−0.57, 0.27) | 0.49 |
| Kynurenine | −0.59 (−0.86, −0.32) | 0.25 (−0.87, 1.37) | −0.07 (−0.67, 0.52) | 0.54 (−0.06, 1.14) | 0.23 | 0.39 (0.17, 0.60) | <0.001 |
| Kynurenic acid | −0.62 (−1.06, −0.17) | 0.45 (−0.21, 1.11) | 0.18 (−0.26, 0.63) | −0.09 (−0.45, 0.27) | 0.23 | 0.31 (−0.07, 0.69) | 0.13 |
| Quinolinic acid | −0.41 (−0.61, −0.21) | −0.12 (−0.49, 0.25) | −0.15 (−0.86, 0.56) | 0.71 (−0.02, 1.45) | 0.24 | 0.49 (0.18, 0.80) | 0.003 |
| 3−HAA | −0.18 (−0.50, 0.15) | 0.13 (−0.85, 1.10) | 0.19 (−0.70, 1.08) | 0.16 (−0.22, 0.54) | 0.23 | 0.23(0.17, 0.30) | <0.001 |
| Kyn/Trp ratio | −0.33 (−0.55, −0.11) | 0.14 (−1.07, 1.35) | −0.14 (−0.58, 0.29) | 0.32 (−0.10, 0.73) | 0.23 | 0.23(0.16, 0.31) | <0.001 |
All models stratified by recruitment center and adjusted for respective baseline metabolite values (continuous), age (years), sex (male, female), intervention group (control, MedDiet + EVOO, MedDiet + nuts), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia, hypertension, baseline HOMA-IR, and baseline HOMA-IR × baseline HOMA-IR.
Adjusted with the False Discovery Rate method.
EFFECT MODIFICATION BY DIETARY INTERVENTION AND OTHER VARIABLES
We tested whether a Mediterranean dietary intervention could potentially offset the positive associations of tryptophan with T2D (see Table 1 in the online Data Supplement). Using baseline tryptophan as the primary exposure, we observed no significant effect modification by dietary intervention, sex, age, obesity status, smoking status, baseline hypertension, or baseline dyslipidemia (see Table 2 in the online Data Supplement).
Discussion
In the present case–cohort study, we report that baseline tryptophan and 1-year increase in quinolinic acid were prospectively associated with higher T2D risk. Baseline tryptophan and kynurenic acid additionally predicted significant increases in HOMA-IR after 1 year, while concurrent positive changes in kynurenine, quinolinic acid, 3-HAA, and Kyn/Trp ratio predicted increases in HOMA-IR. Furthermore, we report no significant effect modification by intervention group on the relationship between baseline tryptophan and T2D. Interestingly, in both active intervention groups, but not in the control group, with Mediterranean-type diets we found reduced levels of most metabolites.
Our study adds to the growing body of literature on the tryptophan–kynurenine pathway in T2D pathophysiology. Previous metabolite profiling studies with T2D exist, but to our knowledge, the present study is the first to prospectively examine metabolites in the tryptophan–kynurenine pathway with T2D in the context of a dietary trial. Because tryptophan cannot be synthesized in humans, dietary tryptophan intake is the primary determinant of tryptophan availability (28). Wang et al. previously reported in a large prospective study of 2422 normoglycemic individuals that baseline plasma tryptophan concentrations were positively associated with later incident T2D (5). In a cross-sectional analysis of 213 participants, Chen et al. similarly reported that serum tryptophan concentrations were significantly increased in diabetics compared with nondiabetic controls (2). In another case–control study of 40 participants, tryptophan was found to be metabolized more quickly, and concentrations of kynurenic acid and 3-HAA were increased in T2D patients (4). While we confirm findings by Wang et al. and Chen et al. for increased tryptophan levels in T2D patients, we did not find significant associations for any other baseline metabolite with incident T2D. Differences between results from previous studies and our study may be explained by both the study design (i.e., cross-sectional vs prospective) and subsequent definition of case status (i.e., prevalent vs incident cases), wherein longitudinal studies tended to report positive associations of tryptophan with T2D whereas cross-sectional studies tended to report null or inverse associations. The positive association between tryptophan and T2D became highly significant after adjusting for baseline glucose, possibly owing to mediating effect of glycemic level. Our initial hypothesis that plasma tryptophan concentrations would be inversely associated with T2D (as we had previously observed with cardiovascular disease (16)) was not supported when using baseline data alone. However, analyses using 1-year changes suggested a more dynamic picture: there may be initial compensatory increases in tryptophan at diabetes onset and then later depletion as diabetes progresses in severity.
Current knowledge of the role of the tryptophan– kynurenine pathway in T2D pathophysiology is unsettled. IDO1, a rate-limiting enzyme for the conversion of tryptophan to kynurenine, is upregulated in response to inflammatory cytokines (29) and may act as a local compensatory mechanism to limit obesity-induced inflammation (30). Thus, in inflammatory states such as obesity, the tryptophan–kynurenine pathway may shift in favor of breakdown of tryptophan into downstream products (31, 32). This hypothesis is supported by our finding that the baseline Kyn/Trp ratio was positively associated with insulin resistance but that 1-year changes in the same ratio was inversely associated with insulin resistance. The resulting increases in downstream products (i.e., kynurenine, xanthurenic acid, 3-hydroxykynurenine) in insulin-resistant individuals have been reported in previous publications (33–35). Overproduction of these products in the context of an inflammatory milieu may shift the metabolism of 3-hydroxykynurenine in favor of excessive xanthurenic acid production, which has been implicated in diabetes pathology (36). P5P, the active form of vitamin B6, is a vital cofactor in this pathway, and disturbances in P5P balance in conjunction with upregulation of tryptophan metabolism have been proposed as a contributor to insulin resistance (9). Dysregulation of the tryptophan–kynurenine pathway has also been proposed as a possible explanation for the observed comorbidity of depression and T2D (37, 38), as alterations in this pathway may affect both serotonin balance and inflammatory pathways (38, 39). Our results suggest that tryptophan levels may initially be increased during prediabetes and then depleted in the transition into a full diabetic state. Furthermore, we found consistent results for both baseline quinolinic acid predicting T2D and changes in quinolinic acid predicting concurrent increases in HOMA-IR. In the Framingham Heart Study, kynurenine and quinolinic acid were both found to predict higher HOMA-IR (34). This may be explained by the interferon –induced activation of IDO1 (evidence by our observed association of HOMA-IR with Kyn/Trp ratio, which is an indicator of IDO1 or tryptophan 2, 3-dioxygenase 2 activity (40, 41)), which leads to increased production of toxic quinolinic acid (42).
We did not find significant effect modification by the assigned dietary treatment group on the association between baseline tryptophan and subsequent T2D. Richard et al. indicate that dietary modification for up to 10 days can lead to 15% to 20% changes in levels of plasma tryptophan (28). In a previous study of tryptophan metabolites and cardiovascular disease, we reported effect modification by diet, with a Mediterranean dietary pattern appearing to offset the effects of an unfavorable tryptophan profile (16). One possible explanation is that nuts are a rich source of vitamin B6 (43), which may compensate for potential P5P depletion. In both the US and Spain, around 24% of individuals had insufficient plasma vitamin B6 concentrations (44, 45). Thus, we continue to support a Mediterranean dietary pattern characterized by high intake of fruits, vegetables, whole grains, healthy oils, nuts, and legumes. Lastly, our finding that the intervention arms tended to decrease tryptophan metabolite concentrations while the control arm increased concentrations suggests that changes in these metabolites may partially explain the protective effect of the interventions on T2D.
Our study has several strengths and limitations. We used an efficient case - cohort design to study a clinical endpoint and its association with multiple plasma metabolites with repeated measures quantified by a validated LC-MS platform. Sampling at baseline preserved the prospective nature of the study and reduced the possibility of differential misclassification. The study power may be limited, as we did not assay blood specimens for the entire PREDIMED cohort, although this would be cost prohibitive. Collection of data on various lifestyle factors allowed us to adjust for potential confounders, but the possibility of residual or unmeasured confounding cannot be discounted and weakens our ability to draw causal conclusions. Because our study sample consisted mostly of white participants in the Mediterranean region, there is likely minimal confounding by ethnicity or geographical region. At the same time, our findings may not be generalizable to other populations with different characteristics. Finally, we could not study several metabolites of interest, such as hydroxykynurenine or xanthurenic acid, as we did not target these molecules a priori.
We conclude from the present prospective study that among a population of individuals at high risk of cardiovascular disease, baseline tryptophan and 1-year changes in quinolinic acid were both positively associated with greater risk of incident T2D. Furthermore, baseline tryptophan and kynurenic acid and positive 1-year changes in kynurenine, quinolinic acid, 3-HAA, and Kyn/Trp ratio were associated with greater 1-year changes in HOMA-IR. Tryptophan may initially increase in early diabetes onset and then deplete as diabetes severity progresses.
Acknowledgment:
The authors thank the contributions of Monica Bullo, Amy Diek, Dong Wang, and Yan Zheng, who provided invaluable assistance throughout the acquisition of data, analysis of data, and preparation of the manuscript.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or final approval of manuscript.
Research Funding: This study was funded by the National Institutes of Health (R01DK102896, F31DK114938), the Spanish Ministry of Health (Instituto de Salud Carlos III, The PREDIMED Network grant RD 06/0045, 2006–2013, coordinated by M.A. Martínez-González; and a previous network grant RTIC-G03/140, 2003–2005, coordinated R. Estruch). Additional grants were received from the Ministerio de Economía y Competitividad-Fondo Europeo de Desarrollo Regional (Projects CNIC-06/2007, CIBER 06/03, PI06–1326, PI070954, PI11/02505, SAF2009–12304 and AGL2010–22319-C03–03) and by the Generalitat Valenciana (ACOMP2010–181, AP111/10, AP-042/11, ACOM2011/145, ACOMP/2012/190, ACOMP/2013/ 159 and ACOMP/213/165). C. Papandreou, postdoctoral fellowship granted by the Autonomous Government of Catalonia (PERIS 2016– 2020 Incorporació de Científics I Tecnòlegs, SLT002/0016/00428); M. Guasch-Ferré, fellowship granted by the Private Foundation Daniel Bravo (Spain); E. Ros, the California Walnut Commission; J. SalasSalvadó, grants from the International Nut and Dried Fruit Foundation.
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: J. Salas-Salvadó, the International Nut and Dried Fruit Foundation; E. Ros, Alexion, the California Walnut Commission.
Stock Ownership: None declared.
Honoraria: E. Ros, Alexion, California Walnut Commission, Merck, Sanofi.
Expert Testimony: None declared.
Patents: None declared.
Nonstandard abbreviations: T2D, type 2 diabetes; IDO, indoleamine 2,3-dioxygenase; P5P, pyridoxal-5-phosphate; PREDIMED, Prevención con Dieta Mediterrànea; HOMAIR, homeostatic model assessment of insulin resistance; MedDiet, Mediterranean Diet; EVOO, extra virgin olive oil; Kyn, kynurenine; Trp, tryptophan; MD, mean difference; 3-HAA, 3-hydroxyanthranilic acid.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–49. [DOI] [PubMed] [Google Scholar]
- 2.Chen T, Zheng X, Ma X, Bao Y, Ni Y, Hu C, et al. Tryptophan predicts the risk for future type 2 diabetes. PLoS One 2016;11:e0162192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost H-G, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuoka K, Kato K, Takao T, Ogawa M, Ishii Y, Shimizu F, et al. Concentrations of various tryptophan metabolites are higher in patients with diabetes mellitus than in healthy aged male adults. Diabetol Int 2017;8:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarcz R The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 2004;4: 12–7. [DOI] [PubMed] [Google Scholar]
- 7.Oxenkrug G, van der Hart M, Summergrad P. Elevated anthranilic acid plasma concentrations in type 1 but not type 2 diabetes mellitus. Integr Mol Med 2015;2: 365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartoli M, Lamoke F, Baban B. Preventing hyperglycemia and tissue injury in diabetes: the dynamic role of 2,3 indoleamine dioxygenase (ido) in diabetes and its complications. In: Mozaffari MS, editor. New strategies to advance pre/diabetes care: integrative approach by PPPM. Vol. 3 Dordrecht (Netherlands): Springer; 2013. p. 265–82. [Google Scholar]
- 9.Oxenkrug G Insulin resistance and dysregulation of tryptophan–kynurenine and kynurenine–nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol 2013;48:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol 2015;52:805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxenkrug G, Ratner R, Summergrad P. Kynurenines and vitamin B6: link between diabetes and depression. J Bioinform Diabetes 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxenkrug G 3-hydroxykynurenic acid and type 2 diabetes: Implications for aging, obesity, depression, Parkinson’s disease, and schizophrenia In: Engin A, Engin AB, editors. Tryptophan metabolism: implications for biological processes, health and disease. Cham (Switzerland): Humana Press; 2015. p. 173–95. [Google Scholar]
- 13.Muzik O, Burghardt P, Yi Z, Kumar A, Seyoum B. Successful metformin treatment of insulin resistance is associated with down-regulation of the kynurenine pathway. Biochem Biophys Res Commun 2017;488: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxenkrug GF, Turski WA, Zgrajka W, Weinstock JV, Summergrad P. Tryptophan-kynurenine metabolism and insulin resistance in hepatitis C patients. Hepat Res Treat 2013;2013:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebnord EW, Strand E, Midttun Ø, Svingen GFT, Christensen MHE, Ueland PM, et al. The kynurenine: tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia 2017;60:1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu E, Ruiz-Canela M, Guasch-Ferre M, Zheng Y, Toledo E, Clish CB, et al. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevención con Dieta Mediterránea (PREDIMED) study. J Nutr 2017;147:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zazpe I, Sanchez-Tainta A, Estruch R, Lamuela-Raventos RM, Schroder H, Salas-Salvado J, et al. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: the PREDIMED study. J Am Diet Assoc 2008;108:1134–44, discussion 45. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Salvado J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014; 160:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Richardson MT, Leon AS, Jacobs DR Jr., Ainsworth BE, Serfass R. Comprehensive evaluation of the Minnesota leisure time physical activity questionnaire. J Clin Epidemiol 1994;47:271–81. [DOI] [PubMed] [Google Scholar]
- 20.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013;59:1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest 2017;127:4394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, et al. Metabolic predictors of incident coronary heart disease in women. Circulation 2018;137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP,Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment:insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom G Statistical estimates and transformed betavariables. New York (NY): Wiley; 1958. [Google Scholar]
- 26.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;289–300. [Google Scholar]
- 28.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res 2009;2:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated psychiatric and medical disorders. J Neural Transm (Vienna) 2011;118:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolowczuk I, Hennart B, Leloire A, Bessede A, Soichot M, Taront S, et al. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol 2012;303:R135–R143. [DOI] [PubMed] [Google Scholar]
- 31.Brandacher G, Hoeller E, Fuchs D, Weiss HG. Chronic immune activation underlies morbid obesity: is ido a key player? Curr Drug Metab 2007;8:289–95. [DOI] [PubMed] [Google Scholar]
- 32.Unluturk U, Erbas T. Diabetes and tryptophan metabolism In: Engin A, Engin AB, editors. Tryptophan metabolism: implications for biological processes, health and disease. Cham (Switzerland): Humana Press; 2015. p. 147–72. [Google Scholar]
- 33.Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015;23: 2066–74. [DOI] [PubMed] [Google Scholar]
- 34.Ho JE, Larson MG, Ghorbani A, Cheng S, Chen M-H, Keyes M, et al. Metabolomic profiles of body mass index in the Framingham Heart Study reveal distinct cardiometabolic phenotypes. PLoS One 2016;11: e0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reginaldo C, Jacques P, Scott T, Oxenkrug G, Selhub J, Paul L. Xanthurenic acid is associated with higher insulin resistance and higher odds of diabetes. FASEB J 2015;29. [Google Scholar]
- 36.Oxenkrug GF. Role of kynurenine pathway in insulin resistance: toward kynurenine hypothesis of insulin resistance and diabetes In: Mittal S, editor. Targeting the broadly pathogenic kynurenine pathway. Cham (Switzerland): Springer; 2015;169–78. [Google Scholar]
- 37.Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol 2015;3:461–71. [DOI] [PubMed] [Google Scholar]
- 38.Stuart MJ, Baune BT. Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci Biobehav Rev 2012;36:658–76. [DOI] [PubMed] [Google Scholar]
- 39.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan—kynurenine metabolism. Ann NY Acad Sci 2010; 1199:1–14. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci 1992;29:307–41. [DOI] [PubMed] [Google Scholar]
- 41.Badawy AAB. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res 2017;10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutinho LG, Christen S, Bellac CL, Fontes FL, de Souza FRS, Grandgirard D, et al. The kynurenine pathway is involved in bacterial meningitis. J Neuroinflammation 2014;11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dreher ML, Maher CV, Kearney P. The traditional and emergingroleofnutsinhealthfuldiets.NutrRev 1996; 54:241–5. [DOI] [PubMed] [Google Scholar]
- 44.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5`-phosphate in the US population: the national health and nutrition examination survey, 2003– 2004. Am J Clin Nutr 2008;87:1446–54. [DOI] [PubMed] [Google Scholar]
- 45.Planells E, Sanchez C, Montellano MA, Mataix J, Llopis J. Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur J Clin Nutr 2003;57: 777–85. [DOI] [PubMed] [Google Scholar]